Abstract
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin lymphoma (NHL) confined to the brain, leptomeninges, eyes, or spinal cord. The majority of PCNSL cases occur in the immunocompetent host, the focus of this review. The prognosis of PCNSL is inferior to that of other NHL subtypes including other organ-specific subtypes of extranodal NHL. The 5- and 10-year survival proportions for PCNSL are 29.3% and 21.6%, respectively. The diagnosis and management of PCNSL differs from that of other primary brain cancers and NHL in other parts of the body.
Learning Objectives
The reader will become familiar with the consensus criteria for the diagnosis of primary PCNSL
The reader will become familiar with the consensus criteria for assessment of treatment response in PCNSL
The reader will become familiar with the treatment options for newly diagnosed PCNSL as defined in randomized clinical trials
The reader will understand the options for the treatment of relapsed PCNSL
Epidemiology
Primary central nervous system lymphoma (PCNSL) is an extranodal non-Hodgkin lymphoma (NHL) confined to the brain, leptomeninges, eyes, or spinal cord.1 PCNSL accounts for ∼2% of all primary central nervous system tumors, with patients’ median age of 65 years at diagnosis.2 Since 2000, there has been an increase in the overall incidence of PCNSL, especially in the elderly.
Pathology
Approximately 90% of PCNSL cases are diffuse large B-cell lymphomas (DLBCL), with the remainder consisting of T-cell lymphomas, poorly characterized low-grade lymphomas, or Burkitt lymphomas.3 Although the incidence of Epstein-Barr Virus (EBV) is high in immunocompromised hosts, virtually all tumor specimens from immunocompetent hosts are EBV-negative. More than 90% of primary CNS DLBCL closely resemble the activated B cell–like (ABC) subtype. Like systemic DLBCL, PCNSL harbors chromosomal translocations of the BCL6 gene, deletions in 6q, and aberrant somatic hypermutation in proto-oncogenes including MYC and PAX5. Inactivation of CDKN2A is also commonly observed in both entities. Gene expression profiles demonstrate that PCNSL is characterized by differential expression of genes related to adhesion and extracellular matrix pathways, including MUM1, CXCL13, and CHI3L1. The ongoing somatic hypermutation with biased use of VH gene segments that has been observed in PCNSL is suggestive of an antigen-dependent proliferation. These observations are consistent with the hypothesis that PCNSL arises after antigen-dependent activation of circulating B cells, which subsequently localize to the CNS by expression of various adhesion and extracellular matrix–related genes. Genomic analysis of 19 tumor specimens obtained from 19 immunocompetent PCNSL patients demonstrated a high prevalence of MYD88 mutations and other genetic alterations consistent with activation of the B-cell receptor (BCR), toll-like receptor and nuclear factor-κβ (NF-κB) pathways in >90% of cases.4,5 These observations provide insight into potential therapeutic targets for future clinical trials in PCNSL.6
Diagnosis
Neurocognitive symptoms are the most common presenting clinical features of PCNSL. The International PCNSL Collaborative Group (IPCG) has developed guidelines to determine extent of disease.7 A gadolinium-enhanced brain magnetic resonance imaging (MRI) scan is the most sensitive radiographic study for the detection of PCNSL (Figure 1). Most PCNSL patients present with a single brain mass. The diagnosis of PCNSL is typically established by stereotactic brain biopsy, cerebrospinal fluid (CSF) analysis, or by analysis of vitreous aspirate in patients with ocular involvement. Given the possible delay in diagnosis and treatment with the latter 2 methods, prompt stereotactic biopsy is advised in almost all cases that are surgically accessible. Corticosteroids have lymphotoxic effects and should be avoided, if possible, before stereotactic biopsy as a histopathologic diagnosis can be difficult or impossible to achieve after exposure to these drugs. Secondary CSF and ocular involvement occurs in ∼15% to 20% and 5% to 20% of PCNSL patients, respectively. Presenting symptoms of ocular involvement include eye pain, blurred vision, and floaters.8 B symptoms such as weight loss, fevers, and night sweats are infrequent in PCNSL. A thorough diagnostic evaluation is needed to establish the extent of the lymphoma and to confirm localization to the CNS. A lumbar puncture should be performed if not contraindicated, and CSF should be assessed by flow cytometry, cytology, and immunoglobulin heavy-chain gene rearrangement. Because extraneural disease must be excluded to establish a diagnosis of primary CNS lymphoma, computed tomography/positron emission tomography scans of the chest, abdomen, and pelvis, and a bone marrow biopsy and aspirate should be performed to exclude occult systemic disease. Involvement of the optic nerve, retina, or vitreous humor should be excluded with a comprehensive eye evaluation by an ophthalmologist that includes a slit-lamp examination. Blood tests should include serum lactate dehydrogenase and HIV serology.7
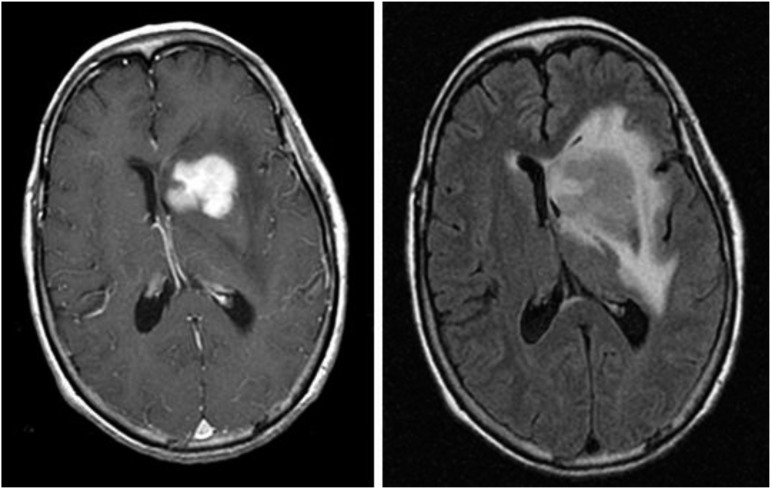
Figure 1.
