Abstract
Individuals with severe hemophilia have benefitted from 5 decades of advances that have led to widespread availability of safe and efficacious factors VIII and IX, a multidisciplinary integrated care model through a network of specialized hemophilia treatment centers, and aggressive introduction of prophylactic replacement therapy to prevent bleeding and preserve joint health. Yet, there are remaining challenges and treatment gaps which have prevented complete abrogation of all joint bleeding, and progressive joint deterioration may continue in some affected individuals over the course of a lifetime. Some of these challenges can now be addressed with recombinant clotting factors with extended half-life that may improve adherence to prophylaxis regimens through more convenient infusion schedules, maintain higher plasma levels for longer when clinically necessary, and allow for better adaptation to individual phenotypic and pharmacokinetic variability. Real-world case studies will be presented that illustrate practical application of these newly approved therapies in clinical practice and the clinical trial data that have demonstrated the potential for improved clinical outcomes by implementing these strategies.
Learning Objectives
Learn the therapeutic strategies that have greatly improved the long-term outcomes in hemophilia
Learn from real-world case studies several practical applications for extended half-life clotting factors to address remaining challenges and treatment gaps that may lead to further advances in long-term outcomes in hemophilia
What are the current clinical outcomes in severe hemophilia?
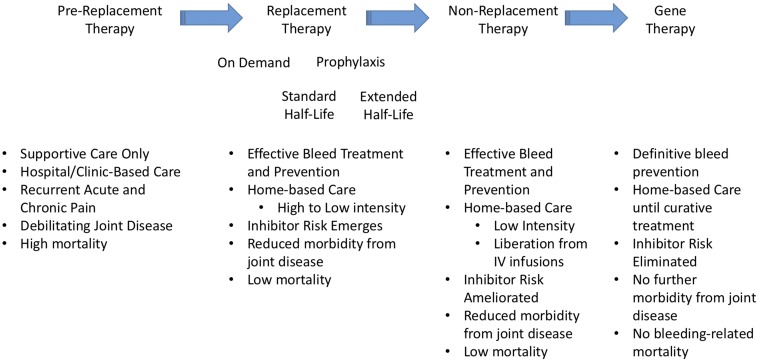
The current generation of individuals with congenital deficiency of factor VIII (FVIII; hemophilia A) or factor IX (FIX; hemophilia B) are benefitting from 5 decades of advances in therapeutics and clinical care that have provided widespread availability of safe and efficacious clotting factor concentrates and a network of centers of excellence (hemophilia treatment centers [HTCs]). The HTCs, consisting of a core team of hematologists, specialized hemophilia nurses, physical therapists, social workers as well as other specialized care providers, have implemented best practices and an evidence-based integrated care model that have produced greatly improved health outcomes. These advances can be seen to have occurred through 2 major waves of advances (Figure 1). The first was the development of cryoprecipitate and then lyophilized purified clotting factor concentrates which allowed for full correction of clotting factor levels and ushered in an era of home infusion therapy. For the first time, bleeding events could be treated quickly and efficiently and persons with hemophilia (PWH) could be liberated from the hospital urgent care and clinic infusion facilities. However, even with prompt effective treatment of bleeds, PWH would go on to develop significant long-term complications, particularly due to recurrent hemarthroses, with progressive arthropathy and negative impacts on physical and social functioning. The second wave occurred through the implementation of prophylaxis strategies.1 The initial rationale for prophylaxis was the observation that those with moderate hemophilia (factor levels of 1%-5%) experienced few spontaneous joint bleeds and rarely developed significant arthropathy. This led to the hypothesis that maintaining a plasma FVIII or FIX level of at least 1% or higher would lead to a more moderate phenotype with a concomitant reduction in spontaneous joint bleeding and subsequent arthropathy. Primary prophylaxis in children (regular, continuous replacement therapy, initiated in the absence of documented joint disease) has been proven to prevent joint bleeding and overall bleeding and can prevent joint disease with health-related quality of life (HRQoL) measures that are indistinguishable from their unaffected peers. Secondary prophylaxis, initiated in individuals who have already established a pattern of recurrent joint bleeds, has also been demonstrated to prevent joint and overall bleeding, slow the progression of joint disease (improved clinical joint status, improved orthopedic joint scores),2 permit increased activity levels including sport participation, reduce school/work absences, and improve HRQoL. Tertiary prophylaxis in adolescents and adults, where prophylaxis is initiated after joint disease has already been established, has also been shown to reduce joint bleeding, maintain mobility, reduce work absences and pain, and improve HRQoL. Thus, prophylaxis, with an emphasis on widespread implementation of primary prophylaxis in the youngest cohort of boys, has been established as the gold standard of modern hemophilia care.
Figure 1.
Major advances in therapy for hemophilia and their associated outcomes.
