Abstract
Background:
The observation that N-methyl-D-aspartate glutamate receptor (NMDAR) antagonists such as ketamine transiently induce schizophrenia-like positive, negative and cognitive symptoms has led to a paradigm shift from dopaminergic to glutamatergic dysfunction in pharmacological models of schizophrenia. NMDAR hypofunction can explain many schizophrenia symptoms directly due to excitatory-to-inhibitory (E/I) imbalance, but also dopaminergic dysfunction itself. However, so far no new drug targeting the NMDAR has been successfully approved. In the search for possible biomarkers it is interesting that ketamine-induced psychopathological changes in healthy participants were accompanied by altered electro-(EEG), magnetoencephalographic (MEG) and functional magnetic resonance imaging (fMRI) signals.
Methods:
We systematically searched PubMed/Medline and Web of Knowledge databases (January 2006 to July 2017) to identify EEG/MEG and fMRI studies of the ketamine model of schizophrenia with human subjects. The search strategy identified 209 citations of which 46 articles met specified eligibility criteria.
Results:
In EEG/MEG studies, ketamine induced changes of event-related potentials, such as the P300 potential and the mismatch negativity, similar to alterations observed in schizophrenia patients. In fMRI studies, alterations of activation were observed in different brain regions, most prominently within the anterior cingulate cortex and limbic structures as well as task-relevant brain regions. These alterations were accompanied by changes in functional connectivity, indicating a balance shift of the underlying brain networks. Pharmacological treatments did alter ketamine-induced changes in EEG/MEG and fMRI studies to different extents.
Conclusion:
This review highlights the potential applicability of the ketamine model for schizophrenia drug development by offering the possibility to assess the effect of pharmacological agents on schizophrenia-like symptoms and to find relevant neurophysiological and neuroimaging biomarkers.
Keywords: EEG, fMRI, ketamine, MEG, NMDA-receptor, schizophrenia
1. INTRODUCTION
The pharmacotherapy of schizophrenia has basically not changed in more than sixty years and the treatment of the disease remains challenging. Pharmacological interventions are representing the main treatment option, nevertheless, high relapse rates and chronic manifestations hinder effective treatment [1]. To date, antipsychotic drugs remain the only approved pharmacological treatment [2]. Despite their classification as typical and atypical drugs or allocation to different generations, all target the dopamine system [3]. Attenuation of this system allows the treatment of positive symptoms like hallucinations or delusions with a satisfactory clinical benefit. This treatment effect is thought to be based on antagonistic effects at dopamine receptors in brain regions showing excessive dopaminergic neurotransmission [4]. Nevertheless, the positive symptoms of one-third of patients with schizophrenia do not respond to antipsychotic medication [1].
Moreover, the impact of the treatment with dopamine antagonists on negative and cognitive symptoms is negligible in most cases [2]. This emphasizes the need for developing new pharmacological agents, preferably targeting other neurotransmitter systems. One system of particular interest is the glutamate system, implicated to be significantly involved in the pathogenesis of schizophrenia [2].
The glutamate hypothesis of schizophrenia is based on findings of increased symptom severity in patients with schizophrenia and the emergence of schizophrenia-like positive and negative symptoms in healthy subjects evoked by N-methyl-D-aspartate glutamate receptor (NMDAR) antagonists [5, 6]. It is further substantiated by findings of an impaired expression of NMDAR-related genes in schizophrenia patients possibly resulting in NMDAR hypofunction [7]. It is suggested that NMDARs are also crucially involved in the excitation of fast-spiking parvalbumin-positive gamma-aminobutyric acid (GABA) interneurons. Thus, an NMDAR hypofunction might result in a reduced excitation of GABAergic interneurons and, subsequently, in a disinhibition of pyramidal cells [8].
Based on the glutamate hypothesis of schizophrenia, several promising drugs targeting the glutamate system were developed, e.g. bitopertin [9], a glycine transporter-1 (GlyT-1) inhibitor, or LY2140023 [10], a positive allosteric modulator of group II metabotropic glutamate receptors [11, 12]. Despite promising pre-clinical studies, all candidates have failed to reach the clinic so far.
Pharmacological models of schizophrenia, such as drug-induced psychosis-like states, could facilitate drug research and development by providing a way to ascertain functional target engagement and the ability to prioritize candidate drugs [13]. Antagonists of glutamatergic neurotransmission such as ketamine are promising substances for this approach.
In the late 1950’s, phencyclidine (PCP), a general anesthetic acting as an antagonist of the NMDAR, has been observed to induce schizophrenia-like symptoms [5]. Subsequently, ketamine, a derivative of PCP, was demonstrated to elicit comparable symptoms while offering an improved safety profile [6]. In addition, ketamine worsened positive, negative as well as cognitive symptoms in unmedicated patients with schizophrenia [14, 15].
The ketamine-induced schizophrenia-like behavioral and psychopathological changes in healthy subjects were accompanied by altered electro- (EEG) and magnetoencephalographic (MEG) biomarkers (e.g. “Mismatch Negativity” (MMN) and the N100 component of the auditory evoked potential), reminiscent of findings observed in patients with schizophrenia [13].
At the molecular level, ketamine has been shown to impact the dopamine-glutamate interplay in the postsynaptic density of glutamatergic excitatory synapses [16]. Recently, treatment strategies modulating glutamatergic neurotransmission have also been implemented in the therapy of mood disorders [17, 18].
In order to assess the effects of promising candidate drugs for new treatment strategies in schizophrenia, there is an urgent need to identify further biomarkers found to be altered both in patients with schizophrenia and in pharmacological models of the disease such as the ketamine model of schizophrenia. This might offer the opportunity to assess the effects of preclinical/clinical drugs on ketamine-induced pathological changes of such biomarkers to identify candidate drugs for the treatment of schizophrenia.
Recent reviews focused on the interspecies applicability of neurophysiological biomarkers elicited by ketamine [19] as well as on the results of ketamine challenges supporting the glutamate hypothesis of schizophrenia [20]. However, pharmacological interventions in the ketamine model have not been reviewed to date.
Therefore, we aim to review results of EEG, MEG and functional magnetic resonance imaging (fMRI) studies using the ketamine model of schizophrenia in order to survey and integrate the literature on possible biomarkers in the ketamine model and the effect of pharmacological interventions thereon. This systematic review includes ketamine studies with healthy human subjects published within the past decade. The objective is to identify potential biomarkers in the ketamine model and compare the findings on the respective biomarkers in patients with schizophrenia. Further, we evaluate the effect of different pharmacological interventions on different biomarkers in the ketamine model to evaluate its applicability. The first section of this review comprises resting state (RS) studies, followed by a section on task-specific activations. The last section focuses on different pharmacological interventions in the ketamine model of schizophrenia and their effect on ketamine-induced alterations of schizophrenia neuroimaging biomarkers.
2. Methods
The review includes experimental studies that fulfilled the following criteria: (i) inclusion of healthy human subjects (ii) treatment with sub-anesthetic doses of ketamine (either racemic or esketamine) (iii) reporting of either EEG/MEG or fMRI results (iv) publication between January 2006 and July 2017 in a peer-reviewed journal. We excluded all studies that did not report their results in English.
The PubMed/Medline and Web of Science search was conducted using the keywords ‘ketamine’ in combination with ‘EEG’ or ‘MEG’ or ‘fMRI’ using the filter ‘humans’. The initial search yielded 448 results (Fig. 1). After removing duplicates, 208 studies remained. The screening of the titles and abstracts for eligibility led to 55 articles that were reviewed in full text of which we had to exclude 10 for the following reasons: acquisition of neuroimages without ketamine challenge (n=3); exposure of major depressive disorder (MDD) patients to ketamine (n=3); applying of pain stimuli during neuroimaging (n=2); use of an aesthetic ketamine dose (n=1); methodological paper (n=1); structural neuroimaging (n=1), This led to the inclusion of 45 articles. In addition, we included one article [21] that met our inclusion criteria and was used as a reference in another included article.
Fig. (1).
PRISMA flowchart detailing selection of studies included in the systematic review.
3. Results
3.1. Resting State
The brain’s resting state (RS) is defined as the baseline brain activity in resting wakefulness and the absence of stimulus- or task-evoked brain activation. In patients with schizophrenia increased high-frequency oscillatory neuronal RS activity (reduced signal-to-noise ratio) [22] and reduced RS functional connectivity strength accompanied by increases in the heterogeneity of the underlying functional connections were found [23]. It is suggested that these observations reflect cortical and hippocampal dysfunctions as well as less strongly integrated functional connectivity in schizophrenia [22, 23].
3.1.1. EEG and MEG
EEG and MEG are non-invasive neuroimaging techniques that rely on voltage fluctuations, elicited by summation of postsynaptic potentials. The examination of the electrical activity of the brain or the emerging magnetic fields, respectively, allows for a high temporal resolution. Both techniques enable the analysis of oscillatory neuronal activity in different frequency ranges (delta [0-4 Hz], theta [4-8 Hz], alpha [8-13 Hz], beta [13-30 Hz] and gamma [above 30 Hz]). There is now increasing and converging evidence from anatomical, physiological and electrophysiological studies suggesting that biological mechanisms that are disturbed in schizophrenia including glutamatergic and GABAergic transmission are directly linked to the generation of neural oscillations [8].
A recent RS MEG study [24] demonstrated that ketamine compared to placebo increases parietal and cingulate gamma-band and medial prefrontal theta-band source power, whereas anterior cingulate, occipital and parietal alpha-band source power was reduced. Additionally, analysis of effective connectivity - investigating directed interactions between different brain areas [25] - demonstrated reduced frontoparietal effective connectivity under ketamine administration. Several models were applied to explain the observed effect. Dynamic causal modelling (DCM) revealed a best fitting model involving reciprocal connections between the precuneus and the prefrontal cortex. In DCM, estimations of effective connectivity between neuronal ensembles in linked brain regions and the alterations of effective connectivity under the application of pharmacological agents are based on biophysical models that are fit to empirical data [26, 27]. Further analyses using this model indicated a ketamine-induced modulation of NMDA- and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated backward connectivity. Functional connectivity analysis is based on correlation or coherence of activation in different brain regions [25]. Applying this technique significant alterations in visual parietal and motor networks under ketamine were observed [24].
