Abstract
Allogeneic hematopoietic stem cell transplantation (HCT) is the only curative therapy for myelodysplastic syndrome (MDS). Broad application is hindered by high risks of transplant-related morbidity and mortality, especially in the older age range represented by the MDS population. However, recent advances in strategies to minimize regimen-related toxicity make HCT a viable option for many more patients. Appropriate selection of patients involves consideration of patient factors, including use of geriatric assessment tools and comorbidity scales, that predict risks of regimen-related toxicity as well as disease factors, including genetic markers, which predict survival with both non-HCT and HCT therapy. Optimal timing of HCT for fit patients must consider MDS risk scores and life-years to be gained, with earlier transplantation indicated for patients with intermediate-2 and high-risk disease but judicious delay for lower risk patients. Selection of suitable conditioning regimens must balance risks of toxicity with opportunity for maximum disease control.
Learning Objectives
To understand which patient-related factors can affect success of transplantation in patients with MDS
To understand which disease-related factors impact the decision of when to transplant a patient with MDS
To become familiar with the relative advantages and disadvantages of different conditioning regimen intensities, donor types, and graft source
Introduction
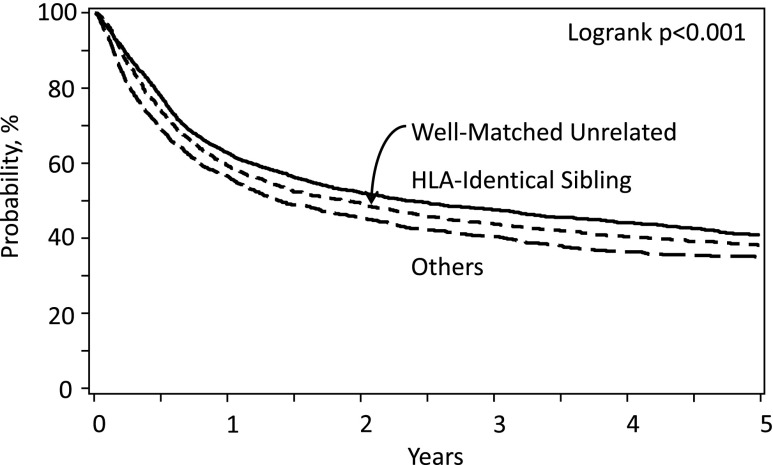
Allogeneic hematopoietic stem cell transplantation (HCT) offers the potential for cure in myelodysplastic syndrome (MDS), largely from potent immune-mediated graft versus tumor effects1,2 with some contribution of the high-dose cytotoxic therapy given for pretransplant conditioning. This contrasts with other MDS therapies that may prolong survival but do not eradicate the disease.3 However, a substantial risk of transplant-related mortality (TRM) offsets the curative advantage of HCT. Additionally, not all patients are cured. Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) indicate TRM and relapse rates of 30% and 30%, respectively, after allogeneic HCT from human leukocyte antigen (HLA)-matched related donors; the 3-year probability of survival is 40%.4 Survival rates improved over the past decade, and recent studies indicate equivalent survival with HLA-matched related and unrelated donors.4,5 Data from CIBMTR (Figure 1) and recent studies suggest that survival rates are lower with other types of donors.4,6-8 Outcomes can be optimized by careful selection of patients, donors, and transplant approaches.
Figure 1.
Overall survival for MDS by donor type; patients registered to CIBMTR, 2000-2014.
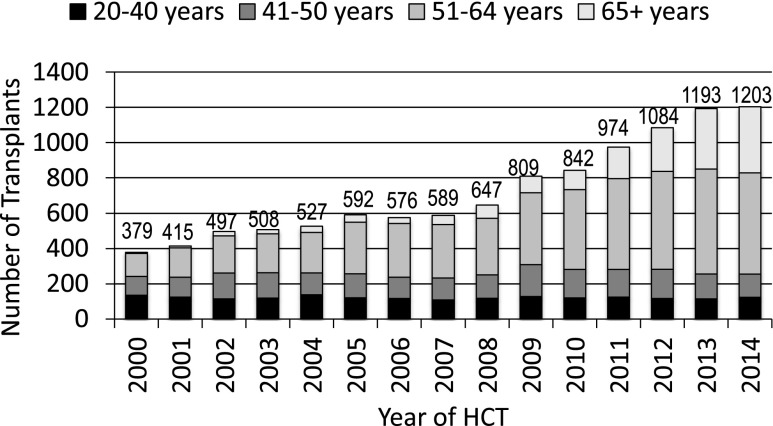
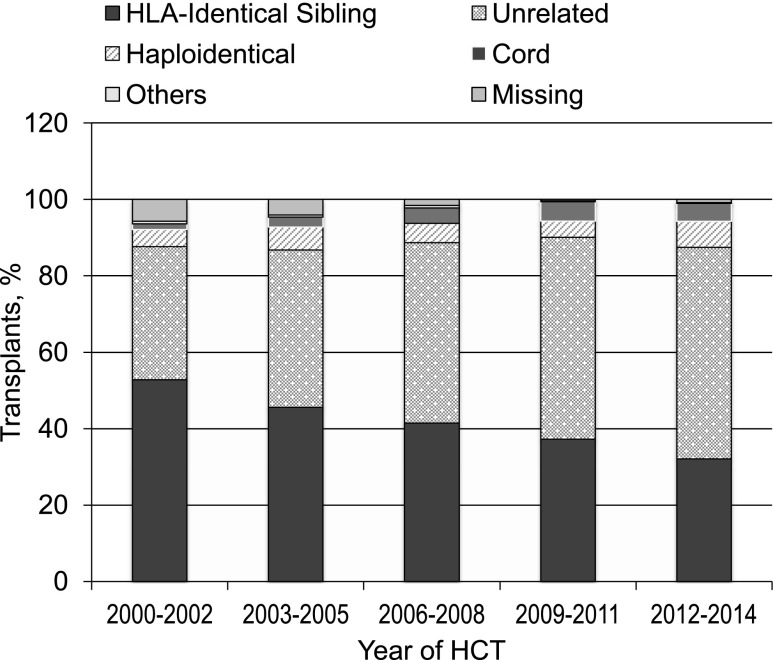
The annual numbers of transplantations for MDS increased dramatically worldwide in the past 15 years (Figure 2). This is a result of expanding donor sources (Figure 3), wider applicability in older, less fit patients with reduced intensity pretransplant conditioning regimens and, most importantly, in the United States, the decision by the Centers for Medicare and Medicaid Services to reimburse centers for MDS transplantation in patients 65 years and older, if done in a study designed to gather evidence on efficacy. The CIBMTR launched such a study in December 2010.9 Almost all HCTs for MDS in US Medicare beneficiaries are part of this study, which prospectively compares outcomes of HCT in patients 50-64 years of age with those in patients 65 and older. Preliminary data from this study were presented at the 2015 American Society of Hematology meetings and suggest that outcomes are similar in older and younger patients.10 However, this study could only address patients who received HCT and, given the advanced age at diagnosis of many MDS patients,11 it is likely that more older than younger patients are deemed ineligible for HCT because of other conditions.
Figure 2.
Number of transplants for MDS by age; patients registered to CIBMTR, 2000-2014.
Figure 3.
Transplants for MDS by donor type; patients registered to CIBMTR, 2000-2014.
