Abstract
Despite the recent substantial improvement of clinical outcome in mantle cell lymphoma (MCL), resistance to immunochemotherapy and common relapses are challenges for long-term tumor control. The assessment of minimal residual disease (MRD) by real-time quantitative polymerase chain reaction has emerged as a widely feasible and standardized tool for direct assessment of therapy-induced reduction of tumor burden and regrowth after cytotoxic treatment in MCL, with much improved sensitivity compared with conventional staging procedures. Several studies have shown that intensification of initial treatment, which has resulted in improved clinical outcome, is immediately reflected in higher molecular remission rates; they have also shown that high-dose consolidation might not be able to compensate for less intensive induction regimens. Persistence or reappearance of MRD in clinical remission proved to be highly predictive for imminent clinical relapse associated with shorter overall survival. Therefore, the investigation of novel MRD-guided treatment strategies aimed at early eradication of MRD and pre-emptive treatment of molecular relapse seems warranted. Furthermore, the integration of MRD assessment into clinical response criteria could result in a more specific and potentially earlier end point for treatment efficacy. New technical developments such as high-throughput sequencing will further enhance the wide applicability of MRD detection in MCL.
Learning Objectives
To interpret the validity and clinical relevance of results on MRD in MCL
To relate MRD to the intensity and efficacy of different treatment strategies
To interpret the prognostic and predictive value of MRD for subsequent clinical progression in different therapeutic settings
To develop strategies for MRD-driven therapeutic concepts in MCL
To examine the potential use of MRD for response assessment in MCL
Introduction
Mantle cell lymphoma (MCL) is a cancer of the lymphatic tissue genetically characterized by the t(11;14) translocation leading to an overexpression of cyclin D1. MCL is considered incurable; relapses are common, and MCL has a shorter overall survival (OS) compared with other lymphoma entities. In the past 20 years, the introduction of immunochemotherapy,1,2 high-dose cytarabine (HA),3,4 high-dose consolidation followed by autologous stem cell transplantation (ASCT),5,6 bendamustine,7 and rituximab maintenance8 have substantially improved the outcome of patients with MCL. Currently, new targeted drugs that aim at long-term control or even cure of MCL are under investigation.
Individual outcome has been shown to be associated with clinical prognostic factors summarized in the MCL International Prognostic Index (MIPI).9 The degree of tumor cell proliferation as measured by the routinely available Ki-67 index is a strong independent biological prognostic factor that, when combined with MIPI, allows a refined risk stratification.10 In recent years, increasing evidence has shown that the presence of minimal residual disease (MRD) detectable in the peripheral blood (PB) or bone marrow (BM) might be an even stronger prognostic and predictive factor (compared with conventional staging procedures) that could be used for treatment decisions to further improve clinical outcome. This review highlights the most important results currently available on the relevance of MRD detection in MCL that give insights into the biology of MCL and the impact of different treatment strategies.
Methods for detecting MCL cells
MCL typically presents with extensive involvement of lymphatic or extralymphatic tissue in advanced Ann Arbor stage II to IV disease, as determined by imaging and clinical work-up. The diagnosis is routinely made by histopathology on a tissue biopsy. BM biopsies are routinely taken for investigation of microscopic BM infiltration, demonstrating that the majority of patients present with histologic BM involvement. A number of more sensitive methods are used to detect the amount of dissemination of MCL cells at diagnosis and of MRD during and after treatment (Table 1).
Table 1.
Comparison of different techniques to detect MRD in MCL
| Method | MFC | Consensus PCR | Nested PCR | RQ-PCR | HTS |
|---|---|---|---|---|---|
| Target | Immunophenotype | IGH rearrangement or t(11;14) | IGH rearrangement or t(11;14) | IGH rearrangement or t(11;14) | IGH rearrangement |
| Reproducible detection limit | 10−3 to 10−4 (4-color MFC) | IGH: 10−2 to 10−3 | 10−5 | 10−5 | Up to 10−6 (dependent on DNA amount) |
| 10−4 (8-color MFC) | t(11;14): 10−4 | ||||
| Level of information | Quantitative (above detection limit) | Qualitative | Qualitative | Quantitative (above limit of quantification) | Quantitative (above detection limit) |
| Patient-specific PCR primer needed | Not applicable | No | Depending on approach | Yes | No |
| Patient applicability in advanced-stage MCL | >85% | >95% | >85% | >85% | No data yet |
| Expertise | High for 6- to 8-color MFC | Lower | High | High | High |
| Standardization | No | No | No | Yes | No |
| Turnaround time | 3-4 h | 3-4 h | Dependent on method; mostly 3-4 h | 2 wk | 1 wk |
| Advantages | Rapid quantification | Rapid and robust | No establishment of serial dilution for quantification needed | High sensitivity; greatest interlaboratory reproducibility; regular quality control rounds | High sensitivity; independent of patient-specific primers; additional information on background B-cell repertoire |
| Disadvantages | Low sensitivity; expertise needed for evaluation; not standardized | Low sensitivity; unspecific amplification possible; no quantification | Not quantitative; not standardized | ASO primer design necessary; time-consuming workflow | Super multiplex PCR (disproportional target amplification possible); discrimination from normal cell background (requires about 5% tumor cell infiltration); complex bioinformatic evaluation |
IGH, immunoglobulin heavy chain gene.