Rivolta et al. [28] reported an increase in frontal, parietal and temporal gamma-band power under ketamine administration compared to placebo in a single-blind RS investigation. Gamma-band sources were found in various cortical (frontal and temporal cortex) and subcortical regions (thalamus and hippocampus). In addition, the increase of gamma power in the right hippocampus correlated negatively with the Positive symptom subscale of the Positive and Negative Syndrome Scale (PANSS). In contrast, central beta-band (13-30 Hz) power was reduced for various sources. Regarding functional connectivity, an increased coupling in a thalamocortical network was observed [28]. Sanacora et al. [29] also demonstrated ketamine-induced increases in RS gamma-band power in a double-blind, placebo-controlled study. In addition, the alpha-wave index (a vigilance-indicative) and theta-cordance (an EEG measure linked to brain perfusion) were decreased. In this study lanicemine, a highly selective NMDA-antagonist, induced increases in gamma-band power without producing significant dissociative symptoms [29]. Theta-cordance reduction under ketamine in comparison to placebo was also reported by another study in prefrontal regions, whereas central regions indicated increased theta-cordance [30]. In a recent randomized, placebo-controlled, double-blind RS EEG-study de la Salle et al. [31] found widespread reductions of activity in scalp surface delta and alpha oscillations measured by means of current source density (CSD) under the influence of ketamine compared to placebo. Theta CSD was only reduced in posterior regions, whereas gamma CSD showed an overall increase (no effect on beta CSD). These findings were accompanied by frequency-specific CSD alterations within hubs of the default mode network (DMN) (ventromedial prefrontal cortex [vmPFC] and posterior cingulate cortex [PCC]), the Central Execution Network (CEN) (posterior parietal cortex [PPC]) and the Salience Network (SN) (anterior cingulate cortex [ACC] and anterior insula). The amount of alpha activity was negatively correlated with the severity of ketamine-induced symptoms [31]. The authors suggest their findings on gamma oscillations to be reminiscent of EEG alterations in schizophrenia and to be based on NMDAR hypofunction. Specifically, inhibition of GABAergic, parvalbumin-positive interneurons and subsequent disinhibition of pyramidal neurons is suggested to be the underlying mechanism of increased RS gamma oscillations following acute ketamine application. The authors discuss this phenomenon in terms of increased “background noise” resulting in a decreased signal-to-noise ratio and, therefore, hindering the ability to estimate the relevance of certain information, which is also known as a common symptom in the early stages of schizophrenia [28, 29, 31]. Furthermore, the authors discuss their results in light of the three-network model of schizophrenia, which suggests a disparity of the normally anti-correlated DMN and CEN, possibly arising from abnormalities in the nodes of the SN, such as the ACC. This disparity could lead to core symptoms of schizophrenia by diminishing the boundaries of internal and external mental contents [31].
In summary, findings of increased gamma band and reduced alpha band power were consistent in the reviewed RS studies. In line with this, patients with schizophrenia demonstrate increased RS gamma-activity [22] and reduced RS alpha-band power [32]. Notably, an increase in resting state gamma band power has been shown to be positively correlated with positive symptom severity in schizophrenia patients [33, 34].
3.1.2. fMRI
fMRI is a neuroimaging technique for measuring brain activity via changes in local blood-oxygenation levels. Increases of the blood-oxygen-level-dependent (bold) response resemble an activation of the respective brain regions.
Compared to placebo, Hoflich et al. [35] observed ketamine-induced bold increases in the dorsal ACC, the midcingulate cortex (MCC), the bilateral thalamus and insula, as well as temporal and frontal regions in a randomized, double-blind RS study. The ketamine-induced activation in the right insula correlated positively with the 5-Dimensional Altered States of Consciousness Rating Scale (5D-ASC) subscales anxious ego-dissolution and auditory alterations. Concurrently, decreased activation was observed in a cluster extending from the gyrus rectus into the subgenual anterior cingulate cortex (sgACC) and orbifrontal cortex (OFC) [35]. In line with these findings, cerebral blood flow (CBF) was increased in the right ACC and right vmPFC under a low ketamine dose (target plasma level: 50-75ng/ml) in an investigation assessing the CBF by arterial spin labeling (ASL) [36]. The observed increases in CBF seemed to be dose-dependent since in the high-dose (target plasma level: 150ng/ml) condition significant increases in CBF were restricted to the subgenual part of the right ACC. Decreases of CBF were revealed in the left retrosubicular hippocampal area for the low ketamine dose. In the high-dose condition decreases reached significance in the right superior temporal cortex. Furthermore, changes of CBF in areas associated with sensory processing correlated with perceptual abnormalities. In contrast, a different study [37] demonstrated a decreased bold response in the sgACC after ketamine administration compared to a baseline scan. Increased activation was observed in multiple brain regions comprising the cingulate gyrus, hippocampus, insula, thalamus and midbrain. bold response in the paracentral lobe correlated positively with Psychotomimetic Scales Inventory (PSI) [38] subscales Perceptual Distortion and Delusional Thinking [37]. De Simoni et al. [39] reported a vast midline activation under the influence of ketamine during the RS comprising e.g. the anterior and posterior cingulate cortex, precuneus, bilateral thalamus and anterior insula. Conversely, decreased activation was shown for the sgACC. For these ketamine-induced changes a robust test-retest reliability was reported [39]. These studies identify the ACC (dorsal and subgenual) as a key structure for ketamine-induced changes in perception. The authors discuss the heterogenous findings in light of the different temporal dynamics of the bold contrast and ASL [35-37]. Utilizing the bold contrast, it is possible to measure changes in activation immediately after the initiation of a ketamine-infusion whereas ASL allows the measurement of steady-state activation [36, 37]. Thus, it could be hypothesized that ketamine induces transitory reduced activation in the sgACC shortly after infusion. Notably, hyper- as well as hypo-connectivity in ACC networks has also been reported for first-episode schizophrenia patients linking ACC dysfunction with schizophrenia symptoms [40].
Focusing on the sgACC, Wong et al. [41] observed a reduced connectivity of this region with a large brain region spanning across the hippocampus, the retrosplenial cortex (RSC) and the thalamus following ketamine administration. A negative correlation was found between ketamine-induced schizophrenia-like symptoms (PANSS general score) and sgACC connectivity with the medial prefrontal cortex as well as the subcallosal gyrus [41]. Complementarily, another double-blind, placebo-controlled study demonstrated increased coupling between the left and right dorsolateral prefrontal cortex (DLPFC) and the left hippocampus after administration of ketamine in comparison to placebo [42].
Moreover, ketamine has been shown to increase thalamus connectivity with a bilateral cluster spanning from the parietal lobule towards the temporal cortex [43]. This double-blind, placebo-controlled study also revealed a ketamine-induced increased functional connectivity for two cortico-thalamic pathways, namely between the somatosensory cortex and ventrolateral thalamus and between temporal cortex and the mediodorsal, anteroventral and anterolateral areas of the thalamus [43].
Compared to placebo in a randomized, single-blind, seed-based investigation, ketamine also increased striatal functional connectivity in two networks connecting (1) the dorsal caudate, the thalamic region and the midbrain and (2) connecting the ventral striatum with the left anterior and vmPFC [44]. These increases in functional connectivity correlated with ketamine-induced psychotic and dissociative symptoms. [44].
Following administration of ketamine, an increased overall functional connectivity, assessed as Global based connectivity (GBC), compared to placebo was shown by Driesen et al. [45]. These findings were accompanied by a negative correlation between negative symptoms assessed by terms of the PANSS and an increase of region-specific GBC in the medial and dorsal anterior striatum as well as the thalamus. Furthermore, a positive correlation between the PANSS positive subscale and the region-specific GBC increase was detected. These effects were most prominent for the right insula, the right planum temporale, the bilateral pulvinar nuclei, the left lingual gyrus and the anterior cerebellar vermis [45].
In line with prior findings, Khalili-Mahani et al. [46] revealed a reduced CBF in the right hippocampus, bilateral putamen and sensorimotor areas, the medial visual cortex and cerebellum after ketamine administration in a single-blind, placebo-controlled RS investigation. Increases in CBF were demonstrated for the medial as well as the dorsolateral prefrontal cortex. Regarding hippocampal functional connectivity, emerging connectivities under ketamine were found between the hippocampal head and the insula as well as the medial visual and posterior parietal cortex. Hyper-connectivity under ketamine was most prominently detected for a network containing the cingulate cortex and the hippocampal head. Further increases in functional connectivity were demonstrated from the hippocampus body to the premotor and the lateral visual cortex as well as the superior precuneus [46]. Contrary to these findings of increased hippocampal connectivity under ketamine, Kraguljac et al. [47] reported a ketamine-induced decrease of hippocampal functional connectivity with a frontal cluster involving the ACC, MCC and medial prefrontal cortex and a large temporo-parietal cluster in a placebo-controlled study. Regarding the frontal cluster, the authors found a negative correlation between hippocampus connectivity and hippocampal glutamate and glutamine (Glx) levels under ketamine in terms of magnetic resonance spectroscopy (MRS) [47]. The authors link the hyperglutamatergic hippocampal state with hippocampal-frontal connectivity, suggesting a crucial role of NMDA-mediated transmission in this region. On the other hand, aberrant thalamo-/striatal-hippocampal connectivity as found in patients with schizophrenia could not be replicated, possibly due to the major involvement of the dopaminergic system [47].
To summarize, most of the RS studies consistently reported a ketamine-induced activation of the dorsal part of the ACC, while the subgenual part of the ACC seems to be deactivated by ketamine. Most of the studies discuss these findings as a possible link between schizophrenia symptomatology and aberrant ACC function. Focusing on subcortical structures, the insula mostly showed an increased activity under ketamine administration while regarding other regions such as the thalamus and the hippocampus inconsistent results have been reported.
Most fMRI studies investigating functional connectivity during the brain’s RS demonstrated an increase of connectivity under ketamine administration, while all studies revealed functional connectivity alterations. Notably, alterations in functional connectivity are also observed in patients with schizophrenia [23, 48-50]. The alterations of connectivity patterns have mainly been interpreted with respect to activation shifts between the DMN and CEN [23, 48]. Generally, the thalamus and striatal regions seem to show a ketamine-induced increase of RS connectivity with several other brain regions, whereas for ketamine-induced changes of the hippocampal connectivity inconsistent results have been reported. These inconsistencies correspond to the heterogeneity of findings on a disturbed hippocampus connectivity in patients with schizophrenia [51].
3.2. Task-specific Activation
3.2.1. EEG and MEG
The Mismatch Negativity (MMN) is an event-related potential (ERP) generated by deviant sensory stimuli after a succession of identical standard stimuli. The deviant stimuli differ from standard stimuli with respect to physical features such as duration or intensity in case of auditory stimuli. Thus, a largely accepted theory of the MMN suggests that the MMN reflects the alignment of the current stimulus with a memory trace of the preceding stimuli. Mainly two prominent cortical generators have been shown to account for the MMN. A generator of the early MMN (110-160ms) has been suggested to be located in the primary auditory cortex (PAC), while the generator of the late MMN (160-210ms) has been localized within the prefrontal cortex [52].