Who Should Be Considered for Transplantation?
Prospective, controlled comparisons of HCT and non-HCT are lacking, although a North American phase 3 study comparing 3-year survival after HCT with reduced intensity conditioning vs nontransplant therapies (using a biologic assignment, donor vs no-donor design) among patients aged 50-75 years with high-risk de novo MDS (as defined by intermediate 2 or high-risk score on the International Prognostic Scoring System [IPSS]12) is currently enrolling patients.6 Targeted accrual is 338 patients by March 2018; as of 1 May 2016, 175 patients have enrolled. Similarly, a European randomized phase 2 biologic assignment trial is comparing HCT with reduced intensity conditioning after 5-azacitidine vs 5-azacitidine alone based on donor availability is in progress; enrollment of 230 patients is planned, with results expected by June 2017.13 Data from these trials will better inform decision-making, although neither is powered sufficiently to compare HCT and non-HCT in all relevant subgroups. In general, the decision-making process requires balancing the risk of TRM, by considering patient and donor features, and the likelihood of disease control, which requires considering disease risk features with HCT vs non-HCT therapy.
Age
The older age distribution of MDS patients is frequently cited as a barrier to HCT. Indeed, in a CIBMTR study of 701 patients with median age 53 years (range, 22-78 years), undergoing HCT with either reduced intensity or high-dose myeloablative conditioning regimens, from 2002 through 2006, older age was associated with significantly poorer survival posttransplant.4 However, the latter finding was largely attributable to the very favorable outcomes in patients younger than age 40.4 Another CIBMTR study, restricted to patients older than age 40 undergoing HCT with reduced intensity conditioning (n = 535), showed that age up to 70 was not a prognostic factor.14 Similarly, preliminary data from the study being done for evidence development for Centers for Medicare and Medicaid Services (see previous) indicate similar survival in patients older than 65 years vs those aged 55-64.10 Lim et al analyzed outcomes of 1333 patients older than 50 years undergoing HCT with either reduced or high intensity conditioning and also found no association of age with post-HCT outcomes.15 In another study of 105 patients older than age 50, all undergoing high-dose conditioning and T-cell–depleted HCT, older age was not a prognostic factor.16 However, it must be acknowledged that these studies included only patients deemed fit enough to undergo transplantation. With the median age of diagnosis being in the 7th decade,11 and given the high prevalence of comorbidities in the elderly, many MDS patients at the time of their diagnosis will not be considered sufficiently fit for the procedure.
How to assess fitness for HCT is uncertain. Aging is a heterogeneous process with changes across many domains—physiologic, physical, functional, social, psychiatric, and cognitive.17 The Comprehensive Geriatric Assessment (CGA) was developed to unmask vulnerabilities in these domains.18 In a pilot study of 166 transplantation candidates aged 50 years or older, the CGA revealed high prevalence of impairments in functional status, frailty, disability, and mental health.19 In a subsequent study by Muffly et al among 203 patients with a median age of 58 years (range, 50-73) who underwent allogeneic HCT for myeloid (n = 128 [30 had MDS]) and lymphoid malignancies, CGA domains were independently prognostic of overall survival.20 The authors then developed a simple 3-point risk score combining comorbidities assessment at HCT (see the following section) and one domain in the CGA – Instrumental Activities of Daily Living. Among patients aged 60 years or greater, this risk score identified a group of older HCT recipients with a 2-year survival of 0%, suggesting complete lack of benefit from HCT.20 Future studies are needed to further refine instruments such as the CGA to guide clinical decision-making in the older age population.