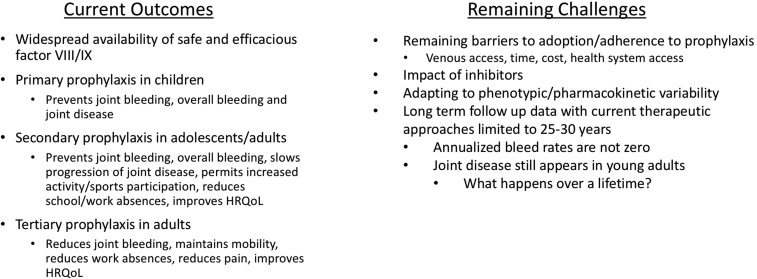
However, there remain several challenges and unmet needs (Figure 2). There continue to be barriers to adoption and adherence to prophylaxis. Venous access can be a significant challenge affecting the timing of initiation of prophylaxis in infants or driving the need for implantable central venous catheters. Regular prophylaxis requires a significant time commitment for PWH and their families. Costs for prophylaxis can be between $150 000 and $300 000 per year (US dollars). Current therapeutic products have not eliminated the risk for inhibitors. These antibodies that develop in response to the infused clotting factors neutralizing their efficacy occur in up to 30% of those with severe hemophilia A. The impact of these challenges has been recently highlighted in a birth cohort anlysis from a longitudinal data set within the US HTCs in cooperation with the Centers for Disease Control and Prevention.3 This study examined 4 birth cohorts (era A with men born prior to 1958; era B men born 1958-1975; era C born 1976-1982, and era D born 1982-1993). Though there was an increasing proportion reporting the use of prophylaxis therapy with each of the successively younger eras (roughly half of those in era D), the results showed that frequent bleeding remained prevalent even in era D despite modern hemophilia care strategies. Notably, 1 in 3 participants with severe hemophilia reported >5 bleeds in 6 months and 1 in 4 reporting a target joint for recurrent hemorrhages. Although the prevalence of disability in this latter cohort is low presently, the concern would be that over a 25- to 30-year time frame, this unacceptable rate of recurrent joint hemorrhages could continue a trajectory of joint disease similar to prior cohorts unless there is continued improvement in strategies for the prevention and treatment of bleeding.4
Figure 2.
Current outcomes and remaining challenges in therapeutic strategies for hemophilia.
Current approaches to prophylaxis have greatly improved the outcomes of PWH, and the annualized bleed rate (ABR) and annualized joint bleed rate have been used as surrogates for the efficiency of prophylaxis regimens. Studies using intensive regimens have shown mean ABRs from 2 to 5 and mean annualized joint bleed rates of 0.5 (∼1 joint bleed every 2 years). However, even with such a low rate of annualized joint bleeding, joint disease may progress slowly over several decades.4 Thus, there remains opportunity to further improve these long-term outcomes.
Aspirationally, it remains the goal to eliminate all bleeding, joint bleeding in particular, in order to better preserve joint function over the course of a lifetime, with the least burden to the PWH or their caregiver. The target for most trough levels for prophylaxis have been ≥1%. Although Collins et al5 showed that the likelihood of having an ABR of 0 steadily declines as the number of hours per week spent with a FVIII level below 1% increases, this remains an arbitrary value above which hemorrhages can still occur and below which bleeding is not inevitable.6 Pharmacokinetic (PK)-guided prophylaxis dosing of FVIII and FIX considers the wide variability in half-life that can be observed across all age ranges and has been shown that it can facilitate reduced frequency of infusion compared with standard programmatic dosing and intervals by targeting the individual to maintain a specific trough level. However, other factors may be influencing bleeding in PWH, such as the musculoskeletal status of their joints and their individual activity level. Thus, even the target trough level may need to be individualized. Such strategies also require laboratory data and PK models to determine an individual’s half-life with strict adherence to the dose/interval. In addition, the half-life of some PWH may not allow for an extended interval between doses without significantly higher dosing, adding to an already costly replacement therapy.
Can EHL products improve on current outcomes?
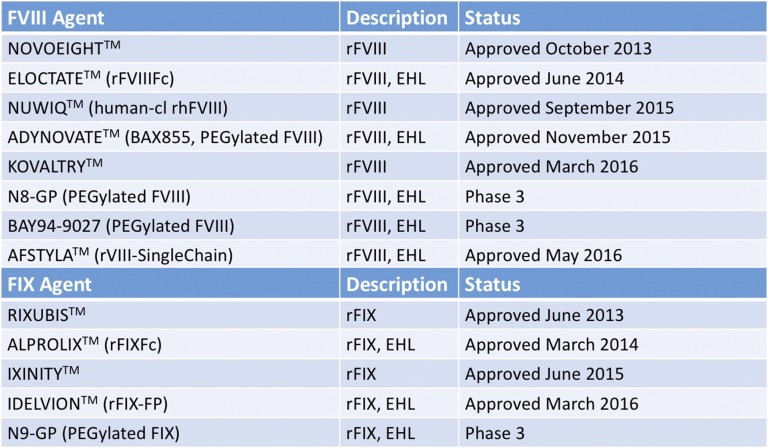
The half-life of conventional FVIII and FIX products (∼12 hours and ∼16-18 hours, respectively) drives the frequency of their infusions for efficient bleed control through prophylaxis (generally 3 times per week or every other day for hemophilia A and 2-3 times per week for hemophilia B). Several new products have been recently approved or are in development with an extended half-life (Figure 3). Products with an extended half-life could allow for prolonged protection from bleeding episodes and reduce the frequency of infusions. The reduced burden of administration could then lead to enhanced compliance with a prophylactic regimen providing still greater prevention of bleeding episodes and, ultimately, may improve long-term outcomes. Alternatively, extended half-life (EHL) factors may allow trough activity levels to remain above key thresholds for longer periods relative to conventional factor products. Longer half-lives and reduced clearance could lead to reduced factor consumption, while maintaining or improving protection from bleeding, leading to reductions in hemophilia-related complications and their associated cost burden.