Multiparameter flow cytometry
Multiparameter flow cytometry (MFC) is a well-established method for diagnosing hematologic malignancies, and it reliably detects dissemination of MCL to PB or BM with a detection limit of 8 × 10−4 when a 4-color MFC assay is used.11 As shown by results from the European MCL Network MCL Younger4 or MCL Elderly8 trials, more than 85% of patients with MCL of Ann Arbor stages II to IV have disseminated disease at primary diagnosis that is detectable in PB or BM by MFC.11,12 Although MFC is an assay that is suitable for use at MCL diagnosis, there are no established MFC panels for MRD quantification during follow-up. In a comparative analysis using surface light chain restriction in the CD19+CD5+ subpopulation, 4-color MFC lacked sensitivity for quantifying MRD, being inferior to consensus qualitative polymerase chain reaction (PCR).11 A recent publication showed that a single, 8-color 10-antibody MFC tube allowed specific MRD assessment with a robust sensitivity of 0.01% in all patients; however, even 8-color MFC approaches rarely exceed this limit of sensitivity.12,13 By using the cutoff level of 0.01%, MFC detected MRD in only 80% of the patients who were MRD positive by real-time quantitative PCR (RQ-PCR).12 Because it has become apparent that even very low levels of MRD positivity could be used for therapeutic intervention in clinical trials, there is a need for optimized MFC strategies that use new antibody combinations or new bioinformatics tools for evaluating greater numbers of cells to achieve a sensitivity comparable to or even higher than that of RQ-PCR.
RQ-PCR
RQ-PCR is currently the method of choice for detecting MRD in MCL because it is the most standardized and sensitive technique. The most broadly applicable marker for MRD studies in malignant B-cell lymphomas, detectable in about 80% to 95% of MCLs, is the immunoglobulin heavy chain (IGH) gene rearrangement. Identification of the clonal IGH rearrangement in diagnostic samples by consensus PCR and subsequent sequencing of the junctional region allows the design of an allele-specific oligonucleotide (ASO) assay for RQ-PCR (ASO-RQ-PCR) that targets the tumor-specific hypervariable complementary determining region (Figure 1A). The t(11;14) translocation as a specific chromosomal translocation in MCL represents a second suitable target for RQ-PCR because of its high stability and lack of somatic mutations; however, it is detectable by PCR in only 25% to 40% of patients14 because breakpoints on chromosome 11 often scatter outside breakpoint cluster regions and are not detected by consensus chromosome 11 primers.
Figure 1.
Workflow for MRD assessment by RQ-PCR and high-throughput sequencing targeting the clonal immunoglobulin heavy chain gene (IGH) rearrangement. (A) Consensus PCR for assessment of clonal IGH gene rearrangements is followed by Sanger sequencing to identify the clonal VH-N-DH-N-JH region for each patient. Allele-specific primers are used for sensitive quantification of residual tumor cells by RQ-PCR. (B) An amplicon-sequencing strategy is used for identification of clonal IGH gene rearrangement in a 2-step PCR approach, in which the first PCR is performed by using multiplex gene-specific primers tailed with a universal linker sequence. The universal tailed amplicons can be used for second-round PCR, in which next-generation sequencing platform-specific adapters can be introduced, depending on the platform used for sequencing.
Nonquantitative PCR strategies that use consensus primers for the variable or the joining regions of the IGH locus have a detection limit of about 1% to 2% of lymphoma cells in a polyclonal background, and thus they are limited in their suitability for MRD detection.11 In contrast, qualitative nested PCR or RQ-PCR that use ASO primers targeting the IGH rearrangement or the t(11;14) translocation reach reproducible detection limits of 1 MCL cell among up to 100 000 white blood cells.15,16 RQ-PCR for MRD assessment is applicable in the vast majority of patients with MCL as demonstrated by results from the MCL Younger and MCL Elderly trials: in 96% of 641 patients with MCL who had a diagnostic PB or BM sample, a clonal IGH signal or the t(11;14) translocation was detectable by PCR. The leukemic nature of MCL was further underscored by the high median infiltration level of MCL cells in PB at primary diagnosis of 6.3 × 10−2.17 However, RQ-PCR for both targets is restricted to those patients with junctional regions suited to reach sufficient sensitivity by applying the published guidelines.18 By applying the guidelines, 86% of the MCL Younger and MCL Elderly trial cohorts were reliably quantifiable by RQ-PCR. In that investigation, results for ASO-RQ-PCR targeting either the t(11;14) translocation or IGH were highly comparable.
Standardization of MRD assessment and regular quality controls are essential for high interlaboratory comparability of MRD and for treatment guided by MRD. The European Study Group on MRD as a division of the European Scientific Foundation for Laboratory HematoOncology has developed guidelines for analyzing and interpreting RQ-PCR MRD data18 in acute lymphoblastic leukemia (ALL) that can be directly applied to MCL.
