Abstract
Heparin-induced thrombocytopenia (HIT) remains an important diagnosis to consider in hospitalized patients developing thrombocytopenia. HIT is an immune-mediated prothrombotic disorder caused by antibodies to platelet factor 4 (PF4) and heparin. Recent basic scientific studies have advanced our understanding of disease pathogenesis through studies of the PF4/heparin structure, immune mechanisms, and cellular basis of thrombosis. Clinical advances have also occurred in areas of HIT prevention, description of disease variants, and diagnostic strategies. Emerging anticoagulants with the potential to change HIT treatment are evolving, although with limited data. This review will provide a current perspective on HIT pathogenesis, disease features, diagnostic strategies, and role of emerging therapies for the management of HIT.
Learning Objectives
Understand new developments in the pathogenesis, epidemiology, clinical presentation, and new therapeutics for HIT
Understand diagnostic strategies for HIT
Understand the role of emerging therapeutics in the management of HIT
Introduction
Until recently, unfractionated heparin (UFH) and low-molecular weight heparins (LMWHs) have served as the cornerstones of anticoagulant therapy in hospitalized patients. Notwithstanding the clinical introduction of several novel oral anticoagulant therapies in recent years, these drugs are likely to remain in our therapeutic armamentarium because of their favorable pharmacologic profiles, ability to inhibit multiple coagulation proteins, and therapeutic efficacy for several indications for which there are no suitable alternatives (eg, cardiac surgery, treatment of cancer-associated thrombosis). Consequently, heparin-induced thrombocytopenia (HIT), an immune complication of heparin therapy, remains a highly relevant complication for the hematology practitioner to diagnose and manage. This review will update the reader on recent developments in HIT pathogenesis, disease prevention, clinical features, testing, and novel therapies. For comprehensive reviews on the clinical presentation, diagnosis, and management of HIT, the reader is referred to recent reviews.1,2
Updates on HIT pathogenesis
HIT is an iatrogenic complication of UFH or LMWH therapy caused by antibodies that recognize complexes of platelet factor 4 (PF4) and heparin within 5 to 14 days of drug exposure.2 In some sensitized patients, high-titer anti-PF4/heparin antibodies of the immunoglobulin G (IgG) isotype bind FcγRIIA receptor bearing cells, trigger cellular activation, and elicit a profound hypercoagulable state that may result in arterial and/or venous thrombosis. Recent developments in elucidating the pathogenesis of HIT have occurred in areas related to the crystal structure of the PF4/heparin complex, immune basis of HIT, and mechanisms of thrombosis.
The PF4/heparin antigenic complex
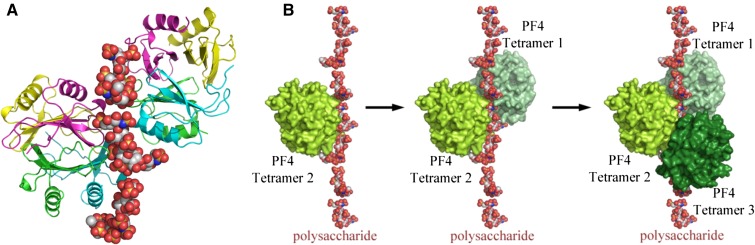
PF4 is a highly cationic protein stored in platelet α granules and released upon platelet activation. PF4 binds to the negatively charged heparin through electrostatic interactions, leading to the generation of ultralarge complexes. Structural studies by Brandt et al and Kreimann et al suggest that neoepitopes are expressed on PF4/heparin only under energetically favorable conditions that are dually met when PF4 binds to heparins of minimal chain length (>11 saccharides) and exceeds a threshold of energy to induce a conformational change.3,4 Cai et al extended these findings in 2015 through crystallization of the PF4/heparin complex using the synthetic fondaparinux as a heparin analog rather than the biologically heterogeneous UFH/LMWH.5 These studies reveal that heparin stabilizes the PF4 molecule in its tetrameric conformation and, in the process, linearizes itself, allowing additional PF4 tetramers to join the growing complex (Figure 1) and exposing neoepitopes on PF4 recognized by HIT antibodies. HIT antibodies further stabilize this antigenic complex. Inhibition of tetramer formation using a monoclonal antibody to the PF4 monomer interferes with heparin and HIT antibody binding, pointing the way to development of therapeutic targets.5
Figure 1.
Crystal structure of the PF4/heparin complex and formation of ultralarge complexes. (A) Overall structure of the PF4/fondaparinux complex. Fondaparinux makes contact with a single PF4 tetramer in the groove among the monomers on one side of the asymmetric tetramer. Monomers A, B, C, and D in one PF4 tetramer are colored in green, cyan, magenta, and yellow, respectively. (B) Analysis of crystal lattice reveals a molecular pathway for the formation of antigenic complexes. A fragment of heparin first binds within the groove of one PF4 tetramer (limon, left); binding of the first PF4 tetramer imparts a local linearized structure on heparin, which enhances the binding of a second tetramer (pale green, middle); and progression of this process eventuates in the formation of ultralarge antigenic complexes (right). Reprinted from Cai et al5 with permission.