Figure 3.
FVIII and FIX therapeutic agents recently approved or in late-phase clinical development. Approved agents are presented with their trademarked names as TM. Agents in the late-phase trials discussed in this review are indicated by their clinical names. Fc fusion and albumin fusion denoted by Fc and FP. Human-cl rh indicates human cell line recombinant human. EHL, extended half-life. Adapted from Impact Education, LLC/National Hemophilia Foundation/Postgraduate Institute for Medicine with permission.43
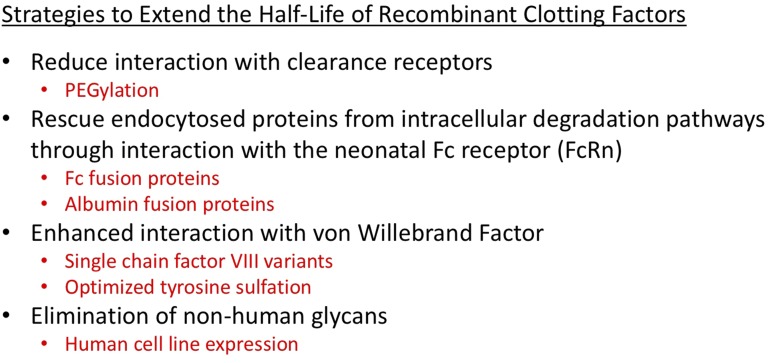
Four broad strategies have been explored to date7 (Figure 4). PEGylation involves the covalent attachment of polyethylene glycol to FVIII or FIX to enhance the PK, pharmacodynamic, and immunogenic properties of the molecules. PEGylation increases the circulating half-life for FVIII and FIX potentially by reducing the binding capacity of the PEGylated protein for their clearance receptors. PEGylation of therapeutic molecules has generally been considered to have a low risk of immunogenicity and may even reduce their immunogenicity. Fusion of the Fc domain of human immunoglobulin G1 (IgG1) to therapeutic proteins through recombinant technology takes advantage of a natural biological pathway centering on the neonatal Fc receptor (FcRn) that prolongs the half-life of circulating IgG. Fc fusion molecules are taken up by the cell via pinocytosis and/or endocytosis. The Fc domain subsequently binds to FcRn, which resides within endosomal compartments, at acidic pH. The FcRn then shuttles the Fc fusion molecule back to the plasma membrane and releases it into circulation at neutral pH, thereby diverting away from lysosomal degradation, thus delaying clearance and extending the functional plasma half-life. This FcRn-mediated protective pathway is also used by endogenous albumin, and recombinant fusion with albumin is another strategy that has been applied to prolong the half-life of FIX. The third strategy has been directed at optimizing the protein structure and posttranslational modifications of recombinant FVIII (rFVIII) such that it has enhanced interaction with von Willebrand factor (VWF). This may be achieved with single-chain forms of rFVIII as well as enhanced cell lines to optimize tyrosine sulfation. Finally, expression of rFVIII in human cell lines may also lead to improved PK properties by the elimination of nonhuman glycans.8
Figure 4.
Strategies to extend the half-life of recombinant clotting factors.
What have we learned from the EHL factor clinical trials?
The published phase 1 through 3 programs for the EHL factors to date have all demonstrated that they:
improved PK properties of the bioengineered molecules (range of half-life extension of 1.2- to 1.5-fold for FVIII and 3- to 5-fold for FIX),
were well tolerated with no inhibitors in previously treated patient populations, and
were efficacious in the treatment and prevention of bleeding episodes (see comprehensive reviews9-12).
The prophylaxis regimens that were part of these pivotal clinical trial programs followed 4 strategies:
Programmatic prophylaxis (fixed dose and interval): once weekly for FIX and twice weekly for FVIII,
PK-driven (dosed to a target trough, fixed interval),
Phenotypic-driven (variable dose and interval according to bleeding pattern and activity), and
Convenience-driven (higher dose, longer interval).
This has led to regulatory approval in the United States for 2 EHL FVIII products (rFVIIIFc, PEGylated FVIII) and 2 EHL FIX products (rFIXFc, rFIX-FP) within the past 2 years (Figure 3). This has given clinicians within the United States an opportunity to use such regimens within their patient population. What follows is a series of clinical cases adapted from real experiences at our HTC that demonstrate how implementation of EHL factors within the population is having a significant impact on clinical outcomes.
How I treat with EHL factors for nonadherence
Case 1
A 6-year-old boy with severe hemophilia A was transferred to our HTC at age 3 years. He had been managed outside of the HTC network with on-demand infusions and had a clinical history of recurrent bleeding primarily into the left ankle (ie, target joint). He was placed initially on a rFVIII at 50 IU/kg every other day and the family was trained in peripheral venipuncture. The family struggled with adherence over 3 years and had continued problems with recurrent hemarthroses into bilateral ankles. His mother was typically managing 2 infusions per week. He was placed on rFVIIIFc at 50 IU/kg twice weekly. Within 6 months, he had complete resolution of target joint bleeding and, following an additional 6 months on this regimen, his clinical manifestations of ankle synovitis had also resolved. A 72-hour trough FVIII activity was 7% by 1-stage clotting assay and 5% by chromogenic assay.