To summarize, RQ-PCR targeting the IGH locus or the t(11;14) translocation is considered the gold standard for MRD assessment in MCL because it yields the highest sensitivity, gives quantitative results, is highly standardized, and is widely applicable.14
High-throughput sequencing
High-throughput technologies may provide a novel approach for MRD detection in the future that could overcome the disadvantages of classical ASO-RQ-PCR–based MRD approaches.19 Amplicon-based high-throughput sequencing (HTS) of clonal IGH rearrangements (Figure 1B) avoids the laborious design and testing of patient-specific assays, and the readout is more specific than that of RQ-PCR, in which false-positive results may occur because of nonspecific binding of ASO primers. However, because HTS is based on PCR amplification of clonal IGH rearrangements, the same technical restrictions as for standard PCR also apply for HTS-based target identification.
HTS was compared with RQ-PCR in 3 different types of B-cell malignancies, including a series of 30 patients with MCL.20 In 4 patients, HTS quantified MRD whereas no suitable MRD marker could be developed for RQ-PCR because of the failure of marker sequencing or an unsuitable junctional region for primer design. Of 156 follow-up samples, 82% were concordant between the 2 methods. Discordances were major qualitative in 1%, borderline qualitative in 13%, and quantitative in 3%; RQ-PCR yielded a positive or 1-log higher result compared with HTS in 10% whereas the opposite occurred in 8%. These results are very encouraging; however, HTS-based MRD quantification is also dependent on the amount of input DNA, which limits sensitivity. In addition, the assumption that specificity of HTS sequence detection is absolute must be critically discussed, because background amplification of unrelated clones might occur, limiting the sensitivity of HTS. Furthermore, as with RQ-PCR, the degree of tumor cell infiltration of the diagnostic sample is critical for determining the clonotype followed for MRD by HTS. As shown in ALL, a frequency threshold of 5% is used to assign a clonotype originating from the tumor clone.21 Results regarding the prognostic value of MRD detection by HTS have been published for ALL21 and multiple myeloma22; however, no data are currently available for MCL. Overall, standardization, quality control, and validation of HTS in a multicenter setting as well as guidelines for bioinformatic evaluation and data interpretation have to be implemented before HTS is routinely used for MRD detection in MCL. Ideally, the integrated use of standardized MFC, RQ-PCR, and HTS will provide MRD assessment for the majority of patients with MCL in clinical trials.
MRD during and after induction treatment
During and after treatment, the detection and quantification of MRD by RQ-PCR offers the possibility of investigating the effect of different treatment strategies on tumor burden and detecting residual tumor cells with high sensitivity. Chemotherapy using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) does not seem to result in a relevant reduction of tumor load in PB, BM, or PB stem cell products, even among patients who achieve a clinical remission, as reported in the Nordic MCL1 study5 and a single-center series of homogeneously treated patients (Table 2).16 Immunochemotherapy with rituximab plus CHOP (R-CHOP) in the MCL Younger and MCL Elderly trials showed (for the first time) relevant rates of molecular remission (MRD negativity) of 40%, with 21% of patients MRD negative already at midterm induction.4,23 Further intensification of induction treatment with HA in the arm of the MCL Younger trial, which used alternating R-CHOP and rituximab plus dexamethasone, HA, and cisplatin (R-DHAP), was associated with 66% molecular remissions, with 39% of patients MRD negative already at midterm induction.24 The impact of further induction treatment cycles on lymphoma cell reduction midterm induction were well documented for both treatment arms by an increase in the molecular remission rate after full induction. In the Nordic MCL3 study, similar to the MCL Younger trial, 53% molecular remissions were observed by qualitative nested PCR in patients with clinical remission after 5 alternating R-CHOP/R-HA cycles.25
Table 2.
Overview of studies that investigated the molecular remission rates among previously untreated patients with Ann Arbor stage II to IV MCL
| MRD negativity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Reference | Induction | Consolidation/maintenance | MRD detection method | At end of induction | After consolidation | ||||
| PB or BM | PB | BM | PB or BM | PB | BM | |||||
| Nordic MCL1 | 5 | 6 × maxiCHOP | ASCT | MFC or PCR | NR (0 of 16 stem cell products) | NR | NR | NR | NR | 38% of 13 |
| Kiel single-center report | 16 | 6 × CHOP-like | ASCT | RQ-PCR | 0 of 9 (6% of 17 stem cell products) | NR | NR | 52% of 27 | NR | NR |
| MCL Younger and MCL elderly | 4, 24, 25 | 6-8 × R-CHOP | ASCT or rituximab maintenance or interferon alpha maintenance | RQ-PCR | 40% of 225 | 53% of 216 | 34% of 156 | 48% of 209 | 58% of 205 | 56% of 117 |
| MCL Younger | 4, 25 | 6 × R-CHOP | ASCT | RQ-PCR | 34% of 126 | 47% of 119 | 26% of 91 | 54% of 119 | 68% of 117 | 59% of 81 |
| MCL Younger | 4, 25 | 3 × R-CHOP/R-DHAP | ASCT | RQ-PCR | 66% of 109 | 79% of 103 | 61% of 77 | 77% of 114 | 85% of 112 | 79% of 71 |
| Nordic MCL2 | 3, 34, 36 | 3 × R-maxiCHOP/R-HA | ASCT | Nested PCR | NR (88% of 42 stem cell products) | NR | NR | 92% of 79 | NR | NR |
| Nordic MCL3 | 26 | 3 × R-maxiCHOP/R-HA | Ibritumomab-tiuxetan + ASCT | Nested PCR | 53% of 99 | NR | NR | 83% of 107 | NR | NR |
| LYMA | 29 | 4 × R-DHAP | ASCT + rituximab maintenance or observation | RQ-PCR | NR | 80% of 120 | 66% of 98 | NR | 95% of 162 | 82% of 122 |
| CALGB 59909 | 30 | 2 × methotrexate + R-maxiCHOP | ASCT | RQ-PCR | NR | 46% of 39 | 36% of 39 | 74% of 27 | NR | NR |
| CALGB 50403 | 31, 32 | 2-3 × methotrexate + R-maxiCHOP | ASCT + bortezomib | RQ-PCR | NR | NR | 32% of 47 | NR | NR | NR |
| Seattle single-center report | 33 | R-hyperCVAD or R-CHOP-like | ASCT | MFC or PCR | 89% of 75 | NR | NR | NR | NR | NR |
Numbers indicate % MRD negative among evaluable patients in the respective compartment at the respective time point.