In a double-blind, placebo-controlled EEG ketamine study, Schmidt et al. [53] used a roving auditory oddball paradigm to elicit the MMN. A sequence of sinusoidal tones of the same frequency was followed by a sequence of tones of a different frequency. The first stimulus of each train represents the frequency deviant stimulus. This procedure revealed an increase of MMN amplitudes with an increasing length of the pre-deviant sequence. This finding is referred to as “MMN memory trace effect” and was most pronounced over the frontal electrodes. The authors report that ketamine reduced the MMN amplitude and disrupted the MMN memory trace effect over the frontal electrodes. Moreover, a positive correlation between the baseline MMN slope and the impaired control and cognition subscale of the ASC-R under ketamine was demonstrated [53]. Using a model-based analysis approach Schmidt et al. [54] applied dynamic causal modelling and Bayesian model selection (BMS) on EEG scalp data. These models focused on assumed MMN generators within the bilateral primary auditory cortex and superior temporal gyrus for the early MMN time frame and within the right inferior frontal gyrus for the late MMN. The best fitting model incorporated adaptation in the primary auditory cortices as well as short-term synaptic plasticity in the forward and backward hierarchic auditory connections. In the selected model short-term plasticity but not the adaption was affected by ketamine. The only connection that was altered by ketamine was found in the forward connection from the left primary auditory cortex to the superior temporal gyrus. Furthermore, a negative correlation between the effects on short-term plasticity under ketamine compared to placebo and control and cognition ratings in the subscale of the ASC-R was demonstrated for this connection [54]. In line with these findings, Heekeren et al. [55] also reported a reduction of the auditory MMN amplitude under the influence of ketamine compared to baseline using a double-blind study design. This reduction of the MMN was due to reduced activity in a priori defined sources: the bilateral superior temporal lobes for the early MMN time frame and the right inferior temporal gyrus as well as the anterior cingulate gyrus for the late MMN time frame [55]. In another randomized, placebo-controlled, single-blind EEG-study, Thiebes et al. [56] demonstrated comparable results. Ketamine decreased the auditory MMN amplitude for duration as well as frequency deviants. The extent of ketamine-induced schizophrenia-like negative symptoms predicted the MMN amplitude in the ketamine condition. A MMN source localization approach revealed reduced activity within the STG (whole MMN interval), the PAC (early MMN) and the middle frontal and posterior cingulate gyrus (late MMN) [56].
The P300 component of ERPs is a large positive deflection typically found around 300ms after the presentation of an auditory or visual target-stimulus. It is thought to be elicited by processes of decision making or categorizing as a novel (P3a) or target (P3b) stimuli [57].
In a double-blind, placebo-controlled visual oddball study Watson et al. [58] revealed ketamine-induced reductions of the P3a and the P3b amplitude at parietal electrode sites [58]. Musso et al. [59] also investigated the ketamine effect on the P300 component in a visual oddball task in a randomized, double-blind, placebo-controlled combined EEG and fMRI study. As expected, a reduction of the P300 amplitude was demonstrated after the application of ketamine. Suggested generators of the P300 (current density maxima) were mainly located in the parietooccipital cortex with extensions to medial frontal brain regions [59].
A passive auditory sensory-gating paradigm was used by Boeijinga et al. [60] to assess ketamine effects on the prepulse inhibition in a double-blind, placebo-controlled combined EEG and MEG study. The prepulse inhibition is thought to reflect the processing of sensory information and is disrupted in schizophrenia. In this study, the signal of interest was defined as ∆M100, the difference between M100 with and without a prestimulus click (interstimulus intervals of 100ms or 500ms). A ∆M100 disruption in the bilateral temporoparietal scalp regions was only found after the administration of a high ketamine dose (bolus: 0.27mg/kg, maintenance infusion: 0.135mg/kg/h) and only appeared after the short 100ms inter click-pulse interval. In the EEG ∆N100, as EEG equivalent to the ∆M100, was reduced by ketamine predominantly in left posterior temporal and parietal regions. Furthermore, a weak positive correlation between ∆N100 in the left temporal region and the BPRS rating was revealed [60]. Focusing on the N100 component, Kort et al. investigated ketamine-induced alterations of the N100-suppression during vocalization, a putative measure of auditory predictive coding [61]. Ketamine attenuated N00-suppression during vocalization without affecting N100 amplitudes during listening. Notably, the degree of the N100-suppression under ketamine correlated positively with dissociative symptoms. These results paralleled the disturbances of predictive coding in schizophrenia as seen in the second set of experiments [61].
Gamma oscillations are generated by the synchronized inhibition of pyramidal cells by parvalbumin-positive, gamma-aminobutyric acid (GABA) interneurons and are thought to play a crucial role in cognitive and perceptual functions [62]. The attenuated task-evoked gamma-band response has been demonstrated across all stages of schizophrenia [63-66] and is suggested to constitute a pathomechanism of cognitive dysfunction in schizophrenia [67, 68]. Notably, a recent study demonstrated a relationship between increased interhemispheric gamma-band connectivity and the emergence of auditory verbal distortions and hallucinations under ketamine [69]. Using a visuomotor paradigm in a single-blind, placebo-controlled MEG study, Shaw et al. [70] revealed increased amplitudes of movement-related gamma synchrony and ipsilateral movement-related beta desynchrony elicited by ketamine within the motor cortex. In the visual cortex, an increase in induced gamma-band power was observed for a low contrast visual stimulation. For both contrast conditions, event-related desynchronization in the beta-band was significantly reduced after the administration of ketamine [70]. In line with this finding, there are further studies reporting a ketamine-induced increase of task-induced gamma activity: Hong et al. [71] examined ketamine effects in an auditory click-stimulus paradigm with a double-blind, placebo-controlled design. After a single click (S1) ketamine increased gamma but reduced delta power spectrum density (PSD) at central and frontal sites compared to placebo. Both changes were related to an increase of the withdrawal symptom factor of the BPRS [71].
To conclude, the MMN and P300 paradigms indicate very clear and consistent results. Studies focusing on P300 indicated uniform ketamine-induced reductions of the P3a and P3b amplitude, respectively. Ketamine likewise reduced the MMN amplitude, though not all results reached significance. Notably, components of the MMN correlated with negative and cognitive symptom severity induced by ketamine. These findings are in line with results of schizophrenia studies that have demonstrated reduced P300 and MMN amplitudes in patients [72-75]. Findings of disrupted prepulse inhibition are consistently reported in patients with schizophrenia and have been linked to deficient social perception as well as the severity of auditory hallucinations, in parallel with the results found in the ketamine model [76].
3.2.2. fMRI
Musso et al. [59] revealed decreased bold-responses in the visual cortex, the ACC and the temporoparietal cortex under ketamine during the performance of a visual oddball task. The authors suggest that the distinct activation pattern resembles that of patients with schizophrenia, possibly qualifying the ketamine-induced state as a schizophrenia model [59].
In a recent study, Steffens et al. [77] investigated the influence of ketamine in a smooth-pursuit eye movement (SPEM) task. bold responses found in the placebo condition (primary visual cortex, frontal eye field, motion processing areas, superior parietal lobule and bilateral thalamus) were diminished during ketamine administration within visual and motion-related areas. The authors discuss their findings in light of the notion that dysfunctional connectivity in a frontal-thalamic-cerebellar network causes a deficit in SPEM in schizophrenia patients. The reported results further underline the importance of glutamatergic neurotransmission in smooth-pursuit performance since early visual processing deficits are discussed as underlying causes of many different cognitive deficits in NMDA models of schizophrenia [77].
Using a reward-anticipation task, Francois et al. demonstrated a reduction of activity of the nucleus accumbens (NAc) under the influence of ketamine compared to placebo [78].
Driesen et al. [79] assessed the effects of ketamine on brain activation and functional connectivity in a WM task. During the encoding and maintenance period of the paradigm, ketamine reduced the activity of the bilateral DLPFC. The connectivity between the DLPFC and different brain areas known to be related to WM processes, such as medial and inferior frontal regions, was reduced in the ketamine compared to the placebo condition. Furthermore, moderate negative correlations between WM performance and connectivity changes within DLPFC networks were reported. The authors discuss their findings with respect to evidence showing that NMDAR stimulation is crucially involved in the recurrent excitation of pyramidal cells in the DLPFC. The authors argue that blocking NMDAR most likely led to a dysfunction of the whole WM network [79].
Another placebo-controlled ketamine WM work has been published by Anticevic et al. [80]. During the first set of experiments, regions with either task-based activation or deactivation were identified to second test ketamine-induced changes to these identified regions. During the encoding and delay phase of the WM task, ketamine in comparison to placebo diminished the activity of several WM-related brain regions, whereas activity in regions of the DMN was increased. Activation in DMN regions is typically suppressed during demanding cognitive tasks. In a computational model including these data the inhibition of GABA interneurons by ketamine appeared to account for diminished task-based activation as well as missing DMN deactivation. Local microcircuits seemed to play the dominant role in this model function. Further task-based functional connectivity was assessed in a seed-based analysis focusing on the frontoparietal network and the DMN. A modulation of connectivity between these networks was found during the WM delay phase. Notably, a negative correlation between ketamine-induced schizophrenia-like negative symptoms and the DMN suppression was demonstrated in this work [80]. Honey et al. studied ketamine-induced alterations of brain activation during a verbal WM as well as a continuous performance and sentence completion task in a placebo-controlled investigation [81]. In the verbal WM task ketamine elicited increased activation in the basal ganglia and the thalamus compared to placebo. The degree of activation of the left thalamus and bilateral foci within the prefrontal cortex in the placebo condition was associated with increased ketamine-induced negative symptoms. The continuous performance task revealed a correlation between ketamine-induced negative symptoms and increased task-related activation in the bilateral inferior frontal gyri and the right middle frontal gyrus in the placebo condition. A comparable association was found in the sentence completion task: activation in the left middle and superior temporal gyri as well as left inferior frontal gyrus in the placebo condition was positively correlated with an increased severity of ketamine-induced thought disorders. Furthermore, the degree of activation in the left middle temporal, anterior cingulate and right inferior frontal gyri in a verbal self-monitoring task in the placebo condition was correlated with the occurrence of ketamine-induced auditory illusions. The activation patterns reported in this study are comparable to results from schizophrenia studies and linked to changes in perception resembling schizophrenia symptoms. Thus, the authors state, their results may provide a biomarker applicable to predict individual psychotic symptoms [81].