This case illustrates common challenges when prophylaxis is not instituted early in life. There is increased likelihood of nonadherence, a failure to adapt to the changing needs of the child related to increased physical activity, and reduced efficacy of prophylaxis once joint disease has been established.13 Without a significant intervention, this boy would be destined to progressive joint disease and likely debilitating arthropathy in early adulthood. Nonadherence in this case was not related to difficulty with venous access, thus a central venous access device would likely not have altered the clinical course. Even doubling the dose of the prior rFVIII would have added only 1 additional half-life of time with FVIII levels in a potentially hemostatic range and may not have provided sufficient trough levels to suppress target joint bleeding. The switch to a programmatic prophylaxis regimen, patterned after the clinical trial program with rFVIIIFc, allowed for continuing a twice-weekly regimen and resulted in trough levels sufficient to suppress target joint bleeding. In our clinical experience, the efficacy of programmatic prophylaxis with the reduced intervals that can be realized with the EHL factors has also proven effective at converting adults who have remained on on-demand treatment protocols onto tertiary prophylaxis. This has been particularly true with EHL FIX where a once-weekly regimen has been well received as a starting regimen with further interval extension as the patient’s clinical response allows. The long-term safety and efficacy of rFVIIIFc and rFIXFc in children and adults is being collected in the ASPIRE14 and B-YOND (NCT01425723) extension studies. No inhibitors to FVIII or FIX have been observed. In an interim analysis, 86.9% of the subjects remain on twice-weekly dosing with rFVIIIFc with a median average weekly consumption of 99.9 IU (interquartile range: 88.6, 114.2) in children <6 years and 91.2 (81, 107.9) in children 6 to <12 years of age. Adolescents and adults were able to achieve low ABRs with a fixed weekly dosing arm (20-100 IU/kg every 7 days) and 13.7 days in an individualized prophylaxis arm (100 IU/kg every 8-16 days or twice monthly). A retrospective analysis from a database of specialty pharmacy provider records15 included 118 individuals with hemophilia B, median age 20 years (2-63 years) and 520 individuals with hemophilia A, median age 18 years (1-77 years). From this early real-world utilization data, it was observed that 76.1% were using rFIXFc with a dosing frequency of every 7 days and 23.9% >7 days. There was more variability observed for rFVIIIFc with 65.7% of adults dosing between twice weekly and every 5 days and 11.9% dosing weekly.
How I treat new-onset target joint bleeding with EHL factors
Case 2
An 8-year-old boy with severe hemophilia A had been been on primary prophylaxis since the first year of life. His ABR had been 0 to 1 on rFVIII 50 IU/kg every other day with FVIII trough level of 2 IU/dL. However, recently he had been having recurrent bleeding into his elbows bilaterally (up to once per month). This correlated with increased competitive participation in basketball. Physical examination demonstrated mild bilateral elbow synovitis with mild loss of range of motion bilaterally. He was transitioned to PEGylated FVIII at 50 IU/kg every other day. He had no further elbow bleeds over the subsequent 6 months and prior clinical manifestations of mild synovitis also resolved.
This boy was already on an aggressive prophylaxis regimen. Doubling his dose of rFVIII likely would have raised his trough level to ∼4 IU/dL. The family was not interested in switching to a daily dosing regimen nor was he willing to withdraw from his sport participation. Following the switch to an EHL FVIII, his 48-hour trough level was 15 IU/dL. This case highlights an important observation from long-term outcomes of PWH across the spectrum of severity. Den Uijl et al showed that patients with severe hemophilia (<1%) experience more joint bleeding than do those with moderate (1%-5%) and mild disease (>5%) with a particularly steep reduction in ABR to ∼1 to 2 for PWH with baseline levels approaching 3% to 5% and essentially no observed joint bleeding with baseline levels >12% to 15%.16 In addition, the prophylactic regimens in the pivotal trials for several of the EHL factors have shown their significant impact on joint bleeding and this correlates with substantially higher trough levels. In the phase 3 trial for N8-GP, on a prophylaxis regimen of 50 IU/kg every 4 days, the mean plasma half-life was 18.4 hours with a mean trough level of 8% and subjects maintained an ABR of 1.3 (median).17 The phase 3 trial for N9-GP examined prophylaxis regimens of 10 IU/kg and 40 IU/kg weekly.18 Subjects had a mean half-life of 110 hours with mean FIX trough levels of 9.8% (95% confidence interval, 8.0%-11.9%) and 21.3% (95% confidence interval, 18.9%-24.1%), respectively. Notably, on the 40 IU/kg per week regimen, two-thirds of subjects had complete resolution of target joints. Subjects in the phase 3 trial for rFIX-FP treated with a regimen of 40 IU/kg weekly exhibited a mean half-life of 102 hours, mean trough level of 20% (interquartile range, 17%-26%), and had 100% resolution of target joints.19 In addition, there were 13 subjects in the pediatric phase 3 trial of rFVIIIFc with target joints at baseline.20 Twelve of the 13 subjects did not continue to meet the prespecified definition for target joints while on rFVIIIFc prophylaxis and 7 subjects had no target joint rebleeding while on study. Broderick et al studied the association of physical activity and factor level with risk of bleeding in children.21 They observed that bleeding incidence was lower by 2% for every 1% increase in clotting factor level. Although several other studies would seem to confirm a poor correlation between trough factor levels and risk of bleeds while on prophylaxis,6 those studies have examined predicted trough levels that are typically <5 IU/dL. The improved PK properties of the EHL factors can now allow for much higher targeted trough levels than could be practically achieved previously. With further follow-up from case 2 patient’s intervention, and resolution of his target joint manifestations, it is likely that he would be able to reduce his dosing interval toward an even more cost-effective prophylaxis regimen.