PF4/heparin immune response
Recent investigations of the HIT immune response suggest that innate immune mechanisms, caused either by bacterial infection and/or antigen-mediated complement activation, may contribute significantly to the development of anti-PF4/heparin antibodies. Population studies show a correlation of periodontal disease with anti-PF4/heparin antibody reactivity, irrespective of Ig isotype.6 Several studies have documented interactions of PF4 with both gram-positive and gram-negative bacteria,7 as well as to short chain polyphosphates released from platelets.8 Other studies implicate direct complement activation by PF4/heparin complexes. In these latter studies, C3/C4 activation by PF4/heparin complexes results in antigen binding to complement receptor 2/CD21 on B cells.9 Because antigen binding to CD21 significantly enhances immunogenicity, these studies implicate CD21-mediated binding of PF4/heparin complexes as an initial step for subsequent antibody formation.
Mechanisms of thrombosis
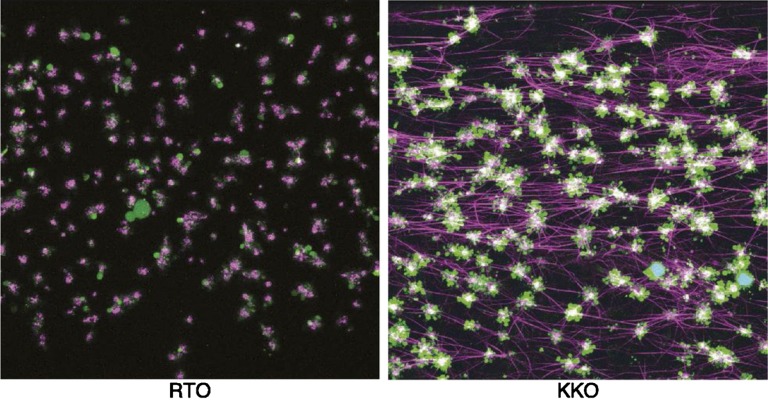
Although it has been long appreciated that platelets are a prime target for HIT antibodies, the mechanisms underlying thrombin generation have been poorly understood. Recent studies by Tutwiler et al have shown a prominent role for monocytes in mediating the profound hypercoagulable state in HIT.10 These studies show that HIT antibodies activate both platelets and monocytes primarily by FcγRIIA and confirm prior observations on the contribution of monocytes to tissue factor expression and thrombin generation (Figure 2). In addition to direct platelet FcγRIIA signaling by HIT antibodies, monocyte-derived thrombin provides additional platelet signaling via cleavage of protease-activated receptor 1.10 Depletion of monocytes or inhibition of FcγRIIA signaling markedly impairs platelet aggregation and fibrin deposition in this experimental system, thus providing an explanation for the intense thrombin generation seen in association with HIT.
Figure 2.
Visualization of thrombus formation by a HIT-like monoclonal antibody. Confocal microscopy images of fixed thrombi formed in human whole blood perfused with a control antibody (RTO) or a HIT-like antibody (KKO), and PF4. Platelet aggregates are shown in green, fibrin fibers visualized by adding of Alexa 647-labeled fibrinogen are purple, and white blood cells are shown in cyan (overlap of blue nuclear dye is Hoechst and green is calcein AM). The attachment points of fibrin to platelets are white because of the superposition of purple and green colors. Reprinted from Tutweiler et al10 with permission.
Updates in HIT epidemiology
Anti-PF4/heparin antibodies are rare in healthy individuals. In 2 large surveys of healthy donors (n = 40297) and blood bank donors (n = 380011), anti- PF4/heparin antibodies were detected in ∼3% to 4% of patients, using a low cutoff for antibody positivity (optical density [OD] >0.4), and in 0.3% to 0.5% of healthy subjects, using a higher cutoff for antibody positivity (OD >1).
Although antibody formation is unusual in healthy subjects, there is evidence that anti-PF4/heparin autoantibodies develop in the context of inflammation and/or orthopedic surgery. An intriguing study by Bito et al12 suggests that surgical inflammation may provide sufficient inflammatory signals for triggering PF4/heparin antibody formation in the absence of heparin. In this prospective study of ∼2000 patients who were either treated with pharmacologic thromboprophylaxis (n = 1125; UFH, LMWH, or fondaparinux) or mechanical compression stockings (n = 944; dynamic or static compression), seroconversion rates were surprisingly high in patients receiving only mechanical prophylaxis: ∼15% in patients receiving dynamic compression vs 6.5% for static compressions. This effect of dynamic compression was additive in patients receiving fondaparinux as compared with fondaparinux-alone–treated patients without compression stockings (21% vs 5.7%; P = .025).12 The authors did not examine whether PF4/heparin “autoantibodies” developing in the absence of heparin exposure have similar platelet-activating antibodies as HIT antibodies or carry a similar propensity for causing disease. As well, the study did not examine the mechanism of PF4/heparin autoantibody formation in patients receiving disease-modifying therapies. The authors speculate that mechanical compression compounds the tissue damage caused by surgery leading to enhanced platelet activation, polyanion, and/or nucleic acid release. Additional studies are clearly needed to confirm these findings and characterize the biologic risk posed by these autoantibodies.
For the majority of patients, however, HIT occurs as a complication of heparin exposure. Epidemiologic data indicate that UFH is associated with higher rates of seroconversion and clinical disease than LMWH13 or fondaparinux.14 The observation that LMWHs have a 10-fold lower rate of immunogenicity than UFH has prompted studies of disease prevention. In a recent study, investigators at Sunnybrook Health Sciences Center implemented an “Avoid Heparin” initiative in 2006.15 This initiative consisted of systematic replacement of UFH with LMWH for thromboprophylaxis and/or treatment, saline catheter flushes rather than UFH, and removal of UFH from the nursing units. Comparison of outcomes of HIT testing, diagnosis, and costs before (2003-2005) and after full implementation of this program (2007-2012) revealed a dramatic reduction in the cases of suspected HIT (85.5 vs 49.0 cases per 10 000 admissions; P < .001), positive HIT enzyme-linked immunosorbent assay (ELISA) (16.5 vs 6.1 cases per 10 000 admissions; P < .001), and adjudicated cases of HIT with and without thrombosis (10.7/4.6 vs 2.2/0.4 cases per 10 000 admissions; P < .001).15 These reductions were accompanied by significant costs savings associated with caring for HIT patients from $322 321 to $55 383.15 This study and related studies of computerized support tools employing 4Ts HIT scoring16 suggest that disease burden can be considerably lowered through use of LMWH.
