Abstract
Background:
Kidney disease is a serious problem that adversely affects human health, but critical knowledge is lacking on how to effectively treat established chronic kidney disease. Mounting evidence from animal and clinical studies has suggested that Vitamin D Re-ceptor (VDR) activation has beneficial effects on various renal diseases.
Methods:
A structured search of published research literature regarding VDR structure and function, VDR in various renal diseases (e.g., IgA nephropathy, idiopathic nephrotic syndrome, renal cell carcinoma, diabetic nephropathy, lupus nephritis) and therapies targeting VDR was performed for several databases.
Result:
Included in this study are the results from 177 published research articles. Evidence from these papers indicates that VDR activation is involved in the protection against renal inju-ry in kidney diseases by a variety of mechanisms, including suppression of RAS activation, an-ti-inflammation, inhibiting renal fibrogenesis, restoring mitochondrial function, suppression of autoimmunity and renal cell apoptosis.
Conclusion:
VDR offers an attractive druggable target for renal diseases. Increasing our under-standing of VDR in the kidney is a fertile area of research and may provide effective weapons in the fight against kidney diseases
Keywords: Vitamin D receptor, renal injury, renal tubular epithelial cell, chronic kidney disease, renal osteodystrophy, Acute kidney injury
1. INTRODUCTION
Since the discovery of vitamin D in 1920, the vitamin D endocrine system has achieved prominence as a central regulator to control bone and calcium homeostasis. In addition, connections between vitamin D and a broad array of pathological processes (e.g., inflammation, immunity, apoptosis, autophagy) have been reported in a large number of studies [1, 2]. The biological effect of vitamin D is mediated by the vitamin D receptor (VDR). VDR expression is found in most tissues, including those participating in the classic actions of vitamin D, such as the bone, gut and kidney, and other non-classic tissues [3, 4]. Mounting evidence from animal and clinical studies suggests that vitamin D is involved in various renal diseases (e.g., acute kidney injury and diabetic nephropathy) [5, 6]. The therapeutic application of vitamin D includes treatment of renal osteodystrophy and diabetic nephropathy and prevention of graft rejection [7, 8]. The purpose of this review is to summarize the role of VDR in kidney diseases and discuss the function of VDR as a therapeutic target in the treatment of kidney diseases.
2. STRUCTURE AND FUNCTION OF VDR
The VDR is a single-chain polypeptide of approximately 50,000 Da [3]. VDR is present in over 30 classic tissues (e.g., intestine, kidney, cartilage, bone) and non-classical tissues (e.g., activated B and T lymphocytes). To date, VDR cDNAs have been cloned from various species, including human, rat, mouse, chicken, Japanese quail, and frog, and these cDNA sequences share a significant similarity [9]. The structure of VDR consists of an N-terminal domain, a conserved DNA-binding domain, a flexible hinge region and a conserved ligand-binding domain [3].
The VDR is a ligand-activated transcription factor that belongs to the nuclear receptor superfamily. 1,25-Dihydroxyvitamin D (1,25(OH)2D3), the hormonal metabolite of vitamin D, is the natural ligand of the VDR. After binding to 1,25(OH)2D3, VDR enters the nucleus and form a heterodimer with retinoid X receptor (RXR), which interacts with response elements in target gene promoters to regulate gene transcription [3]. It is believed that 1,25(OH)2D3 can exert both genomic and rapid non-genomic actions by interacting with VDR. The rapid non-genomic activities are mediated by cell membrane-associated VDR that is activated by 1,25(OH)2D3 with different confirmations, which results in opening of the voltage-gated calcium channels or activation of second messengers (e.g., protein kinase C) [10].
The VDR gene is located on chromosome 12 and contains 11 exons that span ~75 kb [3, 4]. More than 470 single nucleotide polymorphisms (SNPs) have been reported in the human VDR gene [11]. Most studies of VDR polymorphisms focus on five SNPs (FokI, TaqI, BsmI, ApaI, TruI). Some of these polymorphic forms of VDR are associated with modified effectiveness of vitamin D, which may lead to a higher risk of endocrine and kidney diseases, such as osteoporosis, urolithiasis, type 1 diabetes mellitus, and diabetic nephropathy [12-15]. In addition, ethnic and genetic differences in the frequency of the VDR polymorphic genotypes have been reported in different populations, which may result in different disease susceptibility.
3. VDR AND KIDNEY DISEASES
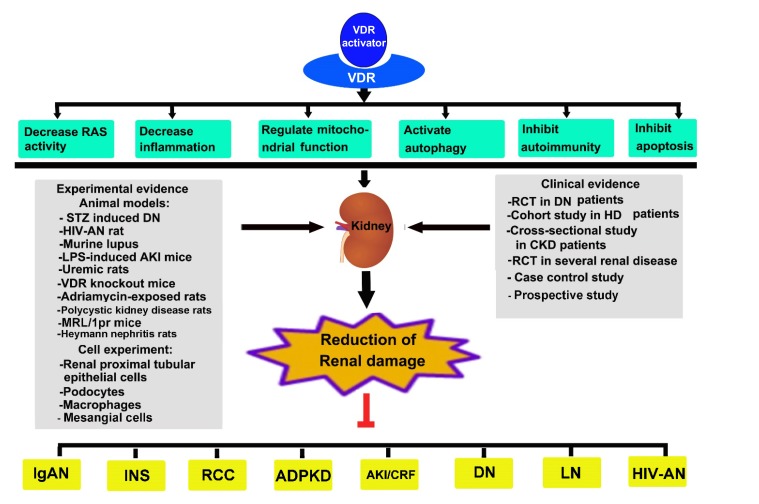
Growing evidence suggests that kidney diseases are closely tied to inadequate vitamin D levels. The prevalence of vitamin D deficiency is very high in patients with kidney disease, mainly due to decreased CYP27b1 activity for 1,25(OH)2D3 synthesis, impaired reabsorption of 25(OH)D in the proximal tubular cells, and increased levels of fibroblast growth factor 23 that suppresses the biosynthesis of 1,25(OH)2D3. As the only nuclear receptor that mediates the biological activity of 1,25(OH)2D3, VDR activity is compromised in vitamin D deficiency, which is believed to contribute to the pathogenesis of kidney disease. In the kidney, VDR is mainly expressed in proximal and distal tubular epithelial cells, podocytes, and collecting duct epithelial cells. In addition, VDR is found in the macula densa of the juxtaglomerular apparatus, but its expression is low in glomerular mesangial cells [16, 17]. Treatment with VDR agonists has been shown to reduce albuminuria and alleviate glomerular injury in experimental models of kidney diseases (Fig. 1).
Fig. (1).
Various mechanisms relevant to vitamin D receptor in kidney diseases.
4. VDR in primary kidney diseases
4.1. VDR in IgA Nephropathy (IgAN)
IgAN is the most common cause of primary glomerulonephritis in Asia. Vitamin D and its analogs are known to have profound effects on immune cell balance [18]; thus, supplementation with vitamin D may have renoprotective effects in IgAN patients. The relationship between vitamin D levels and IgAN prognosis has attracted nephrologists’ attention. A study by Yuan et al. found that a combination therapy of vitamin D and tacrolimus can effectively alleviate renal tissue damage in IgAN rats by regulating immune response and the NF-κB/TLR4 pathway [19]. Li et al. found that 25(OH)D deficiency is significantly correlated with poorer clinical outcomes, more serious renal pathological features and increased risk of renal progression in IgAN [20]. Recent clinical trials demonstrated that oral calcitriol can decrease proteinuria in IgAN patients with only mild side effects [21-23]. This clinical data suggests that vitamin D therapy may exert beneficial effects through targeting cytokines, immunoglobulin receptors and leukotriene metabolism.
4.2. VDR in Idiopathic Nephrotic Syndrome (INS)
INS is the most common glomerular disorder in children. The pathogenesis of INS remains inconclusive and controversial. It has been reported that the vitamin D status is insufficient in most subjects with INS [24]. Vitamin D supplementation can decrease the odds of 25(OH)D deficiency, prevent bone loss and increase the remission rate of nephrotic syndrome [25, 26]. Response to initial treatment with glucocorticoid is an indicator of prognosis for INS. Bennett et al. found that urinary vitamin D-binding protein is significant associated with steroid-resistant nephrotic syndrome [27, 28]. Some studies have also reported VDR gene polymorphisms in INS [29], indicating that VDR gene polymorphisms may be used as a predictor for steroid responsiveness in children with INS.
4.3. VDR in Renal Cell Carcinoma (RCC)
Approximately 90% of all kidney cancers are RCC. Previous studies show that VDR expression is decreased in RCC [30], and altered VDR expression may be associated with RCC carcinogenesis [31]. In a case-control study, the association between VDR variants and RCC risk was analyzed in the Chinese Han population, and the results showed that the VDR ApaI variant AA and AC genotypes were associated with a significantly increased risk of RCC [32]. The association between VDR gene polymorphism and RCC susceptibility has also been reported by Pospiech et al. who observed that the haplotype in VDR comprising positions BglI, TaqI, ApaI and BsmI is a significant risk factor for RCC [33]. The relationship between VDR gene polymorphism and RCC may be variable among different races. The FokI and BsmI genotypes of VDR gene is implicated in the pathogenesis of RCC in the North Indian population [34], but in Central and Eastern Europe subjects, the overall associations between VDR gene polymorphisms (BsmI, FokI, TaqI) and RCC risk are not observed [35]. In the Japanese population the AA genotype at the ApaI site of the VDR gene may be a risk factor for RCC and contribute to a poor prognosis [36]. Therefore, the association between VDR gene polymorphisms and RCC is still controversial.
In various pathogenic conditions, VDRA / VDR could alleviate RAS activity, inhibit EMT, inhibit apoptosis, decrease inflammation, activate autophagy, regulate mitochondrial function and induce immune tolerance through various signaling pathways. Experimental and clinical evidence showed that pharmacological activation of VDR may offer an attractive druggable target for various renal diseases. Note: VDR, Vitamin D Receptor; RAS, Renin Angiotensin System; STZ, Streptozocin; HIV, Human Immunodeficiency Virus; LPS, Lipopolysaccharide; AKI, Acute Kidney Injury; DN, Diabetic Nephropathy; HD, Hemodialysis; CKD, Chronic Kidney Disease; RCT, Randomized Controlled Trial; IgAN, IgA Nephropathy; INS, Idiopathic Membranous Nephropathy; RCC, Renal Cell Carcinoma ; ADPKD, Autosomal Aominant Polycystic Kidney Disease ; CRF, Chronic Renal Failure; LN, Lupus Nephritis; HIV-AN, Human Immunodeficiency Virus-Associated Nephropathy.
Preclinical experiments suggest that vitamin D may have therapeutic potential for RCC. Nagakura et al. showed that 1,25(OH)2D3 can inhibit the growth of a RCC cell line in vitro [37]. In addition, Dormoy et al. found that cholecalciferol supplementation can suppress RCC cell proliferation and stimulate cancer cell death by inhibition of the SHH-Gli pathway in vitro and in vivo [38]. Similarly, 1,25(OH)2D3 can inhibit Akt phosphorylation and phosphorylation of its downstream target, caspase-9, in a mouse xenograft model of renal tumor and prevent renal cancer cell proliferation [39].
4.4. VDR in Autosomal Dominant Polycystic Kidney Disease (ADPKD)
ADPKD is the most common genetic cause of renal failure in the world. Currently, there are no effective treatments for ADPKD. Kugita et al. performed a global gene expression analysis in a spontaneous rat model of polycystic kidney disease and found that VDR/RXR-mediated signaling is significantly altered in developing kidneys with polycystic kidney disease [40], suggesting that vitamin D/VDR might play an important role in the progression of polycystic kidney disease. Furthermore, in an open-label observational study, Rangan et al. supplemented ADPKD patients who were vitamin D deficient or insufficient with oral cholecalciferol for 6 months, and showed that cholecalciferol can attenuate hypertension and proteinuria and delay the progression of ADPKD [41]. This clinical outcome indicates that vitamin D repletion might be a novel treatment to prevent renal failure in ADPKD.
