Abstract
Since 1998, the National Institutes of Health has funded 5 randomized controlled trials (RCTs) for primary and secondary prevention of strokes in children with sickle cell anemia (SCA). In a systematic fashion, these trials have significantly advanced the care of children with SCA. In the absence of an RCT, clinicians are often compelled to make decisions at the bedside, based on experience, observational studies, and principles of hematology. We will provide an initial example that describes how a team-based, learning collaborative developed a multisite standard care protocol with a low budget (<$10 000 per year) to overcome the intrinsic limitations of advancing the care of neurologic complications in sickle cell disease (SCD). The critical components of this approach include: (1) regular meetings with the multidisciplinary team from multiple sites; (2) consensus regarding the best evidence-based neurologic management in multiple SCD centers; (3) an Institutional Review Board-approved protocol based on consensus standard care; (4) minimizing and ensuring accurate data collection; and most importantly, (5) a spirit of collaboration to improve the care of individuals with SCD. Four common neurologic problems and strategies for management in children and adults with SCD will be discussed: (1) secondary stroke prevention in high-income countries; (2) primary stroke prevention in low- and middle-income countries (LMICs); (3) poor academic performance in students; and (4) cognitive disability in adults. With a commitment to a team-based learning collaborative, incremental advances are possible for the neurologic care of children and adults with SCD.
Learning Objectives
To describe the benefit of a multidisciplinary team-based learning collaborative for the management of acute and chronic neurologic sequelae in SCD
To describe potential strategies for improved clinical management in the absence of RCTs for: (1) secondary stroke prevention in high-income countries; (2) primary stroke prevention in LMICs; (3) poor academic performance in students with SCD; and (4) cognitive disability in adults with SCD
Introduction
Over the last 20 years, 5 randomized controlled trials (RCTs) have been completed for primary and secondary stroke prevention in children with sickle cell anemia (SCA). The 5 completed trials are: (1) Stroke Prevention in Sickle Cell Anemia Trial (STOP, #NCT00000592)1; (2) Stroke Prevention in Sickle Cell Anemia Trial II (STOP II, #NCT00006182)2; (3) Stroke With Transfusions Changing to Hydroxyurea Trial (SWiTCH, #NCT00122980)3; (4) Silent Cerebral Infarct Multi-Center Clinical Trial (SIT, #NCT00072761)4; and recently, (5) Transcranial Doppler (TCD) With Transfusions Changing to Hydroxyurea Trial (TWiTCH, #NCT01425307).5 In high-income countries, where resources are typically not the main limitation in health care delivery, the collective impact of these 5 trials is to prevent new cerebral infarcts or progression of cerebral infarcts for individuals with SCA. Unfortunately, the information gained from these trials does not inform the management of many acute and chronic neurologic complications occurring in children and adults with sickle cell disease (SCD).
In the absence of RCTs and large prospective studies, clinicians must revert to unproven strategies that they believe are in the best interest of their patients. However, without systematic evaluation of the benefits and risks of such management strategies, health care providers have well-described intrinsic biases when making clinical decisions (Table 1).6 Given these well-established cognitive biases in managing common diseases, the rates of errors are likely higher when health care providers make decisions in rare diseases, like SCD, where few RCTs to support clinical decisions have been performed.
Table 1.
List and definition of biases that occur in the diagnosis and management of disease
| Definition | |
|---|---|
| Diagnostic biases | |
| Confirmation | Selectively gathering and interpreting evidence that confirms a diagnosis and ignoring evidence that might disconfirm it |
| Representativeness | Overemphasizing evidence that strongly resembles a class of events. Can lead to undervaluing of relevant base rates, ignoring regression to the mean, and gambler’s fallacy |
| Availability | Overestimating the probability of a diagnosis when instances are relatively easy to recall |
| Hindsight | Overestimating the probability of a diagnosis when the correct diagnosis is already known |
| Regret | Overestimating the probability of a diagnosis with severe possible outcome because of anticipated regret if diagnosis were missed |
| Treatment biases | |
| Omission | Determining that detrimental actions are more harmful than inactions |
| Framing | Choosing riskier treatments when they are described in negative (eg, mortality) rather than positive (eg, survival) terms |
| Number of alternatives |
Choosing a given treatment option more often when there are additional alternatives |
Adapted from Bornstein and Emler6 with permission. These biases are common in the practice of medicine, and likely more frequent in a rare disease, such as SCD.
A team-based learning collaborative is a well-established approach for advancing medical care. Based on the significant variability in the management of common acute and chronic neurologic diseases in children and adults with SCA, reaching consensus on a standard care protocol that can be followed at more than 1 site is an arduous, but feasible task.7 Here, we set the stage for future clinical investigation of SCD by providing an explicit example of a multisite, multidisciplinary team-based learning collaborative, and then outlining 4 SCD-related neurologic problems that will likely benefit from this approach.