hyper CVAD, fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; maxiCHOP, dose-intensified CHOP; NR, not reported.
The investigation of MRD during induction treatment indicates that the higher intensity of modern regimens is reflected in a rapid tumor cell clearance in PB and BM. Therefore, MRD assessment seems to be well suited for precisely monitoring the impact of different treatment schemes or individual drugs on tumor cell clearance.
Currently, only very limited data are available that describe the impact of less intensive treatments such as bendamustine-based immunochemotherapy on MRD. In the Nordic MCL4 study, after 6 cycles of rituximab, bendamustine, and lenalidomide, the percentage of patients among 32 evaluable patients who were MRD positive by qualitative nested PCR was 56% in BM and 61% in PB.26 In a smaller phase 2 study, clonotypes for MRD assessment were identified by HTS in 19 of 23 patients, and 77% of 13 patients with PB samples were MRD negative by HTS after 3 cycles of R-bendamustine.27 To the best of our knowledge, there are no currently available data regarding the effects of new targeted treatment drugs such as ibrutinib on MRD in MCL.
Prognostic value of MRD before high-dose consolidation
Several studies have provided consistent evidence showing the strong prognostic value of MRD status in clinical remission at the end of induction and before high-dose consolidation on subsequent progression-free survival (PFS). In the MCL Younger trial, MRD positivity before ASCT was strongly prognostic for shorter PFS (hazard ratio [HR] in PB, 2.7; in BM, 1.8), independently of the induction treatment arm.4 Thus, the prolonged PFS in the HA-containing R-CHOP/R-DHAP arm compared with the R-CHOP arm might be a result of the higher molecular remission rate at the end of induction; this adds evidence to the hypothesis that intensification of induction treatment as proposed by the Nordic Lymphoma Group and the European MCL Network 15 years ago has resulted in improved outcome in younger patients with MCL.
In the Nordic MCL3 study, patients in molecular remission who were given transplants after receiving augmented R-CHOP/R-HA therapy had prolonged PFS (∼82% vs 62% at 4 years).25 Similarly, in an interim analysis of the LYMA trial by the Lymphoma Study Association (LYSA), patients in molecular remission after 4 cycles of R-DHAP who received transplants had superior PFS compared with patients who were MRD positive before ASCT; this prognostic effect seemed to be more prominent in the patients observed after ASCT (3-year PFS of MRD-negative vs MRD-positive patients before ASCT: 87% vs 52% in PB; 84% vs 62% in BM) compared with patients who were treated with rituximab maintenance after ASCT (93% vs 80% in PB; 92% vs 86% in BM).28 Several smaller studies have also shown the prognostic value of MRD status after various induction immunochemotherapies and before high-dose consolidation.29-32
As far as ASCT is concerned, the question arises whether the presence of MRD in the stem cell product re-transfused is associated with inferior PFS. Four studies consistently reported that the presence of or the relative content of tumor cells in the stem cell product did not affect further clinical outcome.5,16,29,31 This could indicate that tumor cells contaminating the re-infused stem cells might not be able to cause or promote clinical progression, and that cytoreduction in the involved tissues by induction and consolidation treatment has the strongest impact on tumor control.