HST-Comorbidity Index (HCT-CI)
Comorbidities evaluated at the time of HCT are powerful prognostic markers of TRM independent of age.21 The HCT-CI was developed to quantitate this association and is now validated in many HCT settings, including HCT for MDS.22,23 Whether the HCT-CI identifies a subset of patients whose comorbidity burden leads to TRM rates that are too high to consider transplantation was addressed in a large analysis of more than 3000 patients with a median age of 45 years (range, 0.1-74), 40% of whom received reduced intensity conditioning.24 The authors concluded that mortality rates increased with increasing HCT-CI scores, but a level at which the HCT-CI score indicated a complete lack of benefit from the HCT was not identified regardless of the diagnosis category.24 Reduced intensity conditioning allows many older and less fit patients to undergo transplantation with acceptable risks of TRM. However, as mentioned previously, a recent study in older patients with hematologic malignancies showed that combining the HCT-CI with a geriatric assessment tool in patients older than 60 years could identify a group of patients with very poor survival after transplantation.20 Validation of this approach in larger groups of patients is warranted.
Disease risk as determined by scoring systems
The IPSS, the Revised IPSS (IPSS-R), and the World Health Organization classification-based Prognostic Scoring System were developed and validated in cohorts of patients who mostly did not undergo transplantation.12,25,26 These scores provide reliable predictors of life expectancy after diagnosis using several hematologic and cytogenetic features of the disease. For the IPSS-R, median life expectancies range from >8 years for patients with very low-risk disease to <1 year for patients with very high-risk disease. The ability of these scores to predict HCT outcomes is less well-studied. Della Porta et al analyzed outcomes of 519 patients with MDS who underwent transplantation from 2000 to 2011. In their analysis, IPSS-R performed better as a prognostic factor than IPSS.27 In their model, IPSS-R was prognostic of outcomes of patients in the high- and very high-risk groups, but not in the low- and intermediate-risk groups.27 By combining IPSS-R with other variables that correlated with post-HCT survival in multivariate analysis, such as patient age and comorbidities, presence of monosomal karyotype and pre-HCT response to induction chemotherapy, the authors successfully developed an MDS transplantation risk index. The transplantation risk index stratified the cohort into 4 groups with significantly different 5-year survival probabilities (low risk: 76%; intermediate: 48%; high: 18%; very high: 5%). However, the MDS transplantation risk index has not yet been validated in an independent data set.27
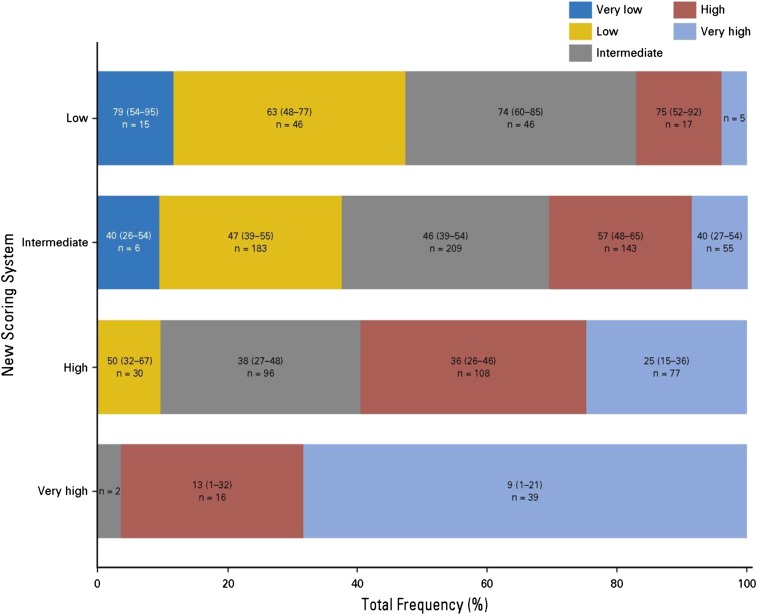
A large CIBMTR study analyzed outcomes of 2133 MDS patients transplanted from 2000 to 2012.28 This study used data collected at the time of HCT to develop a novel transplant-specific risk scoring system in a training set of 1151 patients undergoing HCT from HLA-matched related donors or well-matched unrelated donors. Five prognostic factors (age, peripheral blood blasts percentage, cytogenetic findings, performance status, and platelet count) were identified. Based on the magnitude of risk, these factors were combined into a risk score that divided patients into 4 risk groups (low, intermediate, high, and very high risk). The new score was highly prognostic of survival, disease-free survival, TRM, and relapse. The score was successfully validated in an independent cohort of 577 MDS patients receiving HCT from HLA-matched donors and further evaluated in an independent cohort of 405 MDS patients receiving HCT from HLA-mismatched unrelated donors. In the latter group, the score was only prognostic of relapse. Unlike IPSS-R at HCT, which could not sufficiently discriminate between posttransplant survival in the low and intermediate IPSS-R risk groups,26 all 4 risk groups in the CIBMTR risk score had significantly different survival from one another posttransplant, including the low- and intermediate-risk groups. This was further shown by comparing the performance of the new score with the performance of IPSS-R in a subset of 699 patients undergoing HCT from HLA-matched donors. Figure 4 illustrates that the 2 scores generally agreed in identifying patients at very high risk, but the new CIBMTR score significantly reclassified patients in the low-, intermediate-, and high-risk IPSS-R groups.28 Using the CIBMTR score to assess life expectancy after HCT and the IPSS-R to assess life expectancy without HCT may allow a better estimation of the relative benefits of the 2 approaches in individual patients. A caveat to this is that the prognostic value of the IPSS-R in the non-HCT setting is validated primarily when used at the time of diagnosis. Its value later in the course of the disease, after intervening therapy and when HCT is often being considered, is less well-understood.