Team-based learning collaborative for secondary stroke prevention in children with SCA
Example: A 14-year-old child with SCA receiving regular blood transfusion therapy for secondary stroke prevention has a recurrent stroke after 4 years of transfusion therapy. What is the optimal therapy?
Secondary stroke prevention in children with SCA has evolved since 1979 when Sarnaik et al demonstrated, in a single-center retrospective cohort analysis, that blood transfusion therapy was superior for the prevention of recurrent stroke when compared to observation.8 Subsequently, when 60 children with SCA receiving regular blood transfusion therapy for previous strokes were observed for 191.7 patient-years, Pegelow et al demonstrated the superiority of blood transfusion when compared with historical controls (P < .001).9 However, this retrospective cohort study had several limitations, including the lack of central record review. Scothorn et al validated and extended the results of the Pegelow study. In this retrospective cohort study, 137 children with strokes who received regular blood transfusions were followed for ∼1300 patient-years from 14 pediatric clinical centers.10 The study design improved on the Pegelow et al study with a larger number of children, adolescents, and young adults, followed for a greater period of time (mean follow up was 10.1 years) and central review of all records. For the first time, the study demonstrated that children receiving regular blood transfusion therapy for secondary stroke prevention were experiencing stroke recurrence with hemoglobin S levels <30% at the time of stroke recurrence.10 The stroke recurrence rate was 2.2 events per 100, and approximately one-third of the children with stroke recurrence had a third stroke.10 Recognizing the limitations of these prior retrospective studies, a group of hematologists, psychologists, neurologists, and neuroradiologists from 7 sites elected to meet annually to establish standard care guidelines for the management of strokes in children with SCA (hemoglobin SS and hemoglobin S-β thalassemia zero). Although initially the team did not come together to form a multidisciplinary team-based learning collaborative, the essential components of the collaborative developed and were met.11 Each team agreed to travel to 1 site for 24 hours, where travel costs were borne by the team traveling, and the room and board costs were covered by the hosting team. After a series of annual meetings at each site, the team agreed to enroll participants into a prospective cohort study based on the consensus standard care. They also agreed to obtain local Institutional Review Board (IRB) approval to provide regular blood transfusions with an initial goal of maintaining the maximum hemoglobin S levels below 30%. The team enlisted a panel of neuroradiologists to perform surveillance magnetic resonance imaging (MRI) and magnetic resonance angiography, approximately every 1 to 2 years. The median duration of follow up was 5.5 years with 40 children.7 The prospective cohort demonstrated that 45% (18 of 40 children) had progressive cerebral infarcts (stroke recurrence, 3.1 events per 100 patient-years; silent cerebral infarcts [SCIs], 5.0 events per 100 patient-years; or either recurrent stroke or SCIs, 8.1 events per 100 patient-years). The greatest risk factor for infarct recurrence was progressive vasculopathy, with a relative risk of 12.7; 95% confidence interval (CI), 2.65-60.5; P = .001.
The success of the prospective cohort study was heavily contingent on the commitment of the research teams at all sites, in addition to neuroradiologists donating their time to review the MRI and magnetic resonance angiography scans of the brain. Data collection was kept to the minimum required addressing the study hypotheses. All clinical care was considered standard, with clear justification for the benefit in the IRB-approved protocol. Equally important, no salary was paid to any staff, fellow, or faculty member to complete this project. The only cost (<$10 000 per year) was associated with travel expenses for a 24-hour trip for team members to travel to the host city and travel to each site to review the source documents for central data collection. Six of 7 teams, at each site, enrolled participants. Ultimately, the completion of the study served as a springboard for future investigation.