Magnetic resonance images from a patient with PCNSL. A T1-weighted, axial, postcontrast scan (left) demonstrates intense, homogenous enhancement of the tumor in the region of the left caudate nucleus. An axial T2/FLAIR scan at the same anatomical level (right) demonstrates hyperintense signal surrounding the tumor, reflecting vasogenic cerebral edema. (Courtesy Priscilla K. Brastianos, M.D.)
Prognostic models
Two prognostic scoring systems have been developed specifically for PCNSL.9,10 In a retrospective review of 105 PCNSL patients, the International Extranodal Lymphoma Study Group (IELSG) identified age >60, Eastern Cooperative Oncology Group (ECOG) performance status >1, elevated serum LDH level, elevated CSF protein concentration, and involvement of deep regions of the brain as independent predictors of poor prognosis. In patients with 0 to 1 factors, 2 to 3 factors, and 4 to 5 factors, the 2-year survival proportions were 80%, 48%, and 15%, respectively. In another prognostic model, PCNSL patients were divided into 3 groups based on age and performance status: (1) <50 years old, (2) ≥50 years old with a KPS ≥70, and (3) ≥50 years old with a KPS <70. Based on these 3 divisions significant differences in overall and failure-free survival were observed.
Treatment
Newly diagnosed PCNSL
Defining response to treatment in PCNSL requires assessment of all documented sites (brain, CSF, eye) of involvement on the baseline assessment. The IPCG has established response criteria that have been adopted into most prospective clinical trials of PCNSL (Table 1).7
Table 1.
International PCNSL Collaborative Group Consensus Guidelines for the Assessment of Response in PCNSL7
| Response | Brain imaging | Steroid dose | Ophthalmologic examination | CSF cytology |
|---|---|---|---|---|
| Complete response | No contrast enhancing disease | None | Normal | Negative |
| Unconfirmed complete response | No contrast enhancing disease | Any | Normal | Negative |
| Minimal enhancing disease | Any | Minor RPE abnormality | Negative | |
| Partial response | 50% decrease in enhancement | NA | Normal or minor RPE abnormality | Negative |
| No contrast-enhancing disease | NA | Decrease in vitreous cells or retinal infiltrate | Persistent or suspicious | |
| Progressive disease | 25% increase in enhancing disease | NA | Recurrent or new disease | Recurrent or positive |
| Any new site of disease | ||||
| Stable disease | All scenarios not covered by responses above |
Corticosteroids decrease tumor-associated edema and may result in partial radiographic regression of PCNSL. An initial response to corticosteroids is associated with a favorable outcome in PCNSL.11 However, after an initial response to corticosteroids, almost all patients quickly relapse. Corticosteroids should be avoided if possible before a biopsy, given the risk of disrupting cellular morphology, resulting in a nondiagnostic pathologic specimen.
Surgical resection is not part of the standard treatment approach for PCNSL given the multifocal nature of this tumor.12 Although in one report a possible benefit of gross total resection in PCNSL patients was suggested, this was a retrospective, subset analysis likely confounded by selection bias.13 Other reports demonstrate no clear benefit. The role of neurosurgery in PCNSL is to establish a diagnosis via stereotactic biopsy.