Figure 4.
Categorization of patients according to the proposed CIBMTR prognostic system vs the IPSS-R. Reprinted from Shaffer et al28 with permission.
Minimal residual disease/molecular markers
There are few data on the prognostic value of minimal residual disease before transplantation or as a variable to consider when deciding on conditioning regimen intensity. In fact, only a minority of patients with MDS is in hematologic remission before HCT. When one uses sensitive techniques such as next-generation sequencing, most of these patients will have evidence of clonal hematopoiesis.29 A recent study evaluated the prognostic value of minimally identifiable disease (MID) among 287 patients who underwent transplantation from 2004 through 2013.30 MID was determined by multiparameter flow cytometry and cytogenetic assessment on bone marrow aspirates. Among patients in morphologic remission (n = 219), 54% were MID-positive and 23% were MID-negative. The association between MID and survival was dependent on regimen intensity. Among patients receiving high-dose, myeloablative conditioning regimens (n = 138), MID-negative and MID-positive patients had similar risks of post-HCT mortality; however, among those receiving reduced intensity regimens (n = 80), mortality was higher in the MID-positive patients, mainly because of the higher risk of MDS relapse.30
Bejar et al addressed the issue of molecular markers in 87 patients who underwent HCT from 2004 through 2009. Two-thirds underwent HCT with reduced intensity conditioning.29 The investigators sequenced 40 recurrently mutated genes. They detected somatic mutations in 92% of patients. In multivariate analysis adjusting for clinical variables, mutations in TP53, TET2, or DNMT3A were independent predictors for inferior survival. The main cause of death among patients who carried these mutations was disease relapse. Half of the cohort had a somatic mutation in 1 of these 3 genes. The association of these mutations with mortality did not vary by conditioning regimen intensity, but the sample size was relatively small.29
When Should Transplantation Be Done?
Because current non-HCT therapies do not cure MDS, one might reason that all patients with MDS, if fit, should undergo transplantation. However, many patients can live for years with their disease and there is an unavoidable risk of early TRM after HCT, even in low-risk, fit patients. Consequently, the decision about when to do transplantation involves estimating how to maximize pretransplant survival before exposing patients to the high early mortality risk of HCT, while not delaying so long that HCT outcomes are adversely affected. Evidence to inform this decision is available from retrospective studies using Markov model–based decision analyses to identify optimal timing of transplantation while stratifying patients based on IPSS scores31,32 or on IPSS and World Health Organization classification-based Prognostic Scoring System scores.33 These studies are consistent in indicating that life expectancy is maximized when transplantation is delayed for patients with low-risk disease. Conversely, life expectancy is maximized when transplantation is performed without delay for patients with high-risk disease. They disagree regarding the optimal strategy for patients with intermediate 1 IPSS. In 2 studies, delaying transplantation for these patients offered the maximum life expectancy,31,32 whereas in the third study, proceeding immediately to transplantation offered the maximum life expectancy.33 These estimations were similar when quality of life–adjusted life expectancy was considered.
There are subsets of patients with low-risk (by IPSS) MDS in whom early transplantation may still be warranted. Patients with excessive red cell transfusion requirements are at risk for inferior posttransplant outcomes if transplantation is delayed past the point of iron overload.34-37 Patients with intermediate 1 disease who fail hypomethylating agents have poor prognoses and warrant consideration of transplantation.38 Patients with bone marrow fibrosis also may represent a unique group with inferior outcomes despite low-risk disease. In fact, in 1 study, the presence of fibrosis represented a “shift up” in disease risk.39 Patients with therapy-related MDS have poor prognosis with nontransplant therapies. Bally et al analyzed 54 patients with therapy-related MDS (n = 42) or acute myeloid leukemia (AML; n = 12). The 2-year survival was only 14%.40 Given the grim prognosis with nontransplant approaches, these patients may benefit from early referral for allogeneic HCT consideration. Finally, molecular abnormalities present at the time of diagnosis predict survival with non-HCT therapy. In a study of 439 patients, multivariate analysis confirmed the independent prognostic value of 5 genes: TP53, EZH2, ETV6, RUNX1, and ASXL1.41 The incorporation of molecular data produced an important stage shift in the IPSS risk score, which would impact the decision of when to refer a patient for alloHCT.41
How Should Transplantation Be Done?
There are several transplantation approaches used in this and other populations that vary in the agents used to reduce disease burden pretransplant and ensure donor cell engraftment (pretransplant conditioning) and the type of graft infused. We outline a few important considerations in these strategies.