5. VDR in Secondary Kidney Diseases
5.1. VDR in Diabetic Nephropathy (DN)
DN is the most common renal complication of diabetes and a leading cause of chronic renal failure across ethnic groups [42]. A large body of evidence has demonstrated that VDR agonists can effectively attenuate the progression of DN. Podocyte injury is a key event in the development of DN, which results in proteinuria and often leads to progressive renal injury. Verouti et al. showed that calcitriol and paricalcitol can maintain and reactivate the expression of specialized components of podocytes, including podocalyxin, hence providing protection against loss of the permselective renal barrier [43]. Wang et al. reported that 1,25(OH)2D3 suppresses high glucose-induced podocyte apoptosis by blocking p38- and ERK-mediated pro-apoptotic pathways, and podocyte-specific expression of human VDR in transgenic mice protects against diabetic renal injury in experimental models [44]. VDR can also inhibit podocyte apoptosis by targeting the activation of the NF-κB pathway [45]. Other studies further showed that treating DN rats with calcitriol can normalize the expression of podocyte markers including nephrin and podocin [46], and that PI3K/p-Akt signaling pathway participates in calcitriol amelioration of podocyte injury in DN rats [47]. These data provide strong evidence that 1,25(OH)2D3 -VDR signaling in podocytes plays a critical role in the protection of the kidney under diabetes. The renoprotective effect of 1,25(OH)2D3 has also been demonstrated in mesangial cells. Zhang et al. reported that 1,25(OH)2D3 blocks high glucose-induced MCP-1 expression in mesangial cells by blunting NF-κB activation [48]. Consistent with these findings, Wang et al. found that 1,25(OH)2D3 can effectively inhibit rat mesangial cell proliferation induced by high glucose via inhibiting the DDIT4/TSC2/mTOR pathway [49].
Zhang et al. first reported that a combination therapy with paricalcitol and losartan can achieve a synergistic efficacy in preventing renal injury compared to monotherapies, including reduction of glomerulosclerosis and albuminuria in diabetic mice [50]. Wang et al. demonstrated that 1,25(OH)2D3 treatment can significantly reduce albumin excretion, mean glomerular volume, glomerular basement membrane and total kidney volume in DN rats, whereas intravenous injection of a recombinant lentivirus carrying shRNAs targeting the rat VDR significantly worsens renal injury in STZ-induced diabetic rats [49]. An increase in heparanase can induce proteinuria in experimental DN. Garsen et al. showed that VDR can directly bind to the heparanase promoter and suppresses heparanase gene promoter activity in podocytes, leading to decreased proteinuria in DN models [51].
We have investigated the relationship between BsmI and ApaI polymorphisms in VDR gene and DN in a Han Chinese population and found that the allele B (BB or Bb genotype) in VDR gene is correlated with albuminuria in type 2 diabetes, which may represent a risk factor for early-onset DN [52]. Similarly, Bucan et al. found that the VDR gene BsmI genotype is significantly associated with cumulative prevalence of DN [53]. Comparison of DN and healthy subjects identified a statistically significant difference for the FokI polymorphism in the VDR gene in DN patients, and the BBFFAATt combination is more frequent in DN than in healthy subjects [54]. While a case-control study showed that the rare VDR gene AGT haplotype is a significantly protective factor against DN in individuals with type 1 diabetes [55], some meta-analysis studies further identified the associations between VDR gene polymorphisms and DN risk [56, 57]. These observations suggest that analyzing VDR genetic markers in order to identifying the risk of DN could be important.
5.2. VDR in Lupus Nephritis (LN)
LN is a common complication of Systemic Lupus Erythematosus (SLE), which is associated with autoimmune disorders manifesting by proteinuria and renal dysfunction, but the exact pathogenesis of LN is still unclear. The presence of VDR on immune cells suggests that vitamin D has modulating effects on both the innate and adaptive immunity [58]. The suppressive immunologic properties of vitamin D have raised speculation for the involvement of vitamin D/VDR in SLE/LN pathogenesis. Lemire et.al. found that treatment with 1,25(OH)2D3 can reduce the severity of SLE and the level of serum anti-ssDNA antibodies in MRL/l mice [59]. These antibodies can induce serious LN. Indeed, vitamin D deficiency is common in SLE/LN patients. Sumethkul et al. showed that SLE patients with LN have significantly lower vitamin D levels compared with inactive SLE and active SLE without LN. Hence, vitamin D deficiency may be a significant predictor of nephritis in SLE [60]. In line with this, Abdel et.al. found that deficiency of 25(OH)D3 has a direct relationship with increased disease activity and nephritis in Egyptian SLE patients [61]. It is well known that heavy exposure to sunlight has deleterious effects on SLE, and a traditional explanation is that ultraviolet radiation damages DNA, which then becomes an immunogenic substance. However, Vaisberg et al. suggested that ultraviolet radiation from sunlight might aggravate the course of SLE by promoting vitamin D3 synthesis [62]. Thus, the relationship between vitamin D3 and SLE susceptibility needs further investigation.
Research suggests that VDR genotypes might be predictive factors for SLE/LN susceptibility. A case-control study in Egyptian children and adolescents showed that the VDR FokI FF genotype and F allele are overexpressed among childhood-onset SLE patients compared with the controls. In addition, a significant association between VDR FokI FF genotype and LN has been found, suggesting that the VDR FokI polymorphism might contribute to the susceptibility of SLE [63]. However, the association of VDR polymorphisms and risk of SLE has often produced conflicting results in different ethnic backgrounds. In the Han Chinese population Luo et al. found that the frequency of VDR B allele is significantly increased in SLE subjects and associated with the development of nephritis [64]. Similarly, it has been reported that the VDR BB genotype is associated with SLE in Taiwanese and Japanese patients [65, 66], and there is a significant association between VDR BsmI BB genotype and LN, suggesting that the VDR BsmI BB genotype is a risk factor for the development of nephritis among SLE subjects [67].
5.3. VDR in Human Immunodeficiency Virus-Associated Nephropathy (HIV-AN)
Glomerular podocytes are highly specialized cells that play a pivotal role in the pathogenesis of glomerular sclerosis and the collapsing variant of this entity [68], frequently encountered in HIV-AN [69]. Altered podocyte phenotypes, reduction in number and effacement of foot processes are associated with HIV-AN. Chandel et al. reported that HIV compromises the integrity of actin cytoskeleton in podocytes by enhancing podocyte cathepsin L expression and cleavage of dynamin, and VDR activation can effectively protect podocyte cytoskeletal integrity [70]. Interestingly, emerging reports indicate that African American patients with HIV infection are vitamin D deficient [71]. Rai et al. found that the renal VDR expression is downregulated in HIV milieu both in vivo and in vitro [72]. Salhan et al. showed that renal tubular cells display oxidative stress and DNA damage in the HIV milieu, and pretreatment with EB-1089, a VDR agonist, can prevent tubular cell DNA injury [73]. These findings suggest that VDR might serve as a novel therapeutic target for HIV-AN.
5.4. VDR in Acute Kidney Injury (AKI)
AKI, defined as a rapid renal dysfunction with severe tubular damage, is a frequent and serious complication of critical patients. Lai et al. assessed the relationship between serum vitamin D levels and the prognosis of AKI and showed that the degree of 1,25 (OH)2D3 deficiency is associated with the severity of AKI [74]. Ischemia/reperfusion (IR) is an important pathogenic factor for AKI, and it has been shown that therapy with the VDR activator paricalcitol can attenuate renal IR injury by improving tubular necrosis and medullar congestion [75]. Furthermore, Gonçalves et al. reported that vitamin D deficiency aggravates the tubulointerstitial damage and renal fibrosis progression in IR-induced AKI as a result of activation of pro-inflammatory pathways and upregulation of TGF-ß1 [76]. On the other hand, vitamin D supplementation can relieve glomerular podocyte loss and reduce proteinuria via inhibiting heparanase expression in podocytes in an Adriamycin induced AKI rat model [51]. Reactive oxygen species (ROS) plays important roles in AKI. Xu et al. demonstrated that vitamin D pretreatment can attenuate renal oxidative stress through regulating NADPH oxidase and nitric oxide synthase in an AKI mouse model [77]. In addition, studies by Luchi et al. suggest that vitamin D deficiency is a risk factor for contrast-induced AKI due to imbalance in intrarenal vasoactive substances and oxidative stress [78].
5.5. VDR in Chronic Renal Failure (CRF)
CRF is a common outcome of many renal pathological changes, which progresses to End-Stage Renal Disease (ESRD) if left untreated. Among CRF patients with and without dialysis, reduced levels of vitamin D and VDR have been reported [79]. Recent research indicates that vitamin D deficiency is associated with vascular calcification, anemia and cardiovascular mortality in patients with ESRD. Although the primary purpose for administration of VDR activators is for treating secondary hyperparathyroidism in patients with ESRD, studies have shown that the active form of vitamin D agonist calcitriol can improve hematopoiesis [80-82]. The relationship between VDR BsmI gene polymorphism, hemoglobin levels, and erythropoietin (EPO) has been reported among maintenance HD patients [83, 84]. Left ventricular hypertrophy is a strong cardiovascular mortality risk factor in CKD patients, the B alleles of VDR gene BsmI polymorphism has been correlated with left ventricular hypertrophy [85]. Peritoneal dialysis is another type of renal replacement therapy for ESRD, and peritoneal fibrosis is a significant problem associated with continuous peritoneal dialysis. Animal studies show that paricalcitol treatment can significantly reduce peritoneal dialysis-induced ECM thickening and prevent peritoneal fibrosis [86, 87].
6. RENOPROTECTIVE MECHANISMS OF VDR
Numerous reports based on animal and clinical studies have shown beneficial effects of VDR activation on renal diseases, and the underlying renoprotective mechanisms of VDR-mediated signaling are under intense investigation (Fig. 1).
6.1. Renin Angiotensin System (RAS)
Inappropriate activation of the Renin Angiotensin System (RAS) is a major risk factor responsible for the progression of kidney diseases, and the finding that vitamin D hormone inhibits the RAS generated much excitement in the field. Li et al. first reported that renin expression and plasma angiotensin II production are greatly increased in VDR-null mutant mice, leading to hypertension and cardiac hypertrophy, whereas 1,25(OH)2D3 treatment suppresses renin expression [88]. VDR-null mice with unilateral ureteral obstruction (UUO) develop severe renal damage with marked tubular atrophy, interstitial fibrosis, increased renin expression, and angiotensin II accumulation in the kidney [89]. Consistent with the VDR-null mice, 1-alpha-hydroxylase [1α(OH)ase] knockout mice also develop hypertension due to increased levels of renin and angiotensin II, and these abnormalities can be corrected by administration of exogenous 1,25(OH)2D3 [90]. Vitamin D deficiency aggravates tenofovir-induced nephrotoxicity because of enhanced activation of the RAS in the kidney [91]. In vitro studies confirm that VDR knockout podocytes display upregulation of angiotensinogen [92]. In rats with diabetes, calcitriol treatment attenuates renal pathological abnormalities by blocking the activation of the local RAS [93-95]. In 5/6 nephrectomy rat models, treatment with VDR activator paricalcitol is able to ameliorate the glomerular and tubulointerstitial damage and attenuate cardiac hypertrophy via blocking the activation of renal and myocardial RAS [96, 97]. Moreover, vitamin D hormone can also regulate the RAS in organs other than the kidney. For example, in the mouse model of glucocorticoid-induced osteoporosis, 1,25(OH)2D3 is shown to modulate bone metabolism by downregulating the local bone RAS [98]. Chronic vitamin D deficiency can promote lung fibrosis through activating the RAS [99].
The inverse relationship between vitamin D status and the RAS has also been reported in humans [100]. Tiryaki et al. showed that administration of VDR activator can blunt albuminuria by reducing urinary angiotensinogen levels reflecting intra-renal RAS status [101]. In a large cohort of 3,316 patients with cardiovascular problems, serum 25(OH)D3 and 1,25(OH)2D3 concentrations were found to be independently and inversely correlated to plasma renin activity and angiotensin II levels [102]. In addition, a study performed in 50 newly detected hypertensive patients showed that vitamin D deficiency was associated with stimulation of the RAS activity. Vitamin D supplementation can reduce the tissue RAS activity [103]. Taken together, these results demonstrate that VDR provides renoprotection through inhibition of the RAS. This is an important mechanism that guides clinical studies to treat kidney diseases using vitamin D and its analogues.