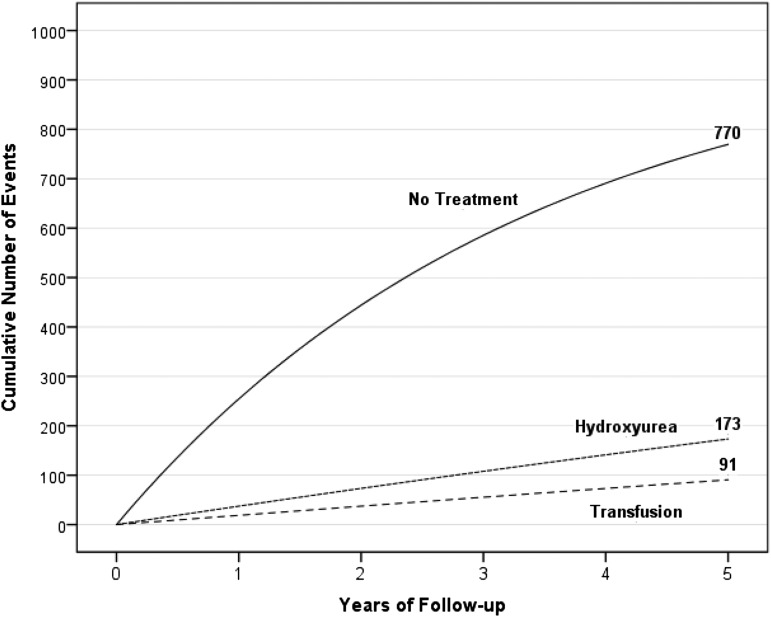
Based on prior experience from the collaborative efforts demonstrating the progressive nature of strokes in children,7,9,10 coupled with a pooled analysis of all published studies since 1998 (Figure 1),12,13 validating the limited benefits of blood transfusion therapy, alternative strategies were considered for decreasing stroke recurrence. Three unproven, but reasonable alternative strategies include hematopoietic stem cell transplant (HSCT), revascularization procedures, or both. No prospective multicenter trial has been initiated to determine the efficacy of any of these alternative strategies for secondary stroke prevention. With prior experience of consortiums addressing the optimal therapy for secondary stroke prevention, we elected to form a new 4-site consortium. Site physicians followed preexisting protocols for a phase 2 HLA haplo-identical SCD clinical trial. The purpose of the consortium was to determine the optimal conditioning regimen for haplo-identical HSCT for severe SCD in adults and for secondary stroke prevention in children (#NCT01850108). The consortium was formally constructed, so that each site had an independent IRB-approved protocol and de-identified data could be shared in the public domain. However, the Data Safety Monitoring Board (DSMB) would evaluate whether stopping rules applied for the first 5 and 10 participants from sites following the same protocol. The main advantages of this strategy were: (1) monthly phone calls creating a shared learning experience for an uncommon treatment of a rare disease; (2) increased pace of recruitment with subsequent alteration of the conditioning regimen or potentially holding the trial enrollment based on predefined stopping rules; (3) assessment of the external DSMB for safety and futility; (4) decreased the bias of estimating the treatment effect compared with single-center trials because of the multi-center rather than a single center trial design14; and (5) meeting all criteria for the conduct of ethical clinical research.15 The combined preliminary results of the consortium ultimately led to the approval of a national phase 2 HLA haplo-identical trial supported by the Blood and Marrow Transplant Clinical Trials Network funded by the National Institutes of Health.
Figure 1.
A hypothetical cohort of 1000 children with SCA and strokes followed for 5 years who either received no therapy, hydroxyurea therapy, or regular blood transfusion therapy. The figure depicts the number of children in the cohort with stroke recurrence in the no treatment group, hydroxyurea therapy group, and regular blood transfusion therapy group with expected incidence rates of 29.1 (95% CI, 19.2-38.9), 3.8 (95% CI, 1.9-5.7), and 1.9 (95 CI, 1.0-2.9) events per 100 patient-years, respectively.12 Figure reproduced from DeBaun and Kirkham.13
Given that hematologists offer options for secondary stroke prevention to families without the benefit of RCT, we strongly recommend that these options be offered in the context of a multidisciplinary, team-based learning collaborative, along with IRB-approved protocols, registration on www.clinicaltrials.gov, a DSMB with formal stopping rules for safety and futility, and adherence to conduct of ethical research. Without these formal strategies, the clinical care of children and adults with SCD and neurologic complications will not likely advance beyond what we have been providing for the last 30 years.
Primary stroke prevention in low- and middle-income countries (LMICs)
Example: A 7-year-old with SCA living in Nigeria has a transcranial Doppler measurement greater than the transfusion threshold. What is the best option for primary stroke prevention?
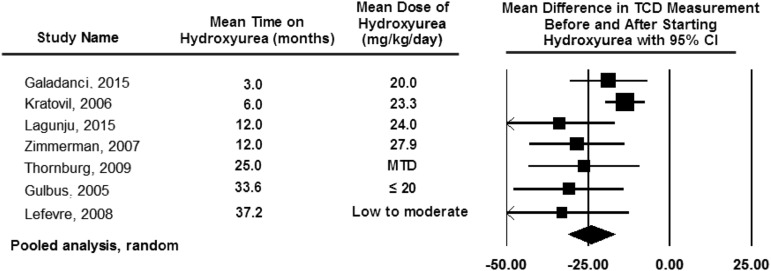
More than 90% of children with SCA are born in LMICs,16 where no RCT has been completed for primary stroke prevention. Without TCD screening, coupled with regular blood transfusion therapy for those with elevated TCD measurements, ∼11% of children with SCA will develop strokes.17 With TCD screening and regular blood transfusion therapy for those with elevated measurements, the incidence rate of strokes can decrease log-fold.18 However, regular blood transfusion therapy combined with eventual iron chelation is not feasible for most families living in sub-Saharan Africa.19 Thus, providers in LMICs must make decisions that are not informed by high-quality data from their setting for primary stroke prevention in an enlarging pediatric population. Based on the pressing challenges to prevent strokes in children with SCA and elevated TCD measurements, Lagunju et al developed an institutional standard care protocol and subsequently demonstrated that hydroxyurea therapy was a reasonable option to consider for children with elevated TCD velocities.20 This strategy was further endorsed by a National Institutes of Health-funded feasibility trial demonstrating the ability to conduct a stroke prevention trial in Nigeria21 and a pooled analysis of 7 studies demonstrating that hydroxyurea therapy decreases TCD velocities by ∼25 cm per second (Figure 2).13,22-25 Collectively, these data led to a phase 3 National Institutes of Health funded RCT (#NCT02560935) comparing fixed doses of hydroxyurea (10 mg/kg per day to 20 mg/kg per day) for the primary prevention of strokes in children with elevated TCD measurements.