Updates in disease presentation
The hallmark of HIT is the development of a mild to moderate absolute (50-70 × 109/L) or relative thrombocytopenia (platelet count fall of >30% to 50%), which develops 5 to 14 days after heparin exposure and a median of 2 days after development of anti-PF4/heparin antibodies17,18 in heparin-naïve individuals. In patients with more proximate heparin exposure (<100 days), thrombocytopenia can develop acutely within 24 hours due to the presence of circulating anti-PF4/heparin antibodies. Thrombocytopenia as the only manifestation of HIT is termed “isolated” HIT. “Isolated” HIT is considered a prothrombotic condition, due to high rates of subsequent thrombosis (20% to 50%).19,20
Two clinical variants of HIT, delayed-onset HIT and spontaneous HIT, exhibit autoimmune features due to occurrence of antibodies that bind PF4 and heparin-like glycosaminoglycans (GAGs). To date, both variants have only been described in case reports and/or series, and their incidence remains poorly defined. Delayed-onset HIT, first described by Warkentin and Kelton in 2001,21 occurs after heparin exposure, but complications of thrombocytopenia and thrombosis may be delayed for days to weeks after heparin discontinuation. Antibodies causing delayed-onset HIT are usually cross-reactive with PF4/GAG complexes found on cell surfaces and characteristically elicit heparin-independent platelet activation in functional assays.21 Spontaneous HIT, on the other hand, is not associated with antecedent heparin exposure and is considered a true autoimmune manifestation caused by autoantibodies to PF4/GAG complexes. All the reported cases to date are associated with findings of thrombocytopenia and thrombosis in association with high-titer platelet activating anti-PF4/heparin antibodies.22,23 As with delayed-onset HIT, anti-PF4/heparin antibodies bind to PF4/GAGs on platelets and, thus, have the distinctive feature of heparin-independent platelet activation. Diagnostic criteria for spontaneous HIT have been proposed based on clinical findings of thrombocytopenia and thrombosis without prior heparin exposure, and stringent laboratory criteria that include both serologic assays and functional assays of heparin-dependent platelet activation.22
Updates in diagnosis
Diagnosis of HIT is established using a combination of clinical and laboratory criteria. Clinical features of the disease, as discussed above, rely on showing a temporal association of heparin therapy with thrombocytopenia and/or thrombosis, and excluding other causes of thrombocytopenia. The 4Ts clinical scoring system, a commonly used risk stratification tool,24 remains a valuable clinical prediction rule for patients suspected of HIT. In a recent meta-analysis of studies using the 4Ts scoring system, a low probability 4Ts score (0-3) was found to reliably exclude HIT in patients. Low 4Ts scores were associated with a high negative predictive value (NPV) (0.998; 95% confidence interval [CI], 0.970-1.000).25 On the other hand, intermediate (4-5) and high (>6) scores had poor positive predictive values for HIT (0.14; CI, 0.09-0.22) and (0.64; CI, 0.40-0.82), respectively, in large part due to subjective differences of individuals using the scoring systems.25 Based on these findings, the “Choosing Wisely” campaign of the American Society of Hematology recommends against testing for HIT in patients with a low pretest probability.26 However, recent studies indicate that a “low score” assessment can also be subject to operator error in ∼2% of cases.24 In a prospective multicenter study, 526 patients suspected of HIT were assessed with a 4Ts score, a rapid particle assay (PF4/H-PaGIA), and serotonin release assay (SRA), and managed based on the results of a 4Ts score and the rapid particle assay while awaiting the results of the SRA. Patients with a low 4Ts score, irrespective of the PF4/H-PaGIA result, or intermediate score and a negative PF4/H-PaGIA assay, were assigned to receive danaparoid/fondaparinux or allowed to continue LMWH therapy. Patients in all other categories (intermediate 4Ts with positive PF4/H-PaGIA or high 4Ts with positive/negative PF4/H-PaGIA) were switched to alternative therapies. A positive SRA was considered diagnostic of HIT in this study. Of the 321 patients with a low pretest score, 39 were positive by the PF4/H-PaGIA and 6 of this latter group were SRA+ (1.9% of low score cohort). Review of these 6 cases assigned to a “low score” indicated that 2 patients were incorrectly scored and 4 cases were complex patients in whom concurrent causes of thrombocytopenia were present. Although the vast majority of the “low score” patients were true negative for HIT (∼98% were SRA negative), this study and similar studies (Table 1)24,27,28 suggest that clinicians should assign a “low score” with certainty; if there is uncertainty regarding the clinical criteria, then patients should be assigned higher points to merit an intermediate level of suspicion.
Table 1.