Cytoreductive therapy before HCT
Several multicenter retrospective analyses attempt to address whether using cytoreductive therapies to reduce pre-HCT disease burden can improve post-HCT outcomes. Some studies suggest a beneficial effect,42,43 whereas others suggest no difference.44 Few studies compare different types of cytoreduction before HCT. Gerds et al concluded that hypomethylating agents given before HCT gave outcomes similar to more intensive induction chemotherapy.45 A study by Damaj et al made similar conclusions.46 However, these studies were retrospective and assessed only patients receiving HCT, making them subject to considerable selection bias. Consequently, the European LeukemiaNet recommended that this question be studied in well-designed prospective trials.47 Such a study is currently under way.48
Conditioning regimen intensity
The purpose of the pretransplant conditioning regimen in hematologic malignancies and similar conditions is to reduce or eliminate neoplastic cells as well as to ensure sufficient immune ablation to allow donor cell engraftment. Historically, high-dose, myeloablative regimens were used to maximize neoplastic cell killing. However, as stated previously, MDS is a disease of the elderly,11 and older patients often tolerate high-dose conditioning poorly with excessive regimen-related morbidity and mortality. Introduction of reduced intensity regimens, which provide some cytotoxic effects and also sufficient immune suppression to facilitate engraftment, allows allogeneic HCT to be undertaken in older and less fit patients. Because of concerns regarding excessive TRM, reduced intensity conditioning is the preferred choice in most patients older than 65-70 years of age and among those with high HCT-CI scores. However, among patients who are to fit enough to tolerate higher dose regimens, the decision is not clear. Two recently completed randomized clinical trials might inform the decision. The first study enrolled patients 18-65 years of age (median, 58 years) with MDS (N = 54) or AML (N = 218). The primary endpoint was 18 month postrandomization overall survival. The reduced intensity regimens were fludarabine with busulfan (n = 110) or melphalan (n = 27). The myeloablative regimens were busulfan with cyclophosphamide (N = 40); or fludarabine (N = 87); or cyclophosphamide and total body irradiation (N = 8). The study was stopped prematurely because of a presumed relapse-free survival benefit with the higher intensity approach, as assessed by an independent Data and Safety Monitoring Board review. Overall survival was not significantly different between the 2 arms. Relapse-free survival for patients on the reduced intensity arm was 47% vs 68% on the myeloablative arm; the difference was statistically significant (P < .01).49 Subset analysis of the MDS patients was not possible because of the small sample size. The second study compared myeloablative doses of busulfan with fludarabine with reduced doses of busulfan with fludarabine. Between 2004 and 2012, 129 patients (median age, 51.4; range, 19-64 years) with MDS or secondary acute leukemia (< 20% blasts) were enrolled. One-year TRM, which was the primary endpoint, was similar between the 2 arms (P = .18). The 2-year probabilities of relapse, disease-free survival, and overall survival were also not significantly different between the 2 arms (P = .5, P = .5, and P = .07, respectively).50 Taken together, the results of these 2 randomized trials49,50 unfortunately do not end the debate, and longer follow-up of both cohorts will be important. However, neither study showed a difference in mortality by regimen intensity for patients deemed sufficiently fit to receive full dose conditioning. It seems prudent to offer the maximum intensity approach for these patients to afford the greatest chance for cure.
Novel conditioning regimens that can provide better disease control with reduced toxicity are urgently needed. In a phase 2 trial enrolling 45 MDS patients conditioned with treosulfan and fludarabine, after a median follow-up of 780 days, the 2-year cumulative incidences of nonrelapse mortality and relapse were 17% and 16%, respectively. The 2-year overall and disease-free survival estimates were 71% and 67%, respectively.51 These are early promising data and further investigations of treosulfan-based conditioning are underway.
Stem cell source
Most of the large studies addressing the question of optimal graft source in HCT involve comparisons of bone marrow and peripheral blood grafts in patients receiving myeloablative conditioning regimens. Retrospective studies and patient-level data meta-analysis suggest that, in the HLA-identical sibling setting, a peripheral blood stem cell source is superior to bone marrow in patients with MDS.52-54 However, in the unrelated donor setting, a phase 3 randomized clinical trial comparing peripheral blood stem cells with bone marrow in 550 transplants using myeloablative conditioning and including 93 patients with early and advanced MDS,55 demonstrated similar survival, disease-free survival, relapse, and TRM. Peripheral blood stem cell recipients were substantially more likely to develop chronic graft versus host disease (GVHD).55 Given the similar survival and lower risk of chronic GVHD in the phase 3 randomized clinical trial, which was conducted in the unrelated donor setting,55 we recommend that bone marrow be considered as the best stem cell source when unrelated donors are used.
Most transplantations using reduced intensity conditioning use peripheral blood grafts, which allow higher cell doses, to ensure engraftment. Eapen et al compared these 2 graft sources in the reduced intensity setting. Patients with AML, MDS, or non-Hodgkin lymphoma undergoing allogeneic HCTs from 2000 through 2008 with either bone marrow (n = 219) or peripheral blood stem cells (n = 887) were included.56 In multivariate analysis, 5-year survival was similar between the 2 graft sources.56 However, a recent study in patients with acute leukemia compared outcomes after reduced intensity allogeneic HCTs using bone marrow (n = 837) vs peripheral blood stem cells (n = 9011).57 After a median follow-up of 2 years, the use of peripheral blood stem cells was associated with superior leukemia-free and overall survival.57 Longer follow-up is needed to fully understand the impact of graft source on posttransplant outcomes in the reduced intensity setting. Use of cord blood grafts is discussed later.
