Abstract
Thrombosis and bleeding are among the most common causes of morbidity and mortality in patients with renal disease or liver disease. The pathophysiology underlying the increased risk for venous thromboembolism and bleeding in these 2 populations is distinct, as are considerations for anticoagulation. Anticoagulation in patients with kidney or liver disease increases the risk of bleeding; this risk is correlated with the degree of impairment of anticoagulant elimination by the kidneys and/or liver. Despite being in the same pharmacologic category, anticoagulant agents may have varied degrees of renal and liver metabolism. Therefore, specific anticoagulants may require dose reductions or be contraindicated in renal impairment and liver disease, whereas other drugs in the same class may not be subject to such restrictions. To minimize the risk of bleeding, while ensuring an adequate therapeutic effect, both appropriate anticoagulant drug choices and dose reductions are necessary. Renal and hepatic function may fluctuate, further complicating anticoagulation in these high-risk patient groups.
Learning Objectives
Identify the risk for thromboembolism and bleeding in patients with kidney disease or liver disease
Describe the pharmacological differences between anticoagulant agents
Discuss thrombotic complications and treatment strategies for patients with renal or liver disease
Bleeding and thrombosis in patients with renal or liver disease
Renal disease
Patients with chronic kidney disease are at increased risk for both arterial and venous thromboembolism (VTE), as well as bleeding. Incremental decreases in renal function increase both VTE and bleeding risk, with the highest risk for fatal pulmonary embolism (PE) and bleeding events in those with an estimated glomerular filtration rate (eGFR) of less than 30 mL/min.1-4 The age- and sex-standardized mortality rate ratio of PE has been reported to be 12 or more times higher in patients receiving dialysis compared with the general population.4,5-7
The pathophysiology of increased thrombosis risk in this population is thought to be secondary to the chronic activation of the coagulation cascade, as evidenced by elevated levels of thrombin–antithrombin complexes and decreased levels of endogenous anticoagulants such as protein C and protein S and antithrombin.8 Many patients with renal disease also have elevated homocysteine values, which likely contribute to the risk for both arterial and venous thrombosis. The risk for thrombosis in patients with end-stage renal disease (ESRD) is further increased as a result of coagulation and platelet activation occurring within the extracorporeal hemodialysis device.9 Bleeding risk is increased as a result of an acquired defect of primary hemostasis caused by platelet dysfunction and altered platelet–vessel wall interactions; in patients receiving dialysis, widespread heparin use and the need for frequent large vessel access further increases this risk, as does highly prevalent use of medications (such as aspirin or warfarin) that are associated with bleeding4,10 The risk of bleeding is increased up to 2-fold if the eGFR falls below 30 mL/min (odds ratio [OR], 2.14; 95% confidence interval [CI], 1.44-3.2).8,9,11-13 The annual risk for intracerebral hemorrhage in patients with ESRD has been reported to range from 6.2 to 10.2 per 1000 individuals, which is well above the population frequency.8,14,15 The incidence rates of major hemorrhage in patients with ESRD range from 8.0 to 10.8 per 100 patient years in those also receiving warfarin.16 In a large, retrospective study with 1028 person-years of follow-up, the annual risk of major bleeding in patients with ESRD was 0.8%, 3.1%, 4.4%, and 6.3% while taking neither warfarin nor aspirin, taking warfarin alone, taking aspirin alone, or taking the combination of warfarin and aspirin, respectively.17 Although major bleeds such as retroperitoneal hemorrhage are rare, being quoted as occurring at rate of 8/10 000 patients per annum, the morbidity and mortality associated with these events are high.18
Minor bleeds are also more common and are also a source of morbidity for patients with ESRD and a cost to the healthcare system. Patients with minor bleeding may require longer time spent at the dialysis unit to manage access bleeding, rescheduling of patient transportation, and added nursing time. It is calculated that the average weekly loss in patients on a thrice-weekly dialysis schedule is 110 mL blood, with a monthly loss of 438 mL; this contributes significantly to anemia in this at-risk population.19
In addition to their increased risk of bleeding, patients with kidney disease are at increased risk for all forms of thrombosis. Despite this, anticoagulant prophylaxis is only used in 44.3% of patients who have chronic kidney disease or are maintained on dialysis in the acute hospital care setting.20 Patients with more severe forms of kidney disease (eGFR of less than 30 mL/min) are also less likely to be discharged home on anticoagulant therapy when indicated.21 VTE and the need for therapeutic anticoagulation in patients with creatinine clearance of less than 30 mL/min are risk factors for subsequent major bleeding and fatal bleeding events, and many of the anticoagulants that are used in patients with normal renal function are contraindicated in patients with renal failure, further complicating treatment.22