Pre- and posttest probability of HIT using a combined approach of the 4Ts and EIAs
| Reference | Design | N | Pretest probability of 4Ts | Posttest probability of 4Ts (+ assay/− assay) | ||||
|---|---|---|---|---|---|---|---|---|
| Low (0-3) | Intermediate (4-5) | High (6-8) | Low (0-3) | Intermediate (4-5) | High (6-8) | |||
| 27 | Single center, retrospective | 1291 | 0.8% | 14% | 53% | NR | 65%/0% | 95%/0% |
| 28 | Multicenter, prospective | 380 | 2.1% | 11% | 47% | 11%/0.06% | 42%/0.4% | 83%/2.6% |
| 24 | Multicenter, prospective | 526 | 1.9% | 7% | 37% | 15%/0% | 42%/0% | 88%/0% |
EIA, enzyme immunoassay; NR, not reported.
Approximately 40% of patients evaluated by the 4Ts will have intermediate or high clinical scores that require additional laboratory testing to establish diagnosis.24 Laboratory testing for HIT can be performed using immunologic or functional assays of platelet activation.
Functional assays identify pathogenic IgG antibodies capable of binding platelet FcγRIIA and eliciting platelet activation. These assays offer the advantage of a high specificity (>95%)29 and positive predictive values (89% to 100%),30 but have the disadvantages of lower sensitivity and being technically cumbersome (56% to 100%).29,31 Variations in assay sensitivity largely derive from differences in the functional end points of the assay. Radioactive assays are generally more sensitive than other detection methods.29 Functional assays are also affected by technical variables, such as differences in platelet reactivity, specialized reagents, and assay expertise. Recent studies indicate that the sensitivity of functional assays, whether the SRA or flow cytometry-based assays, can be improved by the addition of PF4, which alters the stoichiometry of PF4/heparin or PF4/GAG complexes.32,33 One such flow cytometric assay, the PF4-dependent P-selectin expression assay (PEA), was recently compared with the SRA in 91 clinical samples annotated by a 4Ts score. These authors showed improved accuracy of the PEA with the SRA, and reported a higher sensitivity of the PEA (96% as compared with 56% for the SRA) and a specificity (85% as compared with 92% for the SRA).33 The lower sensitivity of the SRA reported in this study as compared with other studies29,31 could be explained by methodologic differences in disease ascertainment. Additional prospective studies are needed to validate these findings whether this nonradioactive assay or other functional assays under development,34 could supplant the radioactive SRA.
Immunoassays rely on measuring anti-PF4/heparin antibodies of all isotypes (IgG, IgA, IgM, or polyclonal or polyspecific assays) or IgG isotypes, and do not distinguish platelet activating from nonplatelet activating antibodies. There are a number of assay platforms that have been developed for serologic detection of antibodies, ranging from original ELISA (polyspecific), which takes 2 to 3 hours to perform to rapid immunoassays with turnaround times of <30 minutes. The reader is referred to recent comprehensive analyses on the performance characteristics of various HIT immunoassays.35,36
Immunoassays are the mainstay of laboratory diagnosis of HIT, because they are widely available, offer a rapid turnaround time, and are highly sensitive (>99%).1 Their main drawback is their lack of specificity (30% to 70%). However, recent studies indicate that specificity can be improved through detection of IgG antibodies and quantification of results, expressed either as an OD or titers (for most rapid immunoassays). In a pooled analysis of tests performed on 3366 patients, IgG-specific ELISAs were associated with greater specificity when compared with polyspecific ELISAs (93.5% vs 89.4%) at the expense of slightly lower sensitivity (95.8% vs 98.1%).37 For ELISA assays, studies have shown that high ODs significantly correlate with the presence of platelet activating antibodies, thrombotic risk, and the likelihood of having HIT.38,39
Studies are increasingly validating the approach of combining clinical and immunoassays for risk stratification, real-time management, and establishing a diagnosis of HIT.24,27,28 In both retrospective27 and prospective studies,24,28 when a clinical 4Ts score is applied alongside immunoassay results, the posttest probabilities are sufficiently high or low such that functional assays are needed only for a subset of patients in the intermediate category with positive immunoassays (Table 1). As recommended by American Society of Hematology “Choosing Wisely” guidelines, if patients are assessed with certainty to have a low clinical suspicion for HIT based on the 4Ts scoring system, the high NPV of scoring systems (NPV = 0.998) is sufficiently high such that laboratory testing is not recommended.1 Similarly, for patients with a high clinical suspicion (eg, 4Ts >6), whose immunoassays show the presence of anti-PF4/heparin antibodies, the likelihood of positive functional assays is sufficiently high enough (83% to 95%) that HIT can be considered confirmed without the need for further functional assays. These patients should be managed according to guidelines using nonheparin anticoagulants.40,41 Although rare in clinical experience and published literature, some patients can present with a high clinical suspicion but have negative immunoassays. In this latter group, platelet-activating antibodies can be demonstrated in ∼3% of patients, possibly due to the presence of non-PF4/heparin antibodies. If suspicion for HIT remains high in these patients despite negative testing, then patients should be continued on alternative anticoagulants. For patients in the “intermediate” category (4Ts = 4-5) of clinical suspicion for HIT who have a pretest probability of 7% to 14%, a negative immunoassay virtually eliminates the possibility of HIT due to posttest probabilities of ∼0% to 0.4% (Table 1). On the other hand, a positive test in this intermediate patient group increases the posttest probability of HIT to 40% to 64%. It is in this category of patients where functional assays may be informative.