6.2. Inflammation
Inflammation is a universal initial response of an organism to any injurious agent. NF-κB regulates a wide range of genes involved in inflammation, including many involved in the development of kidney disease. Xu et al. reported that vitamin D-VDR specifically represses LPS-induced nuclear translocation of NF-κB p65 subunit in the renal tubules and attenuates LPS-induced renal inflammatory cytokines [104]. Studies using the mouse model of UUO showed that paricalcitol administration attenuates inflammation through abolishing the binding of NF-κB p65 to its cognate cis-acting element in the RANTES gene promoter. VDR and p65 form a complex in tubular cells after paricalcitol treatment, which inhibits the ability of p65 to transactivate gene transcription [105]. It has also been reported that 1,25(OH)2D3 induces VDR expression and promotes VDR interaction with p50 subunit of NF-κB, thus exerting anti-inflammatory effect on macrophages [106].
Macrophages are closely related to the progression of renal disease. Classically activated macrophage (M1) is a pro-inflammatory effector, whereas alternatively activated macrophage (M2) exhibits anti-inflammatory properties. Increased M1 macrophage infiltration in the glomeruli and renal interstitium was reported in DN rats, which was attenuated after calcitriol treatment [107]. A study by Zhang et al. showed that active vitamin D promotes M1 phenotype switching to M2 via the VDR-PPARγ pathway [108]. In addition, both calcitriol and paricalcitol can inhibit IL-6, monocyte chemoattractant protein (MCP)-1 and IL-18 in the kidney, independent of albuminuria reduction [109]. In line with these observations, Wu et al. reported that 1,25(OH)2D3 can reverse LPS-induced pro-inflammatory cytokines in mononuclear cells from type 2 diabetic and uremia patients [110]. Yang et al. reported that the anti-inflammatory effect of VDR is associated with crosstalk between STAT5 and VDR, which induces monocyte cytoskeletal rearrangement [111].
We have shown that TNF-α suppresses VDR expression by upregulating miR-346 in HK2 cells [112], confirming that inflammation per se can inhibit VDR expression. In clinical studies, administration of VDR agonist can decrease inflammation in patients with kidney disease. Several lines of evidence suggest a potential anti-inflammatory role for vitamin D in HD patients [113, 114], and vitamin D or paricalcitol may prevent EPO hyporesponsiveness through an anti-inflammatory mechanism in ESRD patients [115]. Oral paricalcitol treatment in kidney transplant recipients can significantly decrease serum IL-6 levels [116]. Mansouri et al. found that paricalcitol treatment for 12 weeks in CKD patients reduces cytokines involved in atherosclerosis and inflammation [117].
6.3. Epithelial–Mesenchymal Transition (EMT)
Most chronic renal diseases are accompanied by accumulation of extracellular matrix (ECM) proteins in the glomerular region and the renal interstitial compartment [118]. When renal interstitial fibrosis develops, the renal interstitium is occupied predominantly by ECM proteins and fibroblasts [119]. It is believed that EMT is a major mechanism responsible for the abnormal accumulation of ECM, leading to loss of epithelial cell-to-cell basement membrane and acquisition of a fibroblastic phenotype [120, 121]. Recent research suggests that VDR may play an important role in inhibiting EMT. In human renal proximal tubular epithelial cells, paricalcitol has been shown to attenuate 4-hydroxy-2-hexenal-induced renal tubular cell injury by suppressing EMT processes through targeting the Wnt/beta-catenin signaling pathway [122]. Vitamin D analogs can suppress matrix-producing myofibroblast activation by upregulating the expression of anti-fibrotic hepatocyte growth factor in renal interstitial fibroblasts [123]. In human peritoneal mesothelial cells, inhibitory effects of VDR agonists on EMT have also been reported [124, 125].
In the UUO model, VDR-null mice develop more severe renal damage in the obstructed kidney compared with wild-type mice, with marked tubular interstitial fibrosis [89], whereas paricalcitol treatment can block progressive renal tubulointerstitial fibrosis [126]. Both peritubular inflammation and tubular EMT are critical events during the pathogenesis of renal fibrosis, but the relationship between these two processes is unclear. Xiong et al. found that the early loss of VDR expression in the UUO mouse kidney is likely mediated by pro-inflammatory TNF-α, which renders tubular cells susceptible to EMT, suggesting that the loss of VDR couples these two events in renal fibrogenesis [127]. Bienaim et al. reported that patients with lower serum 25(OH)D3 concentrations at 3 months after transplantation exhibit a higher risk for the progression of renal interstitial fibrosis [128]. EMT also plays an important role in the development of DN. We found that 1,25(OH)2D3 and its analog BXL-628 can attenuate high glucose-induced EMT and extracellular matrix accumulation in HK-2 cells by suppressing the RhoA/ROCK signaling pathway [129]. An interesting study performed by Wan et al. showed that a GSK 3β inhibitor effectively inhibits high glucose-induced EMT in podocytes and the renal cortex due to activation of VDR [130]. Thus, vitamin D supplementation may be a rational strategy for renal interstitial fibrosis therapy.
6.4. Autoimmunity
Autoimmune disorders are an important pathogenic factor for kidney disease (e.g., glomerulonephritis). Since the discovery of the VDR on blood lymphocytes, the effect of vitamin D on immune-related diseases has been extensively studied [131]. In Lewis rats of the Heymann nephritis model, administration of 1,25(OH)2D3 can significantly reduce the level of autoantibodies in serum, and the immune deposits in kidney is markedly suppressed [132]. Lucisano et al. found that 25(OH)D3 can regulate immune processes through down-regulation of IL-17, IL-6, and IFN-γ in cultured human peripheral blood mononuclear cells [133].
Dendritic cells are a major target of 1,25(OH)2D3-induced immunosuppressive activity. Calcitriol can inhibit the differentiation and maturation of dendritic cells and promote their apoptosis, resulting in suppression of T-cell activation [134]. Ferreira et al. suggested that a metabolic switch toward glycolysis and activation of the PI3K-Akt-mTOR pathway are the first steps for the generation of tolerogenic dendritic cells by 1,25(OH)2D3 [135]. It has been found that active vitamin D can promote an M1 phenotype switch to M2 via the VDR-PPARγ pathway, consequently causing an anti-inflammatory effect [108]. As pro-inflammatory factors secreted by M1 macrophages (e.g., IL-1, IL-6, and TNFα) are involved in various autoimmune diseases, 1,25(OH)2D3 may play a role in the inhibition of immune responses via regulating macrophage phenotype switching. Reports also showed that 1,25(OH)2D3 can reduce B-cell proliferation and induce their apoptosis by preventing nuclear translocation of p65 subunit of NF-κB [136, 137]. In addition, 1,25(OH)2D3 can modulate cytokine secretion and differentiation of T cells [138]. These studies indicate that vitamin D/VDR signaling regulates immunoreaction by targeting all major immune cells.
6.5. Apoptosis
Apoptosis is a process of programmed cell death characterized by volume reduction, chromatin condensation and formation of apoptotic bodies. Apoptosis is a key pathogenic factor for various renal diseases (e.g., AKI, DN) [42, 139]. Experimental evidence supports an inhibiting effect of VDR on apoptosis. García et al. found that VDR activation by paricalcitol can decrease the number of TUNEL-positive apoptotic cells in the obstructed renal cortex in a UUO rat model [140], and vitamin D treatment can decrease LPS-induced apoptosis of podocytes via inhibiting the signaling of NF-κB pathway in cultured mouse podocytes [45]. Additionally, 1,25(OH)2D3 treatment can attenuate apoptosis in tubular epithelial cells of the cortices of uteroplacental perfusion rat models [141]. Therefore, VDR activation can alleviate renal injury via inhibiting apoptosis.
6.6. Autophagy
Autophagy is an intracellular pathway that delivers long-lived proteins and cytoplasmic organelles for lysosomal degradation and recycling in response to various stress conditions, including cell starvation, hypoxia, nutrient deprivation and oxidative injury. It is generally believed that autophagy is crucial in maintaining normal cellular function and morphology [142]. Basal autophagy in the kidneys is vital for the normal homeostasis of the renal cells, suggesting that autophagy deficiency may contribute to the pathogenesis of renal diseases [143-145]. Numerous studies have reported a relationship between vitamin D signaling and the autophagy pathway in inflammatory bowel diseases, traumatic brain injury and breast cancer [146-148]. It has been found that VDR regulates autophagic activity through ATG16L1 [149]. In STZ-induced rats, 1,25(OH)2D3 attenuates myocardial hypertrophy and interstitial fibrosis, improved cardiac function and restored impaired cardiac autophagy. Furthermore, it has been disclosed that 1,25(OH)2D3 improves diabetic cardiomyopathy in DN rats by activating autophagy through the β-catenin/TCF4/GSK-3β and mTOR pathways [150]. In active SLE, which is a common cause of LN, Zhao et al. found that vitamin D deficiency affects the expression of autophagy-related genes in the peripheral blood mononuclear cells [151]. These studies suggest that VDR may affect renal pathophysiology via regulating autophagy.
6.7. Mitochondria
Increasing evidence shows that mitochondrial dysfunction plays an important role in the progression of kidney diseases [152-154]. Mitochondria participate in numerous cellular functions, including steroid synthesis, lipid metabolism, ion homeostasis, calcium signaling transduction and apoptosis [155, 156]. Mitochondrial localization of VDR has been recently reported [157], and VDR appears to be imported into mitochondria through the permeability transition pore (PTP) in a ligand-independent manner [158]. Since the mitochondrial PTP plays a vital role in mitochondrial generation of ROS and mitochondria-induced apoptosis [159], this finding opens new perspectives for VDR’s roles in mitochondria functions. García et al. reported that in a UUO rat model, electron microscopy revealed mitochondrial swelling between the cristae; when the animals were treated with paricalcitol, mitochondrial expansion, dilated crests and wider gaps were inhibited, suggesting that vitamin D exerts beneficial effects on the kidney in part by protective actions on the mitochondria [140]. Indeed, vitamin D induces changes in mitochondrial morphology in the renal tissue of spontaneously hypertensive rats [160]. The mitochondrial respiration chain is an important site for the production of ROS. 1,25(OH)2D3/VDR has been shown to suppress brown adipocyte mitochondrial respiration [161], indicating that VDR may play a role in inhibiting mitochondrial ROS production.
7. THERAPEUTIC EFFECTS OF VDR ACTIVATION
VDR agonists (VDRA) are commonly used in patients with CKD and paricalcitol is the most commonly used vitamin D analog in clinical practice. Recently, novel VDRAs have been reported. Sawada et al. showed that VS-105 exerts a rapid repressive effect on serum PTH, and modulates PTH and VDR gene expression more effectively than paricalcitol [162]. 2MD is another novel and potent VDRA that has a wider therapeutic margin in the uremic rat model than calcitriol or paricalcitol, and suppresses PTH without hypercalcemia in HD patients [163]. A recent study showed that maxacalcitol can prevent the development of cardiac damage in DN rats, independent of RAS inhibition [164]. However, a pooled analysis revealed that there are no differences on the efficacy and safety between paricalcitol and other VDRAs for treating dialysis patients [165].