Figure 2.
Pooled analysis of the 7 studies documenting TCD measurement before and after hydroxyurea therapy from DeBaun and Kirkham.13 The pooled analysis based on the random effect model demonstrating the average drop in TCD measurement after starting hydroxyurea therapy of 25 cm per second. The table also includes the observation that the decrease in TCD measurements can be seen as early as 3 months after starting hydroxyurea therapy, with a sustained impact of hydroxyurea therapy on decreasing TCD measurements for at least 36 months. The black diamond represents the results of random effect model. The edges of the diamond represent the 95% CI of the meta-analyses for the random effect model.13,22-25 Figure reproduced from DeBaun and Kirkham.13
Until the National Institutes of Health funded RCT trial is completed, which is anticipated in 2020, clinicians in LMICs must make decisions to implement a primary stroke prevention strategy. For children living in LMICs, with an elevated TCD measurement, a fixed dose of 20 mg/kg per day (moderate) of hydroxyurea therapy is a reasonable starting point. This fixed moderate dose of hydroxyurea therapy is a compromise between the maximum tolerated dose (typically 25 to 35 mg/kg per day), demonstrated by the TWITCH trial to be as efficacious as regular blood transfusion therapy in preventing strokes after at least 1 year of blood transfusion therapy,5 and low dose (10 mg/kg per day), based on the experience in India that this dose significantly lowers the incidence of vaso-occlusive pain, acute chest syndrome, and blood transfusion therapy.26 Regardless of the hydroxyurea dose selected, a multicenter multidisciplinary learning collaborative could be initiated for primary stroke prevention with development of an IRB-approved protocol, registration on www.clinicaltrials.gov, and the formation of a DSMB with stopping rules.
Decline in educational achievement in students with SCD
Example: A 12-year-old boy with SCA tells you that he has started to fail 2 subjects in school. He previously earned all As and Bs. He denies headaches, acute neurologic changes, or head trauma. How do you proceed with your evaluation and treatment?
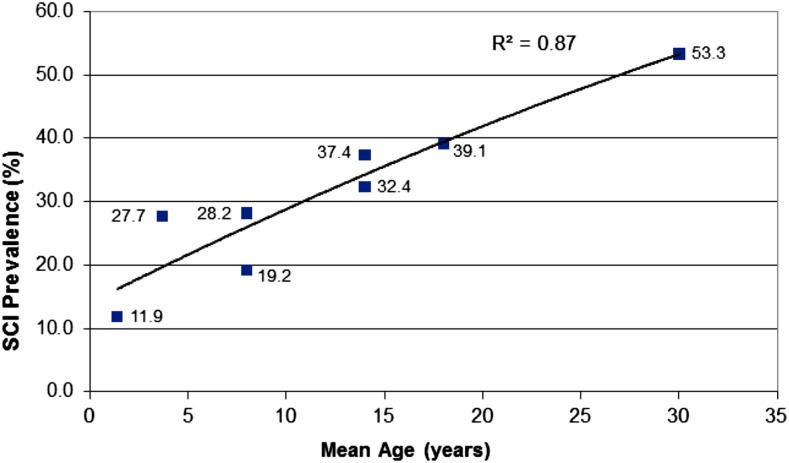
Approximately 39% of children with SCA have SCIs (Figure 3).27-32 In children with SCA and preexisting SCIs, the results of the SIT Trial established regular blood transfusion therapy as the standard of care to prevent progressive infarcts.4 Families should at least be made aware of regular blood transfusion therapy, which provides 58% relative risk reduction in cerebral infarct recurrence (overt and SCI). In addition to preventing infarct recurrence, regular blood transfusions are also associated with improved quality of life and reduced incidence rates of painful vaso-occlusive episodes, hospitalizations, and acute chest syndrome.4 Alternatively families may elect to use hydroxyurea therapy in lieu of blood transfusion therapy. However, the rate of cerebral infarct recurrence (overt stroke or SCI) is so low with regular blood transfusion therapy, 2 events per 100 patient-years (observation of 4.8 events per 100 patient-years),4 that a non-inferiority trial of hydroxyurea therapy with an upper boundary of 3.5 events per 100 patient-years is unlikely to be undertaken because over 1200 participants would be required (Figure 4A).4,7,33,34 Figure 4 also includes 2 additional sample size calculations for potential secondary stroke prevention superiority trials comparing blood transfusion therapy (standard therapy) to HSCT (experimental therapy) to prevent cerebral infarct recurrence for individuals with preexisting strokes (Figure 4B), and to prevent cerebral infarct recurrence for individuals with preexisting SCIs (Figure 4C).