Standardized induction and consolidation treatment of PCNSL has yet to be defined. Historically, PCNSL was treated only with whole-brain radiation therapy (WBRT) at doses ranging from 36 to 45 Gy, which resulted in a high proportion of radiographic responses but also early relapse. In a multicenter, phase 2 trial, 41 patients were treated with WBRT to 40-Gy plus a 20-Gy tumor boost and achieved a median overall survival (OS) of 12 months.14 Given the lack of durable responses to radiation and the risk of neurotoxicity associated with this modality of therapy, WBRT alone is no longer a recommended treatment of patients with newly diagnosed PCNSL. Moreover, because PCNSL is an infiltrative, multifocal disease, focal radiation or radiosurgery is not recommended. The most effective treatment of PCNSL at this time is IV, high-dose methotrexate (HD-MTX) (1-8 g/m2), typically used in combination with other chemotherapeutic agents and/or WBRT. However, there is no consensus on the optimal dose of HD-MTX or on the role of WBRT in combination with methotrexate in the management of newly diagnosed PCNSL. Several randomized trials have been developed to address these issues. Doses of methotrexate ≥3 g/m2 result in therapeutic concentrations in the brain parenchyma and CSF and, when combined with WBRT, lead to more durable treatment responses.15-17 In a phase 2 trial, 79 PCNSL patients were randomly selected to receive induction therapy with either (1) HD-MTX or (2) HD-MTX + cytarabine. Each chemotherapy cycle was 21 days. All patients underwent consolidative WBRT after induction chemotherapy. The HD-MTX + cytarabine arm had a higher proportion of complete radiographic responses (46% vs 18%) and a superior 3-year OS.18 However, it is now widely recognized that there is a high incidence of neurotoxicity with combined modality treatment that includes standard-dose WBRT, especially in elderly patients.19 The latter observation has prompted studies using lower doses of consolidative WBRT. In a multicenter phase 2 trial, no significant neurocognitive decline was observed after consolidative reduced-dose WBRT (23.4 Gy) and cytarabine in patients who had achieved a complete response (CR) to induction chemotherapy including HD-MTX.20 However, further study and longer neuropsychological follow-up of these patients is necessary to definitively assess the safety of this regimen because numerous studies have demonstrated the delayed neurotoxic effects of WBRT in the PCNSL population and the reduced risk of neurotoxicity in regimens consisting of chemotherapy alone.21,22 This has led many experts to advise deferral of WBRT in favor of chemotherapy alone for the induction and consolidation of newly diagnosed PCNSL patients. These approaches are based on a foundation of HD-MTX. Variable doses and schedules of HD-MTX have been used, but in general, doses ≥3 g/m2 delivered as an initial bolus followed by an infusion over 3 hours, administered every 10 to 21 days, is recommended for optimal outcomes and adequate CSF concentrations.16 Multiple phase 2 studies have demonstrated the safety, efficacy, and relatively preserved cognition of HD-MTX–based chemotherapy regimens.23,24 Moreover, longer duration of induction chemotherapy with HD-MTX (>6 cycles) results in higher CR proportions. The IELSG conducted a follow-up, randomized, phase 2 trial in newly diagnosed PCNSL patients using the MTX + cytarabine combination from the IELSG20 study as a control arm. In this study, IELSG32, 3 different induction chemotherapy regimens were compared: arm A, MTX + cytarabine; arm B, MTX + cytarabine + rituximab; and arm C, MTX + cytarabine + rituximab + thiotepa (MATRix). In this study the combination of the 4 drugs (arm C, MATRix) was superior to the other arms in terms of CR and overall response (OR) proportions.25 There are other induction chemotherapy regimens currently under study in randomized, multicenter trials including the methotrexate, temozolomide, rituximab (MTR) regimen24; the rituximab, procarbazine, methotrexate, vincristine (R-MPV) regimen20; and the rituximab, methotrexate, teniposide, biodegradable carmustine (BCNU), prednisolone (R-MBVP) regimen.26 In addition to different chemotherapeutic agents, these different induction regimens also include different doses and schedules of methotrexate. Because there have been no head-to-head comparisons of these induction chemotherapy regimens in randomized trials for newly diagnosed PCNSL, there is no compelling rationale at this time to select one over the other. Future randomized trials will likely test different induction regimens against one another to identify the optimal chemotherapy combination to use in patients with newly diagnosed PCNSL.
Several first-generation chemotherapy regimens for PCNSL included intrathecal chemotherapy. However, in nonrandomized studies that included intrathecal chemotherapy, there was no improvement in outcomes vs regimens that did not include intrathecal injections of chemotherapy.27,28 Thus, intrathecal chemotherapy is not used in the induction regimens commonly in use today.