How I treat using PK tailoring with EHL factors
Case 3
A 21-year-old with severe hemophilia A transferred to our center at age 19 years after mostly on-demand therapy throughout childhood. He had started prophylaxis at 40 IU/kg every other day with rFVIII but struggled with adherence and had continued bleeding. Target joints had been bilateral ankles and elbows with progressive loss of range of motion and chronic pain consistent with established arthropathy. His 48-hour trough level was <1 IU/dL. Notably, his baseline VWF antigen (Ag) level was 52%. Increasing his dose to 50 IU/kg reduced his breakthrough bleeding. However, the patient was motivated to reduce the frequency of his infusions. He was transitioned to rFVIIIFc at 50 IU/kg 3 days per week achieving a trough FVIII level of 12 IU/dL. This was adjusted down to 30 IU/kg 3 days per week with a trough FVIII level of 8 IU/dL. At a follow-up visit on this regimen, he had no reported bleeds in the preceding 3 months and indicated that his “pain was more manageable.”
PK-tailored dosing was explored in several of the phase 3 clinical trials with EHL factors. In the phase 3 study of rFVIIIFc in adolescents and adults,22 1 of the interventions was an individualized prophylaxis arm in which the subject’s PK parameters were used to guide individual adjustments to the dosing interval (down to 3 days or up to 5 days) as well as dose (up to 65 IU/kg) to target a trough FVIII level of 1 to 3 IU/dL or higher as needed to maintain good control of breakthrough bleeding. This led to a median dosing interval of 3.5 days with 30% of the subjects on every 5 days’ dosing by the last 3 months of the trial, with subjects maintaining a median ABR of 1.6. In the phase 3 trial of rFIXFc,23 a similar prophylaxis strategy was evaluated in which subjects were initially started at 100 IU/kg every 10 days and the dose was adjusted as needed. The median dosing interval was 12.5 days with 53.8% of the patients who were in the study for at least 6 months having a dosing interval of 14 or more days during the final 3 months of the study. In the pediatric (<12 years old) phase 3 trial of rFVIIIFc,24 all patients were initially placed on a starting regimen of twice-weekly prophylaxis (day 1, 25 IU/kg; day 4, 50 IU/kg) and the dose (≤80 IU/kg) and dosing interval (≥2 days) adjusted based on the subject’s available PK data and observed bleeding patterns. The median dosing interval was 3.5 days, however, ∼90% of the subjects were on twice-weekly dosing at the end of the trial with a median average weekly rFVIIIFc dose over the course of the study of 88.11 IU/kg per week. In the phase 2/3 pivotal trial for BAX855,25 a PEGylated full-length rFVIII, patients were assigned a dose of 45 IU/kg ± 5 IU/kg twice weekly in the prophylaxis arm based on insights from the subjects’ PK parameters that that dose would ensure that the majority of subjects would maintain FVIII levels above 1% at all times. The median ABR was 1.9 for the prophylaxis study arm with 39.6% of subjects experiencing zero bleeds during the study period. These observations on PK-guided prophylaxis were also confirmed by the Advate Prophylaxis Study26 in which standard prophylaxis (20-40 IU/kg every other day) was compared with a PK-tailored prophylaxis (20-80 IU/kg every 3 days). Both regimens were intended to maintain FVIII trough levels at or above 1%. Median ABRs were comparable for the 2 prophylaxis regimens (1.0 and 2.0 for standard and PK-tailored prophylaxis, respectively). Thus, PK tailoring shows good efficacy in bleed control in these prospective studies. There are several challenges with this approach.27 First, the impact on long-term outcomes still requires using a low ABR as a surrogate for the likelihood of either preserving healthy joints with primary prophylaxis or delaying the progression of joint disease in secondary prophylaxis. Confirming this in a long-term study would be very difficult and require considerable resources. Second, it has generally been practically difficult to conduct PK evaluations in individual patients outside of clinical studies given the number of sampling points required. Clinicians may rely on trough levels, however, with trough levels typically close to 1%, this introduces analytical challenges.6 PK estimation by Bayesian analysis based on population PK models allows for improved confidence from just a few sampling points earlier in the PK curve when FVIII or FIX plasma levels are in a more suitable range.28 Knowledge of individual PK with the EHL factors is likely to be even more important given the extended intervals between dosing and the unpredictable impact of the bioengineering approach within individual patients. Population PK models with the EHL factors are not yet available for routine clinical use to help guide PK tailoring, though a database has been launched by a research group at McMaster University (the Web-based Application for the Population Pharmacokinetic Service [WAPPS]-Hemo Project; http://www.wapps-hemo.org) to develop these.