Figure 3.
Prevalence of SCIs in unselected children and adults with SCA, and the prevalence of SCIs in children and young adults with SCA. The figure displays the cumulative prevalence of SCIs in children and young adults based on 6 studies.27-32 The cumulative prevalence of SCIs suggests that the incidence of SCI does not plateau, but rather steadily increases through 30 years of age.27 Figure reproduced from Kassim et al.27
Figure 4.
Projected sample size calculations for various secondary stroke prevention trials. (A) In children with sickle cell anemia and silent cerebral infarcts, a hypothetical non-inferior trial of secondary cerebral infarct recurrence (stroke or silent cerebral infarct), comparing standard therapy (blood transfusion) to anticipated benefits of hydroxyurea. (B) Sample size calculation for individuals with sickle cell anemia and strokes, in a superiority trial with two arms being compared: standard therapy (blood transfusion) to a hematopoietic stem cell transplant (experimental arm). (C) In children with sickle cell anemia and silent cerebral infarcts, a superiority trial with two arms being compared: standard therapy (blood transfusion) to a hematopoietic stem cell transplant (experimental arm). *Incidence of infarct recurrence in children with preexisting SCIs receiving blood transfusion therapy.4 †Estimated upper boundary non-inferiority threshold is intermediate between the treatment and observation arms in the Silent Cerebral Infarct Trial (2 + 4.8 events per 100 patient-years/2). ‡Incidence of stroke recurrence (silent and overt) in children and young adults with overt stroke receiving regular blood transfusion therapy.7 §Incidence of stroke recurrence (silent and overt stroke) in children and young adults after an HSCT.33,34 ||Estimated incidence of infarct recurrence (silent and overt) in children with SCIs after an HSCT (actual incidence in 10 patients was 0, but incidence estimate is not calculable).
The disease-related cognitive impairments in SCD are compounded by the challenge of poverty, with ∼60% of these children in the United States receiving public health insurance based on family income.35,36 An SCI is predicted to decrease a child’s Full Scale Intelligence Quotient (FSIQ) by ∼5.2 points. If a child’s parent has not obtained any college education, then the FSIQ of the child would be predicted to decrease by an additional 6.2 points.37 Given the overwhelming prevalence of poverty and lower levels of education in families of children with SCD, the home environment has at least the same impact on FSIQ as the presence of SCIs.36
All students with academic challenges should receive a full medical evaluation for poor school performance, including an MRI of the brain and cognitive testing. Without screening for the presence of SCIs, coupled with cognitive assessments if SCIs are present, students are denied the opportunity to reach their fullest academic potential. Further, children4 and young adults with untreated SCIs are at risk for ongoing neurologic injury (Figure 3). Although the burden of blood transfusion therapy to prevent the progression of SCIs is significant, students and their parents should be aware of their SCI status, risk of future neurologic events with and without treatment, and given the opportunity to make an informed decision regarding preventative therapy. If faced with a struggling student in the classroom, most health care providers would seek for an explanation, remedial strategies, and options to prevent further injury to the brain. Parents of students with SCA are no different in wanting the best educational opportunity for their children.
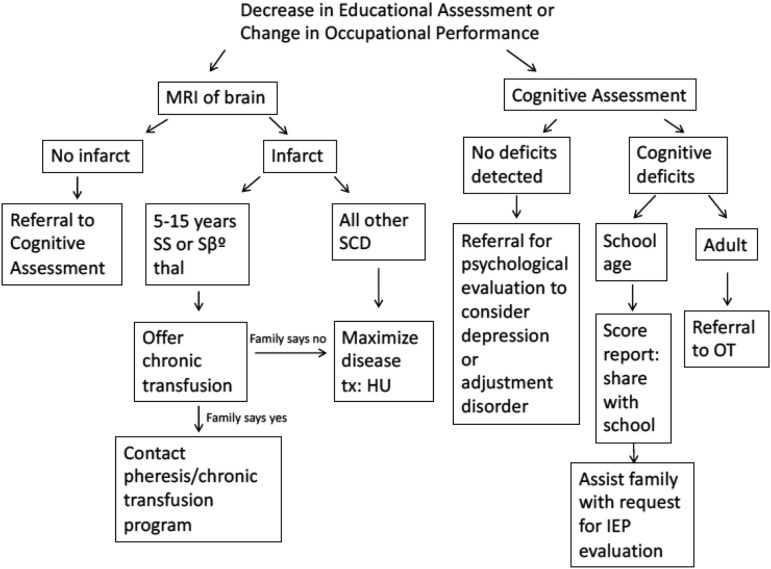
In the United States, the importance of identifying cognitive deficits is described in two Federal laws: the Individuals with Disabilities Education Act (IDEA)38 and Section 504 of the Rehabilitation Act of 1973.39 For students with disabilities who require specialized instruction, IDEA controls the procedural requirements. An Individualized Education Program is developed to meet the educational needs of the child.40 The IDEA process requires documentation of measurable improvement in cognitive milestones. A 504 Plan should be considered for all students with SCD who do not require specialized instruction but need assurance they will receive equal access to public education and services, particularly with increased days missed from school for vaso-occlusive pain events. A document is created to outline their specific requirements. Students with 504 Plans should be reassessed annually to ensure the most effective accommodations for their specific circumstances. Implementing an Individualized Education Program and 504 Plans are evidence-based practices in the school setting, but must be requested based on a cognitive evaluation or medical diagnosis. Examples of how these plans attempt to compensate for students’ weaknesses include: allowing for additional time for examinations, providing accommodations for prolonged school absences secondary to hospitalizations, and obtaining class notes ahead of time. Figure 5 outlines a recommended approach to evaluating poor academic performance. Given the significant lack of evidence-based data regarding the optimal strategy for cognitive remediation, opportunities exist for multiple providers from different sites to provide incremental knowledge through a team-based learning collaborative.