In a double-blind, placebo-controlled study Nagels et al. [82] investigated the ketamine effect in an overt word generation task. The pattern of activation in the fronto-temporal language network was found to be similar but enhanced under ketamine compared to placebo. This network encompasses the left superior temporal as well as the inferior parietal lobe. In the ketamine condition, conceptual disorganization as assessed by means of the PANSS was correlated with an increased bold response in the left temporo-parietal region, the right middle and inferior frontal region as well as the precuneus. Furthermore, the PANSS abstract thinking subscore was positively correlated with increased activations within the right anterior cingulate gyrus and the left superior frontal gyrus. Finally, a positive correlation between the bold response in the left superior temporal gyrus and the lack of spontaneity and flow of conversation subscore was demonstrated [82]. Nagels et al. [83] also examined ketamine-induced changes in brain activation during a continuous overt verbal fluency (VF) task (lexical, phonologic, and semantic) in a counterbalanced, double-blind, placebo-controlled setting. Under placebo, task-specific activations were found within the left inferior frontal gyrus, the left middle and superior temporal gyrus, the left occipital lobe as well as the bilateral motor cortex and the cerebellum. Ketamine administration led to a slightly attenuated activation pattern. On the other hand, an increased activation was observed in frontal and supramarginal cortical regions during lexical and phonologic but not semantic VF [83]. The authors discuss the strong increase of involvement of the frontal regions under the influence of ketamine as a possible compensatory mechanism referred to as hyperfrontality, which is also known to be present in schizophrenia patients [82, 83]. The compensation strategy hypothesis is further supported by the fact, that the most challenging task (semantic VF) did not reveal an increase of frontal activation, which might be the reason for the ketamine-induced impairment in performance [83].
Stone et al. [84] examined ketamine-induced changes in a randomized, double-blind, placebo-controlled verbal self-monitoring investigation. Participants were asked to read adjectives aloud and received an immediate verbal feedback either by their own voice or by another voice. Both voices were presented either unchanged or distorted (lowered in pitch). In the ketamine condition, the bold response within the left superior temporal gyrus was reduced during the self-distorted speech. Misidentifying the feedback as the alien was associated with a reduced activation in the retrosplenial cortex. A lowered bold response in the PCC was found during correctly identified trials. Furthermore, correct attribution of self-distorted feedback was associated with increased activation in the bilateral temporal cortices and in the left ventrolateral cortex. The authors reported findings of increased lateral temporal activation to be associated with the external misattribution of self-distorted speech resembling findings in acutely psychotic patients with schizophrenia [85]. The ketamine-induced reduced activation within the left superior temporal gyrus during self-distorted trials is interpreted by the authors as an impaired response to unexpected stimuli. In light of these results, the authors suggest that impaired verbal self-monitoring underlies acute hallucinations and delusions in patients with schizophrenia, possibly resulting from a disrupted glutamatergic neurotransmission at NMDARs [84].
Becker et al. [86] investigated ketamine effects on the encoding of negative, neutral and positive images in a randomized, double-blind, placebo-controlled study. Regardless of the emotional content of stimuli ketamine decreased the activation of the right para-hippocampal gyrus and the mPFC during encoding. Differential deactivations with respect to the emotional content are reported for the left amygdala, the right orbifrontal cortex and the right parahippocampal region. In particular, ketamine increased the activation of the left amygdala for negative stimuli. Further, activity in the left amygdala during encoding of positive stimuli was positively correlated with arousal ratings. Functional connectivity between left amygdala and the ipsilateral frontal orbital cortex was reduced during encoding of negative stimuli. On the other hand, coupling between the left mPFC and right hippocampus was increased irrespective of the emotional content [86]. Scheidegger et al. [87] investigated ketamine effects under emotional stimulation compared to baseline. Ketamine decreased bold-responses in the amygdala-hippocampal complex. The degree of ketamine-induced psychedelic alterations of consciousness was positively correlated with the extent of this reduction. Counterintuitively, no changes in cortico-limbic resting-state functional connectivity in seed regions for the bilateral amygdala and hippocampus as well as the pregenual anterior cingulate cortex (pgACC) were found [87].
Daumann et al. [88] assessed the effects of ketamine in a randomized, double-blind, placebo-controlled study using a visual target detection task with visually or auditorily cued or uncued trials. The analysis of ketamine effects focused on brain regions showing a task-specific activation in the placebo condition. In the auditory modality, ketamine increased activation within the left insula and the left precentral gyrus. Since ketamine administration did not impair task performance, the authors speculate that the observed hyperactivation might be a compensatory mechanism [88].
In a second randomized, double-blind, placebo-controlled design Daumann [89] et al. examined the effects of ketamine compared to placebo in a spatial attention-orienting task. The task investigated the inhibition of return (IOR), a phenomenon thought to prevent subjects from redirecting attention to regions previously identified as insignificant. Ketamine infusion led to an increased activation in the right superior frontal gyrus, the left superior temporal gyrus and the right midfrontal gyrus in the IOR condition, whereas IOR was not blunted. Notably, findings of IOR in patients with schizophrenia are heterogenous and might point to differential mechanisms causing symptoms in schizophrenia [89].
Taken together, most studies observed ketamine-induced patterns of deactivation in task-relevant brain regions. The deactivations were accompanied by changes in functional connectivity of the respective brain regions. The reason might be a balance-shift of different brain networks such as the DMN, the executive network and the salience network. Notably, the activity and the functional connectivity within the DMN are reported to be aberrant also in patients with schizophrenia [90]. Schizophrenia patients demonstrate impairments in the activation or structural integrity of prefrontal regions, a phenomenon referred to as hypofrontality [91, 92].
3.3. Pharmacological Challenges
Based on different considerations, several pharmacological agents were tested within the ketamine model in recent studies. For instance, nicotine has been used to investigate the smoking behavior of schizophrenia patients as an adaptive response to a deficit in glutamatergic neurotransmission [21, 93, 94]. Furthermore, the effects of different antipsychotics, e.g. haloperidol and risperidone [95-97], have been tested in the ketamine model of schizophrenia. Rimonabant, an antagonist of the CB1 cannabinoid receptor, was examined with respect to the hypothesized involvement of the endogenous cannabinoid system in schizophrenia-related cognitive deficits [98]. Other drugs such as N-acetylcysteine (NAC) [99] and lamotrigine [96, 97, 100] were investigated because of their effects on the glutamatergic system.
3.3.1. EEG and MEG
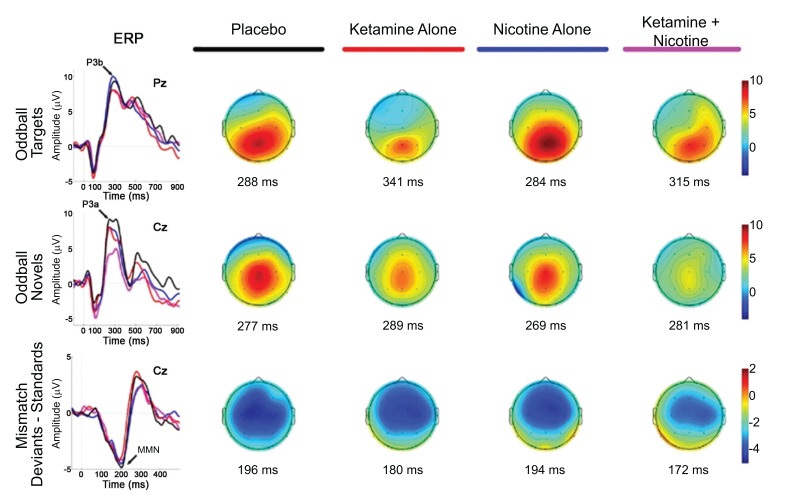
Knott et al. [93] investigated the effects of nicotine pretreatment on the RS EEG during infusion of sub-psychotomimetic ketamine doses (0,04mg/kg as bolus) in regular smokers and non-smokers in a double-blind, placebo-controlled study. Nicotine is a nicotinic acetylcholine receptor (nAchR) agonist. It is used to investigate, whether the smoking predisposition in schizophrenia patients is a form of self-treatment of cognitive deficits. Improvements in cognition have been reported after activation of nAchRs, which are crucially involved in attention, working and recognition memory and sensory processing. The authors reported reduced resting-state power of fast (beta) and slow (delta, theta) oscillations under ketamine. Delta wave power reductions were evident only for non-smokers, suggesting an interaction between long-term nicotine abuse and glutamatergic neurotransmission. On the other hand, acute nicotine application did not affect the ketamine-induced reduction of delta-wave power in non-smokers [93]. In a second double-blind, placebo-controlled study Knott et al. [94] investigated nicotine effects on the P300 ERP in a visual information processing task during administration of a sub-psychotomimetic ketamine dose. Non-smokers showed a reduction of the P300 amplitude under the influence of ketamine. A time-dependent potentiation of this ketamine effect was observed for non-smokers after consumption of nicotine-gum. The application of ketamine did not affect P300 latency [94]. A ketamine-induced latency delay and amplitude decrease for novel (P3a) and target stimuli (P3b) compared to placebo was revealed by Mathalon et al. [21] in a double-blind, placebo-controlled investigation. These effects were most prominent for central electrode sites; intravenous nicotine pretreatment did not affect these ketamine-induced changes (Fig. 2). In contrast to prior findings, this study did not find significant ketamine-induced alterations of the MMN amplitude (duration deviants). Counterintuitively, a shortened MMN latency was reported. The isolated application of nicotine led to a trend level reduction of the MMN amplitude [21]. Discordant with the hypotheses applied in the reported nicotine studies, nicotine did not abate ketamine-induced cognitive or neurophysiological impairments. According to the authors the doses of nicotine might not have been sufficient to overcome the impairments induced by ketamine. It is further suggested by the authors that nicotine leads to excessive prefrontal glutamate release further amplifying the aberrant non-NMDAR glutamatergic neurotransmissions, which are thought to negatively affect cortical attention networks. Moreover, the findings with chronic nicotine exposure (smoker status) are interpreted as a long-term alteration of glutamatergic neurotransmission promoting nicotine dependence.
Fig. (2).
Event-related brain potential grand average waveforms (left) and corresponding topographic maps (right) are shown for placebo (black), ketamine alone (red), nicotine alone (blue), and ketamine + nicotine (magenta) days. ERPs, overlaid for each test day, are shown to oddball targets at Pz (top row), to oddball novels at Cz (middle row), and to difference waveforms (deviants-standards) at Cz. The oddball target elicited a P3b, the oddball novel elicited a P3a, and the deviant elicited an MMN, with each peak denoted by an arrow on the ERP waveforms. Amplitude (in microvolts) is on the y-axis, and latency (in milliseconds) is on the x-axis. Stimulus onset is at 0 ms. Negativity is plotted down. Scalp topography maps are shown for each test day for each stimulus, at the peak latency for P3b (top), P3a (middle), and MMN (bottom). Hot colors indicate positive voltage; cool colors indicate negative voltage [21].