Donor type
Data from CIBMTR and from single-center analyses independently confirm that contemporary rates of posttransplant survival in patients with MDS are similar after HCT from either HLA-identical sibling donors or 8/8 well-matched (HLA-A, B, C, DRB1) unrelated donors.4,5 However, HCTs from 7/8 HLA-matched unrelated donors are associated with 10% to 15% lower survival rates compared with HLA-identical sibling and unrelated donor HCTs.4,7
Recent analyses demonstrate that the 3-year survival rates after HCT using unrelated umbilical cord blood stem cells in patients with MDS are about 30%,8 which is lower than the 3-year survival rates with HLA-identical siblings and 8/8 well-matched unrelated donors.4 A recent comparative analysis from Eurocord and European Group for Blood and Marrow Transplantation confirms that, in MDS, outcomes after umbilical cord blood HCTs are inferior to outcomes after 8/8 well-matched unrelated donor HCTs.7 Only a handful of HCTs from haploidentical donors for patients with MDS have been performed to date, and very few were performed using the novel platform of posttransplant cyclophosphamide.58 Saber et al reported 3-year survival of only 33% on a cohort of 95 MDS patients after alloHCT from a haploidentical donor, using variety of approaches.6 Although survival rates with HLA-mismatched donors and with unrelated cord blood are lower than with HLA-matched donors, these transplant approaches provide a reasonable option for patients who have failed hypomethylating agents or who have other very high-risk features.
Given these data, we recommend that transplantation from HLA-identical siblings and 8/8 matched unrelated donors be considered standard therapy for MDS, with optimal timing determined by the considerations outlined here. HCT from alternative donors should also be considered in very high-risk and fit patients, again considering the tradeoff between life expectancy with non-HCT treatment and risk for HCT-related mortality. Finally, as advances in both non–HCT- and HCT-based therapeutic strategies take place, it will be critical to systematically evaluate quality of life (QOL) with different therapies. QOL is indeed extremely important for MDS patients, and high incidences of chronic GVHD can lead to sustained impairments in QOL after HCT.59 However, few studies to date address QOL in this patient population.60,61 Fortunately, MDS-specific QOL instruments were recently developed and validated.62,63 These instruments are now being incorporated in the design of the ongoing US natural history study of MDS.64
Acknowledgments
This work was supported by the National Institutes of Health: U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute, and U24-CA076518 from the National Cancer Institute, the National Heart, Lung, and Blood Institute, and the National Institute of Allergy and Infectious Diseases; and contract HHSH250201200016C with Health Resources and Services Administration.
References
- 1.Stern M, de Wreede LC, Brand R, et al. . Sensitivity of hematological malignancies to graft-versus-host effects: an EBMT megafile analysis. Leukemia. 2014;28(11):2235-2240. [DOI] [PubMed] [Google Scholar]
- 2.Porter DL, Antin JH. Donor leukocyte infusions in myeloid malignancies: new strategies. Best Pract Res Clin Haematol. 2006;19(4):737-755. [DOI] [PubMed] [Google Scholar]
- 3.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. ; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saber W, Cutler CS, Nakamura R, et al. . Impact of donor source on hematopoietic cell transplantation outcomes for patients with myelodysplastic syndromes (MDS). Blood. 2013;122(11):1974-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robin M, Porcher R, Adès L, et al. . Matched unrelated or matched sibling donors result in comparable outcomes after non-myeloablative HSCT in patients with AML or MDS. Bone Marrow Transplant. 2013;48(10):1296-1301. [DOI] [PubMed] [Google Scholar]
- 6.Saber W, Le Rademacher J, Sekeres M, et al. . Multicenter biologic assignment trial comparing reduced-intensity allogeneic hematopoietic cell transplant to hypomethylating therapy or best supportive care in patients aged 50 to 75 with intermediate-2 and high-risk myelodysplastic syndrome: Blood and Marrow Transplant Clinical Trials Network #1102 study rationale, design, and methods. Biol Blood Marrow Transplant. 2014;20(10):1566-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robin M, Ruggeri A, Labopin M, et al. . Comparison of unrelated cord blood and peripheral blood stem cell transplantation in adults with myelodysplastic syndrome after reduced-intensity conditioning regimen: a collaborative study from Eurocord (Cord blood Committee of Cellular Therapy & Immunobiology Working Party of EBMT) and Chronic Malignancies Working Party. Biol Blood Marrow Transplant. 2015;21(3):489-495. [DOI] [PubMed] [Google Scholar]
- 8.Gerds AT, Kalaycio ME, Ahn KW, et al. Outcomes after umbilical cord blood transplantation for myelodysplastic syndromes: a Center for International Blood and Marrow Transplant Registry (CIBMTR) Study [abstract]. Blood. 2015;126(23). Abstract 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Atallah E, Rizzo JD HCT for MDS Medicare Study (CMS). https://www.cibmtr.org/studies/clinicaltrials/hct-mds/Pages/index.aspx. Accessed 8 May 2016.
- 10.Atallah E, Horowitz MM, Logan B, et al. Outcome of patients 65 years and older with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation compared to patients 55-64 years of age [abstract]. Blood. 2015;126(23). Abstract 193. [Google Scholar]
- 11.Rollison DE, Howlader N, Smith MT, et al. . Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45-52. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg P, Cox C, LeBeau MM, et al. . International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088. [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov. 5-azacytidine treatment versus 5-azacytidine followed by allogeneic stem cell transplantation in elderly patients with myelodysplastic syndrome (MDS). https://clinicaltrials.gov/ct2/show/NCT01404741. Accessed 26 April 2016.