Emerging evidence in CKD patients shows that vitamin D can effectively reduce proteinuria. Our previous study showed that VDR mRNA and protein levels in peripheral blood mononuclear cells of T2DM patients are negatively correlated with urinary albumin levels [112]. In a prospective study that enrolled 18 patients with PD who were given oral paricalcitol treatment at a dose of 1-2 µg/d, the patients whose doses of paricalcitol were maintained after 3 months had significantly reduced proteinuria [166]. The ViRTUE study is a prospective, multicenter, randomized, double-blind trial targeting CKD patients with albuminuria of >300 mg/day due to non-diabetic kidney disease, and the results showed that paricalcitol therapy can reduce albuminuria [167]. VITAL trial is another large multinational trial evaluating the effect of paricalcitol in DN patients, and the result showed that treatment with 2 µg/day paricalcitol in patients already on RAS inhibitors can safely and significantly reduce residual urinary albumin levels. This suggests that a combination therapy with a RAS inhibitor and a VDR agonist is a more effective therapeutic option for patients with kidney diseases [168]. Other small clinical trials have also validated the renal protective effect both in patients with diabetes and non-diabetic kidney diseases [169, 170]. However, Keyzer et al. analyzed the resluts of ViRTUE-CKD trial and found that 45 nondiabetic CKD patients with albuminuria >300 mg/24 h were treated for four 8-week periods with paricalcitol (2 µg/d) or placebo, each combined with a low-sodium or regular sodium diet, and the intention-to-treat analysis and per-protocol analysis showed that the sodium restriction diet substantially reduced albuminuria, while the additional effect of paricalcitol on albuminuria was small and non-significant [171]. Therefore, more long-term clinical trials are needed to confirm the benefits of vitamin D analogs in patients with different kidney diseases.
Left Ventricular Hypertrophy (LVH) is a strong cardiovascular risk marker in ESRD patients; it has been found that vitamin D signaling and VDR gene polymorphisms are associated with LVH in ESRD patients [85, 172]. Studies in various clinical trials have demonstrated that VDR stimulation by VDRAs protects against cardiovascular death risk in CKD patients. A multicenter study with 1,516 patients showed that administration of VDRA during predialysis can decrease the incidence of cardiovascular disease onset after dialysis initiation [173]. Oral calcitriol, even in low doses, can reduce overall and cardiovascular mortality and increase the survival rates in dialyzed patients [174, 175]. In a nationwide cohort study including 8,675 dialysis patients, the pleiotropic effects of VDRA on cardiovascular disease, malignancy and infections in dialysis patients were evaluated, and multivariable survival analyses revealed that VDRA use was significantly associated with lower rates of infection- and malignancy-related deaths but not with cardiovascular death [176]. In addition, Naves-Díaz et al. assessed the effects of oral calcitriol use on the survival of HD patients from six Latin American countries with a median 16-month follow-up. Time-dependent Cox regression analysis showed that the 7,203 patients who received oral active vitamin D had significant reductions in overall, cardiovascular, infectious and neoplastic mortality compared to the 8,801 patients who had not received vitamin D [177]. Collectively, these clinical studies indicate that targeting the VDR via VDR activators or agonists can produce significant therapeutic benefits in patients with kidney diseases (Fig. 1).
CONCLUSION
Evidence from both animal and clinical studies has demonstrated that VDR activation protects against renal injury in various kidney diseases by a variety of mechanisms, including suppression of RAS activation, renal fibrogenesis, autoimmunity, and renal cell apoptosis. Therefore, VDR offers an attractive druggable target for renal diseases. Future studies are warranted to develop new classes of pharmacological activators of VDR that have better therapeutic efficacy but reduced side effects for the treatment of kidney diseases.
Acknowledgements
Supported by grants from the New Xiangya Talent Project, Third Xiangya Hosipital of Central South University (JY201521).
List of ABBREVIATIONS
- VDR
Vitamin D Receptor
- cDNA
Complementary Deoxyribonucleic Acid
- RXR
Retinoid X Receptor
- SNPs
Single Nucleotide Polymorphisms
- IgAN
Immunoglobulin A Nephropathy
- INS
Idiopathic Nephrotic Syndrome
- RCC
Renal Cell Carcinomas
- ADPKD
Autosomal Dominant Polycystic Kidney Disease
- DN
Diabetic Nephropathy
- ERK
Extracellular Signal-regulated Kinase
- NF-κB
Nuclear Factor -kappaB
- MCP-1
Monocyte Chemoattractant Protein-1
- STZ
Streptozocin
- LN
Lupus Nephritis
- SLE
Systemic Lupus Erythematosus
- HIV-AN
Human Immunodeficiency Virus -Associated Nephropathy
- AKI
Acute Kidney Injury
- CRF
Chronic Renal Failure
- TGF-ß1
Transforming Growth Factor-ß1
- ROS
Reactive Oxygen Species
- ESRD
End - Stage Renal Disease
- EPO
Erythropoietin
- RAS
Renin Angiotensin System
- UUO
Unilateral Ureteral Occlusion
- EMT
Epithelial–Mesenchymal Transition
- ECM
Extracellular Matrix
- IL
Interleukin
- IFN
Interferon
- mTOR
Mammalian Target of Rapamycin
- PTP
permeability Transition Pore
- VDRA
VDR Agonists
- HD
Hemodialysis
- LVH
Left Ventricular Hypertrophy
Consent for Publication
Not applicable.
conflict of interest
The authors declare no conflict of interest, financial or otherwise.
The authors alone are responsible for the content and writing of the paper.
References
- 1.Christakos S., Seth T., Hirsch J., Porta A., Moulas A., Dhawan P. Vitamin D biology revealed through the study of knockout and transgenic mouse models. Annu. Rev. Nutr. 2013;33:71–85. doi: 10.1146/annurev-nutr-071812-161249. [DOI] [PubMed] [Google Scholar]
- 2.Griffin M.D., Xing N., Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu. Rev. Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 3.Pike J.W. Vitamin D3 receptors: Structure and function in transcription. Annu. Rev. Nutr. 1991;11:189–216. doi: 10.1146/annurev.nu.11.070191.001201. [DOI] [PubMed] [Google Scholar]
- 4.Haussler M.R. Vitamin D receptors: Nature and function. Annu. Rev. Nutr. 1986;6:527–562. doi: 10.1146/annurev.nu.06.070186.002523. [DOI] [PubMed] [Google Scholar]
- 5.Lucisano S., Buemi M., Passantino A., Aloisi C., Cernaro V., Santoro D. New insights on the role of vitamin D in the progression of renal damage. Kidney Blood Press. Res. 2013;37(6):667–678. doi: 10.1159/000355747. [DOI] [PubMed] [Google Scholar]
- 6.Santoro D., Gitto L., Ferraro A., Satta E., Savica V., Bellinghieri G. Vitamin D status and mortality risk in patients with chronic kidney disease. Ren. Fail. 2011;33(2):184–191. doi: 10.3109/0886022X.2011.553303. [DOI] [PubMed] [Google Scholar]
- 7.Momeni A., Mirhosseini M., Kabiri M., Kheiri S. Effect of vitamin D on proteinuria in type 2 diabetic patients. J. Nephropathol. 2017;6(1):10–14. doi: 10.15171/jnp.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGregor R., Li G., Penny H., Lombardi G., Afzali B., Goldsmith D.J. Vitamin D in renal transplantation - from biological mechanisms to clinical benefits. Am. J. Transplant. 2014;14(6):1259–1270. doi: 10.1111/ajt.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haussler M.R., Whitfield G.K., Haussler C.A., Hsieh J.C., Thompson P.D., Selznick S.H., Dominguez C.E., Jurutka P.W. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998;13(3):325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 10.Norman A.W. Minireview: Vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006;147(12):5542–5548. doi: 10.1210/en.2006-0946. [DOI] [PubMed] [Google Scholar]
- 11.Yang L., Ma J., Zhang X., Fan Y., Wang L. Protective role of the vitamin D receptor. Cell. Immunol. 2012;279(2):160–166. doi: 10.1016/j.cellimm.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Melhus H., Kindmark A., Amer S., Wilen B., Lindh E., Ljunghall S. Vitamin D receptor genotypes in osteoporosis. Lancet. 1994;344(8927):949–950. doi: 10.1016/s0140-6736(94)92297-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Wang X., Wu J., Lin Y., Chen H., Zheng X., Zhou C., Xie L. Association of vitamin D receptor gene polymorphism and calcium urolithiasis in the Chinese Han population. Urol. Res. 2012;40(4):277–284. doi: 10.1007/s00240-011-0438-y. [DOI] [PubMed] [Google Scholar]
- 14.Cooper J.D., Smyth D.J., Walker N.M., Stevens H., Burren O.S., Wallace C., Greissl C., Ramos-Lopez E., Hypponen E., Dunger D.B., Spector T.D., Ouwehand W.H., Wang T.J., Badenhoop K., Todd J.A. Inherited variation in vitamin D genes is associated with predisposition to autoimmune disease type 1 diabetes. Diabetes. 2011;60(5):1624–1631. doi: 10.2337/db10-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santoro D., Caccamo D., Gagliostro G., Ientile R., Benvenga S., Bellinghieri G., Savica V. Vitamin D metabolism and activity as well as genetic variants of the Vitamin D Receptor (VDR) in chronic kidney disease patients. J. Nephrol. 2013;26(4):636–644. doi: 10.5301/jn.5000203. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R., Schaefer J., Grande J.P., Roche P.C. Immunolocalization of calcitriol receptor, 24-hydroxylase cytochrome P-450, and calbindin D28k in human kidney. Am. J. Physiol. 1994;266(3 Pt 2):F477–F485. doi: 10.1152/ajprenal.1994.266.3.F477. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Borchert M.L., Deluca H.F. Identification of the vitamin D receptor in various cells of the mouse kidney. Kidney Int. 2012;81(10):993–1001. doi: 10.1038/ki.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X., Wu X., Xiong L., Yi Z., He Q., He X., Mo S. Role of vitamin D3 in regulation of T helper cell 17 and regulatory T-cell balance in rats with immunoglobulin a nephropathy. Iran. J. Kidney Dis. 2014;8(5):363–370. [PubMed] [Google Scholar]
- 19.Yuan D., Fang Z., Sun F., Chang J., Teng J., Lin S., Liu X. Effect of vitamin D and tacrolimus combination therapy on IgA nephropathy. Med. Sci. Monit. 2017;23:3170–3177. doi: 10.12659/MSM.905073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X.H., Huang X.P., Pan L., Wang C.Y., Qin J., Nong F.W., Luo Y.Z., Wu Y., Huang Y.M., Peng X., Yang Z.H., Liao Y.H. Vitamin D deficiency may predict a poorer outcome of IgA nephropathy. BMC Nephrol. 2016;17(1):164. doi: 10.1186/s12882-016-0378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szeto C.C., Chow K.M., Kwan B.C., Chung K.Y., Leung C.B., Li P.K. Oral calcitriol for the treatment of persistent proteinuria in immunoglobulin A nephropathy: An uncontrolled trial. Am. J. Kidney Dis. 2008;51(5):724–731. doi: 10.1053/j.ajkd.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 22.Liu L.J., Lv J.C., Shi S.F., Chen Y.Q., Zhang H., Wang H.Y. Oral calcitriol for reduction of proteinuria in patients with IgA nephropathy: A randomized controlled trial. Am. J. Kidney Dis. 2012;59(1):67–74. doi: 10.1053/j.ajkd.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Deng J., Zheng X., Xie H., Chen L. Calcitriol in the treatment of IgA nephropathy with non-nephrotic range proteinuria: a meta-analysis of randomized controlled trials. 2017. [DOI] [PubMed]