Oranje et al. [95] investigated the effects of haloperidol pretreatment on ketamine-induced alterations of the P300 amplitude and processing negativity in a double-blind, placebo-controlled study. They hypothesized that changes of these neurophysiological markers could be due to ketamine acting as a direct agonist on dopaminergic D2-receptors. Haloperidol is a potent antagonist of D2-receptors and could disrupt this effect. The authors report a reduction of the P300 amplitude and of the processing negativity during application of ketamine in an auditory selective-attention paradigm. Pretreatment with Haloperidol, a 1st generation antipsychotic, averted the latter effect, but not the reduction of the P300 amplitude. The results point to an involvement of dopaminergic D2-antagonism in the ketamine-induced reduction of processing negativity, while the reduction of the P300 amplitude is promoted by NMDAR antagonism [95].
The effects of Rimonabant on ketamine-induced MMN deficits were assessed in a randomized, placebo-controlled study by Roser et al. [98]. The endogenous cannabinoid system is assumed to be involved in the emergence of cognitive impairments in schizophrenia. Agonists of the CB1-receptor, which is located at high density on the presynaptic sites of glutamatergic neurons, have been found to induce cognitive impairments in healthy subjects. Rimonabant acts as a potent antagonist of the cannabinoid CB1-receptor. Thus, Roser et al. hypothesized, that it is able to reverse the ketamine-induced reduction of the MMN amplitude. Counterintuitively, in this study ketamine did not significantly reduce the MMN amplitude, either for frequency or for duration deviants, but the MMN latency was delayed for frequency deviants in the ketamine condition. The additional application of rimonabant did not affect the MMN latency but reduced the MMN amplitude, at odds with the hypothesis for both types of deviants. The authors speculate, that the modulation of CB1-receptors through Rimonabant enhances glutamatergic neurotransmission by means of limiting the effect of the endogenous CB1-receptor-ligand 2-arachidonoylglycerol (2-AG), which acts as a retrograde inhibitory neurotransmitter at glutamatergic synapses [98].
The impact of NAC, an inducer of the cysteine-glutamate exchanger, on ketamine effects was investigated by Gunduz-Bruce et al. [99] in a single-blind, placebo-controlled study. The authors assumed that the induction of the cysteine-glutamate exchanger abates ketamine-related neurophysiological impairments by enhancing the non-vesicular glutamate release from glial cells. As expected, ketamine reduced the MMN and the P300 amplitudes. Ketamine only affected MMN amplitudes induced by intensity and frequency deviants while MMN amplitudes elicited by duration deviants were not affected. The reduction of the P300 amplitude affected P3a and P3b components similarly. NAC alone caused reductions of the frequency deviant MMN amplitude, as well as an increase of the P300 amplitude, while NAC pretreatment did not attenuate ketamine-induced ERP effects despite a significant NAC ketamine interaction. The results of a ketamine-induced MMN amplitude reduction for frequency and intensity deviants, but not for duration deviants, point to the involvement of different underlying generators. Furthermore, the missing effect of the NAC pretreatment on ketamine-related neurophysiological changes is suggested to depend on the local density and distribution of the cysteine-glutamate exchanger. This hypothesis is supported by the finding that the generation of the P300 was enhanced, whereas the MMN-generation was disabled [99].
In summary, most ketamine-related findings reported in these studies match the observed task-specific ketamine effects in studies without further pharmacological intervention. However, the MMN amplitude was largely unaffected by the administration of ketamine. This might be a reason for the finding that no effects of the application of nicotine, antipsychotics or NAC prior to the ketamine challenge on the MMN amplitude have been observed so far. Regarding the P300 ERP component, results after the isolated application of ketamine are in line with previous studies. However, neither nicotine nor haloperidol has been reported to reverse these ketamine effects.
In patients with schizophrenia, nicotine increased the MMN amplitude elicited by duration deviants in smokers [101], whereas no effect on the MMN amplitude but a shortened latency for frequency deviants was observed in non-smoking patients [102, 103]. NAC, on the other hand, tended to increase MMN amplitude in schizophrenia patients without reaching significance [104]. Interestingly, one study demonstrated normalization of the P300 amplitude as well as latency for schizophrenia patients after haloperidol treatment [105]. Regarding antipsychotic medication in general, contradictory results were found for effects on the P300 ERP, despite clinical improvement [73, 106].
3.3.2. fMRI
Doyle et al. [96] examined effects of risperidone, a second-generation antipsychotic drug, and lamotrigine, an antiepileptic that attenuates glutamate release, on RS bold changes after ketamine administration in a randomized, double-blind, placebo-controlled design. Both pharmacological agents are hypothesized to attenuate the increased glutamate concentration in the synaptic cleft following acute ketamine application - in the case of risperidone by antagonizing serotonin 5-HT2A-receptors, which are crucially involved in stimulating glutamate release in pyramidal cells, and in the case of lamotrigine by prolonging the refractory phase of over-reactive glutamatergic neurons by blocking voltage-dependent sodium-channels, which stabilizes the membrane potential. In line with previous findings, ketamine increased the resting state activity in widespread brain regions including the ACC, posterior cingulate cortex, MPC, right DLPFC and ventrolateral prefrontal cortex (vlPFC), bilateral parahippocampal gyrus and insula, MCC as well as the striatum. Decreased activity was observed within the sgACC and the vmPFC. Pretreatment with risperidone led to an attenuation of ketamine-induced resting state network changes (increases and decreases of activity), whereas lamotrigine only diminished the ketamine-induced increase of resting state activity. According to the authors their findings indicate, that the ketamine effects were successfully antagonized by risperidone as well as lamotrigine [96].
In a double-blind, placebo-controlled RS study Joules et al. [97] examined the impact of risperidone and lamotrigine on functional connectivity under ketamine. Functional connectivity was assessed by means of degree centrality (DC). The authors hypothesized that only risperidone affects ketamine-induced connectivity changes if these ketamine effects were solely based on direct NMDAR antagonism. The authors suggested the same underlying mechanisms of both pharmacological agents as assumed by Doyle et al. [96]. Ketamine altered DC and shifted the pattern from cortically to subcortically centered connections. Pretreatment with risperidone led to significantly different connectivity patterns after ketamine application, which was distinct to both, the placebo and the ketamine condition. An increased DC was revealed in frontal and temporal sites, whereas a decreased DC was found in the occipital and parietal cortices and the basal ganglia, counteracting the ketamine effects. Lamotrigine pretreatment, on the other hand, exerted no effect on ketamine-induced DC patterns, but attenuated signal amplitudes. According to the authors, these results point to an NMDAR-blockade rather than downstream glutamatergic signaling at non-NMDA receptors as the major mechanism underlying ketamine-induced connectivity changes [97].
In another counterbalanced, double-blind, placebo-controlled RS study by Deakin et al. [100] ketamine evoked increased bold response in several brain regions compared to placebo. These regions included the precuneus, mid posterior cingulate gyrus, motor cortex, superior frontal gyrus, superior and inferior temporal gyrus as well as hippocampus. The authors report a positive correlation between activation in the frontal pole (most strongly), the parahippocampal gyrus and the posterior cingulate with psychotic symptoms, whereas only activation in the posterior cingulate correlated positively with dissociative symptoms. Ketamine-induced reductions in bold response were observed in the bilateral medial orbifrontal cortex (OFC) and subgenual cingulate cortex as well as the temporal pole. Activation within these regions correlated negatively with dissociative symptoms. The authors further report a negative correlation between ketamine-induced psychosis symptoms and activity within the OFC. In the second set of experiments, the effects of lamotrigine pretreatment were tested and found to attenuate the ketamine-induced bold changes as well as psychosis and dissociative symptoms. On the other hand, the authors report unchanged euphoria rating despite lamotrigine-pretreatment. The authors suggest that these results point to an aberrant non-NMDA neurotransmission as the underlying mechanism of bold-changes and schizophrenia-like symptoms in the ketamine model of schizophrenia. The results on euphoria ratings suggest the blockade of NMDAR as a crucial mechanism [100].
The observed ketamine effects in these studies are in line with the results of the reviewed studies that did not include pharmacological interventions. Pretreatment with risperidone tended to attenuate ketamine-induced alterations in the respective studies. In fMRI studies with schizophrenia patients, short-term treatment with risperidone led to inconsistent results [107, 108]. Lamotrigine likewise reduced bold-changes in the ketamine model, whereas functional connectivity was not affected. However, its effect on schizophrenia patients has not been assessed in a fMRI study to date.
CONCLUSION
With regard to electrophysiological studies, the current review suggests that ketamine-induced changes of ERPs, such as the P300 potential and the MMN, could serve as biomarkers in a schizophrenia model because similar changes have been observed in schizophrenia patients. Further research is needed to substantiate the applicability of these results. With respect to the MMN, paradigms focusing on frequency deviants yielded the most consistent results and might be implemented as a standard in future research. With respect to the reduction of MMN amplitudes, ketamine-induced symptoms resembling chronic schizophrenia [109]. This is further substantiated by findings in individuals at high risk of developing schizophrenia [110] and first-episode patients [111] showing deficits in the generation of the MMN due to duration, but not frequency deviants.
Most pharmacological treatments did not alter ketamine-induced changes in EEG. It might be speculated, that the lack of effect of the tested substances is due to their lack of impact on negative symptoms. This underscores the need to examine multiple pharmacological agents within the ketamine model of schizophrenia. Second-generation antipsychotics and drugs that affect glutamatergic neurotransmission, e.g. bitopertin and glycine, should especially be considered in this context.
In fMRI studies, alterations of activation were observed in different brain regions, most prominently within the ACC and limbic structures as well as task-relevant brain regions. These alterations were accompanied by changes in functional connectivity, indicating a balance shift of the underlying brain networks. Further evidence regarding the link between these results and ketamine-induced symptoms is necessary in order to demonstrate their applicability as schizophrenia biomarkers. Nevertheless, in light of ketamine’s mode of action, the results of pretreatment with risperidone and lamotrigine demonstrated the possible use of ketamine as a schizophrenia model applicable for the investigation of the effects of schizophrenia treatment candidate pharmacological agents in fMRI studies.
With respect to this review, small sample sizes and methodological differences place some limits on the informative value of the included studies. Furthermore, it has to be taken into account, that including only PubMed/Medline and Web of Science listed papers might reduce the explanatory power of the review.
The ketamine model itself, despite offering strong explanatory value, has several shortcomings. Most prominently, ketamine’s mode of action is not restricted to NMDAR but involves various neurotransmitter systems such as the dopamine (partial D2-receptor agonism) and the serotonin (5-HT2A-receptor agonism) systems, most possibly influencing EEG/MEG and fMRI signaling. Mutually influencing effects on synaptic neurotransmission between the tested pharmacological agents and ketamine, due to ketamine’s rich pharmacodynamics, might have further modulated the observed effects. In addition, there is a need for the integration of the ketamine model with alternative pharmacological models of schizophrenia, such as the Δ-9-tetrahydrocannabinol-model [20].