Updates in emerging therapies for HIT
Management decisions in HIT must often be made prior to availability of laboratory results. The direct thrombin inhibitors (DTI), argatroban and bivalirudin, are US Food and Drug Administration-approved agents for the treatment of HIT (argatroban) and use in HIT patients undergoing percutaneous coronary intervention or cardiac surgery (bivalirudin). Use of either agent should be guided by the half-life of therapeutic agent and co-existing hepatic and/or renal dysfunction. For discussion of the safety and efficacy of parenteral nonheparin anticoagulants in the management of HIT, the reader is referred to recent guidelines and reviews.40,42
DTI therapy, and eventual bridging to warfarin, requires inpatient administration and prolongs hospital stay. DTI alternatives such as fondaparinux, and emerging therapies such as the direct oral anticoagulants (DOACs), are clinically appealing because they have the potential to shorten hospital stay, decrease cost, and enable outpatient anticoagulant treatment. Although these agents have not been subject to formal testing against parenteral DTIs in HIT, data suggest that they may be efficacious. In the following sections, we will highlight newer therapies for the treatment of HIT, including fondaparinux and DOACs, and discuss an evolving role for applications of plasma exchange to reduce antibody burden in patients with acute and subacute HIT.
Fondaparinux
Fondaparinux is increasingly being used for the management of HIT in patients, because fondaparinux does not cross-react with HIT antibodies.43 In a minority of patients, fondaparinux has been reported to cause HIT.44 Given the number of limited reports, the incidence of fondaparinux-induced HIT remains unknown. Several retrospective series, however, have demonstrated the safety and efficacy of fondaparinux in the treatment of HIT. In a small retrospective series of 16 SRA+ HIT patients treated with fondaparinux, half of whom had thrombosis, there were no new or progressive thromboses noted and treatment was associated with a reduction in thrombin-antithrombin complexes within 24 hours of treatment.45 A retrospective multicenter registry of 195 patients found that fondaparinux was the most commonly used anticoagulant occurring in 40% of patients.46 In another retrospective study of 133 patients receiving fondaparinux for HIT, Kang et al found no significant differences in complications of thrombosis and/or bleeding in patients as compared with propensity matched controls on DTI.47 To date, there is only 1 prospective study of fondaparinux for the treatment of HIT. In this small study, 7 patients treated with fondaparinux showed similar rates of platelet recovery without new thrombosis as compared with historical controls treated with DTI.48 Because increased bleeding rates (∼10% to 22%) have been noted in small series,49,50 caution must be exercised for use in critically-ill patients due to the drug’s long half-life and renal clearance. British guidelines recommend the use of therapeutic dose fondaparinux for the management of HIT based on level 2C evidence.41
DAOCs
Rivaroxaban use in HIT has been reported in a small number of case reports, 1 case series,51 and a multicenter single arm prospective study.52 The latter prospective single arm study closed early due to poor enrollment,52 but reported outcomes on 22 enrolled patients, 12 of whom were diagnosed with HIT. In this small cohort of HIT patients, rivaroxaban appeared to be safe and effective with 9/10 thrombocytopenic patients achieving full platelet count recovery during therapy. Although there were no new/recurrent thromboses or bleeding complications in patients diagnosed with HIT, some complications were noted, including the progression of a previously diagnosed catheter-associated thrombosis in 1 patient, amputation being required for another patient with worsening ischemia, and major bleeding occurring >1 week after drug discontinuation in another patient. In a case series by Sharifi et al, 11 patients were initially treated with a parenteral DTI, followed by rivaroxaban. The authors reported no recurrent arterial or venous thrombosis or bleeding complications in this cohort. However, a lack of study details and heterogenous treatment assignment limit conclusions from this study.51 Other than the case series reported by Sharifi et al,51 there are only case reports on the use of apixaban and dabigatran for HIT (Table 2).51-53
Table 2.
DAOCs in HIT
| Reference | Study (N) | HIT Dx | DOAC | Dose (mg) | Other AC | Outcomes |
|---|---|---|---|---|---|---|
| 51 | Case series* (22) | ELISA/SRA+ (20) | Dabigatran (6) | 150 twice daily | Initial argatroban | New DVT (5) |
| Clinical Dx (2) | Rivaroxaban (11) | 20 daily | SVT (2) | |||
| — | Apixaban (5) | 5 twice daily | Deaths (6)† | |||
| 52 | Prospective cohort (12) | SRA+ | Rivaroxaban | 15 twice daily → 20 daily | Initial fondaparinux (6) | VTE (1) |
| Transitioned to fondaparinux‡ (1) | BKA (1) | |||||
| Deaths (4)† | ||||||
| Major bleeding‡ (1) | ||||||
| 53 | Case series (3) | ELISA+ | Rivaroxaban | 15 twice daily → 20 daily | Transitioned to warfarin (1) | Platelet recovery, no thrombosis |
| 15 twice daily | ||||||
| 10 daily§ |
AC, anticoagulant; BKA, below-knee amputation; DVT, deep vein thrombosis; DX, diagnosis; SVT, supraventricular tachycardia; VTE, venous thromboembolism.
*Retrospective case series with prospective determination of outcomes.
†Deaths were not due to thrombosis but to the underlying disease states, including cancer, heart failure, renal failure, systemic sclerosis, sepsis, and end-stage chronic obstructive pulmonary disease.
‡One patient transitioned to fondaparinux due to elevated hepatic enzymes.
§Renal-adjusted dose in a dialysis-dependent patient.
Based on the difficulties encountered by Canadian investigators of the multicenter study of rivaroxaban for HIT,52 it is unlikely that DOACs will undergo rigorous or systematic investigation in HIT. For now, there is insufficient clinical data or experience to recommend the use of these drugs as stand-alone therapy for HIT.