- 14.McClune BL, Weisdorf DJ, Pedersen TL, et al. . Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28(11):1878-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim Z, Brand R, Martino R, et al. . Allogeneic hematopoietic stem-cell transplantation for patients 50 years or older with myelodysplastic syndromes or secondary acute myeloid leukemia. J Clin Oncol. 2010;28(3):405-411. [DOI] [PubMed] [Google Scholar]
- 16.Tamari R, Chung SS, Papadopoulos EB, et al. . CD34-selected hematopoietic stem cell transplant conditioned with myeloablative regimens and antithymocyte gobulin for advanced myelodysplastic syndrome: limited graft-versus-host disease without increased relapse. Biol Blood Marrow Transplant. 2015;21(12):2106-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atallah E, Bylow K, Troy J, Saber W. Treatment of older patients with high-risk myelodysplastic syndromes (MDS): the emerging role of allogeneic hematopoietic stem cell transplantation (Allo HSCT). Curr Hematol Malig Rep. 2014;9(1):57-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodin MB, Mohile SG. A practical approach to geriatric assessment in oncology. J Clin Oncol. 2007;25(14):1936-1944. [DOI] [PubMed] [Google Scholar]
- 19.Muffly LS, Boulukos M, Swanson K, et al. . Pilot study of comprehensive geriatric assessment (CGA) in allogeneic transplant: CGA captures a high prevalence of vulnerabilities in older transplant recipients. Biol Blood Marrow Transplant. 2013;19(3):429-434. [DOI] [PubMed] [Google Scholar]
- 20.Muffly LS, Kocherginsky M, Stock W, et al. . Geriatric assessment to predict survival in older allogeneic hematopoietic cell transplantation recipients. Haematologica. 2014;99(8):1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorror ML, Maris MB, Storer B, et al. . Comparing morbidity and mortality of HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative and myeloablative conditioning: influence of pretransplantation comorbidities. Blood. 2004;104(4):961-968. [DOI] [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. . Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorror ML, Sandmaier BM, Storer BE, et al. . Comorbidity and disease status based risk stratification of outcomes among patients with acute myeloid leukemia or myelodysplasia receiving allogeneic hematopoietic cell transplantation. J Clin Oncol. 2007;25(27):4246-4254. [DOI] [PubMed] [Google Scholar]
- 24.Sorror ML, Storb R, Sandmaier BM, et al. . Impact of comorbidities on early and late mortalities after allogeneic hematopoietic cell transplantation (HCT) [abstract]. Blood. 2011;118(21). Abstract 326. [Google Scholar]
- 25.Malcovati L, Porta MG, Pascutto C, et al. . Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23(30):7594-7603. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg PL, Tuechler H, Schanz J, et al. . Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Della Porta MG, Alessandrino EP, Bacigalupo A, et al. ; Gruppo Italiano Trapianto di Midollo Osseo. Predictive factors for the outcome of allogeneic transplantation in patients with MDS stratified according to the revised IPSS-R. Blood. 2014;123(15):2333-2342. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer BC, Ahn KW, Hu ZH, et al. . Scoring system prognostic of outcome in patients undergoing allogeneic hematopoietic cell transplantation for myelodysplastic syndrome. J Clin Oncol. 2016;34(16):1864-1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bejar R, Stevenson KE, Caughey B, et al. . Somatic mutations predict poor outcome in patients with myelodysplastic syndrome after hematopoietic stem-cell transplantation. J Clin Oncol. 2014;32(25):2691-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Festuccia M, Deeg HJ, Gooley TA, et al. . Minimal identifiable disease and the role of conditioning intensity in hematopoietic cell transplantation for MDS and AML evolving from MDS. Biol Blood Marrow Transplant. 2016;22(7):1227-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutler CS, Lee SJ, Greenberg P, et al. . A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104(2):579-585. [DOI] [PubMed] [Google Scholar]
- 32.Koreth J, Pidala J, Perez WS, et al. . Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013;31(21):2662-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alessandrino EP, Porta MG, Malcovati L, et al. ; Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Optimal timing of allogeneic hematopoietic stem cell transplantation in patients with myelodysplastic syndrome. Am J Hematol. 2013;88(7):581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busca A, Falda M, Manzini P, et al. . Iron overload in patients receiving allogeneic hematopoietic stem cell transplantation: quantification of iron burden by a superconducting quantum interference device (SQUID) and therapeutic effectiveness of phlebotomy. Biol Blood Marrow Transplant. 2010;16(1):115-122. [DOI] [PubMed] [Google Scholar]
- 35.Armand P, Kim HT, Cutler CS, et al. . Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem cell transplantation. Blood. 2007;109(10):4586-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kataoka K, Nannya Y, Hangaishi A, et al. . Influence of pretransplantation serum ferritin on nonrelapse mortality after myeloablative and nonmyeloablative allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(2):195-204. [DOI] [PubMed] [Google Scholar]
- 37.Mahindra A, Bolwell B, Sobecks R, et al. . Elevated pretransplant ferritin is associated with a lower incidence of chronic graft-versus-host disease and inferior survival after myeloablative allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2009;146(3):310-316. [DOI] [PubMed] [Google Scholar]
- 38.Jabbour E, Garcia-Manero G, Batty N, et al. . Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 2010;116(16):3830-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Porta MG, Malcovati L, Boveri E, et al. . Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol. 2009;27(5):754-762. [DOI] [PubMed] [Google Scholar]