Figure 5.
Flow diagram of how to approach children and adults with decreased educational achievement or occupational performance.
Decline in work performance in an adult with SCA
Example: A 30-year-old with SCA presents with complaints of relatively new onset of poor work performance, missed medical appointments, and poor adherence to daily medication. How do you proceed with your evaluation and treatment?
Young adults with SCA have one of the highest rates of cerebral infarcts compared with most other chronic disease populations (Figure 3). Recently, Kassim et al published data demonstrating >50% of individuals with SCD will have an SCI by 30 years of age.27 Although the SIT trial provides evidence for screening and blood transfusion therapy for individuals between 5 and 16 years of age, guidance for adults with SCD is unfortunately lacking. Given the lack of evidence-based guidelines for the management of adults with SCD with cognitive disabilities, these patients should be managed similarly to adults with acquired brain injury. Cognitive deficits are underappreciated in adults with SCD because fewer studies have been completed. Part of the management strategy should include the evaluation by occupational therapy as to who can provide recommendations to facilitate adjustment to their environment and behavioral modifications to compensate or overcome functional challenges. In the Cognitive Orientation to Daily Occupational Performance approach,41,42 participants are trained to use the global strategy, GOAL-PLAN-DO-CHECK, to develop a specific plan to improve performance of a self-selected goal, to review performance, and to modify the plan accordingly if performance is not satisfactory. For example, if deficits are present in executive functions, suggestions are made so that the affected individual may perform better in a quiet area with fewer interruptions.
Other potential sequelae associated with SCIs in adults include, but are not limited to, the inability to follow complex medical management instructions and adherence to medical interventions, which is a common challenge for adolescents and adults with SCD.43,44 Memory deficits decrease the chances that a patient remembers to take a medication or attend an appointment, which results in suboptimal clinical outcomes.44 Executive function deficits are associated with lesions in the frontal lobe where many SCIs occur. Impairment in executive function makes multitasking, and multistep commands difficult to complete. Interventions that serve as memory prompts or break-down multistep processes are helpful in both the school, work, and clinic setting. However, interventions for improving learning and job performance are rarely offered if cognitive impairments are not identified.
Despite the evidence that untreated SCIs continue to occur throughout young adulthood, no evidence or even consensus treatment strategy has been developed for this high-risk population. Clearly, sufficient evidence exists to establish a multidisciplinary team-based learning collaborative for adults with SCD, which includes SCI screening, cognitive assessments, occupational therapy assessments, an educational transition plan (as required by law at 16 years of age), and rehabilitation interventions, such as Cognitive Orientation to Daily Occupational Performance in a more uniform fashion. With a multidisciplinary team and an organized effort, we will have a better understanding of the current achievement, performance, and malleability of outcomes for adolescents and young adults in this vulnerable population.
Conclusion
Without the benefit of a completed RCT, health care providers in both high and LMICs have common challenges in making informed decisions about the management of acute and chronic neurologic disease in SCD. For secondary stroke prevention in children and adults in high-income countries where SCD is rare, health care providers typically default to ingrained institutional practices, without systematic and ongoing assessment of the benefits and risks of their treatment algorithms. For secondary stroke prevention in LMICs where SCD is common, treatment strategies have been nonsystematic in part due to the lack of evidence. This review limited its discussion to only 4 common management challenges of acute and chronic neurologic complications in SCD. However, we could have easily selected others, such as acute management of transient ischemic attacks, the utility of isovolemic hemodilution-red cell exchange for secondary stroke prevention, treatment of cerebral sinus venous nonsystematic, or secondary prevention of strokes in individuals with hemoglobin SC disease. Despite the added value of multidisciplinary and multisite participation with prospective evaluation of neurologic complications in SCD, such approaches are rarely used. To offset inherent biases in medical decision making, the team-based collaborative research approach11,45 can be introduced to optimize standard care for SCD. Ideally, the strategy will include requirements for ethical conduct of clinical research,15 which is conducted with limited institutional resources and may provide preliminary data for competitive federal funding. Without such efforts, there will be continued slow progress, if any, in advancing the neurologic management of children and adults with SCD in both high and LMICs. To this end, the authors have recently initiated a non-funded team based-learning collaborative for secondary prevention of strokes and SCI in adults with SCD. Hematologists interested in joining the learning collaborative may contact the authors.