- 24.Nielsen C.A., Jensen J.E., Cortes D. Vitamin D status is insufficient in the majority of children at diagnosis of nephrotic syndrome. Dan. Med. J. 2015;62(2):A5017. [PubMed] [Google Scholar]
- 25.Banerjee S., Basu S., Sengupta J. Vitamin D in nephrotic syndrome remission: A case-control study. Pediatr. Nephrol. 2013;28(10):1983–1989. doi: 10.1007/s00467-013-2511-y. [DOI] [PubMed] [Google Scholar]
- 26.Bak M., Serdaroglu E., Guclu R. Prophylactic calcium and vitamin D treatments in steroid-treated children with nephrotic syndrome. Pediatr. Nephrol. 2006;21(3):350–354. doi: 10.1007/s00467-005-2118-z. [DOI] [PubMed] [Google Scholar]
- 27.Bennett M.R., Pleasant L., Haffner C., Ma Q., Haffey W.D., Ying J., Wagner M., Greis K.D., Devarajan P. A novel biomarker panel to identify steroid resistance in childhood idiopathic nephrotic syndrome. Biomark. Insights. 2017;12:1177271917695832. doi: 10.1177/1177271917695832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bennett M.R., Pordal A., Haffner C., Pleasant L., Ma Q., Devarajan P. Urinary Vitamin D-binding protein as a biomarker of steroid-resistant nephrotic syndrome. Biomark. Insights. 2016;11:1–6. doi: 10.4137/BMI.S31633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Eisa A.A., Haider M.Z. Vitamin D receptor gene TaqI and Apal polymorphisms and steroid responsiveness in childhood idiopathic nephrotic syndrome. Int. J. Nephrol. Renovasc. Dis. 2016;9:187–192. doi: 10.2147/IJNRD.S111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blomberg Jensen M., Andersen C.B., Nielsen J.E., Bagi P., Jorgensen A., Juul A., Leffers H. Expression of the vitamin D receptor, 25-hydroxylases, 1alpha-hydroxylase and 24-hydroxylase in the human kidney and renal clear cell cancer. J. Steroid Biochem. Mol. Biol. 2010;121(1-2):376–382. doi: 10.1016/j.jsbmb.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y., Miyamoto T., Li K., Nakagomi H., Sawada N., Kira S., Kobayashi H., Zakohji H., Tsuchida T., Fukazawa M., Araki I., Takeda M. Decreased expression of the epithelial Ca2+ channel TRPV5 and TRPV6 in human renal cell carcinoma associated with vitamin D receptor. J. Urol. 2011;186(6):2419–2425. doi: 10.1016/j.juro.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 32.Yang C., Li J., Li Y., Wu D., Sui C., Jiang Y., Meng F. The vitamin D receptor gene ApaI polymorphism is associated with increased risk of renal cell carcinoma in Chinese population. Sci. Rep. 2016;6:25987. doi: 10.1038/srep25987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pospiech E., Ligeza J., Wilk W., Golas A., Jaszczynski J., Stelmach A., Rys J., Blecharczyk A., Wojas-Pelc A., Jura J., Branicki W. Variants of SCARB1 and VDR involved in complex genetic interactions may be implicated in the genetic susceptibility to clear cell renal cell carcinoma. BioMed Res. Int. 2015;2015:860405. doi: 10.1155/2015/860405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arjumand W., Ahmad S.T., Seth A., Saini A.K., Sultana S. Vitamin D receptor FokI and BsmI gene polymorphism and its association with grade and stage of renal cell carcinoma in North Indian population. Tumour Biol. 2012;33(1):23–31. doi: 10.1007/s13277-011-0236-8. [DOI] [PubMed] [Google Scholar]
- 35.Karami S., Brennan P., Hung R.J., Boffetta P., Toro J., Wilson R.T., Zaridze D., Navratilova M., Chatterjee N., Mates D., Janout V., Kollarova H., Bencko V., Szeszenia-Dabrowska N., Holcatova I., Moukeria A., Welch R., Chanock S., Rothman N., Chow W.H., Moore L.E. Vitamin D receptor polymorphisms and renal cancer risk in Central and Eastern Europe. J. Toxicol. Environ. Health A. 2008;71(6):367–372. doi: 10.1080/15287390701798685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obara W., Suzuki Y., Kato K., Tanji S., Konda R., Fujioka T. Vitamin D receptor gene polymorphisms are associated with increased risk and progression of renal cell carcinoma in a Japanese population. Int. J. Urol. 2007;14(6):483–487. doi: 10.1111/j.1442-2042.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 37.Nagakura K., Abe E., Suda T., Hayakawa M., Nakamura H., Tazaki H. Inhibitory effect of 1 alpha,25-dihydroxyvitamin D3 on the growth of the renal carcinoma cell line. Kidney Int. 1986;29(4):834–840. doi: 10.1038/ki.1986.74. [DOI] [PubMed] [Google Scholar]
- 38.Dormoy V., Beraud C., Lindner V., Coquard C., Barthelmebs M., Brasse D., Jacqmin D., Lang H., Massfelder T. Vitamin D3 triggers antitumor activity through targeting hedgehog signaling in human renal cell carcinoma. Carcinogenesis. 2012;33(11):2084–2093. doi: 10.1093/carcin/bgs255. [DOI] [PubMed] [Google Scholar]
- 39.Lambert J.R., Eddy V.J., Young C.D., Persons K.S., Sarkar S., Kelly J.A., Genova E., Lucia M.S., Faller D.V., Ray R. A vitamin D receptor-alkylating derivative of 1alpha, 25-dihydroxyvitamin D3 inhibits growth of human kidney cancer cells and suppresses tumor growth. Cancer Prev. Res. (Phila.) 2010;3(12):1596–1607. doi: 10.1158/1940-6207.CAPR-10-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kugita M., Nishii K., Morita M., Yoshihara D., Kowa-Sugiyama H., Yamada K., Yamaguchi T., Wallace D.P., Calvet J.P., Kurahashi H., Nagao S. Global gene expression profiling in early-stage polycystic kidney disease in the Han: SPRD Cy rat identifies a role for RXR signaling. Am. J. Physiol. Renal Physiol. 2011;300(1):F177–F188. doi: 10.1152/ajprenal.00470.2010. [DOI] [PubMed] [Google Scholar]
- 41.Rangan G.K., Harris D.C. Rationale and design of an observational study to determine the effects of cholecalciferol on hypertension, proteinuria and urinary MCP-1 in ADPKD. Curr. Hypertens. Rev. 2013;9(2):115–120. doi: 10.2174/15734021113099990006. [DOI] [PubMed] [Google Scholar]
- 42.Kanwar Y.S., Sun L., Xie P., Liu F.Y., Chen S. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu. Rev. Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verouti S.N., Tsilibary E.C., Fragopoulou E., Iatrou C., Demopoulos C.A., Charonis A.S., Charonis S.A., Drossopoulou G.I. Vitamin D receptor activators upregulate and rescue podocalyxin expression in high glucose-treated human podocytes. Nephron, Exp. Nephrol. 2012;122(1-2):36–50. doi: 10.1159/000346562. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y., Deb D.K., Zhang Z., Sun T., Liu W., Yoon D., Kong J., Chen Y., Chang A., Li Y.C. Vitamin D receptor signaling in podocytes protects against diabetic nephropathy. J. Am. Soc. Nephrol. 2012;23(12):1977–1986. doi: 10.1681/ASN.2012040383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu L., Zhang P., Guan H., Huang Z., He X., Wan X., Xiao H., Li Y. Vitamin D and its receptor regulate lipopolysaccharide-induced transforming growth factor-beta, angiotensinogen expression and podocytes apoptosis through the nuclear factor-kappaB pathway. J. Diabetes Investig. 2016;7(5):680–688. doi: 10.1111/jdi.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X., Song Z., Guo Y., Zhou M. The novel role of TRPC6 in vitamin D ameliorating podocyte injury in STZ-induced diabetic rats. Mol. Cell. Biochem. 2015;399(1-2):155–165. doi: 10.1007/s11010-014-2242-9. [DOI] [PubMed] [Google Scholar]
- 47.Song Z., Guo Y., Zhou M., Zhang X. The PI3K/p-Akt signaling pathway participates in calcitriol ameliorating podocyte injury in DN rats. Metabolism. 2014;63(10):1324–1333. doi: 10.1016/j.metabol.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Z., Yuan W., Sun L., Szeto F.L., Wong K.E., Li X., Kong J., Li Y.C. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72(2):193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 49.Wang H., Wang J., Qu H., Wei H., Ji B., Yang Z., Wu J., He Q., Luo Y., Liu D., Duan Y., Liu F., Deng H. In vitro and in vivo inhibition of mTOR by 1,25-dihydroxyvitamin D3 to improve early diabetic nephropathy via the DDIT4/TSC2/mTOR pathway. Endocrine. 2016;54(2):348–359. doi: 10.1007/s12020-016-0999-1. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z., Zhang Y., Ning G., Deb D.K., Kong J., Li Y.C. Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: blockade of compensatory renin increase. Proc. Natl. Acad. Sci. USA. 2008;105(41):15896–15901. doi: 10.1073/pnas.0803751105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garsen M., Sonneveld R., Rops A.L., Huntink S., van Kuppevelt T.H., Rabelink T.J., Hoenderop J.G., Berden J.H., Nijenhuis T., van der Vlag J. Vitamin D attenuates proteinuria by inhibition of heparanase expression in the podocyte. J. Pathol. 2015;237(4):472–481. doi: 10.1002/path.4593. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H., Wang J., Yi B., Zhao Y., Liu Y., Zhang K., Cai X., Sun J., Huang L., Liao Q. BsmI polymorphisms in vitamin D receptor gene are associated with diabetic nephropathy in type 2 diabetes in the Han Chinese population. Gene. 2012;495(2):183–188. doi: 10.1016/j.gene.2011.12.049. [DOI] [PubMed] [Google Scholar]
- 53.Bucan K., Ivanisevic M., Zemunik T., Boraska V., Skrabic V., Vatavuk Z., Galetovic D., Znaor L. Retinopathy and nephropathy in type 1 diabetic patients--association with polymorphysms of vitamin D-receptor, TNF, Neuro-D and IL-1 receptor 1 genes. Coll. Antropol. 2009;33(Suppl. 2):99–105. [PubMed] [Google Scholar]
- 54.Vedralova M., Kotrbova-Kozak A., Zeleznikova V., Zoubkova H., Rychlik I., Cerna M. Polymorphisms in the vitamin D receptor gene and parathyroid hormone gene in the development and progression of diabetes mellitus and its chronic complications, diabetic nephropathy and non-diabetic renal disease. Kidney Blood Press. Res. 2012;36(1):1–9. doi: 10.1159/000339021. [DOI] [PubMed] [Google Scholar]
- 55.Martin R.J., McKnight A.J., Patterson C.C., Sadlier D.M., Maxwell A.P. A rare haplotype of the vitamin D receptor gene is protective against diabetic nephropathy. Nephrol. Dial. Transplant. 2010;25(2):497–503. doi: 10.1093/ndt/gfp515. [DOI] [PubMed] [Google Scholar]
- 56.Yin F., Liu J., Fan M.X., Zhou X.L., Zhang X.L. Association between the vitamin D receptor gene polymorphisms and diabetic nephropathy risk: a meta-analysis. Nephrology (Carlton) 2017;26(13):1426–1434. doi: 10.1111/nep.13111. [DOI] [PubMed] [Google Scholar]
- 57.Liu Z., Liu L., Chen X., He W., Yu X. Associations study of vitamin D receptor gene polymorphisms with diabetic microvascular complications: A meta-analysis. Gene. 2014;546(1):6–10. doi: 10.1016/j.gene.2014.05.052. [DOI] [PubMed] [Google Scholar]
- 58.Hewison M. Vitamin D and the immune system: New perspectives on an old theme. Rheum. Dis. Clin. North Am. 2012;38(1):125–139. doi: 10.1016/j.rdc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Lemire J.M., Ince A., Takashima M. 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity. 1992;12(2):143–148. doi: 10.3109/08916939209150321. [DOI] [PubMed] [Google Scholar]