Rituximab, a chimeric monoclonal antibody targeting the CD20 antigen, is being incorporated into induction chemotherapy regimens for PCNSL as noted previously. When rituximab is administered IV at doses of 375-800 mg/m2, CSF levels from 0.1% to 4.4% of serum levels are achieved. Despite limited CSF penetration, radiographic responses have been observed in relapsed PCNSL patients treated with rituximab monotherapy.29 Moreover, in historical comparisons, the complete radiographic response rates are higher with induction regimens that include rituximab vs those in which there is no rituximab.30
The optimal consolidative therapy for PCNSL has not been identified. As noted, options include WBRT, chemotherapy, or high-dose-chemotherapy followed by autologous stem cell transplantation (HDT/ASCT). Given the risk of clinical neurotoxicity, several trials have assessed whether WBRT can be eliminated from the management of PCNSL. In a multicenter, phase 3 trial, patients were randomized to receive HD-MTX–based chemotherapy with or without consolidative WBRT.31 Five hundred fifty-one patients were enrolled, of whom 318 were treated per protocol. Intent-to-treat analysis revealed that the patients randomly selected for the chemotherapy + WBRT arm achieved prolonged progression-free survival (PFS) but no improvement in OS, demonstrating that the elimination of WBRT from the treatment regimen did not compromise OS. There are also initiatives to reduce the dose of consolidative WBRT in an effort to mitigate the risk of neurotoxicity as noted previously.20 Using chemotherapy alone in the consolidation phase of therapy is also being studied. In a cooperative group, multicenter, phase 2 study, 44 PCNSL patients were treated with induction chemotherapy consisting of HD-MTX, rituximab, and temozolomide, all drugs with demonstrated efficacy as monotherapy in PCNSL.24 This induction chemotherapy was followed by consolidative chemotherapy consisting of IV etoposide and cytarabine. Sixty-six percent of these patients achieved CR to induction chemotherapy, median PFS of the entire group was 2.4 years, and median OS was not observed at the time of publication. These outcomes are comparable with regimens that include consolidative WBRT. It is noteworthy that in this study, PFS was significantly shorter in PCNSL patients in whom chemotherapy was delayed >1 month after diagnosis compared with patients who promptly initiated chemotherapy (3-year PFS of 20% vs 59%, respectively). This observation highlights the importance of early diagnosis and prompt initiation of chemotherapy in PCNSL patients. Given the success of high-dose chemotherapy followed by ASCT in relapsed or refractory NHL and PCNSL, there is also interest in this approach as consolidative therapy for newly diagnosed PCNSL.32 Conditioning regimens including thiotepa have demonstrated the most encouraging results. In a multicenter, phase 2 study, 79 patients were treated with induction HD-MTX, cytarabine, rituximab, and thiotepa, followed by carmustine and thiotepa conditioning before ASCT. The OR rate was 91%, 2-year OS was 87%, and treatment-related deaths occurred in <10% of enrolled patients. The toxicities, mostly cytopenias, were manageable. There are 3 ongoing, multicenter, randomized trials comparing the efficacy of consolidative HDT/ASCT vs chemotherapy or WBRT for newly diagnosed PCNSL (Table 2).33,34
Table 2.
Randomized trials in PCNSL
| Induction | Consolidation |
|---|---|
| Completed trials | Completed trials |
| Medical Research Council | G-PCNSL-SG-1 – NCT00153530 |
| Phase 2, n = 53 (stopped early) | Phase 3, n = 551, ages ≥18 y |
| CHOP vs WBRT followed by CHOP33 | Arm 1: Methotrexate ± ifosfamide = > WBRT |
| IELSG 20—NCT00210314 | Arm 2: Methotrexate ± ifosfamide31 |
| Ongoing trials | |
| Phase 2; n = 79, ages 18-75 y | IESLG 32—NCT01011920 |
| Induction arm 1: Methotrexate + Cytarabine = > WBRT | Phase 2, n = 104, ages 18-70 y |
| Induction arm 2: Methotrexate = > WBRT18 | Consolidation arm 1: WBRT |
| ANOCEF-GOELAMS—NCT00503594 | Consolidation arm 1: HDT/ASCT |
| Phase 2, n = 95, ages ≥60 y | ANOCEF-GOELAMS—NCT00863460 |
| Arm 1: Methotrexate, procarbazine, vincristine, cytarabine | Phase 2, n = 100, ages 18-60 y |
| Arm 2: Methotrexate, temozolomide34 | (R-MBVP = > |
| IESLG 32—NCT01011920 | Consolidation arm 1: HDT/ASCT |
| Phase 2, n = 227, ages 18-70 y | Consolidation arm 2: WBRT |