Acknowledgments
The authors thank members of the DeBaun and King research laboratories for review of the manuscript, and Mark Rodoghier for sample size calculations.
References
- 1.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5-11. [DOI] [PubMed] [Google Scholar]
- 2.Adams RJ, Brambilla D; Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353(26):2769-2778. [DOI] [PubMed] [Google Scholar]
- 3.Ware RE, Helms RW; SWiTCH Investigators. Stroke with transfusions changing to hydroxyurea (SWiTCH). Blood. 2012;119(17):3925-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371(8):699-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ware RE, Davis BR, Schultz WH, et al. Hydroxycarbamide versus chronic transfusion for maintenance of transcranial doppler flow velocities in children with sickle cell anaemia-TCD With Transfusions Changing to Hydroxyurea (TWiTCH): a multicentre, open-label, phase 3, non-inferiority trial. Lancet. 2016;387(10019):661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornstein BH, Emler AC. Rationality in medical decision making: a review of the literature on doctors’ decision-making biases. J Eval Clin Pract. 2001;7(2):97-107. [DOI] [PubMed] [Google Scholar]
- 7.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117(3):772-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarnaik S, Soorya D, Kim J, Ravindranath Y, Lusher J. Periodic transfusions for sickle cell anemia and CNS infarction. Am J Dis Child. 1979;133(12):1254-1257. [DOI] [PubMed] [Google Scholar]
- 9.Pegelow CH, Adams RJ, McKie V, et al. Risk of recurrent stroke in patients with sickle cell disease treated with erythrocyte transfusions. J Pediatr. 1995;126(6):896-899. [DOI] [PubMed] [Google Scholar]
- 10.Scothorn DJ, Price C, Schwartz D, et al. Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140(3):348-354. [DOI] [PubMed] [Google Scholar]
- 11.Campbell-Voytal K, Daly JM, Nagykaldi ZJ, et al. Team science approach to developing consensus on research good practices for practice-based research networks: a case study. Clin Transl Sci. 2015;8(6):632-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassim AA, Galadanci NA, Pruthi S, DeBaun MR. How I treat and manage strokes in sickle cell disease. Blood. 2015;125(22):3401-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeBaun MR, Kirkham FJ. Central nervous system complications and management in sickle cell disease. Blood. 2016;127(7):829-838. [DOI] [PubMed] [Google Scholar]
- 14.Dechartres A, Boutron I, Trinquart L, Charles P, Ravaud P. Single-center trials show larger treatment effects than multicenter trials: evidence from a meta-epidemiologic study. Ann Intern Med. 2011;155(1):39-51. [DOI] [PubMed] [Google Scholar]
- 15.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283(20):2701-2711. [DOI] [PubMed] [Google Scholar]
- 16.Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Globa burden of sickle cell anaemia in children under five, 2010-2050: modelling based on demographics, excess mortality, and interventions. PLoS Med. 2013;10(7):e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288-294. [PubMed] [Google Scholar]
- 18.Enninful-Eghan H, Moore RH, Ichord R, Smith-Whitley K, Kwiatkowski JL. Transcranial Doppler ultrasonography and prophylactic transfusion program is effective in preventing overt stroke in children with sickle cell disease. J Pediatr. 2010;157(3):479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagunju IA, Brown BJ, Sodeinde OO. Chronic blood transfusion for primary and secondary stroke prevention in Nigerian children with sickle cell disease: a 5-year appraisal. Pediatr Blood Cancer. 2013;60(12):1940-1945. [DOI] [PubMed] [Google Scholar]
- 20.Lagunju I, Brown BJ, Sodeinde O. Hydroxyurea lowers transcranial Doppler flow velocities in children with sickle cell anaemia in a Nigerian cohort. Pediatr Blood Cancer. 2015;62(9):1587-1591. [DOI] [PubMed] [Google Scholar]
- 21.Galadanci NA, Abdullahi SU, Tabari MA, et al. Primary stroke prevention in Nigerian children with sickle cell disease (SPIN): challenges of conducting a feasibility trial. Pediatr Blood Cancer. 2015;62(3):395-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kratovil T, Bulas D, Driscoll MC, Speller-Brown B, McCarter R, Minniti CP. Hydroxyurea therapy lowers TCD velocities in children with sickle cell disease. Pediatr Blood Cancer. 2006;47(7):894-900. [DOI] [PubMed] [Google Scholar]
- 23.Zimmerman SA, Schultz WH, Burgett S, Mortier NA, Ware RE. Hydroxyurea therapy lowers transcranial Doppler flow velocities in children with sickle cell anemia. Blood. 2007;110(3):1043-1047. [DOI] [PubMed] [Google Scholar]