This review highlights the applicability of the ketamine model in schizophrenia drug development. However, further research is needed to establish the transferability of the results of ketamine studies and to link the normalization of ketamine-induced changes of schizophrenia biomarkers achieved by treatment with schizophrenia treatment candidate drugs with the improvement of ketamine-induced schizophrenia-like symptoms. Notably, the ketamine model could offer the possibility to test functional target engagement of various pharmacological agents relatively rapidly and help improve our understanding of glutamatergic neurotransmission. Therefore, it could facilitate the development of urgently needed agents to improve negative and cognitive symptoms.
Consent for Publication
Not applicable.
Acknowledgements
This work was funded by the German Research Foundation (SFB 936/C6 to C.M.).
The authors thank Stephanie Thiebes, Alberto Grignolo and Ralph Bültmann for their help in proofreading. This work was prepared as part of Moritz Haaf’s dissertation at the University of Hamburg.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Miyamoto S., Miyake N., Jarskog L.F., Fleischhacker W.W., Lieberman J.A. Pharmacological treatment of schizophrenia: A critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry. 2012;17(12):1206–1227. doi: 10.1038/mp.2012.47. [DOI] [PubMed] [Google Scholar]
- 2.Poels E.M., Kegeles L.S., Kantrowitz J.T., Slifstein M., Javitt D.C., Lieberman J.A., Abi-Dargham A., Girgis R.R. Imaging glutamate in schizophrenia: Review of findings and implications for drug discovery. Mol. Psychiatry. 2014;19(1):20–29. doi: 10.1038/mp.2013.136. [DOI] [PubMed] [Google Scholar]
- 3.Miyamoto S., Duncan G.E., Marx C.E., Lieberman J.A. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry. 2005;10(1):79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- 4.Howes O., McCutcheon R., Stone J. Glutamate and dopamine in schizophrenia: An update for the 21st century. J. Psychopharmacol. 2015;29(2):97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luby E.D., Gottlieb J.S., Cohen B.D., Rosenbaum G., Domino E.F. Model psychoses and schizophrenia. Am. J. Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- 6.Krystal J.H., Karper L.P., Seibyl J.P., Freeman G.K., Delaney R., Bremner J.D., Heninger G.R., Bowers M.B., Jr, Charney D.S. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 7.Woo T.U., Walsh J.P., Benes F.M. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch. Gen. Psychiatry. 2004;61(7):649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 8.Lisman J.E., Coyle J.T., Green R.W., Javitt D.C., Benes F.M., Heckers S., Grace A.A. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31(5):234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Umbricht D., Alberati D., Martin-Facklam M., Borroni E., Youssef E.A., Ostland M., Wallace T.L., Knoflach F., Dorflinger E., Wettstein J.G., Bausch A., Garibaldi G., Santarelli L. Effect of bitopertin, a glycine reuptake inhibitor, on negative symptoms of schizophrenia: A randomized, double-blind, proof-of-concept study. JAMA Psychiatry. 2014;71(6):637–646. doi: 10.1001/jamapsychiatry.2014.163. [DOI] [PubMed] [Google Scholar]
- 10.Patil S.T., Zhang L., Martenyi F., Lowe S.L., Jackson K.A., Andreev B.V., Avedisova A.S., Bardenstein L.M., Gurovich I.Y., Morozova M.A., Mosolov S.N., Neznanov N.G., Reznik A.M., Smulevich A.B., Tochilov V.A., Johnson B.G., Monn J.A., Schoepp D.D. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized Phase 2 clinical trial. Nat. Med. 2007;13(9):1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 11.Dunlop J., Brandon N.J. Schizophrenia drug discovery and development in an evolving era: Are new drug targets fulfilling expectations? J. Psychopharmacol. 2015;29(2):230–238. doi: 10.1177/0269881114565806. [DOI] [PubMed] [Google Scholar]
- 12.Nicoletti F., Bruno V., Ngomba R.T., Gradini R., Battaglia G. Metabotropic glutamate receptors as drug targets: What’s new? Curr. Opin. Pharmacol. 2015;20:89–94. doi: 10.1016/j.coph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Javitt D.C., Schoepp D., Kalivas P.W., Volkow N.D., Zarate C., Merchant K., Bear M.F., Umbricht D., Hajos M., Potter W.Z., Lee C.M. 2011. [DOI] [PMC free article] [PubMed]
- 14.Lahti A.C., Koffel B., LaPorte D., Tamminga C.A. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13(1):9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra A.K., Pinals D.A., Adler C.M., Elman I., Clifton A., Pickar D., Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- 16.Tomasetti C., Iasevoli F., Buonaguro E.F., De Berardis D., Fornaro M., Fiengo A.L., Martinotti G., Orsolini L., Valchera A., Di Giannantonio M., de Bartolomeis A. Treating the synapse in major psychiatric disorders: The role of postsynaptic density network in dopamine-glutamate interplay and psychopharmacologic drugs molecular actions. Int. J. Mol. Sci. 2017;18(1):135. doi: 10.3390/ijms18010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanos P., Moaddel R., Morris P.J., Georgiou P., Fischell J., Elmer G.I., Alkondon M., Yuan P., Pribut H.J., Singh N.S., Dossou K.S., Fang Y., Huang X.P., Mayo C.L., Wainer I.W., Albuquerque E.X., Thompson S.M., Thomas C.J., Zarate C.A., Jr, Gould T.D. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serafini G., Hayley S., Pompili M., Dwivedi Y., Brahmachari G., Girardi P., Amore M. Hippocampal neurogenesis, neurotrophic factors and depression: Possible therapeutic targets? CNS Neurol. Disord. Drug Targets. 2014;13(10):1708–1721. doi: 10.2174/1871527313666141130223723. [DOI] [PubMed] [Google Scholar]
- 19.Kocsis B., Brown R.E., McCarley R.W., Hajos M. Impact of ketamine on neuronal network dynamics: Translational modeling of schizophrenia-relevant deficits. CNS Neurosci. Ther. 2013;19(6):437–447. doi: 10.1111/cns.12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frohlich J., Van Horn J.D. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 2014;28(4):287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathalon D.H., Ahn K.H., Perry E.B., Jr, Cho H.S., Roach B.J., Blais R.K., Bhakta S., Ranganathan M., Ford J.M., D’Souza D.C. Effects of nicotine on the neurophysiological and behavioral effects of ketamine in humans. Front. Psychiatry. 2014;5:3. doi: 10.3389/fpsyt.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandal M.J., Edgar J.C., Klook K., Siegel S.J. Gamma synchrony: Towards a translational biomarker for the treatment-resistant symptoms of schizophrenia. Neuropharmacology. 2012;62(3):1504–1518. doi: 10.1016/j.neuropharm.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynall M.E., Bassett D.S., Kerwin R., McKenna P.J., Kitzbichler M., Muller U., Bullmore E. Functional connectivity and brain networks in schizophrenia. J. Neurosci. 2010;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthukumaraswamy S.D., Shaw A.D., Jackson L.E., Hall J., Moran R., Saxena N. Evidence that subanesthetic doses of ketamine cause sustained disruptions of NMDA and AMPA-mediated frontoparietal connectivity in humans. J. Neurosci. 2015;35(33):11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friston K.J. Functional and effective connectivity: A review. Brain Connect. 2011;1(1):13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 26.Friston K.J., Bastos A., Litvak V., Stephan K.E., Fries P., Moran R.J. DCM for complex-valued data: Cross-spectra, coherence and phase-delays. Neuroimage. 2012;59(1):439–455. doi: 10.1016/j.neuroimage.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moran R.J., Jones M.W., Blockeel A.J., Adams R.A., Stephan K.E., Friston K.J. Losing control under ketamine: Suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology. 2015;40(2):268–277. doi: 10.1038/npp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivolta D., Heidegger T., Scheller B., Sauer A., Schaum M., Birkner K., Singer W., Wibral M., Uhlhaas P.J. Ketamine Dysregulates the amplitude and connectivity of high-frequency oscillations in cortical-subcortical networks in humans: Evidence from resting-state magnetoencephalography-recordings. Schizophr. Bull. 2015;41(5):1105–1114. doi: 10.1093/schbul/sbv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanacora G., Smith M.A., Pathak S., Su H.L., Boeijinga P.H., McCarthy D.J., Quirk M.C. Lanicemine: A low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Mol. Psychiatry. 2014;19(9):978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horacek J., Brunovsky M., Novak T., Tislerova B., Palenicek T., Bubenikova-Valesova V., Spaniel F., Koprivova J., Mohr P., Balikova M., Hoschl C. Subanesthetic dose of ketamine decreases prefrontal theta cordance in healthy volunteers: Implications for antidepressant effect. Psychologic. Med. 2010;40(9):1443–1451. doi: 10.1017/S0033291709991619. [DOI] [PubMed] [Google Scholar]
- 31.de la Salle S., Choueiry J., Shah D., Bowers H., McIntosh J., Ilivitsky V., Knott V. Effects of ketamine on resting-state EEG activity and their relationship to perceptual/dissociative symptoms in healthy humans. Front. Pharmacol. 2016;7:348. doi: 10.3389/fphar.2016.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlhaas P.J., Haenschel C., Nikolic D., Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008;34(5):927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat. Rev. Neurosci. 2010;11(2):100–113. doi: 10.1038/nrn2774. [DOI] [PubMed] [Google Scholar]
- 34.Andreou C., Nolte G., Leicht G., Polomac N., Hanganu-Opatz I.L., Lambert M., Engel A.K., Mulert C. Increased resting-state gamma-band connectivity in first-episode schizophrenia. Schizophr. Bull. 2015;41(4):930–939. doi: 10.1093/schbul/sbu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoflich A., Hahn A., Kublbock M., Kranz G.S., Vanicek T., Ganger S., Spies M., Windischberger C., Kasper S., Winkler D., Lanzenberger R. Ketamine-dependent neuronal activation in healthy volunteers. Brain Struct. Funct. 2017;222(3):1533–1542. doi: 10.1007/s00429-016-1291-0. [DOI] [PubMed] [Google Scholar]
- 36.Pollak T.A., De Simoni S., Barimani B., Zelaya F.O., Stone J.M., Mehta M.A. Phenomenologically distinct psychotomimetic effects of ketamine are associated with cerebral blood flow changes in functionally relevant cerebral foci: A continuous arterial spin labelling study. Psychopharmacology (Berl.) 2015;232(24):4515–4524. doi: 10.1007/s00213-015-4078-8. [DOI] [PubMed] [Google Scholar]
- 37.Stone J., Kotoula V., Dietrich C., De Simoni S., Krystal J.H., Mehta M.A. Perceptual distortions and delusional thinking following ketamine administration are related to increased pharmacological MRI signal changes in the parietal lobe. J. Psychopharmacol. 2015;29(9):1025–1028. doi: 10.1177/0269881115592337. [DOI] [PubMed] [Google Scholar]