- 60.Sumethkul K., Boonyaratavej S., Kitumnuaypong T., Angthararuk S., Cheewasat P., Manadee N., Sumethkul V. The predictive factors of low serum 25-hydroxyvitamin D and vitamin D deficiency in patients with systemic lupus erythematosus. Rheumatol. Int. 2013;33(6):1461–1467. doi: 10.1007/s00296-012-2537-7. [DOI] [PubMed] [Google Scholar]
- 61.Abdel Galil S.M., El-Shafey A.M., Abdul-Maksoud R.S., El-Boshy M. Interferon alpha gene expression and serum level association with low vitamin D levels in Egyptian female patients with systemic lupus erythematosus. Lupus. 2018;27(2):199–209. doi: 10.1177/0961203317716321. [DOI] [PubMed] [Google Scholar]
- 62.Vaisberg M.W., Kaneno R., Franco M.F., Mendes N.F. Influence of cholecalciferol (vitamin D3) on the course of experimental systemic lupus erythematosus in F1 (NZBxW) mice. J. Clin. Lab. Anal. 2000;14(3):91–96. doi: 10.1002/(SICI)1098-2825(2000)14:3<91::AID-JCLA2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imam A.A., Ibrahim H.E., Farghaly M.A.A., Alkholy U.M., Gawish H.H., Abdalmonem N., Sherif A.M., Ali Y.F., Hamed M.E., Waked N.M., Fathy M.M., Khalil A.M., Noah M.A., Hegab M.S., Ibrahim B.R., Nabil R.M., Fattah L.A. Vitamin D receptor gene FokI polymorphism in Egyptian children and adolescents with SLE: A case-control study. Lupus. 2017;26(13):1426–1434. doi: 10.1177/0961203317725588. [DOI] [PubMed] [Google Scholar]
- 64.Luo X.Y., Yang M.H., Wu F.X., Wu L.J., Chen L., Tang Z., Liu N.T., Zeng X.F., Guan J.L., Yuan G.H. Vitamin D receptor gene BsmI polymorphism B allele, but not BB genotype, is associated with systemic lupus erythematosus in a Han Chinese population. Lupus. 2012;21(1):53–59. doi: 10.1177/0961203311422709. [DOI] [PubMed] [Google Scholar]
- 65.Huang C.M., Wu M.C., Wu J.Y., Tsai F.J. Association of vitamin D receptor gene BsmI polymorphisms in Chinese patients with systemic lupus erythematosus. Lupus. 2002;11(1):31–34. doi: 10.1191/0961203302lu143oa. [DOI] [PubMed] [Google Scholar]
- 66.Ozaki Y., Nomura S., Nagahama M., Yoshimura C., Kagawa H., Fukuhara S. Vitamin-D receptor genotype and renal disorder in Japanese patients with systemic lupus erythematosus. Nephron. 2000;85(1):86–91. doi: 10.1159/000045635. [DOI] [PubMed] [Google Scholar]
- 67.Azab S.F., Ali Y.F., Farghaly M.A., Hamed M.E., Allah M.A., Emam A.A., Abdelsalam N.I., Hashem M.I., Gawish H.H., Nabil R.M., Kamel L.M., Fahmy D.S., Alsayed S.F., Al Azizi N.M., Al-Akad G.M., Noah M.A., Abdelrahman H.M., Ahmed A.R., Bendary E.A. Vitamin D receptor gene BsmI polymorphisms in Egyptian children and adolescents with systemic lupus erythematosus: A case-control study. Medicine (Baltimore) 2016;95(46):e5233. doi: 10.1097/MD.0000000000005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kriz W., Gretz N., Lemley K.V. Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int. 1998;54(3):687–697. doi: 10.1046/j.1523-1755.1998.00044.x. [DOI] [PubMed] [Google Scholar]
- 69.Barisoni L., Kriz W., Mundel P., D’Agati V. The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J. Am. Soc. Nephrol. 1999;10(1):51–61. doi: 10.1681/ASN.V10151. [DOI] [PubMed] [Google Scholar]
- 70.Chandel N., Sharma B., Husain M., Salhan D., Singh T., Rai P., Mathieson P.W., Saleem M.A., Malhotra A., Singhal P.C. HIV compromises integrity of the podocyte actin cytoskeleton through downregulation of the vitamin D receptor. Am. J. Physiol. Renal Physiol. 2013;304(11):F1347–F1357. doi: 10.1152/ajprenal.00717.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lake J.E., Adams J.S. Vitamin D in HIV-Infected Patients. Curr. HIV/AIDS Rep. 2011;8(3):133–141. doi: 10.1007/s11904-011-0082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rai P., Singh T., Lederman R., Chawla A., Kumar D., Cheng K., Valecha G., Mathieson P.W., Saleem M.A., Malhotra A., Singhal P.C. Hyperglycemia enhances kidney cell injury in HIVAN through down-regulation of vitamin D receptors. Cell. Signal. 2015;27(3):460–469. doi: 10.1016/j.cellsig.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Salhan D., Husain M., Subrati A., Goyal R., Singh T., Rai P., Malhotra A., Singhal P.C. HIV-induced kidney cell injury: Role of ROS-induced downregulated vitamin D receptor. Am. J. Physiol. Renal Physiol. 2012;303(4):F503–F514. doi: 10.1152/ajprenal.00170.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai L., Qian J., Yang Y., Xie Q., You H., Zhou Y., Ma S., Hao C., Gu Y., Ding F. Is the serum vitamin D level at the time of hospital-acquired acute kidney injury diagnosis associated with prognosis? PLoS One. 2013;8(5):e64964. doi: 10.1371/journal.pone.0064964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Azak A., Huddam B., Haberal N., Kocak G., Ortabozkoyun L., Senes M., Akdogan M.F., Denizli N., Duranay M. Effect of novel vitamin D receptor activator paricalcitol on renal ischaemia/reperfusion injury in rats. Ann. R. Coll. Surg. Engl. 2013;95(7):489–494. doi: 10.1308/003588413X13629960049117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goncalves J.G., de Braganca A.C., Canale D., Shimizu M.H., Sanches T.R., Moyses R.M., Andrade L., Seguro A.C., Volpini R.A. Vitamin D deficiency aggravates chronic kidney disease progression after ischemic acute kidney injury. PLoS One. 2014;9(9):e107228. doi: 10.1371/journal.pone.0107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu S., Chen Y.H., Tan Z.X., Xie D.D., Zhang C., Xia M.Z., Wang H., Zhao H., Xu D.X., Yu D.X. Vitamin D3 pretreatment alleviates renal oxidative stress in lipopolysaccharide-induced acute kidney injury. J. Steroid Biochem. Mol. Biol. 2015;152:133–141. doi: 10.1016/j.jsbmb.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 78.Luchi W.M., Shimizu M.H., Canale D., Gois P.H., de Braganca A.C., Volpini R.A., Girardi A.C., Seguro A.C. Vitamin D deficiency is a potential risk factor for contrast-induced nephropathy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;309(3):R215–R222. doi: 10.1152/ajpregu.00526.2014. [DOI] [PubMed] [Google Scholar]
- 79.Ye J.J., Zhou T.B., Zhang Y.F., Wang Q., Su Y.Y., Tang J.M., Li H.Y. Levels of vitamin D receptor and CYP24A1 in patients with end-stage renal disease. Afr. Health Sci. 2016;16(2):462–467. doi: 10.4314/ahs.v16i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith E.M., Tangpricha V. Vitamin D and anemia: insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015;22(6):432–438. doi: 10.1097/MED.0000000000000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cortes M., Chen M.J., Stachura D.L., Liu S.Y., Kwan W., Wright F., Vo L.T., Theodore L.N., Esain V., Frost I.M., Schlaeger T.M., Goessling W., Daley G.Q., North T.E. Developmental vitamin D availability impacts hematopoietic stem cell production. Cell Reports. 2016;17(2):458–468. doi: 10.1016/j.celrep.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lucisano S., Di Mauro E., Montalto G., Cernaro V., Buemi M., Santoro D. Vitamin D and anemia. J. Ren. Nutr. 2014;24(1):61–62. doi: 10.1053/j.jrn.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 83.Erturk S., Kutlay S., Karabulut H.G., Keven K., Nergizoglu G., Ates K., Bokesoy I., Duman N. The impact of vitamin D receptor genotype on the management of anemia in hemodialysis patients. Am. J. Kidney Dis. 2002;40(4):816–823. doi: 10.1053/ajkd.2002.35694. [DOI] [PubMed] [Google Scholar]
- 84.Sezer S., Tutal E., Bilgic A., Ozdemir F.N., Haberal M. Possible influence of vitamin D receptor gene polymorphisms on recombinant human erythropoietin requirements in dialysis patients. Transplant. Proc. 2007;39(1):40–44. doi: 10.1016/j.transproceed.2006.10.214. [DOI] [PubMed] [Google Scholar]
- 85.Testa A., Mallamaci F., Benedetto F.A., Pisano A., Tripepi G., Malatino L., Thadhani R., Zoccali C., Vitamin D. Receptor (VDR) Gene Polymorphism is Associated with Left Ventricular (LV) Mass and Predicts Left Ventricular Hypertrophy (LVH) progression in End-Stage Renal Disease (ESRD) patients. J. Bone Miner. Res. 2010;25(2):313–319. doi: 10.1359/jbmr.090717. [DOI] [PubMed] [Google Scholar]
- 86.Stavenuiter A.W., Farhat K., Vila Cuenca M., Schilte M.N., Keuning E.D., Paauw N.J., ter Wee P.M., Beelen R.H., Vervloet M.G. Protective effects of paricalcitol on peritoneal remodeling during peritoneal dialysis. BioMed Res. Int. 2015;2015:468574. doi: 10.1155/2015/468574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Mateo G.T., Fernandez-Millara V., Bellon T., Liappas G., Ruiz-Ortega M., Lopez-Cabrera M., Selgas R., Aroeira L.S. Paricalcitol reduces peritoneal fibrosis in mice through the activation of regulatory T cells and reduction in IL-17 production. PLoS One. 2014;9(10):e108477. doi: 10.1371/journal.pone.0108477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Y.C., Kong J., Wei M., Chen Z.F., Liu S.Q., Cao L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y., Kong J., Deb D.K., Chang A., Li Y.C. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J. Am. Soc. Nephrol. 2010;21(6):966–973. doi: 10.1681/ASN.2009080872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang W., Chen L., Zhang L., Xiao M., Ding J., Goltzman D., Miao D. Administration of exogenous 1,25(OH)2D3 normalizes overactivation of the central renin-angiotensin system in 1alpha(OH)ase knockout mice. Neurosci. Lett. 2015;588:184–189. doi: 10.1016/j.neulet.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 91.Canale D., de Braganca A.C., Goncalves J.G., Shimizu M.H., Sanches T.R., Andrade L., Volpini R.A., Seguro A.C. Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: Role of oxidative stress and renin-angiotensin system. PLoS One. 2014;9(7):e103055. doi: 10.1371/journal.pone.0103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chandel N., Ayasolla K., Wen H., Lan X., Haque S., Saleem M.A., Malhotra A., Singhal P.C. Vitamin D receptor deficit induces activation of renin angiotensin system via SIRT1 modulation in podocytes. Exp. Mol. Pathol. 2017;102(1):97–105. doi: 10.1016/j.yexmp.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Deng X., Cheng J., Shen M. Vitamin D improves diabetic nephropathy in rats by inhibiting renin and relieving oxidative stress. J. Endocrinol. Invest. 2016;39(6):657–666. doi: 10.1007/s40618-015-0414-4. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Z., Sun L., Wang Y., Ning G., Minto A.W., Kong J., Quigg R.J., Li Y.C. Renoprotective role of the vitamin D receptor in diabetic nephropathy. Kidney Int. 2008;73(2):163–171. doi: 10.1038/sj.ki.5002572. [DOI] [PubMed] [Google Scholar]
- 95.Klaus G. Renoprotection with vitamin D: specific for diabetic nephropathy? Kidney Int. 2008;73(2):141–143. doi: 10.1038/sj.ki.5002693. [DOI] [PubMed] [Google Scholar]
- 96.Freundlich M., Quiroz Y., Zhang Z., Zhang Y., Bravo Y., Weisinger J.R., Li Y.C., Rodriguez-Iturbe B. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 2008;74(11):1394–1402. doi: 10.1038/ki.2008.408. [DOI] [PubMed] [Google Scholar]