Therapeutic plasma exchange (TPE) for the treatment of HIT
In the pre-DTI era, TPE was used as a primary treatment of acute HIT. A retrospective study of 44 patients with HIT showed that plasma exchange initiated within 4 days of a HIT diagnosis decreased mortality compared with patients who did not receive plasma exchange or in whom treatment was delayed (4.8 vs 32; 57%).54 In the post-DTI era, plasma exchange has been used as a temporizing strategy that decreases anti-PF4/heparin antibody titers sufficiently enough to allow heparin re-exposure without increasing thrombotic events.55 A retrospective study of 11 cardiac surgery patients who underwent a single plasma exchange at the time of surgery showed a reduction in heparin/PF4 titers of up to 84%.56 One patient developed foot ischemia in the setting of cardiogenic shock and 3 patients died of causes unrelated to HIT. A recent case report suggests that TPE efficacy may in part be due to lowering levels of platelet activating antibodies in circulation, even though antibody levels, as measured by immunoassays, may remain high.57 Although the optimal role of TPE in HIT has yet to be defined, its use appears justified in settings where treatment options are either lacking, for example the management of DTI-refractory disease or where there is clinical equipoise (eg, due to bleeding risk associated with DTI use in cardiac surgery).54,56
Conclusion
HIT remains an important clinical problem in hospitalized patients due to the continued need for heparin in certain clinical settings. Basic investigations of HIT biology have provided insights into the pathogenesis of antibody formation and mechanisms of thrombosis. Although HIT remains a challenging diagnosis, studies are validating a diagnostic approach combining the 4Ts with immunoassays to reduce the need for functional assays and to optimize management. Data are currently limited for use of DAOCs in HIT, but with evolving clinical experience, they hold promise for broadening therapeutic options.
Acknowledgments
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (P01HL110860 [G.M.A.] and HL110860-S1 [O.O.]).
References
- 1.Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. [DOI] [PubMed] [Google Scholar]
- 2.Greinacher A. Heparin-induced thrombocytopenia. N Engl J Med. 2015;373(19):1883-1884. [DOI] [PubMed] [Google Scholar]
- 3.Brandt S, Krauel K, Gottschalk KE, et al. . Characterisation of the conformational changes in platelet factor 4 induced by polyanions: towards in vitro prediction of antigenicity. Thromb Haemost. 2014;112(1):53-64. [DOI] [PubMed] [Google Scholar]
- 4.Kreimann M, Brandt S, Krauel K, et al. . Binding of anti-platelet factor 4/heparin antibodies depends on the thermodynamics of conformational changes in platelet factor 4. Blood. 2014;124(15):2442-2449. [DOI] [PubMed] [Google Scholar]
- 5.Cai Z, Yarovoi SV, Zhu Z, et al. . Atomic description of the immune complex involved in heparin-induced thrombocytopenia. Nat Commun. 2015;6:8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greinacher A, Holtfreter B, Krauel K, et al. . Association of natural anti-platelet factor 4/heparin antibodies with periodontal disease. Blood. 2011;118(5):1395-1401. [DOI] [PubMed] [Google Scholar]
- 7.Krauel K, Pötschke C, Weber C, et al. . Platelet factor 4 binds to bacteria, [corrected] inducing antibodies cross-reacting with the major antigen in heparin-induced thrombocytopenia [published correction appears in Blood. 2011;117(21):5783]. Blood. 2011;117(4):1370-1378. [DOI] [PubMed] [Google Scholar]
- 8.Brandt S, Krauel K, Jaax M, et al. . Polyphosphates form antigenic complexes with platelet factor 4 (PF4) and enhance PF4-binding to bacteria. Thromb Haemost. 2015;114(6):1189-1198. [DOI] [PubMed] [Google Scholar]
- 9.Khandelwal S, Lee GM, Hester CG, et al. . The antigenic complex in HIT binds to B-cells via complement and complement receptor 2 (CD21). Blood. 2016;128(14):1789-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tutwiler V, Madeeva D, Ahn HS, et al. . Platelet transactivation by monocytes promotes thrombosis in heparin-induced thrombocytopenia. Blood. 2016;127(4):464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hursting MJ, Pai PJ, McCracken JE, et al. . Platelet factor 4/heparin antibodies in blood bank donors. Am J Clin Pathol. 2010;134(5):774-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bito S, Miyata S, Migita K, et al. . Mechanical prophylaxis is a heparin-independent risk for anti-platelet factor 4/heparin antibody formation after orthopedic surgery. Blood. 2016;127(8):1036-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106(8):2710-2715. [DOI] [PubMed] [Google Scholar]
- 14.Warkentin TE, Maurer BT, Aster RH. Heparin-induced thrombocytopenia associated with fondaparinux. N Engl J Med. 2007;356(25):2653-2655. [DOI] [PubMed] [Google Scholar]