- 38.Mason O.J., Morgan C.J., Stefanovic A., Curran H.V. The psychotomimetic states inventory (PSI): Measuring psychotic-type experiences from ketamine and cannabis. Schizophr. Res. 2008;103(1-3):138–142. doi: 10.1016/j.schres.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 39.De Simoni S., Schwarz A.J., O’Daly O.G., Marquand A.F., Brittain C., Gonzales C., Stephenson S., Williams S.C., Mehta M.A. Test-retest reliability of the BOLD pharmacological MRI response to ketamine in healthy volunteers. Neuroimage. 2013;64:75–90. doi: 10.1016/j.neuroimage.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 40.Cui L.B., Liu J., Wang L.X., Li C., Xi Y.B., Guo F., Wang H.N., Zhang L.C., Liu W.M., He H., Tian P., Yin H., Lu H. Anterior cingulate cortex-related connectivity in first-episode schizophrenia: A spectral dynamic causal modeling study with functional magnetic resonance imaging. Front. Hum. Neurosci. 2015;9:589. doi: 10.3389/fnhum.2015.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong J.J., O’Daly O., Mehta M.A., Young A.H., Stone J.M. Ketamine modulates subgenual cingulate connectivity with the memory-related neural circuit-a mechanism of relevance to resistant depression? PeerJ. 2016;4:e1710. doi: 10.7717/peerj.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grimm O., Gass N., Weber-Fahr W., Sartorius A., Schenker E., Spedding M., Risterucci C., Schweiger J.I., Bohringer A., Zang Z., Tost H., Schwarz A.J., Meyer-Lindenberg A. Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology (Berl.) 2015;232(21-22):4231–4241. doi: 10.1007/s00213-015-4022-y. [DOI] [PubMed] [Google Scholar]
- 43.Hoflich A., Hahn A., Kublbock M., Kranz G.S., Vanicek T., Windischberger C., Saria A., Kasper S., Winkler D., Lanzenberger R. Ketamine-induced modulation of the thalamo-cortical network in healthy volunteers as a model for schizophrenia. 2015. [DOI] [PMC free article] [PubMed]
- 44.Dandash O., Harrison B.J., Adapa R., Gaillard R., Giorlando F., Wood S.J., Fletcher P.C., Fornito A. Selective augmentation of striatal functional connectivity following NMDA receptor antagonism: Implications for psychosis. Neuropsychopharmacology. 2015;40(3):622–631. doi: 10.1038/npp.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Driesen N.R., McCarthy G., Bhagwagar Z., Bloch M., Calhoun V., D’Souza D.C., Gueorguieva R., He G., Ramachandran R., Suckow R.F., Anticevic A., Morgan P.T., Krystal J.H. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol. Psychiatry. 2013;18(11):1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khalili-Mahani N., Niesters M., van Osch M.J., Oitzl M., Veer I., de Rooij M., van Gerven J., van Buchem M.A., Beckmann C.F., Rombouts S.A., Dahan A. Ketamine interactions with biomarkers of stress: A randomized placebo-controlled repeated measures resting-state fMRI and PCASL pilot study in healthy men. Neuroimage. 2015;108:396–409. doi: 10.1016/j.neuroimage.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 47.Kraguljac N.V., Frolich M.A., Tran S., White D.M., Nichols N., Barton-McArdle A., Reid M.A., Bolding M.S., Lahti A.C. Ketamine modulates hippocampal neurochemistry and functional connectivity: A combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Mol. Psychiatry. 2017;22(4):562–569. doi: 10.1038/mp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodward N.D., Rogers B., Heckers S. Functional resting-state networks are differentially affected in schizophrenia. Schizophr. Res. 2011;130(1-3):86–93. doi: 10.1016/j.schres.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: Where are we now? Neurosci. Biobehav. Rev. 2011;35(5):1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Skudlarski P., Jagannathan K., Anderson K., Stevens M.C., Calhoun V.D., Skudlarska B.A., Pearlson G. Brain connectivity is not only lower but different in schizophrenia: A combined anatomical and functional approach. Biol. Psychiatry. 2010;68(1):61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fornito A., Zalesky A., Pantelis C., Bullmore E.T. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62(4):2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 52.Naatanen R., Kujala T., Escera C., Baldeweg T., Kreegipuu K., Carlson S., Ponton C. The mismatch negativity (MMN)--a unique window to disturbed central auditory processing in ageing and different clinical conditions. Clin. Neurophysiol. 2012;123(3):424–458. doi: 10.1016/j.clinph.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt A., Bachmann R., Kometer M., Csomor P.A., Stephan K.E., Seifritz E., Vollenweider F.X. Mismatch negativity encoding of prediction errors predicts S-ketamine-induced cognitive impairments. Neuropsychopharmacology. 2012;37(4):865–875. doi: 10.1038/npp.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt A., Diaconescu A.O., Kometer M., Friston K.J., Stephan K.E., Vollenweider F.X. Modeling ketamine effects on synaptic plasticity during the mismatch negativity. Cereb. Cortex. 2013;23(10):2394–2406. doi: 10.1093/cercor/bhs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heekeren K., Daumann J., Neukirch A., Stock C., Kawohl W., Norra C., Waberski T.D., Gouzoulis-Mayfrank E. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl.) 2008;199(1):77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thiebes S., Leicht G., Curic S., Steinmann S., Polomac N., Andreou C., Eichler I., Eichler L., Zollner C., Gallinat J., Hanganu-Opatz I., Mulert C. Glutamatergic deficit and schizophrenia-like negative symptoms: New evidence from ketamine-induced mismatch negativity alterations in healthy male humans. J. Psychiatry Neurosci. 2017;42(4):160187. doi: 10.1503/jpn.160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Polich J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watson T.D., Petrakis I.L., Edgecombe J., Perrino A., Krystal J.H., Mathalon D.H. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int. J. Neuropsychopharmacol. 2009;12(3):357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Musso F., Brinkmeyer J., Ecker D., London M.K., Thieme G., Warbrick T., Wittsack H.J., Saleh A., Greb W., de Boer P., Winterer G. Ketamine effects on brain function--simultaneous fMRI/EEG during a visual oddball task. Neuroimage. 2011;58(2):508–525. doi: 10.1016/j.neuroimage.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 60.Boeijinga P.H., Soufflet L., Santoro F., Luthringer R. Ketamine effects on CNS responses assessed with MEG/EEG in a passive auditory sensory-gating paradigm: An attempt for modelling some symptoms of psychosis in man. J. Psychopharmacol. 2007;21(3):321–337. doi: 10.1177/0269881107077768. [DOI] [PubMed] [Google Scholar]
- 61.Kort N.S., Ford J.M., Roach B.J., Gunduz-Bruce H., Krystal J.H., Jaeger J., Reinhart R.M., Mathalon D.H. Role of N-methyl-d-aspartate receptors in action-based predictive coding deficits in schizophrenia. Biol. Psychiatry. 2017;81(6):514–524. doi: 10.1016/j.biopsych.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis D.A., Curley A.A., Glausier J.R., Volk D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35(1):57–67. doi: 10.1016/j.tins.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leicht G., Karch S., Karamatskos E., Giegling I., Moller H.J., Hegerl U., Pogarell O., Rujescu D., Mulert C. Alterations of the early auditory evoked gamma-band response in first-degree relatives of patients with schizophrenia: Hints to a new intermediate phenotype. J. Psychiatr. Res. 2011;45(5):699–705. doi: 10.1016/j.jpsychires.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 64.Leicht G., Kirsch V., Giegling I., Karch S., Hantschk I., Moller H.J., Pogarell O., Hegerl U., Rujescu D., Mulert C. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol. Psychiatry. 2010;67(3):224–231. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 65.Leicht G., Andreou C., Polomac N., Lanig C., Schottle D., Lambert M., Mulert C. Reduced auditory evoked gamma band response and cognitive processing deficits in first episode schizophrenia. World J. Biol. Psychiatry. 2015;•••:1–11. doi: 10.3109/15622975.2015.1017605. [DOI] [PubMed] [Google Scholar]
- 66.Leicht G., Vauth S., Polomac N., Andreou C., Rauh J., Mussmann M., Karow A., Mulert C. EEG-informed fMRI reveals a disturbed gamma-band-specific network in subjects at high risk for psychosis. Schizophr. Bull. 2016;42(1):239–249. doi: 10.1093/schbul/sbv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gonzalez-Burgos G., Cho R.Y., Lewis D.A. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol. Psychiatry. 2015;77(12):1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uhlhaas P.J., Singer W. Oscillations and neuronal dynamics in schizophrenia: The search for basic symptoms and translational opportunities. Biol. Psychiatry. 2015;77(12):1001–1009. doi: 10.1016/j.biopsych.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 69.Thiebes S., Steinmann S., Curic S., Polomac N., Andreou C., Eichler I.C., Eichler L., Zollner C., Gallinat J., Leicht G., Mulert C. Alterations in interhemispheric gamma-band connectivity are related to the emergence of auditory verbal hallucinations in healthy subjects during NMDA-receptor blockade. Neuropsychopharmacology. 2018;43(7):1608–1615. doi: 10.1038/s41386-018-0014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw A.D., Saxena N.L.E.J., Hall J.E., Singh K.D., Muthukumaraswamy S.D. Ketamine amplifies induced gamma frequency oscillations in the human cerebral cortex. Eur. Neuropsychopharmacol. 2015;25(8):1136–1146. doi: 10.1016/j.euroneuro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 71.Hong L.E., Summerfelt A., Buchanan R.W., O’Donnell P., Thaker G.K., Weiler M.A., Lahti A.C. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology. 2010;35(3):632–640. doi: 10.1038/npp.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bramon E., Rabe-Hesketh S., Sham P., Murray R.M., Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr. Res. 2004;70(2-3):315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 73.Jeon Y.W., Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40(5):684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- 74.Naatanen R., Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: A review. Int. J. Neuropsychopharmacol. 2009;12(1):125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- 75.Umbricht D., Krljes S. Mismatch negativity in schizophrenia: A meta-analysis. Schizophr. Res. 2005;76(1):1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 76.Javitt D.C., Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am. J. Psychiatry. 