- 24.Thornburg CD, Dixon N, Burgett S, et al. A pilot study of hydroxyurea to prevent chronic organ damage in young children with sickle cell anemia. Pediatr Blood Cancer. 2009;52(5):609-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gulbis B, Haberman D, Dufour D, et al. Hydroxyurea for sickle cell disease in children and for prevention of cerebrovascular events: the Belgian experience. Blood. 2005;105(7):2685-2690. [DOI] [PubMed] [Google Scholar]
- 26.Patel DK, Mashon RS, Patel S, Das BS, Purohit P, Bishwal SC. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin. 2012;36(5):409-420. [DOI] [PubMed] [Google Scholar]
- 27.Kassim AA, Pruthi S, Day M, et al. Silent cerebral infarcts and cerebral aneurysms are prevalent in adults with sickle cell anemia. Blood. 2016;127(16):2038-2040. [DOI] [PubMed] [Google Scholar]
- 28.Kwiatkowski JL, Zimmerman RA, Pollock AN, et al. Silent infarcts in young children with sickle cell disease. Br J Haematol. 2009;146(3):300-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang WC, Langston JW, Steen RG, et al. Abnormalities of the central nervous system in very young children with sickle cell anemia. J Pediatr. 1998;132(6):994-998. [DOI] [PubMed] [Google Scholar]
- 30.Wang WC, Pavlakis SG, Helton KJ, et al. ; BABY HUG Investigators. MRI abnormalities of the brain in one-year-old children with sickle cell anemia. Pediatr Blood Cancer. 2008;51(5):643-646. [DOI] [PubMed] [Google Scholar]
- 31.Bernaudin F, Verlhac S, Arnaud C, et al. Impact of early transcranial Doppler screening and intensive therapy on cerebral vasculopathy outcome in a newborn sickle cell anemia cohort. Blood. 2011;117(4):1130-1140, quiz 1436. [DOI] [PubMed] [Google Scholar]
- 32.Bernaudin F, Verlhac S, Arnaud C, et al. Chronic and acute anemia and extracranial internal carotid stenosis are risk factors for silent cerebral infarcts in sickle cell anemia. Blood. 2015;125(10):1653-1661. [DOI] [PubMed] [Google Scholar]
- 33.Bernaudin F, Socie G, Kuentz M, et al. ; SFGM-TC. Long-term results of related myeloablative stem-cell transplantation to cure sickle cell disease. Blood. 2007;110(7):2749-2756. [DOI] [PubMed] [Google Scholar]
- 34.Walters MC, Hardy K, Edwards S, et al. ; Multicenter Study of Bone Marrow Transplantation for Sickle Cell Disease. Pulmonary, gonadal, and central nervous system status after bone marrow transplantation for sickle cell disease. Biol Blood Marrow Transplant. 2010;16(2):263-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panepinto JA, Owens PL, Mosso AL, Steiner CA, Brousseau DC. Concentration of hospital care for acute sickle cell disease-related visits. Pediatr Blood Cancer. 2012;59(4):685-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King AA, Rodeghier MJ, Panepinto JA, et al. Silent cerebral infarction, income, and grade retention among students with sickle cell anemia. Am J Hematol. 2014;89(10):E188-E192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.King AA, Strouse JJ, Rodeghier MJ, et al. Parent education and biologic factors influence on cognition in sickle cell anemia. Am J Hematol. 2014;89(2):162-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.United States Department of Education - Office of Special Education Programs. Building the Legacy: IDEA 2004. http://idea.ed.gov/explore/home. Accessed 7 October 2016.
- 39.United States Department of Education Office for Civil Rights. Protecting Students with Disabilities Frequently Asked Questions About Section 504 and the Education of Children With Disabilities 2015. http://www2.ed.gov/about/offices/list/ocr/504faq.html. Accessed 7 October 2016.
- 40.Office of Special Education and Rehabilitation Services. A Guide to the Individualized Education Program. United States Department of Education; 2000. http://idea.ed.gov/explore/home. Accessed 7 October 2016. [Google Scholar]
- 41.Polatajko HJ, Mandich AD, Miller LT, Macnab JJ. Cognitive orientation to daily occupational performance (CO-OP): part II--the evidence. Phys Occup Ther Pediatr. 2001;20(2-3):83-106. [PubMed] [Google Scholar]
- 42.Dawson DR, Gaya A, Hunt A, Levine B, Lemsky C, Polatajko HJ. Using the cognitive orientation to occupational performance (CO-OP) with adults with executive dysfunction following traumatic brain injury. Can J Occup Ther. 2009;76(2):115-127. [DOI] [PubMed] [Google Scholar]
- 43.Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics. 2008;122(6):1332-1342. [DOI] [PubMed] [Google Scholar]
- 44.Walsh KE, Cutrona SL, Kavanagh PL, et al. Medication adherence among pediatric patients with sickle cell disease: a systematic review. Pediatrics. 2014;134(6):1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kania J, Kramer M.. Collective impact: Stanford Social Innovation Review. 2011;9(1). [Google Scholar]