MRD after high-dose consolidation
In the 2 series of patients with MCL who were treated with CHOP-like chemotherapy by which virtually no molecular remissions were achieved, molecular remission rates of 38%5 and 52%16 were observed after high-dose consolidation and ASCT. In the MCL Younger trial, high-dose consolidation increased molecular remission rates after R-CHOP from 47% to 68% in PB and from 26% to 59% in BM; after R-CHOP/R-DHAP, rates increased from 79% to 85% in PB and from 61% to 79% in BM.4 The smaller molecular remission rates after ASCT in the treatment arm that did not contain HA suggest that myeloablative treatment could not compensate for the lower molecular remission rates observed before ASCT.4 In the Nordic MCL2 study, after alternating R-CHOP/R-HA therapy and ASCT, the molecular remission rate was high with 92% in PB and/or BM.3,33 In the Nordic MCL3 study, ASCT raised the percentage of MRD-negative patients in PB and/or BM from 53% after alternating R-CHOP/R-HA therapy to 83%.25 In an interim analysis of the LYMA trial, ASCT increased MRD-negative rates among patients in clinical remission after 4 cycles of R-DHAP from 80% to 95% in PB and from 66% to 82% in BM.28
These results reinforce the observation that the additional effect of high-dose consolidation on tumor cell clearance is dependent on the power of the preceding induction treatment. By increasing MRD response before high-dose consolidation, the effect of ASCT on prognosis might be abrogated.
Predictive value of MRD in clinical remission after treatment
In contrast to the investigation of the prognostic value of MRD during cytoreduction or consolidation, in which subsequent treatment might influence a potential association, results regarding the prognostic value of MRD after treatment more closely reflect the biological relationship between MRD and clinical progression and the potential predictive value of MRD status for subsequent outcome. Several larger studies that investigated the prognostic value of MRD after treatment, especially after high-dose consolidation, have consistently shown a high predictive value of MRD for subsequent PFS.
In the single-center report16 of 27 patients treated with myeloablative radiochemotherapy after CHOP-like induction, MRD positivity in PB or BM during the first year after ASCT was highly predictive for clinical progression, with median PFS of 21 months in patients who were MRD positive compared with 92 months in patients who were MRD negative. MRD positivity after ASCT was also prognostic for shorter OS, and the prognostic relevance of MRD positivity for PFS and OS was independent of baseline variables and clinical complete remission (CR).
In the Nordic MCL2 study,3 among 79 patients in clinical remission after ASCT, PFS differed among patients who had MRD-positive PB or BM during the first year after ASCT (median PFS, 1.5 years), patients who were MRD positive at a later time point (median PFS, 5 years), and patients who were consistently MRD negative after ASCT (median PFS, not reached). Similarly, in the Nordic MCL3 study,25 MRD status after ASCT was strongly prognostic for PFS, with 4-year PFS of about 38% (median PFS, ∼3 years) in 18 patients who had MRD-positive PB or BM compared with about 88% in 89 MRD-negative patients. The prognostic value of MRD status after ASCT was stronger than that before ASCT and was also seen after adjustment for MIPI, Ki-67 index, computed tomography status before ASCT and positron emission tomography (PET) status before ASCT, with adjusted HRs of 5.0 for PFS and 4.8 for OS.
In the MCL Younger trial, the MRD status during the first year after ASCT was strongly prognostic for subsequent PFS with HRs of 3.2 in PB and 2.2 in BM.4 The prognostic effect of the MRD status after ASCT was stronger compared with the time point before ASCT, and it was independent of MIPI score, achievement of a clinical CR, and treatment group. In particular, among the patients who received a transplant after therapy with the HA-containing induction regimen R-CHOP/R-DHAP, patients who had MRD-negative PB during the first year after ASCT had prolonged PFS compared with those with at least 1 MRD-positive PB sample during the first year after ASCT (adjusted PFS HR, 3.2).
In results from the MCL Elderly trial, similar to those obtained for the MCL Younger trial, sustained molecular remission in PB during the first year of maintenance was predictive for subsequent PFS (median PFS from end of induction, 2.0 vs 4.2 years; HR, 1.8), and this was consistently seen for patients treated with initial R-CHOP and rituximab plus fludarabine and cyclophosphamide.23
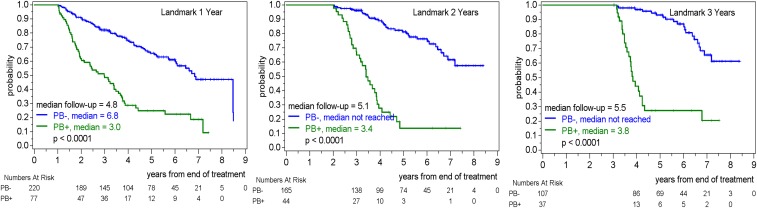
Finally, a combined analysis34 of the MCL Younger and MCL Elderly trials showed that among 406 patients in clinical remission, MRD positivity in the PB at various time points during the follow-up period was strongly predictive for subsequent PFS, with median time from MRD positivity to a PFS event ranging between 1 and 2 years (Figure 2).34 The predictive value of the MRD status in PB during follow-up was independent of clinical prognostic factors (adjusted PFS HRs, 3.3, 7.2, and 8.4 for MRD positivity in PB at 1, 2, and 3 years, respectively) and was similar across the 2 trial cohorts (MCL Younger and MCL Elderly) and across treatment groups within each trial. Furthermore, in patients in clinical remission after treatment, the MRD status in PB was prognostic for OS in a preliminary analysis. Further analyses of this and other larger cohorts of patients with MCL will potentially confirm the prognostic value of MRD not only on subsequent PFS but also OS.
Figure 2.