Liver disease
VTE and bleeding events are increased in patients with liver disease compared with the general population. Cirrhosis and other forms of severe liver injury are associated with baseline increases in international normalized ratio (INR) and activated partial thromboplastin time (aPTT) as a result of reductions in coagulation factor levels. Antithrombin levels have been reported to be 10% to 23% lower in patients with cirrhosis compared with the general population.23 Despite the general acceptance that patients with liver disease are at higher risk of bleeding events, there is a misconception that thrombosis may not be of particular concern in these patients, as they are “auto-anticoagulated” because of reductions in procoagulant factors. Recently, it has been recognized that there is also concomitant increased risk for VTE, probably because of an acquired imbalance in anticoagulant factors.24,25 Impaired clearance of factor VIII, decreased levels of protein C, impaired fibrinolysis, and other liver disease–associated changes may contribute to hypercoagulability in some patients with liver diseases.23,26,27 Data from meta-analyses suggest that the annual incidence of VTE in patients with liver disease ranged from 0.3% to 6.3%, and the prevalence of VTE varied from 0.6% to 4.7%.24 The thrombotic risk is particularly elevated in patients with cirrhosis with higher Child-Pugh scores and those with severe liver injury. Independently, complications of encephalopathy, edema, ascites, immobility, liver cancer, and venous stasis of the portal vein further contribute to the increased risk for thrombosis.23,28,29
Thrombotic complications: special considerations in patients with renal or liver disease
Renal disease
Nephrotic syndrome.
Underlying renal disease secondary to various forms of primary and secondary nephrotic syndromes, systemic lupus erythematosus with or without antiphospholipid syndrome, and pauci-immune antineutrophil cytoplasmic antibody vasculitis, such as granulomatosis with polyangiitis (Wegner's), have all been associated with increased thrombotic risk. Nephrotic syndrome (defined by a urinary protein level exceeding 3.5 g/l.73 m2/d) results in thromboembolic complications resulting from increased urinary loss of antithrombotic factors and increased production of prothrombotic factors by the liver.30 Serum albumin concentrations of less than 2.5 g/dL have been associated with VTE events in up to 40% of patients with membranous nephropathy who are nephrotic, whereas VTE was reported in only ∼3% of their nonnephrotic counterparts.31
Renal vein thrombosis
Renal vein thrombosis (RVT) is also seen in patients with nephrotic syndrome. The incidence of unilateral or bilateral RVT in patients with nephrotic range proteinuria ranges between 25% and 30%.32 The underlying cause of nephrotic syndrome is also predictive for the incidence of RVT with membranous glomerulonephritis, membranoproliferative glomerulonephritis, and minimal change disease having published rates of 37%, 26%, and 24%, respectively.32,33
Hemodialysis access–related thrombosis
As renal disease progresses to end-stage, patients are at particularly high risk for upper extremity thrombosis involving the axillary, subclavian, and brachial veins secondary to central venous catheters. In general, upper extremity DVT rarely causes symptomatic or fatal pulmonary embolism. However, the clot burden in a thrombosed arteriovenous shunt is much higher than would be found in a native vein in the same anatomical location; case reports (which our anecdotal experience support) of symptomatic and fatal pulmonary embolism are found in renal patients with acute arteriovenous shunt thrombosis. The finding of a large thrombosis in or distal to an arteriovenous shunt mandates aggressive anticoagulation, in our opinion. Mechanical declotting or use of fibrinolytic drugs are widely used and may be complicated by symptomatic pulmonary embolism.30 Because patients with hemodialysis access–related thrombosis have, by definition, severely limited renal function, such patients are most often treated with in-hospital intravenous unfractionated heparin (UFH) with aPTT monitoring transition to warfarin administered with a target INR of 2.0 to 3.0. The duration of therapy is unknown; for a single event, 3 months of therapy is widely used; however, in patients with recurrent events, longer-term treatment is frequently employed. Low-intensity warfarin (with a target INR of less than 2.0) has been shown to be ineffective or unsafe for primary prevention of vascular access catheter and graft thrombosis.14,34
Liver disease
Portal vein thrombosis.