- 40.Bally C, Thépot S, Quesnel B, et al. . Azacitidine in the treatment of therapy related myelodysplastic syndrome and acute myeloid leukemia (tMDS/AML): a report on 54 patients by the Groupe Francophone Des Myelodysplasies (GFM). Leuk Res. 2013;37(6):637-640. [DOI] [PubMed] [Google Scholar]
- 41.Bejar R, Stevenson K, Abdel-Wahab O, et al. . Clinical effect of point mutations in myelodysplastic syndromes. N Engl J Med. 2011;364(26):2496-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sierra J, Pérez WS, Rozman C, et al. . Bone marrow transplantation from HLA-identical siblings as treatment for myelodysplasia. Blood. 2002;100(6):1997-2004. [PubMed] [Google Scholar]
- 43.de Witte T, Hermans J, Vossen J, et al. . Haematopoietic stem cell transplantation for patients with myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000;110(3):620-630. [DOI] [PubMed] [Google Scholar]
- 44.Appelbaum FR, Anderson J. Allogeneic bone marrow transplantation for myelodysplastic syndrome: outcomes analysis according to IPSS score. Leukemia. 1998;12(Suppl 1):S25-S29. [PubMed] [Google Scholar]
- 45.Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant. 2012;18(8):1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damaj G, Duhamel A, Robin M, et al. . Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Société Française de Greffe de Moelle et de Thérapie-Cellulaire and the Groupe-Francophone des Myélodysplasies. J Clin Oncol. 2012;30(36):4533-4540. [DOI] [PubMed] [Google Scholar]
- 47.Malcovati L, Hellström-Lindberg E, Bowen D, et al. ; European Leukemia Net. Diagnosis and treatment of primary myelodysplastic syndromes in adults: recommendations from the European LeukemiaNet. Blood. 2013;122(17):2943-2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ClinicalTrials.gov. Chemotherapy in Treating Patients With Myelodysplastic Syndrome Before Donor Stem Cell Transplant (ICT-HCT). https://clinicaltrials.gov/ct2/show/NCT01812252?term=NCT01812252&rank=1. Accessed June 14, 2016.
- 49.Scott BL, Pasquini MC, Logan B, et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901 [abstract]. Blood. 2015;126(23). Abstract LBA-8. [Google Scholar]
- 50.Kröger N, Brand R, Niederwieser D, et al. . Reduced intensity vs. standard conditioning followed by allogeneic stem cell transplantation for patients with MDS or secondary AML: a prospective, randomized phase III study of the Chronic Malignancies Working Party of the EBMT (RICMAC-Trial) [abstract]. Blood. 2014;124(21). Abstract 320. [Google Scholar]
- 51.Ruutu T, Volin L, Beelen DW, et al. . Reduced-toxicity conditioning with treosulfan and fludarabine in allogeneic hematopoietic stem cell transplantation for myelodysplastic syndromes: final results of an international prospective phase II trial. Haematologica. 2011;96(9):1344-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.del Cañizo MC, Martínez C, Conde E, et al. ; Grupo Español de Trasplante Hematopoyético. Peripheral blood is safer than bone marrow as a source of hematopoietic progenitors in patients with myelodysplastic syndromes who receive an allogeneic transplantation. Results from the Spanish registry. Bone Marrow Transplant. 2003;32(10):987-992. [DOI] [PubMed] [Google Scholar]
- 53.Guardiola P, Runde V, Bacigalupo A, et al. ; Subcommittee for Myelodysplastic Syndromes of the Chronic Leukaemia Working Group of the European Blood and Marrow Transplantation Group. Retrospective comparison of bone marrow and granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells for allogeneic stem cell transplantation using HLA identical sibling donors in myelodysplastic syndromes. Blood. 2002;99(12):4370-4378. [DOI] [PubMed] [Google Scholar]
- 54.Stem Cell Trialists’ Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23(22):5074-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anasetti C, Logan BR, Lee SJ, et al. ; Blood and Marrow Transplant Clinical Trials Network. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367(16):1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eapen M, Logan BR, Horowitz MM, et al. . Bone marrow or peripheral blood for reduced-intensity conditioning unrelated donor transplantation. J Clin Oncol. 2015;33(4):364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savani BN, Labopin M, Blaise D, et al. . Peripheral blood stem cell graft compared to bone marrow after reduced intensity conditioning regimens for acute leukemia: a report from the ALWP of the EBMT. Haematologica. 2016;101(2):256-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sekeres MA, Stone RM, Zahrieh D, et al. . Decision-making and quality of life in older adults with acute myeloid leukemia or advanced myelodysplastic syndrome. Leukemia. 2004;18(4):809-816. [DOI] [PubMed] [Google Scholar]
- 60.Heptinstall K, Myelodysplastic Syndromes Foundation I. Quality of life in myelodysplastic syndromes. A special report from the Myelodysplastic Syndromes Foundation, Inc. Oncology (Williston Park). 2008;22(2 Suppl Nurse Ed):13-19. [PubMed] [Google Scholar]
- 61.Caocci G, La Nasa G, Efficace F. Health-related quality of life and symptom assessment in patients with myelodysplastic syndromes. Expert Rev Hematol. 2009;2(1):69-80. [DOI] [PubMed] [Google Scholar]
- 62.Abel GA, Klaassen R, Lee SJ, et al. . Patient-reported outcomes for the myelodysplastic syndromes: a new MDS-specific measure of quality of life. Blood. 2014;123(3):451-452. [DOI] [PubMed] [Google Scholar]
- 63.Abel GA, Efficace F, Buckstein RJ, et al. . Prospective international validation of the Quality of Life in Myelodysplasia Scale (QUALMS). Haematologica. 2016;101(6):781-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.ClinicalTrials.gov. The National Myelodysplastic Syndromes (MDS) Study. https://clinicaltrials.gov/ct2/show/NCT02775383?term=NCT02775383&rank=1. Accessed 14 June 2016.