Case 3 also illustrated the role of endogenous VWF levels on an individual’s PK parameters. His relatively low VWF level is likely contributing to reduced recovery and half-life. VWF:Ag levels have shown correlation in all the EHL FVIII studies consistent with the role of VWF in protecting FVIII from premature clearance from plasma. Thus, this is another consideration in interindividual differences that may drive PK tailoring. This patient was also targeted to a higher trough level than those in the PK-tailored arms of the pivotal trials with EHL FVIII. His clinical course is, however, supported by improved bleed prevention with this strategy. PK tailoring to a higher trough level will now be explored in a phase 3, prospective, randomized, multicenter study (PROPEL, NCT02585960) in adolescents and adults to compare the outcomes of PK-guided treatment with PEGylated full-length FVIII targeting FVIII trough levels to 1% to 3% vs ∼10% (8%-12%). Such a trial would have been difficult to conduct with traditional rFVIII but can now be realized due to the improved PK parameters with EHL FVIII.
How I treat inhibitors by immune-tolerance induction with EHL factors
Case 4
A 6-year-old with severe hemophilia A had a history of developing a high-titer inhibitor within the first year of life. He began daily high-dose immune-tolerance induction (ITI) with rFVIII. However, he had no decline in his inhibitor titer over a 6-month period. He was switched to a VWF-containing plasma-derived FVIII with no improvement over an 18-month period with his inhibitor remaining 41.8 Bethesda units (BU). He was initiated on rFVIIIFc at 100 IU/kg daily. His inhibitor declined within a few weeks of initiation on rFVIIIFc reaching a negative inhibitor titer at 6 months later and normal recovery a few months later. He now maintains effective bleed prevention on a 3-times-per-week regimen of 50 IU/kg.
There have not been any inhibitors observed in the previously treated patient population within the clinical trial programs for rFVIIIFc.14 The trial in previously untreated patients has launched (NCT02234323) and will likely accumulate some experience with ITI over the coming years. However, the use of rFVIIIFc ITI for existing patients is rational based on several lines of preclinical and clinical evidence. Borel demonstrated that coupling of haptens to IgG can induce Ag-specific tolerance with evidence that the Fc portion of IgG was required.29 Zambidis and Scott demonstrated that IgG fusion molecules can modulate cellular and humoral immune responses to immunodominant epitopes and was likely attributable to their prolonged half-life, and mediated by Fc receptor–mediated uptake and presentation by nonprofessional Ag-presenting cells, such as resting B cells, and by mechanisms involving the crosslinking of IgM to FcRs.30 There is experimental evidence that IV immunoglobulin can induce regulatory T cells (Tregs).31 Lei and Scott showed that immunoglobulin engineered with immunogenic regions of FVIII can be used to induce tolerance in a hemophilia mouse model.32 Tregitopes are Treg epitope sequences in immunoglobulin that can trigger expansion of Tregs.33 The potential presence of Tregitopes, the asparagine-linked glycans, and differential fucosylation and sialylation within the Fc region of immunoglobulin may all contribute to the tolerance-inducing effect of IV immunoglobulin therapy.34,35 In addition, FcRn-mediated transfer of Fc-fused immunodominant domains of FVIII conferred Ag-specific tolerance in a hemophilic mouse model by taking advantage of the same mechanism by which immunoglobulin crosses the maternal-fetal circulation.36
There are now case reports of ITI using rFVIIIFc. Groomes et al reported on a 15-month-old African American boy with severe hemophilia and and a high titer inhibitor who was initiated on 3-times-per-week ITI with rFVIIIFc at 50 IU/kg per dose.37 His inhibitor declined from 11 BU to 0.7 BU over 10 months of ITI treatment. Malec et al reported on 3 children with severe hemophilia A and an inhibitor >5 BU.38 The historic peak titers were 16 to 422 BU and this was a salvage ITI in 1 patient. ITI was initiated with rFVIIIFc at 100 to 200 IU/kg every other day. Time to negative BU was 4 to 12 weeks, which is considerably shorter than with current rFVIII ITI regimens. They have now launched a prospective observational study of rFVIIIFc ITI pre- and post-ITI T-cell responses in children with hemophilia and inhibitors, the Hemophilia Inhibitor Response to Eloctate (HIRE) study. In addition, this same team of investigators is leading the INHIBIT study (NCT02196207), a multicenter, phase 2, single-arm, 48-week trial, to determine whether rFVIIIFc reduces inhibitor formation in previously untreated children when begun before a bleed or surgery or trauma (preemptive) and continued once weekly to prevent bleeds (prophylaxis).