| Induction arm 1: Methotrexate, cytarabine | RTOG 1114—NCT01399372 |
| Induction arm 2: Methotrexate, cytarabine, rituximab | Phase 2, n = 84, ages ≥18 y |
| Induction arm 3: Methotrexate, cytarabine, rituximab, thiotepa25 | Methotrexate, procarbazine, vincristine, rituximab = > |
| Ongoing trials | |
| ALLG/HOVON—EudraCT 2009-014722-42 | Consolidation arm 1: WBRT (lower dose) = > cytarabine |
| Phase 3, n = 200, ages 18-70 y | Consolidation arm 2: Cytarabine |
| Arm 1: Methotrexate, BCNU, teniposide, prednisone = > Cytarabine, WBRT | Alliance 51101—NCT01511562 |
| Arm 2: Methotrexate, BCNU, teniposide, prednisone = > Cytarabine, WBRT | Phase 2, n = 160, ages 18-75 y |
| Methotrexate, temozolomide, rituximab, cytarabine = > | |
| Consolidation arm 1: HDT/ASCT | |
| Consolidation arm 2: Etoposide, cytarabine | |
| MATRIX/IELSG43 | |
| Phase 2, n = 220, ages 18-70 y | |
| Methotrexate, cytarabine, thiotepa, rituximab (MATRix) = > | |
| Consolidation arm 1: HDT/ASCT | |
| Consolidation arm 2: Dexamethasone, ifosfamide, VP-16, carboplatin (DEViC) |
ALLG, Australasian Leukaemia and Lymphoma Group; ANOCEF, Association des Neuro-Oncologue d’Expression Française; GOELAMS, Groupe Ouest Est d’Etude des Leucémies et Autres Maladies du Sang; G-PCNSL-SG, German Primary CNS Lymphoma Study Group; HDT/ASCT, high-dose chemotherapy and autologous stem cell transplantation; HOVON, Stichting Hemato-Oncologie voor Volwassenen Nederland (Dutch-Belgian Cooperative Trial Group for Hematology Oncology); IELSG, International Extranodal Lymphoma Study Group; NCT, national clinical trial; RTOG, Radiation Therapy Oncology Group; WBRT, whole-brain radiation therapy.
Elderly patients with PCNSL
Elderly patients account for more than half of all persons diagnosed with PCNSL.2 The risk of neurotoxicity is highest in this population, and in general, chemotherapy alone is the preferred option for this subgroup. The majority of PCNSL patients >60 years of age develop clinical neurotoxicity after treatment with a WBRT-containing regimen and some of these patients die of treatment-related complications, rather than recurrent disease.19 Several studies have indicated that HD-MTX at doses of 3.5-8 g/m2 is well tolerated in elderly patients with manageable grade 3 or 4 renal and hematologic toxicities.35 A meta-analysis of 783 PCNSL patients >60 years of age demonstrated that regimens including HD-MTX are associated with improved survival. Although WBRT was also associated with improved survival, it was also associated with an increased risk of neurologic side effects (OR 5.23).36 In a multicenter, randomized, phase 2 trial of chemotherapy alone in elderly patients with PCNSL, 98 patients were randomly selected to receive three 28-day cycles of either methotrexate, procarbazine, and vincristine (MPV-A) or methotrexate and temozolomide (MT), with 1 additional cycle of cytarabine in the MPV arm only. Although trends favored the MPV-A regimen over the simpler, less toxic MT regimen, with respect to CR rate, PFS, and OS, none of these differences reached statistical difference.34 Subsequent studies suggest that the addition of rituximab to both MPV and MT could increase the radiographic response rate. Other nonrandomized studies have demonstrated the feasibility of high-dose methotrexate (8 g/m2) and multi-agent, immunochemotherapy consisting of rituximab, methotrexate, procarbazine, and lomustine for elderly patients with newly diagnosed PCNSL.35,37 There is no standard of care established for elderly patients (>60 years of age) with newly diagnosed PCNSL but deferral of WBRT and utilization of chemotherapeutic approaches is the primary approach recommended by many experts.
Author's approach to newly diagnosed PCNSL
There are many variables that should be considered in the treatment of newly diagnosed PCNSL patients including patient age, organ function, immunologic status, and neurologic function. As a general approach, the author uses a methotrexate-based induction regimen, typically MTR, in both nonelderly and elderly newly diagnosed PCNSL patients. The methotrexate dose may be adjusted based on patient age and renal function. In nonelderly patients, the author prefers HDT/ASCT for consolidative therapy, whereas in elderly patients, consolidation options include cytarabine, maintenance methotrexate, lenalidomide, or reduced-dose WBRT.