- 15.McGowan KE, Makari J, Diamantouros A, et al. . Reducing the hospital burden of heparin-induced thrombocytopenia: impact of an avoid-heparin program. Blood. 2016;127(16):1954-1959. [DOI] [PubMed] [Google Scholar]
- 16.Samuelson BT, Glynn E, Holmes M, White AA, Martin DB, Garcia D. Use of a computer-based provider order entry (CPOE) intervention to optimize laboratory testing in patients with suspected heparin-induced thrombocytopenia. Thromb Res. 2015;136(5):928-931. [DOI] [PubMed] [Google Scholar]
- 17.Greinacher A, Farner B, Kroll H, Kohlmann T, Warkentin TE, Eichler P. Clinical features of heparin-induced thrombocytopenia including risk factors for thrombosis. A retrospective analysis of 408 patients. Thromb Haemost. 2005;94(1):132-135. [DOI] [PubMed] [Google Scholar]
- 18.Warkentin TE, Sheppard JA, Moore JC, Cook RJ, Kelton JG. Studies of the immune response in heparin-induced thrombocytopenia. Blood. 2009;113(20):4963-4969. [DOI] [PubMed] [Google Scholar]
- 19.Warkentin TE, Kelton JG. A 14-year study of heparin-induced thrombocytopenia. Am J Med. 1996;101(5):502-507. [DOI] [PubMed] [Google Scholar]
- 20.Lewis BE, Wallis DE, Leya F, Hursting MJ, Kelton JG; Argatroban-915 Investigators. Argatroban anticoagulation in patients with heparin-induced thrombocytopenia. Arch Intern Med. 2003;163(15):1849-1856. [DOI] [PubMed] [Google Scholar]
- 21.Warkentin TE, Kelton JG. Delayed-onset heparin-induced thrombocytopenia and thrombosis. Ann Intern Med. 2001;135(7):502-506. [DOI] [PubMed] [Google Scholar]
- 22.Warkentin TE, Basciano PA, Knopman J, Bernstein RA. Spontaneous heparin-induced thrombocytopenia syndrome: 2 new cases and a proposal for defining this disorder. Blood. 2014;123(23):3651-3654. [DOI] [PubMed] [Google Scholar]
- 23.Warkentin TE, Anderson JA. How I treat patients with a history of heparin-induced thrombocytopenia. Blood. 2016;128(3):348-359. [DOI] [PubMed] [Google Scholar]
- 24.Linkins LA, Bates SM, Lee AY, Heddle NM, Wang G, Warkentin TE. Combination of 4Ts score and PF4/H-PaGIA for diagnosis and management of heparin-induced thrombocytopenia: prospective cohort study. Blood. 2015;126(5):597-603. [DOI] [PubMed] [Google Scholar]
- 25.Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hicks LK, Bering H, Carson KR, et al. . Five hematologic tests and treatments to question. Blood. 2014;124(24):3524-3528. [DOI] [PubMed] [Google Scholar]
- 27.Nellen V, Sulzer I, Barizzi G, Lämmle B, Alberio L. Rapid exclusion or confirmation of heparin-induced thrombocytopenia: a single-center experience with 1,291 patients. Haematologica. 2012;97(1):89-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leroux D, Hezard N, Lebreton A, et al. . Prospective evaluation of a rapid nanoparticle-based lateral flow immunoassay (STic Expert(®) HIT) for the diagnosis of heparin-induced thrombocytopenia. Br J Haematol. 2014;166(5):774-782. [DOI] [PubMed] [Google Scholar]
- 29.Warkentin TE, Sheppard JC, Moore KM et al. . Laboratory testing for the antibodies that cause heparin-induced thrombocytopenia: how much class do we need? J Lab Clin Med. 2005;146(6):341-346. [DOI] [PubMed] [Google Scholar]
- 30.Arepally GM, Ortel TL. Clinical practice. Heparin-induced thrombocytopenia. N Engl J Med. 2006;355(8):809-817. [DOI] [PubMed] [Google Scholar]
- 31.Pouplard C, Amiral J, Borg JY, Laporte-Simitsidis S, Delahousse B, Gruel Y. Decision analysis for use of platelet aggregation test, carbon 14-serotonin release assay, and heparin-platelet factor 4 enzyme-linked immunosorbent assay for diagnosis of heparin-induced thrombocytopenia. Am J Clin Pathol. 1999;111(5):700-706. [DOI] [PubMed] [Google Scholar]
- 32.Nazi I, Arnold DM, Warkentin TE, Smith JW, Staibano P, Kelton JG. Distinguishing between anti-platelet factor 4/heparin antibodies that can and cannot cause heparin-induced thrombocytopenia. J Thromb Haemost. 2015;13(10):1900-1907. [DOI] [PubMed] [Google Scholar]
- 33.Padmanabhan A, Jones CG, Curtis BR, et al. . A novel PF4-dependent platelet activation assay identifies patients likely to have heparin-induced thrombocytopenia/thrombosis (HIT). Chest. 2016;150(3):506-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sono-Koree NK, Crist RA, Frank EL, Rodgers GM, Smock KJ. A high-performance liquid chromatography method for the serotonin release assay is equivalent to the radioactive method. Int J Lab Hematol. 2016;38(1):72-80. [DOI] [PubMed] [Google Scholar]
- 35.Sun L, Gimotty PA, Lakshmanan S, Cuker A. Diagnostic accuracy of rapid immunoassays for heparin-induced thrombocytopenia. A systematic review and meta-analysis. Thromb Haemost. 2016;115(5):1044-1055. [DOI] [PubMed] [Google Scholar]
- 36.Nagler M, Bachmann LM, ten Cate H, ten Cate-Hoek A. Diagnostic value of immunoassays for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2016;127(5):546-557. [DOI] [PubMed] [Google Scholar]