The next waves of therapy for hemophilia
It is reasonable to expect that long-term outcomes will continue to improve with continued engagement by PWH with their HTCs, aggressive efforts toward early initiation of continuous lifelong prophylaxis, and integration of EHL factors into routine clinical practice. However, the EHL factors have not addressed all of the treatment gaps for hemophilia care. Liberation from the need for IV access, avoidance of inhibitors, and achievement of steady-state hemostatic correction have still not been realized. The Survey of Inhibitors in Plasma Product-Exposed Toddlers (SIPPET) study reported a higher incidence of inhibitors in previously untreated patients with the rFVIII class of products compared with the VWF-containing plasma-derived FVIII product class.39 This study primarily included first- and second-generation rFVIII products. It is reasonable to explore whether innovations such as PEGylation, fusion proteins, enhanced VWF interaction, and human cell line–derived rFVIII may lead to a reduction in inhibitors among previously untreated patients, although they are unlikely to eliminate this risk. Another wave of therapeutic agents,40 alternative nonreplacement therapies, are likely to bring us closer to eliminating these remaining gaps. These include bispecific antibodies with FVIII mimetic properties, inhibitors of the “brakes” of coagulation such as antitissue factor pathway inhibitors, and antithrombin knockdown via small interfering RNA technology. These will be discussed within this year’s educational program in the context of novel therapeutics for hemophilia complicated by inhibitors, however, the clinical trial programs have also included noninhibitor patients. These have shown to be efficacious in phase 1/2 studies when administered subcutaneously, with relatively low intensity due to their prolonged half-life. With no exposure to FVIII or FIX, there would be no context for alloinhibitor development with these agents. Recent successes with gene therapy for both FVIII and FIX have increased enthusiasm for this approach41,42 to not simply convert severe disease to moderate or mild but perhaps true, durable, curative plasma FVIII and FIX levels. We are likely on the cusp of these next 2 paradigm shifts9 in hemophilia care within the next 2 to 3 years. We can now look forward to additional “How I treat…” sessions with nonreplacement therapies and gene therapy at future educational symposia.
References
- 1.Ljung R, Gretenkort Andersson N. The current status of prophylactic replacement therapy in children and adults with haemophilia. Br J Haematol. 2015;169(6):777-786. [DOI] [PubMed] [Google Scholar]
- 2.Tagliaferri A, Di Perna C, Rivolta GF. Secondary prophylaxis in adolescent and adult haemophiliacs. Blood Transfus. 2008;6(suppl 2):s17-s20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazepa MA, Monahan PE, Baker JR, Riske BK, Soucie JM; US Hemophilia Treatment Center Network. Men with severe hemophilia in the United States: birth cohort analysis of a large national database. Blood. 2016;127(24):3073-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125(13):2038-2044. [DOI] [PubMed] [Google Scholar]
- 5.Collins PW, Blanchette VS, Fischer K, et al. ; rAHF-PFM Study Group. Break-through bleeding in relation to predicted factor VIII levels in patients receiving prophylactic treatment for severe hemophilia A. J Thromb Haemost. 2009;7(3):413-420. [DOI] [PubMed] [Google Scholar]
- 6.Fischer K, Berntorp E. Targeting factor replacement therapy in severe hemophilia: which level is important? Semin Thromb Hemost. 2015;41(8):860-863. [DOI] [PubMed] [Google Scholar]
- 7.Aledort L, Ljung R, Mann K, Pipe S. Factor VIII therapy for hemophilia A: current and future issues. Expert Rev Hematol. 2014;7(3):373-385. [DOI] [PubMed] [Google Scholar]
- 8.Valentino LA, Negrier C, Kohla G, et al. The first recombinant FVIII produced in human cells--an update on its clinical development programme. Haemophilia. 2014;20(suppl 1):1-9. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R, Dunn A, Carcao M. Changing paradigm of hemophilia management: extended half-life factor concentrates and gene therapy. Semin Thromb Hemost. 2016;42(1):18-29. [DOI] [PubMed] [Google Scholar]
- 10.Berntorp E, Andersson NG. Prophylaxis for hemophilia in the era of extended half-life factor VIII/factor IX products. Semin Thromb Hemost. 2016;42(5):518-525. [DOI] [PubMed] [Google Scholar]
- 11.Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388(10040):187-197. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman RJ, Powell JS. Molecular approaches for improved clotting factors for hemophilia. Blood. 2013;122(22):3568-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carcao M, Srivastava A. Factor VIII/factor IX prophylaxis for severe hemophilia. Semin Hematol. 2016;53(1):3-9. [DOI] [PubMed] [Google Scholar]
- 14.Nolan B, Mahlangu J, Perry D, et al. Long-term safety and efficacy of recombinant factor VIII Fc fusion protein (rFVIIIFc) in subjects with haemophilia A. Haemophilia. 2016;22(1):72-80. [DOI] [PubMed] [Google Scholar]
- 15.Buckley B, Livingston T, Krishnan S, Odom T. Real-world dosing of factor VIII in hemophilia A patients. J Manag Care Spec Pharm. 2014;20(10-a):S21. [Google Scholar]
- 16.Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17(6):849-853. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenforth S. N8-GP–a new long-acting factor VIII for the treatment of patients with haemophilia A. Haemophilia. 2014;20(suppl 3):188. [Google Scholar]