Landmark analyses for PFS in clinical remission after ASCT (MCL Younger trial) or end of induction (MCL Elderly trial). For each landmark time point, patients in follow-up at the landmark are included and stratified according to MRD status in PB in the 6-month period preceding the landmark.35
In summary, there is convincing evidence that MRD positivity after currently recommended standard treatment is strongly predictive for imminent clinical relapse within 1 to 2 years, whereas the outcome of patients with MCL in sustained molecular remission seems favorable. Future investigations of novel treatment strategies could elucidate how to improve the dismal outcome of patients not adequately responding to standard treatment as determined by the presence or reappearance of MRD.
Source of material for MRD assessment
At initial diagnosis, the infiltration level in PB and BM was shown to be comparable in the MCL Younger and MCL Elderly trials,17 but it has been consistently observed that during and after treatment, PB is more frequently and more rapidly cleared of MRD than BM. In the MCL Younger and MCL Elderly trials, we observed that analysis of PB alone failed to demonstrate persistent lymphoma cells at the end of induction in about 15% of patients who were simultaneously positive in BM. Indeed it seems that true MRD negativity in the sense of cure and elimination of every MCL cell is not achieved in the majority of patients with current treatment protocols. However, the current results from the MCL Younger and MCL Elderly trials suggest that the prognostic impact of MRD eradication in PB is stronger than that in BM at least at all time points during induction and consolidation treatment.4 Furthermore, because of easier access and patient comfort, MRD analysis of PB is preferable to BM assessment. Therefore, MRD assessment by RQ-PCR might identify patients with the potential to be cured of disease because MRD negativity reflects sensitivity to treatment.
Thus, the choice of material depends on the purpose of MRD assessment. For describing treatment effects on MRD clearance in different compartments, both BM and PB seem to be informative; in contrast, for MRD-guided treatment in clinical remission, the use of PB seems both feasible and justified by the strong predictive and prognostic value of MRD in PB.
Rethinking clinical response assessment in MCL
Standardized response criteria are critical for the optimal management of patients with MCL, particularly in light of new therapies with increasing remission rates. However, conventional imaging techniques may not be sensitive enough to accurately assess response. This triggered the inclusion of [18F]fluorodeoxyglucose-PET (18F-FDG-PET)/computed tomography in staging for FDG-avid lymphomas in the Lugano classification system, which was recently published.35 The evaluation of MRD emerges as an attractive tool for assessing the response to treatment in MCL on a highly sensitive level. Almost all patients with MCL present with PCR-detectable dissemination to PB or BM, and MRD assessment by RQ-PCR is feasible in about 85% of patients presenting with advanced-stage MCL. Quantification of MRD by RQ-PCR is highly standardized and reflects the cytoreductive treatment effect, and MRD response is a strong prognostic and predictive marker for subsequent clinical outcome.
Because molecular remission is an indicator of quality and depth of clinical remission, it seems reasonable to combine clinical staging by imaging with MRD assessment to allow a more specific evaluation of treatment efficacy. Therefore, response criteria should integrate MRD response as an important parameter for optimal response evaluation. During follow-up in clinical remission, the strong predictive power of MRD assessment for clinical progression suggests that molecular monitoring could be additive, or it could be a substitute for currently used regular response monitoring by imaging in MCL. Of note, just as PFS combines clinical progression and death as a result of any cause, a potential future MRD-based end point for treatment efficacy needs to combine the results of MRD assessment with clinical progression and an indicator of relevant toxicity such as survival status.
MRD-driven treatment strategies
At this point, MRD-guided treatment is not recommended in clinical routines. Pre-emptive rituximab treatment of molecular relapse after high-dose consolidation is, to the best of our knowledge, the only MRD-driven strategy that has been investigated in patients with MCL so far. In the Nordic MCL2 study, pre-emptive rituximab monotherapy given to 26 patients in clinical remission after molecular relapse resulted in re-conversion to MRD negativity in 92% of patients with a median duration of re-induced molecular remission of 1.5 years.36 Although no long-term molecular remissions were re-induced, the observed clinical relapse-free survival of more than 50% after 2 years suggested a potential role for treatment with rituximab after ASCT. In 4 patients with molecular relapse with clinical relapse (2 patients) or without (2 patients) after allogeneic stem cell transplant, immunomodulation with rituximab and donor lymphocyte infusion again induced clinical and molecular remissions.37 So far, larger evaluations of pre-emptive treatment of molecular relapse are lacking that compare this MRD-guided strategy to a standard approach to assess long-term outcome.
In principle, there are different options for MRD-based intervention strategies that could be investigated in future clinical trials. In the published multicenter trials, treatment intensification was consistently reflected by higher molecular remission rates predictive for prolonged clinical remission, indicating that early MRD eradication should be a therapeutic goal in the treatment of MCL. It is therefore of major interest to elucidate the MRD response to novel targeted treatments to select new strategies for further investigation. One MRD-driven strategy might then be a treatment modification by intensified induction treatment blocks and the addition of novel drugs in MRD-positive patients and subsequently to challenge high-dose consolidation in MRD-negative clinical remission in favor of a potent maintenance treatment. Furthermore, in the setting of maintenance treatment, given the strong prognostic and predictive value of MRD positivity for subsequent clinical progression, MRD reappearance could be the trigger for switching treatment to novel drugs to improve the long-term outcome in these patients. It should be emphasized that MRD-driven therapy strategies, as with any new therapeutic concept, need to be established in future trials that include a randomized comparison with the standard treatment in relation to a clinically meaningful end point such as OS.