Refractory and relapsed PCNSL
Despite high initial response rates with HD-MTX–based treatment, most patients with PCNSL experience relapse. Moreover, there is a subset of patients who have HD-MTX–refractory disease. Prognosis of relapsed or refractory PCNSL is poor, with a limited number of prospective, phase 2 studies for guidance on the management of this patient population. In a study of 256 PCNSL patients with relapsed or refractory disease after initial therapy, tumor progression was asymptomatic in 25% of patients and was identified on serial surveillance imaging. This highlights the importance of surveillance imaging as recommended by the IPCG guidelines. Survival was worse in those patients who had refractory PCNSL or who relapsed within 1 year vs those who relapsed after 1 year.38 Although relapses in PCNSL are predominantly within the CNS, relapses in extraneural organs are reported in as much as 17% of patients. Late relapses also appear to occur more commonly in primary CNS DLBCL vs systemic DLBCL. In 1 long-term follow-up study, 26% of all relapses occurred after 5 years and 1.7% occurred after 10 years.39 Re-challenge with HD-MTX is effective in patients who had previously responded to this agent. In a multicenter, retrospective study of 22 relapsed PCNSL patients with a history of prior response to HD-MTX, 91% had a radiographic response to the first salvage treatment with HD-MTX, and 100% had response to a second salvage. The median OS from the first salvage was 61.9 months.40 In patients who have not previously been treated with HDT/ASCT, this is also an option at the time of relapse. In a phase 2 trial of 43 patients with relapsed or refractory PCNSL, salvage therapy with high-dose cytarabine and etoposide was followed by HDT/ASCT with a conditioning regimen consisting of thiotepa, busulfan, and cyclophosphamide. Twenty-seven patients ultimately proceeded to transplantation. Twenty-six of 27 patients had a CR and the median PFS and OS in this group were 41.1 and 58.6 months, respectively.41 It is noteworthy that in a small series of patients with relapsed PCNSL after initial HDT/ASCT, a second ASCT was successful as salvage treatment.42 In patients who have not received radiation as a part of their initial treatment, WBRT at the time of relapse can be an effective option, although the risk of neurotoxicity remains significant.43 Many clinicians reserve WBRT for those patients with chemotherapy-refractory disease or at the time of relapse. In a series of 27 relapsed or refractory PCNSL patients treated with WBRT (median dose 36 Gy), 74% achieved an overall radiographic response and the median OS was 10.6 months. Delayed neurotoxicity rates of 15% were noted at doses >36 Gy, even in this setting of short survival.
Novel therapeutics currently under study for primary CNS DLBCL include ibrutinib, lenalidomide, pomalidomide, buparlisib, pemetrexed, temsirolimus, pemetrexed, and bendamustine.6 In light of the fact that >90% of primary CNS DLBCL cases closely resemble the poor prognosis ABC subtype and the importance of BCR signaling in these tumors, a treatment regimen designed to target BCR signal transduction using the Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib is noteworthy. In 6 patients treated with temozolomide, etoposide, doxil, dexamethasone, ibrutinib, and rituximab (TEDDI-R), it was observed that all patients had tumor regression, with 3 achieving CR and 1 achieving PR.44 Temsirolimus, an inhibitor of mammalian target of rapamycin (mTOR), was assessed as a single agent in a phase 2 study of 37 relapsed and refractory PCNSL patients. Although the radiographic response proportion (CR, partial response [PR]) was 54%, the median PFS was only 2.1 months.45 Lenalidomide, an oral, immunomodulatory agent, has antiproliferative properties and is the subject of several ongoing, prospective clinical trials in relapsed and refractory PCNSL. In 1 report of 6 patients with relapsed PCNSL, 2 patients (33%) achieved a CR, including duration of 24+ months in 1 patient.46 In a phase 1 study of dose-escalating lenalidomide, 9 patients with relapsed or refractory CNS lymphoma (7 PCNSL, 2 secondary central nervous system lymphomas [SCNSL]) were treated and 8 of 8 evaluable subjects achieved objective responses (4 CR, 4 PR) after 1 month of lenalidomide monotherapy. In a separate cohort of 10 patients from the same study with relapsed or refractory CNS lymphoma (8 PCNSL, 2 SCNSL), lenalidomide (5-10 mg) was administered as maintenance therapy after first-line salvage treatment, and 5 patients maintained durable responses ≥2 years, suggesting that this agent should be further investigated as a potential maintenance or consolidative therapy.47 Clinical trials of lenalidomide plus rituximab for relapsed or refractory PCNSL are ongoing.