- 18.Young G, Collins PW, Colberg T, et al. Nonacog beta pegol (N9-GP) in haemophilia B: a multinational phase III safety and efficacy extension trial (paradigm™4). Thromb Res. 2016;141:69-76. [DOI] [PubMed] [Google Scholar]
- 19.Santagostino E, Martinowitz U, Lissitchkov T, et al. ; PROLONG-9FP Investigators Study Group. Long-acting recombinant coagulation factor IX albumin fusion protein (rIX-FP) in hemophilia B: results of a phase 3 trial. Blood. 2016;127(14):1761-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nolan B, Young G, Rangarajan S, et al. Analysis of bleeding rates in pediatric subjects with target joints and severe hemophilia A receiving prophylaxis with recombinant factor VIII Fc fusion protein (rFVIIIFC) in Kids A-Long. J Thromb Haemost. 2015;13(S2):347.25557828 [Google Scholar]
- 21.Broderick CR, Herbert RD, Latimer J, et al. Association between physical activity and risk of bleeding in children with hemophilia. JAMA. 2012;308(14):1452-1459. [DOI] [PubMed] [Google Scholar]
- 22.Mahlangu J, Powell JS, Ragni MV, et al. ; A-LONG Investigators. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014;123(3):317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell JS, Pasi KJ, Ragni MV, et al. ; B-LONG Investigators. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323. [DOI] [PubMed] [Google Scholar]
- 24.Young G, Mahlangu J, Kulkarni R, et al. Recombinant factor VIII Fc fusion protein for the prevention and treatment of bleeding in children with severe hemophilia A. J Thromb Haemost. 2015;13(6):967-977. [DOI] [PubMed] [Google Scholar]
- 25.Konkle BA, Stasyshyn O, Chowdary P, et al. Pegylated, full-length, recombinant factor VIII for prophylactic and on-demand treatment of severe hemophilia A. Blood. 2015;126(9):1078-1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valentino LA, Mamonov V, Hellmann A, et al. ; Prophylaxis Study Group. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10(3):359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins PW, Fischer K, Morfini M, Blanchette VS, Bjorkman S. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17(1):2-10. [DOI] [PubMed] [Google Scholar]
- 28.Björkman S. Limited blood sampling for pharmacokinetic dose tailoring of FVIII in the prophylactic treatment of haemophilia A. Haemophilia. 2010;16(4):597-605. [DOI] [PubMed] [Google Scholar]
- 29.Borel Y. Haptens bound to self IgG induce immunologic tolerance, while when coupled to syngeneic spleen cells they induce immune suppression. Immunol Rev. 1980;50:71-104. [DOI] [PubMed] [Google Scholar]
- 30.Zambidis ET, Scott DW. Epitope-specific tolerance induction with an engineered immunoglobulin. Proc Natl Acad Sci USA. 1996;93(10):5019-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ephrem A, Chamat S, Miquel C, et al. Expansion of CD4+CD25+ regulatory T cells by intravenous immunoglobulin: a critical factor in controlling experimental autoimmune encephalomyelitis. Blood. 2008;111(2):715-722. [DOI] [PubMed] [Google Scholar]
- 32.Lei TC, Scott DW. Induction of tolerance to factor VIII inhibitors by gene therapy with immunodominant A2 and C2 domains presented by B cells as Ig fusion proteins. Blood. 2005;105(12):4865-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Groot AS. Do Tregitopes have the potential to impact the current treatment landscape of autoimmune diseases? Expert Rev Clin Immunol. 2013;9(12):1155-1157. [DOI] [PubMed] [Google Scholar]
- 34.Anthony RM, Wermeling F, Ravetch JV. Novel roles for the IgG Fc glycan. Ann N Y Acad Sci. 2012;1253:170-180. [DOI] [PubMed] [Google Scholar]
- 35.Quast I, Lünemann JD. Fc glycan-modulated immunoglobulin G effector functions. J Clin Immunol. 2014;34(suppl 1):S51-S55. [DOI] [PubMed] [Google Scholar]
- 36.Gupta N, Culina S, Meslier Y, et al. Regulation of immune responses to protein therapeutics by transplacental induction of T cell tolerance. Sci Transl Med. 2015;7(275):275ra21. [DOI] [PubMed] [Google Scholar]
- 37.Groomes CL, Gianferante DM, Crouch GD, Parekh DS, Scott DW, Lieuw K. Reduction of factor VIII inhibitor titers during immune tolerance induction with recombinant factor VIII-Fc fusion protein. Pediatr Blood Cancer. 2016;63(5):922-924. [DOI] [PubMed] [Google Scholar]
- 38.Malec LM, Ragni MV, Journeycake JM, Alabek M. Immune tolerance induction using rFVIIIFc (Eloctate) [abstract]. Blood. 2015;126(23). Abstract 3531. [Google Scholar]
- 39.Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374(21):2054-2064. [DOI] [PubMed] [Google Scholar]
- 40.Monahan PE. Emerging genetic and pharmacologic therapies for controlling hemostasis: beyond recombinant clotting factors. Hematology Am Soc Hematol Educ Program. 2015;2015:33-40. [DOI] [PubMed] [Google Scholar]
- 41.Giangrande P. The future of hemophilia treatment: longer-acting factor concentrates versus gene therapy. Semin Thromb Hemost. 2016;42(5):513-517. [DOI] [PubMed] [Google Scholar]
- 42.Ward P, Walsh CE. Current and future prospects for hemophilia gene therapy. Expert Rev Hematol. 2016;9(7):649-659. [DOI] [PubMed] [Google Scholar]
- 43.Impact Education, LLC, National Hemophilia Foundation, Postgraduate Institute for Medicine. Factor VIII and IX therapeutic agents recently approved or in late phase clinical development. Available at: www.managedcarehemo.com. Accessed 20 September, 2016. [Google Scholar]