Summary
MRD assessment has evolved not only as a diagnostic tool but also as powerful predictor of long-term remission and prognosis in MCL. Clinical intervention according to MRD status must be based on robust prospective clinical data that demonstrate a clinical benefit for MRD-driven treatment strategies. Therefore, prospective clinical trials investigating this concept in different scenarios are needed to further improve prognosis in MCL in the long term. New techniques such as HTS or next-generation flow cytometry may compensate for the limitations of current MRD assessment methods if validation is successful. Thus, MRD detection has the potential to improve current treatment strategies for MCL.
References
- 1.Lenz G, Dreyling M, Hoster E, et al. . Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol. 2005;23(9):1984-1992. [DOI] [PubMed] [Google Scholar]
- 2.Hoster E, Unterhalt M, Pfreundschuh M, et al. The addition of rituximab to CHOP improves failure-free and overall survival of mantle-cell lymphoma patients—a pooled trials analysis of the German Low-Grade Lymphoma Study Group (GLSG) [abstract]. Blood 2014;124(21). Abstract 1752. [Google Scholar]
- 3.Geisler CH, Kolstad A, Laurell A, et al. ; Nordic Lymphoma Group. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112(7):2687-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermine O, Hoster E, Walewski J, et al. . Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high-dose cytarabine containing myeloablative regimen and autologous stem cell transplantation versus 6 courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and autologous stem cell transplantation in patients <65years (MCL Younger): a randomised, open-label, phase 3 trial of the European MCL Network. Lancet. In press. [Google Scholar]
- 5.Andersen NS, Pedersen L, Elonen E, et al. ; Nordic Lymphoma Group. Primary treatment with autologous stem cell transplantation in mantle cell lymphoma: outcome related to remission pretransplant. Eur J Haematol. 2003;71(2):73-80. [DOI] [PubMed] [Google Scholar]
- 6.Dreyling M, Lenz G, Hoster E, et al. . Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677-2684. [DOI] [PubMed] [Google Scholar]
- 7.Rummel MJ, Niederle N, Maschmeyer G, et al. ; Study group indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210. [DOI] [PubMed] [Google Scholar]
- 8.Kluin-Nelemans HC, Hoster E, Hermine O, et al. . Treatment of older patients with mantle-cell lymphoma. N Engl J Med. 2012;367(6):520-531. [DOI] [PubMed] [Google Scholar]
- 9.Hoster E, Dreyling M, Klapper W, et al. ; German Low Grade Lymphoma Study Group (GLSG); European Mantle Cell Lymphoma Network. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111(2):558-565. [DOI] [PubMed] [Google Scholar]
- 10.Hoster E, Rosenwald A, Berger F, et al. . Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J Clin Oncol. 2016;34(12):1386-1394. [DOI] [PubMed] [Google Scholar]
- 11.Böttcher S, Ritgen M, Buske S, et al. ; EU MCL MRD Group. Minimal residual disease detection in mantle cell lymphoma: methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica. 2008;93(4):551-559. [DOI] [PubMed] [Google Scholar]
- 12.Cheminant M, Derrieux C, Touzart A, et al. . Minimal residual disease monitoring by 8-color flow cytometry in mantle cell lymphoma: an EU-MCL and LYSA study. Haematologica. 2016;101(3):336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chovancová J, Bernard T, Stehlíková O, et al. . Detection of minimal residual disease in mantle cell lymphoma-establishment of novel eight-color flow cytometry approach. Cytometry B Clin Cytom. 2015;88(2):92-100. [DOI] [PubMed] [Google Scholar]
- 14.Pott C. Minimal residual disease detection in mantle cell lymphoma: technical aspects and clinical relevance. Semin Hematol. 2011;48(3):172-184. [DOI] [PubMed] [Google Scholar]
- 15.Andersen NS, Donovan JW, Zuckerman A, Pedersen L, Geisler C, Gribben JG. Real-time polymerase chain reaction estimation of bone marrow tumor burden using clonal immunoglobulin heavy chain gene and bcl-1/JH rearrangements in mantle cell lymphoma. Exp Hematol. 2002;30(7):703-710. [DOI] [PubMed] [Google Scholar]
- 16.Pott C, Schrader C, Gesk S, et al. . Quantitative assessment of molecular remission after high-dose therapy with autologous stem cell transplantation predicts long-term remission in mantle cell lymphoma. Blood. 2006;107(6):2271-2278. [DOI] [PubMed] [Google Scholar]
- 17.Pott C, Hoster E, Delfau-Larue MH, et al. . Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL intergroup study. Blood. 2010;115(16):3215-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Velden VH, Cazzaniga G, Schrauder A, et al. ; European Study Group on MRD detection in ALL (ESG-MRD-ALL). Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21(4):604-611. [DOI] [PubMed] [Google Scholar]