Neurotoxicity
The most frequent complication in long-term PCNSL survivors is delayed neurotoxicity. Although this risk is high, the exact incidence of delayed neurotoxicity is unclear, because most studies have not systematically assessed neurocognitive function with serial neuropsychological testing. The elderly are at highest risk for this complication, with the majority of patients >60 developing clinical neurotoxicity after combined modality therapy. Treatment with WBRT has been identified as the major risk factor for the development of late neurotoxicity. Common symptoms and signs include deficits in attention, memory, executive function, gait ataxia, and incontinence. These deficits have a detrimental impact on quality of life. Radiographic findings include periventricular white matter changes, ventricular enlargement and cortical atrophy. Pathologic studies reveal demyelination, hippocampal neuronal loss, and large-vessel atherosclerosis.48 Although the pathophysiology is unclear and likely multifactorial, damage to neural progenitor cells has been implicated to play an important role in radiation-related neurotoxicity.49 Currently, there are no treatments to reverse these delayed neurotoxic effects. The IPCG has developed an instrument for monitoring neurocognitive function, which is composed of quality-of-life questionnaires and standardized neuropsychological tests that include assessment of executive function, attention, memory, and psychomotor speed.19
Monitoring and follow-up
As treatment improves for PCNSL, more patients are living longer, emphasizing the need to optimize neurocognitive function and quality of life. The IPCG recommends a schedule of follow-up neuroimaging studies and cognitive assessments in PCNSL survivors.7
References
- 1.Batchelor TT, DeAngelis LM, eds. Lymphoma and Leukemia of the Nervous System. New York: Springer; 2013 [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro-oncol. 2014;16(suppl 4):iv1-iv63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008 [Google Scholar]
- 4.Braggio E, Van Wier S, Ojha J, et al. . Genome-Wide Analysis Uncovers Novel Recurrent Alterations in Primary Central Nervous System Lymphomas. Clin Cancer Res. 2015;21(17):3986-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapuy B, Roemer MG, Stewart C, et al. . Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ponzoni M, Issa S, Batchelor TT, Rubenstein JL. Beyond high-dose methotrexate and brain radiotherapy: novel targets and agents for primary CNS lymphoma. Ann Oncol. 2014;25(2):316-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abrey LE, Batchelor TT, Ferreri AJ, et al. ; International Primary CNS Lymphoma Collaborative Group. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23(22):5034-5043. [DOI] [PubMed] [Google Scholar]
- 8.Chan CC, Rubenstein JL, Coupland SE, et al. . Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16(11):1589-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abrey LE, Ben-Porat L, Panageas KS, et al. . Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24(36):5711-5715. [DOI] [PubMed] [Google Scholar]
- 10.Ferreri AJ, Blay JY, Reni M, et al. . Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21(2):266-272. [DOI] [PubMed] [Google Scholar]
- 11.Mathew BS, Carson KA, Grossman SA. Initial response to glucocorticoids. Cancer. 2006;106(2):383-387. [DOI] [PubMed] [Google Scholar]
- 12.Bellinzona M, Roser F, Ostertag H, Gaab RM, Saini M. Surgical removal of primary central nervous system lymphomas (PCNSL) presenting as space occupying lesions: a series of 33 cases. Eur J Surg Oncol. 2005;31(1):100-105. [DOI] [PubMed] [Google Scholar]
- 13.Weller M, Martus P, Roth P, Thiel E, Korfel A; German PCNSL Study Group. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro-oncol. 2012;14(12):1481-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson DF, Martz KL, Bonner H, et al. . Non-Hodgkin’s lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Radiat Oncol Biol Phys. 1992;23(1):9-17. [DOI] [PubMed] [Google Scholar]
- 15.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ; Radiation Therapy Oncology Group Study 93-10. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20(24):4643-4648. [DOI] [PubMed] [Google Scholar]
- 16.Ferreri AJ, Guerra E, Regazzi M, et al. . Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer. 2004;90(2):353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glantz MJ, Cole BF, Recht L, et al. . High-dose intravenous methotrexate for patients with nonleukemic leptomeningeal cancer: is intrathecal chemotherapy necessary? J Clin Oncol. 1998;16(4):1561-1567. [DOI] [PubMed] [Google Scholar]
- 18.Ferreri AJ, Reni M, Foppoli M, et al. ; International Extranodal Lymphoma Study Group (IELSG). High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet. 2009;374(9700):1512-1520. [DOI] [PubMed] [Google Scholar]
- 19.Correa DD, Maron L, Harder H, et al. . Cognitive functions in primary central nervous system lymphoma: literature review and assessment guidelines. Ann Oncol. 2007;18(7):1145-1151. [DOI] [PubMed] [Google Scholar]
- 20.Morris PG, Correa DD, Yahalom J, et al. . Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol. 2013;31(31):3971-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doolittle ND, Korfel A, Lubow MA, et al. . Long-term cognitive function, neuroimaging, and quality of life in primary CNS lymphoma. Neurology. 2013;81(1):84-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juergens A, Pels H, Rogowski S, et al. . Long-term survival with favorable cognitive outcome after chemotherapy in primary central nervous system lymphoma. Ann Neurol. 2010;67(2):182-189. [DOI] [PubMed] [Google Scholar]