- 97.Freundlich M., Li Y.C., Quiroz Y., Bravo Y., Seeherunvong W., Faul C., Weisinger J.R., Rodriguez-Iturbe B. Paricalcitol downregulates myocardial renin-angiotensin and fibroblast growth factor expression and attenuates cardiac hypertrophy in uremic rats. Am. J. Hypertens. 2014;27(5):720–726. doi: 10.1093/ajh/hpt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shen L., Ma C., Shuai B., Yang Y. Effects of 1,25-dihydroxyvitamin D3 on the local bone renin-angiotensin system in a murine model of glucocorticoid-induced osteoporosis. Exp. Ther. Med. 2017;13(6):3297–3304. doi: 10.3892/etm.2017.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shi Y., Liu T., Yao L., Xing Y., Zhao X., Fu J., Xue X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci. Rep. 2017;7(1):3312. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santoro D., Caccamo D., Lucisano S., Buemi M., Sebekova K., Teta D., De Nicola L. Interplay of vitamin D, erythropoiesis, and the renin-angiotensin system. BioMed Res. Int. 2015;2015:145828. doi: 10.1155/2015/145828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tiryaki O., Usalan C., Sayiner Z.A. Vitamin D receptor activation with calcitriol for reducing urinary angiotensinogen in patients with type 2 diabetic chronic kidney disease. Ren. Fail. 2016;38(2):222–227. doi: 10.3109/0886022X.2015.1128250. [DOI] [PubMed] [Google Scholar]
- 102.Tomaschitz A., Pilz S., Ritz E., Grammer T., Drechsler C., Boehm B.O., Marz W. Independent association between 1,25-dihydroxyvitamin D, 25-hydroxyvitamin D and the renin-angiotensin system: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Clin. Chim. Acta. 2010;411(17-18):1354–1360. doi: 10.1016/j.cca.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 103.Kota S.K., Jammula S., Meher L.K., Panda S., Tripathy P.R., Modi K.D. Renin-angiotensin system activity in vitamin D deficient, obese individuals with hypertension: An urban Indian study. Indian J. Endocrinol. Metab. 2011;15(Suppl. 4):S395–S401. doi: 10.4103/2230-8210.86985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu S., Chen Y.H., Tan Z.X., Xie D.D., Zhang C., Zhang Z.H., Wang H., Zhao H., Yu D.X., Xu D.X. Vitamin D3 pretreatment regulates renal inflammatory responses during lipopolysaccharide-induced acute kidney injury. Sci. Rep. 2015;5:18687. doi: 10.1038/srep18687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tan X., Wen X., Liu Y. Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-kappaB signaling. J. Am. Soc. Nephrol. 2008;19(9):1741–1752. doi: 10.1681/ASN.2007060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma D., Zhang R.N., Wen Y., Yin W.N., Bai D., Zheng G.Y., Li J.S., Zheng B., Wen J.K. 1, 25(OH)2D3-induced interaction of vitamin D receptor with p50 subunit of NF-kappaB suppresses the interaction between KLF5 and p50, contributing to inhibition of LPS-induced macrophage proliferation. Biochem. Biophys. Res. Commun. 2017;482(2):366–374. doi: 10.1016/j.bbrc.2016.11.069. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X.L., Guo Y.F., Song Z.X., Zhou M. Vitamin D prevents podocyte injury via regulation of macrophage M1/M2 phenotype in diabetic nephropathy rats. Endocrinology. 2014;155(12):4939–4950. doi: 10.1210/en.2014-1020. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X., Zhou M., Guo Y., Song Z., Liu B. 1,25-Dihydroxyvitamin D(3) promotes high glucose-induced M1 macrophage switching to M2 via the VDR-PPARgamma signaling pathway. BioMed Res. Int. 2015;2015:157834. doi: 10.1155/2015/157834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanchez-Nino M.D., Bozic M., Cordoba-Lanus E., Valcheva P., Gracia O., Ibarz M., Fernandez E., Navarro-Gonzalez J.F., Ortiz A., Valdivielso J.M. Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2012;302(6):F647–F657. doi: 10.1152/ajprenal.00090.2011. [DOI] [PubMed] [Google Scholar]
- 110.Wu E.L., Cui H.X. Effect of 1,25-(OH)2D3 and lipopolysaccharide on mononuclear cell inflammation in type 2 diabetes mellitus and diabetic nephropathy uremia. Genet. Mol. Res. 2016;15(3) doi: 10.4238/gmr.15038553. [DOI] [PubMed] [Google Scholar]
- 111.Yang M., Yang B.O., Gan H., Li X., Xu J., Yu J., Gao L., Li F. Anti-inflammatory effect of 1,25-dihydroxy-vitamin D3 is associated with crosstalk between signal transducer and activator of transcription 5 and the vitamin D receptor in human monocytes. Exp. Ther. Med. 2015;9(5):1739–1744. doi: 10.3892/etm.2015.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yi B., Huang J., Zhang W., Li A.M., Yang S.K., Sun J., Wang J.W., Li Y.C., Zhang H. Vitamin D receptor down-regulation is associated with severity of albuminuria in type 2 diabetes patients. J. Clin. Endocrinol. Metab. 2016;101(11):4395–4404. doi: 10.1210/jc.2016-1516. [DOI] [PubMed] [Google Scholar]
- 113.Zimmermann J., Herrlinger S., Pruy A., Metzger T., Wanner C. Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int. 1999;55(2):648–658. doi: 10.1046/j.1523-1755.1999.00273.x. [DOI] [PubMed] [Google Scholar]
- 114.Izquierdo M.J., Cavia M., Muniz P., de Francisco A.L., Arias M., Santos J., Abaigar P. Paricalcitol reduces oxidative stress and inflammation in hemodialysis patients. BMC Nephrol. 2012;13:159. doi: 10.1186/1471-2369-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Icardi A., Paoletti E., De Nicola L., Mazzaferro S., Russo R., Cozzolino M. Renal anaemia and EPO hyporesponsiveness associated with vitamin D deficiency: The potential role of inflammation. Nephrol. Dial. Transplant. 2013;28(7):1672–1679. doi: 10.1093/ndt/gft021. [DOI] [PubMed] [Google Scholar]
- 116.Oblak M., Mlinsek G., Kandus A., Buturovic-Ponikvar J., Arnol M. Effects of paricalcitol on biomarkers of inflammation and fibrosis in kidney transplant recipients: results of a randomized controlled trial. Clin. Nephrol. 2017;88(13):119–125. doi: 10.5414/CNP88FX26. [DOI] [PubMed] [Google Scholar]
- 117.Mansouri L., Lundwall K., Moshfegh A., Jacobson S.H., Lundahl J., Spaak J. Vitamin D receptor activation reduces inflammatory cytokines and plasma MicroRNAs in moderate chronic kidney disease - a randomized trial. BMC Nephrol. 2017;18(1):161. doi: 10.1186/s12882-017-0576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Toussaint N.D. Extracellular matrix calcification in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2011;20(4):360–368. doi: 10.1097/MNH.0b013e3283479330. [DOI] [PubMed] [Google Scholar]
- 119.Zeisberg M. Neilson, E.G. Mechanisms of tubulointerstitial fibrosis. J. Am. Soc. Nephrol. 2010;21(11):1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 120.Acloque H., Adams M.S., Fishwick K., Bronner-Fraser M., Nieto M.A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 2009;119(6):1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010;21(2):212–222. doi: 10.1681/ASN.2008121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim C.S., Joo S.Y., Lee K.E., Choi J.S., Bae E.H., Ma S.K., Kim S.H., Lee J., Kim S.W. Paricalcitol attenuates 4-hydroxy-2-hexenal-induced inflammation and epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. PLoS One. 2013;8(5):e63186. doi: 10.1371/journal.pone.0063186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y., Spataro B.C., Yang J., Dai C., Liu Y. 1,25-dihydroxyvitamin D inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005;68(4):1500–1510. doi: 10.1111/j.1523-1755.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 124.Yang L., Fan Y., Zhang X., Huang W., Ma J. 1,25(OH)2D3 treatment attenuates high glucoseinduced peritoneal epithelial to mesenchymal transition in mice. Mol. Med. Rep. 2017;16(4):3817–3824. doi: 10.3892/mmr.2017.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang L., Wu L., Zhang X., Hu Y., Fan Y., Ma J. 1,25(OH)2D3/VDR attenuates high glucoseinduced epithelialmesenchymal transition in human peritoneal mesothelial cells via the TGFbeta/Smad3 pathway. Mol. Med. Rep. 2017;15(4):2273–2279. doi: 10.3892/mmr.2017.6276. [DOI] [PubMed] [Google Scholar]
- 126.Chung S., Kim S., Kim M., Koh E.S., Shin S.J., Park C.W., Chang Y.S., Kim H.S. Treatment combining aliskiren with paricalcitol is effective against progressive renal tubulointerstitial fibrosis via dual blockade of intrarenal renin. PLoS One. 2017;12(7):e0181757. doi: 10.1371/journal.pone.0181757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiong M., Gong J., Liu Y., Xiang R., Tan X. Loss of vitamin D receptor in chronic kidney disease: A potential mechanism linking inflammation to epithelial-to-mesenchymal transition. Am. J. Physiol. Renal Physiol. 2012;303(7):F1107–F1115. doi: 10.1152/ajprenal.00151.2012. [DOI] [PubMed] [Google Scholar]
- 128.Bienaime F., Girard D., Anglicheau D., Canaud G., Souberbielle J.C., Kreis H., Noel L.H., Friedlander G., Elie C., Legendre C., Prie D. Vitamin D status and outcomes after renal transplantation. J. Am. Soc. Nephrol. 2013;24(5):831–841. doi: 10.1681/ASN.2012060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang W., Yi B., Zhang K., Li A., Yang S., Huang J., Liu J., Zhang H. 1,25-(OH)2D3 and its analogue BXL-628 inhibit high glucose-induced activation of RhoA/ROCK pathway in HK-2 cells. Exp. Ther. Med. 2017;13(5):1969–1976. doi: 10.3892/etm.2017.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wan J., Li P., Liu D.W., Chen Y., Mo H.Z., Liu B.G., Chen W.J., Lu X.Q., Guo J., Zhang Q., Qiao Y.J., Liu Z.S., Wan G.R. GSK-3beta inhibitor attenuates urinary albumin excretion in type 2 diabetic db/db mice, and delays epithelial-to-mesenchymal transition in mouse kidneys and podocytes. Mol. Med. Rep. 2016;14(2):1771–1784. doi: 10.3892/mmr.2016.5441. [DOI] [PubMed] [Google Scholar]
- 131.Dankers W., Colin E.M., van Hamburg J.P., Lubberts E. Vitamin D in autoimmunity: Molecular mechanisms and therapeutic potential. Front. Immunol. 2017;7:697. doi: 10.3389/fimmu.2016.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Branisteanu D.D., Leenaerts P., van Damme B., Bouillon R. Partial prevention of active Heymann nephritis by 1 alpha, 25 dihydroxyvitamin D3. Clin. Exp. Immunol. 1993;94(3):412–417. doi: 10.1111/j.1365-2249.1993.tb08210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lucisano S., Arena A., Stassi G., Iannello D., Montalto G., Romeo A., Costantino G., Lupica R., Cernaro V., Santoro D., Buemi M. Role of paricalcitol in modulating the immune response in patients with renal disease. Int. J. Endocrinol. 2015;2015:765364. doi: 10.1155/2015/765364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arnson Y., Amital H., Shoenfeld Y. Vitamin D and autoimmunity: New aetiological and therapeutic considerations. Ann. Rheum. Dis. 2007;66(9):1137–1142. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ferreira G.B., Vanherwegen A.S., Eelen G., Gutierrez A.C., Van Lommel L., Marchal K., Verlinden L., Verstuyf A., Nogueira T., Georgiadou M., Schuit F., Eizirik D.L., Gysemans C., Carmeliet P., Overbergh L., Mathieu C. Vitamin D3 induces tolerance in human dendritic cells by activation of intracellular metabolic pathways. 2015. [DOI] [PubMed]
- 136.Chen S., Sims G.P., Chen X.X., Gu Y.Y., Lipsky P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 137.Geldmeyer-Hilt K., Heine G., Hartmann B., Baumgrass R., Radbruch A., Worm M. 1,25-dihydroxyvitamin D3 impairs NF-kappaB activation in human naive B cells. Biochem. Biophys. Res. Commun. 2011;407(4):699–702. doi: 10.1016/j.bbrc.2011.03.078. [DOI] [PubMed] [Google Scholar]