- 19.Wu D, Sherwood A, Fromm JR, et al. . High-throughput sequencing detects minimal residual disease in acute T lymphoblastic leukemia. Sci Transl Med. 2012;4(134):134ra63. [DOI] [PubMed] [Google Scholar]
- 20.Ladetto M, Brüggemann M, Monitillo L, et al. . Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia. 2014;28(6):1299-1307. [DOI] [PubMed] [Google Scholar]
- 21.Faham M, Zheng J, Moorhead M, et al. . Deep-sequencing approach for minimal residual disease detection in acute lymphoblastic leukemia. Blood. 2012;120(26):5173-5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. . Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123(20):3073-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pott C, Delfau-Larue M, Beldjord K, et al. R-CHOP vs R-FC followed by maintenance with ritzuximab or IFN: First results of MRD assessment within the randomized trial for eldery patients with MCL [abstract]. Ann Oncol. 2011;22(suppl 4). Abstract 233. [Google Scholar]
- 24.Pott C, Hoster E, Beldjord K, et al. R-CHOP/R-DHAP Compared to R-CHOP induction followed by high dose therapy with autologous stem cell transplantation induces higher rates of molecular remission in MCL: results of the MCL Younger Intergroup Trial of the European MCL Network [abstract]. Blood 2010;122(21). Abstract 965. [Google Scholar]
- 25.Kolstad A, Laurell A, Jerkeman M, et al. ; Nordic Lymphoma Group. Nordic MCL3 study: 90Y-ibritumomab-tiuxetan added to BEAM/C in non-CR patients before transplant in mantle cell lymphoma. Blood. 2014;123(19):2953-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albertsson-Lindblad A, Kolstad A, Laurell A, et al. . Lenalidomide-bendamustine-rituximab in untreated mantle cell lymphoma > 65 years, the Nordic Lymphoma Group phase I+II trial NLG-MCL4 [published online ahead of print 27 June 2016] Blood. doi:10.1182/blood-2016-03-704023. [DOI] [PubMed] [Google Scholar]
- 27.Armand P, Redd R, Bsat J, et al. . A phase 2 study of Rituximab-Bendamustine and Rituximab-Cytarabine for transplant-eligible patients with mantle cell lymphoma. Br J Haematol. 2016;173(1):89-95. [DOI] [PubMed] [Google Scholar]
- 28.Callanan MB, Delfau MH, Macintyre E, et al. . Predictive power of early, sequential MRD monitoring in peripheral blood and bone marrow in patients with mantle cell lymphoma following autologous stem cell transplantation with or without rituximab maintenance; interim results from the LyMa-MRD Project, conducted on behalf of the Lysa Group [abstract]. Blood. 2015;126(23). Abstract 338. [Google Scholar]
- 29.Liu H, Johnson JL, Koval G, et al. . Detection of minimal residual disease following induction immunochemotherapy predicts progression free survival in mantle cell lymphoma: final results of CALGB 59909. Haematologica. 2012;97(4):579-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan LD, Jung SH, Stock W, et al. . Bortezomib maintenance (BM) versus consolidation (BC) following aggressive immunochemotherapy and autologous stem cell transplant (ASCT) for untreated mantle cell lymphoma (MCL): CALGB (Alliance) 50403 [abstract]. Blood. 2015;126(23). Abstract 337. [Google Scholar]
- 31.Fulton N, Johnson J, Kaplan LD, et al. . Minimal residual disease (MRD) status following induction chemo-immunotherapy predicts progression-free survival in mantle cell lymphoma (MCL): CALGB 50403 (Alliance) [abstract]. Blood. 2013;122(21). Abstract 3002. [Google Scholar]
- 32.Cowan AJ, Stevenson PA, Cassaday RD, et al. . Pretransplantation Minimal Residual Disease Predicts Survival in Patients with Mantle Cell Lymphoma Undergoing Autologous Stem Cell Transplantation in Complete Remission. Biol Blood Marrow Transplant. 2016;22(2):380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geisler CH, Kolstad A, Laurell A, et al. ; Nordic Lymphoma Group. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158(3):355-362. [DOI] [PubMed] [Google Scholar]
- 34.Pott C, Macintyre E, Delfau-Larue MH, et al. . MRD eradication should be the therapeutic goal in mantle cell lymphoma and may enable tailored treatment approaches: results of the intergroup trials of the European MCL Network [abstract]. Blood. 2014;124(21). Abstract 147. [Google Scholar]
- 35.Cheson BD, Fisher RI, Barrington SF, et al. ; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen NS, Pedersen LB, Laurell A, et al. . Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J Clin Oncol. 2009;27(26):4365-4370. [DOI] [PubMed] [Google Scholar]
- 37.Krüger WH, Hirt C, Basara N, et al. . Allogeneic stem cell transplantation for mantle cell lymphoma--final report from the prospective trials of the East German Study Group Haematology/Oncology (OSHO). Ann Hematol. 2014;93(9):1587-1597. [DOI] [PubMed] [Google Scholar]