- 37.Cuker A, Ortel TL. ASH evidence-based guidelines: is the IgG-specific anti-PF4/heparin ELISA superior to the polyspecific ELISA in the laboratory diagnosis of HIT? Hematology Am Soc Hematol Educ Program. 2009;250-252. [DOI] [PubMed] [Google Scholar]
- 38.Warkentin TE, Greinacher A, Gruel Y, Aster RH, Chong BH; Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Laboratory testing for heparin-induced thrombocytopenia: a conceptual framework and implications for diagnosis. J Thromb Haemost. 2011;9(12):2498-2500. [DOI] [PubMed] [Google Scholar]
- 39.Baroletti S, Hurwitz S, Conti NA, Fanikos J, Piazza G, Goldhaber SZ. Thrombosis in suspected heparin-induced thrombocytopenia occurs more often with high antibody levels. Am J Med. 2012;125(1):44-49. [DOI] [PubMed] [Google Scholar]
- 40.Linkins LA, Dans AL, Moores LK, et al. . Treatment and prevention of heparin-induced thrombocytopenia: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(suppl 2):e495S-e530S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson H, Davidson S, Keeling D; Haemostasis, Thrombosis Task Force of the British Committee for Standards in H. Guidelines on the diagnosis and management of heparin-induced thrombocytopenia: second edition. Br J Haematol. 2012;159(5):528-540. [DOI] [PubMed] [Google Scholar]
- 42.Kelton JG, Arnold DM, Bates SM. Nonheparin anticoagulants for heparin-induced thrombocytopenia. N Engl J Med. 2013;368(8):737-744. [DOI] [PubMed] [Google Scholar]
- 43.Savi P, Chong BH, Greinacher A, et al. . Effect of fondaparinux on platelet activation in the presence of heparin-dependent antibodies: a blinded comparative multicenter study with unfractionated heparin. Blood. 2005;105(1):139-144. [DOI] [PubMed] [Google Scholar]
- 44.Bhatt VR, Aryal MR, Shrestha R, Armitage JO. Fondaparinux-associated heparin-induced thrombocytopenia. Eur J Haematol. 2013;91(5):437-441. [DOI] [PubMed] [Google Scholar]
- 45.Warkentin TE, Pai M, Sheppard JI, Schulman S, Spyropoulos AC, Eikelboom JW. Fondaparinux treatment of acute heparin-induced thrombocytopenia confirmed by the serotonin-release assay: a 30-month, 16-patient case series. J Thromb Haemost. 2011;9(12):2389-2396. [DOI] [PubMed] [Google Scholar]
- 46.Schindewolf M, Steindl J, Beyer-Westendorf J, et al. . Frequent off-label use of fondaparinux in patients with suspected acute heparin-induced thrombocytopenia (HIT)--findings from the GerHIT multi-centre registry study. Thromb Res. 2014;134(1):29-35. [DOI] [PubMed] [Google Scholar]
- 47.Kang M, Alahmadi M, Sawh S, Kovacs MJ, Lazo-Langner A. Fondaparinux for the treatment of suspected heparin-induced thrombocytopenia: a propensity score-matched study. Blood. 2015;125(6):924-929. [DOI] [PubMed] [Google Scholar]
- 48.Lobo B, Finch C, Howard A, Minhas S. Fondaparinux for the treatment of patients with acute heparin-induced thrombocytopenia. Thromb Haemost. 2008;99(1):208-214. [DOI] [PubMed] [Google Scholar]
- 49.Benken ST, Tillman N, Dajani S, Shah A, Thomas T. A retrospective evaluation of fondaparinux for confirmed or suspected heparin-induced thrombocytopenia in left-ventricular-assist device patients. J Cardiothorac Surg. 2014;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cegarra-Sanmartín V, González-Rodríguez R, Paniagua-Iglesias P, et al. . Fondaparinux as a safe alternative for managing heparin-induced thrombocytopenia in postoperative cardiac surgery patients. J Cardiothorac Vasc Anesth. 2014;28(4):1008-1012. [DOI] [PubMed] [Google Scholar]
- 51.Sharifi M, Bay C, Vajo Z, Freeman W, Sharifi M, Schwartz F. New oral anticoagulants in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015;135(4):607-609. [DOI] [PubMed] [Google Scholar]
- 52.Linkins LA, Warkentin TE, Pai M, et al. . Rivaroxaban for treatment of suspected or confirmed heparin-induced thrombocytopenia study. J Thromb Haemost. 2016;14(6):1206-1210. [DOI] [PubMed] [Google Scholar]
- 53.Ng HJ, Than H, Teo EC. First experiences with the use of rivaroxaban in the treatment of heparin-induced thrombocytopenia. Thromb Res. 2015;135(1):205-207. [DOI] [PubMed] [Google Scholar]
- 54.Robinson JA, Lewis BE. Plasmapheresis in the management of heparin-induced thrombocytopenia. Semin Hematol. 1999;36(suppl 1):29-32. [PubMed] [Google Scholar]
- 55.Schwartz J, Winters JL, Padmanabhan A, et al. . Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher. 2013;28(3):187. [DOI] [PubMed] [Google Scholar]
- 56.Welsby IJ, Um J, Milano CA, Ortel TL, Arepally G. Plasmapheresis and heparin reexposure as a management strategy for cardiac surgical patients with heparin-induced thrombocytopenia. Anesth Analg. 2010;110(1):30-35. [DOI] [PubMed] [Google Scholar]
- 57.Warkentin TE, Sheppard JA, Chu FV, Kapoor A, Crowther MA, Gangji A. Plasma exchange to remove HIT antibodies: dissociation between enzyme-immunoassay and platelet activation test reactivities. Blood. 2015;125(1):195-198. [DOI] [PubMed] [Google Scholar]