2015;172(1):17–31. doi: 10.1176/appi.ajp.2014.13121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steffens M., Becker B., Neumann C., Kasparbauer A.M., Meyhofer I., Weber B., Mehta M.A., Hurlemann R., Ettinger U. Effects of ketamine on brain function during smooth pursuit eye movements. Hum. Brain Mapp. 2016;37(11):4047–4060. doi: 10.1002/hbm.23294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Francois J., Grimm O., Schwarz A.J., Schweiger J., Haller L., Risterucci C., Bohringer A., Zang Z., Tost H., Gilmour G., Meyer-Lindenberg A. Ketamine suppresses the ventral striatal response to reward anticipation: A cross-species translational neuroimaging study. Neuropsychopharmacology. 2016;41(5):1386–1394. doi: 10.1038/npp.2015.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Driesen N.R., McCarthy G., Bhagwagar Z., Bloch M.H., Calhoun V.D., D’Souza D.C., Gueorguieva R., He G., Leung H.C., Ramani R., Anticevic A., Suckow R.F., Morgan P.T., Krystal J.H. The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology. 2013;38(13):2613–2622. doi: 10.1038/npp.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anticevic A., Gancsos M., Murray J.D., Repovs G., Driesen N.R., Ennis D.J., Niciu M.J., Morgan P.T., Surti T.S., Bloch M.H., Ramani R., Smith M.A., Wang X.J., Krystal J.H., Corlett P.R. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc. Natl. Acad. Sci. USA. 2012;109(41):16720–16725. doi: 10.1073/pnas.1208494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Honey G.D., Corlett P.R., Absalom A.R., Lee M., Pomarol-Clotet E., Murray G.K., McKenna P.J., Bullmore E.T., Menon D.K., Fletcher P.C. Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J. Neurosci. 2008;28(25):6295–6303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nagels A., Kirner-Veselinovic A., Wiese R., Paulus F.M., Kircher T., Krach S. Effects of ketamine-induced psychopathological symptoms on continuous overt rhyme fluency. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262(5):403–414. doi: 10.1007/s00406-011-0281-8. [DOI] [PubMed] [Google Scholar]
- 83.Nagels A., Kirner-Veselinovic A., Krach S., Kircher T. Neural correlates of S-ketamine induced psychosis during overt continuous verbal fluency. Neuroimage. 2011;54(2):1307–1314. doi: 10.1016/j.neuroimage.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 84.Stone J.M., Abel K.M., Allin M.P., van Haren N., Matsumoto K., McGuire P.K., Fu C.H. Ketamine-induced disruption of verbal self-monitoring linked to superior temporal activation. Pharmacopsychiatry. 2011;44(1):33–48. doi: 10.1055/s-0030-1267942. [DOI] [PubMed] [Google Scholar]
- 85.Fu C.H., Brammer M.J., Yaguez L., Allen P., Matsumoto K., Johns L., Weinstein S., Borgwardt S., Broome M., van Haren N., McGuire P.K. Increased superior temporal activation associated with external misattributions of self-generated speech in schizophrenia. Schizophr. Res. 2008;100(1-3):361–363. doi: 10.1016/j.schres.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 86.Becker B., Steffens M., Zhao Z., Kendrick K.M., Neumann C., Weber B., Schultz J., Mehta M.A., Ettinger U., Hurlemann R. General and emotion-specific neural effects of ketamine during emotional memory formation. Neuroimage. 2017;150:308–317. doi: 10.1016/j.neuroimage.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 87.Scheidegger M., Henning A., Walter M., Lehmann M., Kraehenmann R., Boeker H., Seifritz E., Grimm S. Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation. Hum. Brain Mapp. 2016;37(5):1941–1952. doi: 10.1002/hbm.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daumann J., Wagner D., Heekeren K., Neukirch A., Thiel C.M., Gouzoulis-Mayfrank E. Neuronal correlates of visual and auditory alertness in the DMT and ketamine model of psychosis. J. Psychopharmacol. 2010;24(10):1515–1524. doi: 10.1177/0269881109103227. [DOI] [PubMed] [Google Scholar]
- 89.Daumann J., Heekeren K., Neukirch A., Thiel C.M., Moller-Hartmann W., Gouzoulis-Mayfrank E. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl.) 2008;200(4):573–583. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- 90.Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. Aberrant “default mode” functional connectivity in schizophrenia. Am. J. Psychiatry. 2007;164(3):450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 91.Barch D.M. The cognitive neuroscience of schizophrenia. Annu. Rev. Clin. Psychol. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- 92.Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch. Gen. Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Knott V., McIntosh J., Millar A., Fisher D., Villeneuve C., Ilivitsky V., Horn E. Nicotine and smoker status moderate brain electric and mood activation induced by ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist. Pharmacol. Biochem. Behav. 2006;85(1):228–242. doi: 10.1016/j.pbb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 94.Knott V.J., Millar A.M., McIntosh J.F., Shah D.K., Fisher D.J., Blais C.M., Ilivitsky V., Horn E. Separate and combined effects of low dose ketamine and nicotine on behavioural and neural correlates of sustained attention. Biol. Psychol. 2011;88(1):83–93. doi: 10.1016/j.biopsycho.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Oranje B., Gispen-de Wied C.C., Westenberg H.G., Kemner C., Verbaten M.N., Kahn R.S. Haloperidol counteracts the ketamine-induced disruption of processing negativity, but not that of the P300 amplitude. Int. J. Neuropsychopharmacol. 2009;12(6):823–832. doi: 10.1017/S1461145708009814. [DOI] [PubMed] [Google Scholar]
- 96.Doyle O.M., De Simoni S., Schwarz A.J., Brittain C., O’Daly O.G., Williams S.C., Mehta M.A. Quantifying the attenuation of the ketamine pharmacological magnetic resonance imaging response in humans: A validation using antipsychotic and glutamatergic agents. J. Pharmacol. Experiment. Therapeut. 2013;345(1):151–160. doi: 10.1124/jpet.112.201665. [DOI] [PubMed] [Google Scholar]
- 97.Joules R., Doyle O.M., Schwarz A.J., O’Daly O.G., Brammer M., Williams S.C., Mehta M.A. Ketamine induces a robust whole-brain connectivity pattern that can be differentially modulated by drugs of different mechanism and clinical profile. Psychopharmacology (Berl.) 2015;232(21-22):4205–4218. doi: 10.1007/s00213-015-3951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roser P., Haussleiter I.S., Chong H.J., Maier C., Kawohl W., Norra C., Juckel G. Inhibition of cerebral type 1 cannabinoid receptors is associated with impaired auditory mismatch negativity generation in the ketamine model of schizophrenia. Psychopharmacology (Berl.) 2011;218(4):611–620. doi: 10.1007/s00213-011-2352-y. [DOI] [PubMed] [Google Scholar]
- 99.Gunduz-Bruce H., Reinhart R.M., Roach B.J., Gueorguieva R., Oliver S., D’Souza D.C., Ford J.M., Krystal J.H., Mathalon D.H. Glutamatergic modulation of auditory information processing in the human brain. Biol. Psychiatry. 2012;71(11):969–977. doi: 10.1016/j.biopsych.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deakin J.F., Lees J., McKie S., Hallak J.E., Williams S.R., Dursun S.M. Glutamate and the neural basis of the subjective effects of ketamine: A pharmaco-magnetic resonance imaging study. Arch. Gen. Psychiatry. 2008;65(2):154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- 101.Dulude L., Labelle A., Knott V.J. Acute nicotine alteration of sensory memory impairment in smokers with schizophrenia. J. Clin. Psychopharmacol. 2010;30(5):541–548. doi: 10.1097/JCP.0b013e3181f0c9c6. [DOI] [PubMed] [Google Scholar]
- 102.Fisher D.J., Grant B., Smith D.M., Borracci G., Labelle A., Knott V.J. Nicotine and the hallucinating brain: Effects on mismatch negativity (MMN) in schizophrenia. Psychiatry Res. 2012;196(2-3):181–187. doi: 10.1016/j.psychres.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 103.Inami R., Kirino E., Inoue R., Suzuki T., Arai H. Nicotine effects on mismatch negativity in nonsmoking schizophrenic patients. Neuropsychobiology. 2007;56(2-3):64–72. doi: 10.1159/000111536. [DOI] [PubMed] [Google Scholar]
- 104.Lavoie S., Murray M.M., Deppen P., Knyazeva M.G., Berk M., Boulat O., Bovet P., Bush A.I., Conus P., Copolov D., Fornari E., Meuli R., Solida A., Vianin P., Cuenod M., Buclin T., Do K.Q. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2187–2199. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- 105.Coburn K.L., Shillcutt S.D., Tucker K.A., Estes K.M., Brin F.B., Merai P., Moore N.C. P300 delay and attenuation in schizophrenia: Reversal by neuroleptic medication. Biol. Psychiatry. 1998;44(6):466–474. doi: 10.1016/s0006-3223(97)00402-2. [DOI] [PubMed] [Google Scholar]
- 106.Light G.A., Swerdlow N.R., Thomas M.L., Calkins M.E., Green M.F., Greenwood T.A., Gur R.E., Gur R.C., Lazzeroni L.C., Nuechterlein K.H., Pela M., Radant A.D., Seidman L.J., Sharp R.F., Siever L.J., Silverman J.M., Sprock J., Stone W.S., Sugar C.A., Tsuang D.W., Tsuang M.T., Braff D.L., Turetsky B.I. Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: Characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr. Res. 2015;163(1-3):63–72. doi: 10.1016/j.schres.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hu M.L., Zong X.F., Zheng J.J., Pantazatos S.P., Miller J.M., Li Z.C., Liao Y.H., He Y., Zhou J., Sang D.E., Zhao H.Z., Lv L.X., Tang J.S., Mann J.J., Chen X.G. Short-term effects of risperidone monotherapy on spontaneous brain activity in first-episode treatment-naive schizophrenia patients: A longitudinal fMRI study. Sci. Rep. 2016;6:34287. doi: 10.1038/srep34287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lottman K.K., Kraguljac N.V., White D.M., Morgan C.J., Calhoun V.D., Butt A., Lahti A.C. Risperidone effects on brain dynamic connectivity-a prospective resting-state fMRI study in schizophrenia. Front. Psychiatry. 2017;8:14. doi: 10.3389/fpsyt.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salisbury D.F., Shenton M.E., Griggs C.B., Bonner-Jackson A., McCarley R.W. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Arch. Gen. Psychiatry. 2002;59(8):686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- 110.Bodatsch M., Ruhrmann S., Wagner M., Muller R., Schultze-Lutter F., Frommann I., Brinkmeyer J., Gaebel W., Maier W., Klosterkotter J., Brockhaus-Dumke A. Prediction of psychosis by mismatch negativity. Biol. Psychiatry. 2011;69(10):959–966. doi: 10.1016/j.biopsych.2010.09.057. [DOI] [PubMed] [Google Scholar]
- 111.Haigh S.M., Coffman B.A., Salisbury D.F. Mismatch negativity in first-episode schizophrenia: A meta-analysis. Clin. EEG Neurosci. 2017;48(1):3–10. doi: 10.1177/1550059416645980. [DOI] [PMC free article] [PubMed] [Google Scholar]