- 138.von Essen M.R., Kongsbak M., Schjerling P., Olgaard K., Odum N., Geisler C. Vitamin D controls T cell antigen receptor signaling and activation of human T cells. Nat. Immunol. 2010;11(4):344–349. doi: 10.1038/ni.1851. [DOI] [PubMed] [Google Scholar]
- 139.Havasi A., Borkan S.C. Apoptosis and acute kidney injury. Kidney Int. 2011;80(1):29–40. doi: 10.1038/ki.2011.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia I.M., Altamirano L., Mazzei L., Fornes M., Molina M.N., Ferder L., Manucha W. Role of mitochondria in paricalcitol-mediated cytoprotection during obstructive nephropathy. Am. J. Physiol. Renal Physiol. 2012;302(12):F1595–F1605. doi: 10.1152/ajprenal.00617.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tian X., Ma S., Wang Y., Hou L., Shi Y., Yao M., Wang X., Zhang H., Jiang L. Effects of placental ischemia are attenuated by 1,25-dihydroxyvitamin D treatment and associated with reduced apoptosis and increased autophagy. DNA Cell Biol. 2016;35(2):59–70. doi: 10.1089/dna.2015.2885. [DOI] [PubMed] [Google Scholar]
- 142.Mizushima N., Levine B., Cuervo A.M., Klionsky D.J. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaushal G.P., Shah S.V. Autophagy in acute kidney injury. Kidney Int. 2016;89(4):779–791. doi: 10.1016/j.kint.2015.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kitada M., Ogura Y., Monno I., Koya D. Regulating Autophagy as a Therapeutic Target for Diabetic Nephropathy. Curr. Diab. Rep. 2017;17(7):53. doi: 10.1007/s11892-017-0879-y. [DOI] [PubMed] [Google Scholar]
- 145.Lenoir O., Tharaux P.L., Huber T.B. Autophagy in kidney disease and aging: Lessons from rodent models. Kidney Int. 2016;90(5):950–964. doi: 10.1016/j.kint.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 146.Suh H.W., Kim J.K., Kim T.S., Jo E.K. New insights into vitamin D and autophagy in inflammatory bowel diseases. Curr. Med. Chem. 2017;24(9):898–910. doi: 10.2174/0929867323666161202151856. [DOI] [PubMed] [Google Scholar]
- 147.Cui C., Cui J., Jin F., Cui Y., Li R., Jiang X., Tian Y., Wang K., Jiang P., Gao J. Induction of the vitamin D receptor attenuates autophagy dysfunction-mediated cell death following traumatic brain injury. Cell. Physiol. Biochem. 2017;42(5):1888–1896. doi: 10.1159/000479571. [DOI] [PubMed] [Google Scholar]
- 148.Tavera-Mendoza L.E., Westerling T., Libby E., Marusyk A., Cato L., Cassani R., Cameron L.A., Ficarro S.B., Marto J.A., Klawitter J., Brown M. Vitamin D receptor regulates autophagy in the normal mammary gland and in luminal breast cancer cells. Proc. Natl. Acad. Sci. USA. 2017;114(11):E2186–E2194. doi: 10.1073/pnas.1615015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy. 2016;12(6):1057–1058. doi: 10.1080/15548627.2015.1072670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei H., Qu H., Wang H., Ji B., Ding Y., Liu D., Duan Y., Liang H., Peng C., Xiao X., Deng H. 1,25-Dihydroxyvitamin-D3 prevents the development of diabetic cardiomyopathy in type 1 diabetic rats by enhancing autophagy via inhibiting the beta-catenin/TCF4/GSK-3beta/mTOR pathway. J. Steroid Biochem. Mol. Biol. 2017;168:71–90. doi: 10.1016/j.jsbmb.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 151.Zhao M., Duan X.H., Wu Z.Z., Gao C.C., Wang N., Zheng Z.H. Severe vitamin D deficiency affects the expression of autophagy related genes in PBMCs and T-cell subsets in active systemic lupus erythematosus. Am. J. Clin. Exp. Immunol. 2017;6(4):43–51. [PMC free article] [PubMed] [Google Scholar]
- 152.Yang S., Han Y., Liu J., Song P., Xu X., Zhao L., Hu C., Xiao L., Liu F., Zhang H., Sun L. Mitochondria: A novel therapeutic target in diabetic nephropathy. Curr. Med. Chem. 2017;24(29):3185–3202. doi: 10.2174/0929867324666170509121003. [DOI] [PubMed] [Google Scholar]
- 153.Bhargava P., Schnellmann R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017;13(10):629–646. doi: 10.1038/nrneph.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ralto K.M., Parikh S.M. Mitochondria in acute kidney injury. Semin. Nephrol. 2016;36(1):8–16. doi: 10.1016/j.semnephrol.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hajnoczky G., Csordas G., Das S., Garcia-Perez C., Saotome M., Sinha Roy S., Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40(5-6):553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rossier M.F. T channels and steroid biosynthesis: In search of a link with mitochondria. Cell Calcium. 2006;40(2):155–164. doi: 10.1016/j.ceca.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 157.Silvagno F., De Vivo E., Attanasio A., Gallo V., Mazzucco G., Pescarmona G. Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS One. 2010;5(1):e8670. doi: 10.1371/journal.pone.0008670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Silvagno F., Consiglio M., Foglizzo V., Destefanis M., Pescarmona G. Mitochondrial translocation of vitamin D receptor is mediated by the permeability transition pore in human keratinocyte cell line. PLoS One. 2013;8(1):e54716. doi: 10.1371/journal.pone.0054716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang S.K., Xiao L., Li J., Liu F., Sun L. Oxidative stress, a common molecular pathway for kidney disease: Role of the redox enzyme p66Shc. Ren. Fail. 2014;36(2):313–320. doi: 10.3109/0886022X.2013.846867. [DOI] [PubMed] [Google Scholar]
- 160.Garcia I.M., Altamirano L., Mazzei L., Fornes M., Cuello-Carrion F.D., Ferder L., Manucha W. Vitamin D receptor-modulated Hsp70/AT1 expression may protect the kidneys of SHRs at the structural and functional levels. Cell Stress Chaperones. 2014;19(4):479–491. doi: 10.1007/s12192-013-0474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ricciardi C.J., Bae J., Esposito D., Komarnytsky S., Hu P., Chen J., Zhao L. 1,25-Dihydroxyvitamin D3/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. Eur. J. Nutr. 2015;54(6):1001–1012. doi: 10.1007/s00394-014-0778-9. [DOI] [PubMed] [Google Scholar]
- 162.Sawada K., Wu-Wong J.R., Chen Y.W., Wessale J.L., Kanai G., Kakuta T., Fukagawa M. Vitamin D receptor agonist VS-105 directly modulates parathyroid hormone expression in human parathyroid cells and in 5/6 nephrectomized rats. J. Steroid Biochem. Mol. Biol. 2017;167:48–54. doi: 10.1016/j.jsbmb.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 163.Pandey R., Zella J., Clagett-Dame M., Plum L.A., DeLuca H.F., Coyne D.W. Use of 2MD, a Novel Oral Calcitriol Analog, in Hemodialysis Patients with Secondary Hyperparathyroidism. Am. J. Nephrol. 2016;43(3):213–220. doi: 10.1159/000445756. [DOI] [PubMed] [Google Scholar]
- 164.Fujii H., Nakai K., Yonekura Y., Kono K., Goto S., Hirata M., Shinohara M., Nishi S., Fukagawa M. The vitamin D receptor activator maxacalcitol provides cardioprotective effects in diabetes mellitus. Cardiovasc. Drugs Ther. 2015;29(6):499–507. doi: 10.1007/s10557-015-6629-y. [DOI] [PubMed] [Google Scholar]
- 165.Xie Y., Su P., Sun Y., Zhang H., Zhao R., Li L., Meng L. Comparative efficacy and safety of paricalcitol versus vitamin D receptor activators for dialysis patients with secondary hyperparathyroidism: a meta-analysis of randomized controlled trials. BMC Nephrol. 2017;18(1):272. doi: 10.1186/s12882-017-0691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Coronel F., Rodriguez-Cubillo B., Cigarran S., Gomis A. Effects of oral paricalcitol on hyperparathyroidism and proteinuria in peritoneal dialysis patients. Adv. Perit. Dial. 2011;27:130–133. [PubMed] [Google Scholar]
- 167.Keyzer C.A., de Jong M.A., Fenna van Breda G., Vervloet M.G., Laverman G.D., Hemmelder M., Janssen W.M., Lambers Heerspink H.J., Navis G., de Borst M.H. Vitamin D receptor activator and dietary sodium restriction to reduce residual urinary albumin excretion in chronic kidney disease (ViRTUE study): Rationale and study protocol. Nephrol. Dial. Transplant. 2016;31(7):1081–1087. doi: 10.1093/ndt/gfv033. [DOI] [PubMed] [Google Scholar]
- 168.de Zeeuw D., Agarwal R., Amdahl M., Audhya P., Coyne D., Garimella T., Parving H.H., Pritchett Y., Remuzzi G., Ritz E., Andress D. Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): a randomised controlled trial. Lancet. 2010;376(9752):1543–1551. doi: 10.1016/S0140-6736(10)61032-X. [DOI] [PubMed] [Google Scholar]
- 169.Alborzi P., Patel N.A., Peterson C., Bills J.E., Bekele D.M., Bunaye Z., Light R.P., Agarwal R. Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension. 2008;52(2):249–255. doi: 10.1161/HYPERTENSIONAHA.108.113159. [DOI] [PubMed] [Google Scholar]
- 170.Brown J.M., Secinaro K., Williams J.S., Vaidya A. Evaluating hormonal mechanisms of vitamin D receptor agonist therapy in diabetic kidney disease: The VALIDATE-D study. BMC Endocr. Disord. 2013;13:33. doi: 10.1186/1472-6823-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Keyzer C.A., van Breda G.F., Vervloet M.G., de Jong M.A., Laverman G.D., Hemmelder M.H., Janssen W.M., Lambers Heerspink H.J., Kwakernaak A.J., Bakker S.J., Navis G., de Borst M.H. Effects of vitamin D receptor activation and dietary sodium restriction on residual albuminuria in CKD: The ViRTUE-CKD Trial. J. Am. Soc. Nephrol. 2017;28(4):1296–1305. doi: 10.1681/ASN.2016040407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Santoro D., Gagliostro G., Alibrandi A., Ientile R., Bellinghieri G., Savica V., Buemi M., Caccamo D. Vitamin D receptor gene polymorphism and left ventricular hypertrophy in chronic kidney disease. Nutrients. 2014;6(3):1029–1037. doi: 10.3390/nu6031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Inaguma D., Tanaka A., Shinjo H., Kato A., Murata M. Predialysis vitamin D receptor activator treatment and cardiovascular events after dialysis initiation: A multicenter observational study. Nephron. 2016;133(1):35–43. doi: 10.1159/000445507. [DOI] [PubMed] [Google Scholar]
- 174.Negri A.L. Association of oral calcitriol with improved survival in non-dialysed and dialysed patients with CKD. Nephrol. Dial. Transplant. 2009;24(2):341–344. doi: 10.1093/ndt/gfn624. [DOI] [PubMed] [Google Scholar]
- 175.Messa P., Cozzolino M., Brancaccio D., Cannella G., Malberti F., Costanzo A.M., di Luzio Paparatti U., Festa V., Gualberti G., Mazzaferro S. Effect of VDRA on survival in incident hemodialysis patients: Results of the FARO-2 observational study. BMC Nephrol. 2015;16:11. doi: 10.1186/s12882-015-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Obi Y., Hamano T., Wada A., Tsubakihara Y. Vitamin D receptor activator use and cause-specific death among dialysis patients: A nationwide cohort study using coarsened exact matching. Sci. Rep. 2017;7:41170. doi: 10.1038/srep41170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Naves-Diaz M., Alvarez-Hernandez D., Passlick-Deetjen J., Guinsburg A., Marelli C., Rodriguez-Puyol D., Cannata-Andia J.B. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74(8):1070–1078. doi: 10.1038/ki.2008.343. [DOI] [PubMed] [Google Scholar]