- 23.Batchelor T, Carson K, O’Neill A, et al. . Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol. 2003;21(6):1044-1049. [DOI] [PubMed] [Google Scholar]
- 24.Rubenstein JL, Hsi ED, Johnson JL, et al. . Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol. 2013;31(25):3061-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreri AJ, Cwynarski K, Pulczynski E, et al. . Addition of thiotepa and rituximab to antimetabolites significantly improves outome in primary CNS lymphoma: first randomization of the IELSG32 trial. [abstract] Hematol Oncol. 2015;33:103 Abstract 9. [Google Scholar]
- 26.Poortmans PM, Kluin-Nelemans HC, Haaxma-Reiche H, et al. ; European Organization for Research and Treatment of Cancer Lymphoma Group. High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962. J Clin Oncol. 2003;21(24):4483-4488. [DOI] [PubMed] [Google Scholar]
- 27.Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey LE. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol. 2002;58(2):175-178. [DOI] [PubMed] [Google Scholar]
- 28.Sierra del Rio M, Ricard D, Houillier C, et al. . Prophylactic intrathecal chemotherapy in primary CNS lymphoma. J Neurooncol. 2012;106(1):143-146. [DOI] [PubMed] [Google Scholar]
- 29.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology. 2011;76(10):929-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holdhoff M, Ambady P, Abdelaziz A, et al. . High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology. 2014;83(3):235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thiel E, Korfel A, Martus P, et al. . High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11(11):1036-1047. [DOI] [PubMed] [Google Scholar]
- 32.Illerhaus G, Fritsch K, Egerer G, et al. Sequential High Dose Immuno-Chemotherapy Followed by Autologous Peripheral Blood Stem Cell Transplantation for Patients wtih Untreated Primary Central Nervous System Lymphoma - a Multicentre Study by the Collaborative PCNSL Study Group Freiburg. Blood 2012, 120. Presented at the American Society of Hematology Annual Meeting, 2012. Atlanta, Georgia. [Google Scholar]
- 33.Mead GM, Bleehen NM, Gregor A, et al. . A medical research council randomized trial in patients with primary cerebral non-Hodgkin lymphoma: cerebral radiotherapy with and without cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy. Cancer. 2000;89(6):1359-1370. [PubMed] [Google Scholar]
- 34.Omuro A, Chinot O, Taillandier L, et al. . Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol. 2015;2(6):e251-e259. [DOI] [PubMed] [Google Scholar]
- 35.Zhu JJ, Gerstner ER, Engler DA, et al. . High-dose methotrexate for elderly patients with primary CNS lymphoma. Neuro-oncol. 2009;11(2):211-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasenda B, Ferreri AJ, Marturano E, et al. . First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)--a systematic review and individual patient data meta-analysis. Ann Oncol. 2015;26(7):1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fritsch K, Kasenda B, Hader C, et al. . Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol. 2011;22(9):2080-2085. [DOI] [PubMed] [Google Scholar]
- 38.Langner-Lemercier S, Houillier C, Soussain C, et al. . Primary CNS lymphoma at first relapse/progression: characteristics, management, and outcome of 256 patients from the French LOC network. Neuro-oncol. 2016;18(9):1297-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang N, Gill C, Betensky RA, Batchelor TT. Relapse patterns in primary CNS diffuse large B-cell lymphoma. Neurology. 2015; 84(14S):P3.147.
- 40.Plotkin SR, Betensky RA, Hochberg FH, et al. . Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res. 2004;10(17):5643-5646. [DOI] [PubMed] [Google Scholar]
- 41.Soussain C, Hoang-Xuan K, Taillandier L, et al. ; Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol. 2008;26(15):2512-2518. [DOI] [PubMed] [Google Scholar]
- 42.Kasenda B, Schorb E, Fritsch K, Hader C, Finke J, Illerhaus G. Primary CNS lymphoma--radiation-free salvage therapy by second autologous stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(2):281-283. [DOI] [PubMed] [Google Scholar]
- 43.Nguyen PL, Chakravarti A, Finkelstein DM, Hochberg FH, Batchelor TT, Loeffler JS. Results of whole-brain radiation as salvage of methotrexate failure for immunocompetent patients with primary CNS lymphoma. J Clin Oncol. 2005;23(7):1507-1513. [DOI] [PubMed] [Google Scholar]
- 44.Dunleavey K, Lai C, Roschewski, et al. Phase I/II study of TEDDI-R with Ibrutinib in untreated and relapsed/refractory primary CNS lymphoma [abstract]. Hematol Oncol. 2015;33:174-175. Abstract 136. [Google Scholar]
- 45.Korfel A, Schlegel U, Herrlinger U, et al. . Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol. 2016;34(15):1757-1763. [DOI] [PubMed] [Google Scholar]
- 46.Houillier C, Choquet S, Touitou V, et al. . Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology. 2015;84(3):325-326. [DOI] [PubMed] [Google Scholar]
- 47.Rubenstein JL, Formaker P, Wang X, et al. . Lenalidomide is highly active in recurrent CNS lymphomas: phase I investigation of lenalidomide plus rituximab and outcomes of lenalidomide as maintenance monotherapy [abstract] Hematol Oncol. 2015;33:175 Abstract 137. [Google Scholar]
- 48.Lai R, Abrey LE, Rosenblum MK, DeAngelis LM. Treatment-induced leukoencephalopathy in primary CNS lymphoma: a clinical and autopsy study. Neurology. 2004;62(3):451-456. [DOI] [PubMed] [Google Scholar]
- 49.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62(5):515-520. [DOI] [PubMed] [Google Scholar]