The observation of partial or complete venous obstruction within the portal circulation, oftentimes “asymptomatic,” is an increasingly observed clinical conundrum. As imaging, particularly ultrasound, improves in quality, it is more and more common to receive reports of unexpected portal venous thrombosis, frequently occurring in patients with known hepatic disease. The first stage in the evaluation of these reports is to review the findings with the radiologist because slow, absent, or retrograde flow are commonly seen in patients with liver disease, and may be mistaken for signs of thrombosis by inexperienced technologists or radiologists. If the diagnosis is questionable, an alternate imaging technique should be considered because the implications of anticoagulation in these patients are significant. Findings such as cavernous transformation and large collaterals suggest chronic disease, in which case anticoagulation is probably not indicated. If thrombosis is confirmed, and the patient has compatible clinical symptoms (such as otherwise unexplained abdominal pain), then anticoagulation is probably warranted to prevent clot extension, which may result in venous ischemia of the bowel. Although thrombolytic therapy has been considered in such patients, it is used rarely, and only in patients in whom the benefits of this therapy are likely to outweigh the risks. The etiology of portal vein thrombosis in patients with liver disease is unclear, but is probably a result of an enhanced propensity to clot (as previously discussed) and slow flow or retrograde venous flow resulting from increased trans-hepatic venous pressure. Most patients have coincident risks for bleeding (such as varices), which should be assessed at the time of anticoagulation initialization and treated as needed; anticoagulation should be stopped as soon as felt to be safe, and rarely beyond 3 months from the initial presentation.
Budd–Chiari
Complete occlusion of the hepatic veins is a rare thrombotic complication seen in patients with potent procoagulant states, and appears to be more common with selected thrombophilic states (such as paroxysmal nocturnal hemoglobinuria). It can cause severe hepatic injury and, as these patients tend to be younger, can lead to a need for hepatic transplantation. Because patients generally have good underlying hepatic function and lack long-standing changes of portal hypertension, many patients response to traditional anticoagulant strategies. Because most patients are likely to be hospitalized at the time of diagnosis, initial therapy with unfractionated, or low-molecular-weight, heparin is warranted with a transition to warfarin with a target INR of 2.0 to 3.0. At present, given a lack of information, use of the direct oral anticoagulants in this setting cannot be recommended. The optimal duration of anticoagulation is unknown, but given the clinical seriousness of recurrent thrombosis, it should be probably be extended beyond 3 months, except in patients with a secondary cause of thrombosis in whom the risk factor has been mitigated.
Pharmacologic properties of anticoagulants to consider in patients with renal or liver disease
Anticoagulants are cleared, with only a few exceptions, by the liver and/or kidneys. There can be significant differences in the degree of liver or renal clearance of anticoagulant agents, even if they belong to 1 class of anticoagulant medications. For example, UFH is cleared predominately through the reticuloendothelial system, whereas low-molecular-weight heparins (LMWHs), particularly those with low molecular masses, are cleared (at least in part) through the kidneys.
Anticoagulant treatment options in renal or liver disease
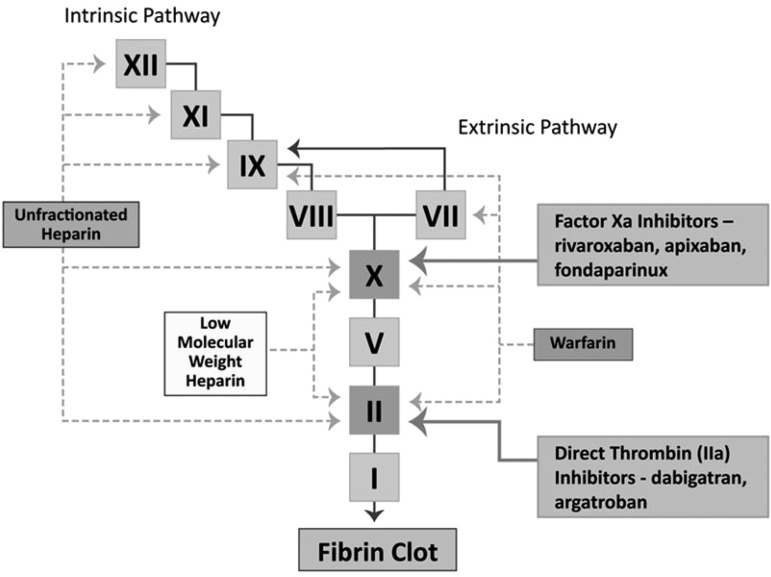
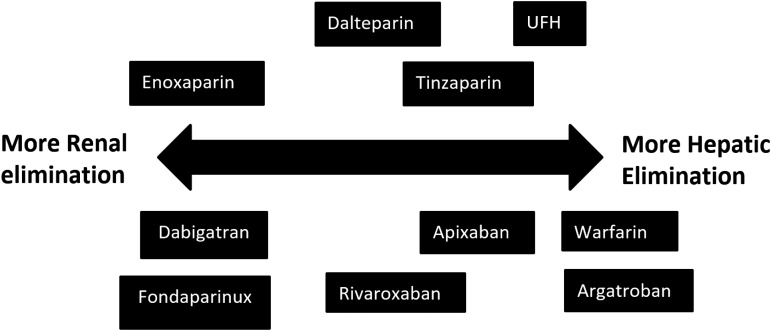
Table 1 provides a broad overview of the most widely used anticoagulants across the spectrum of renal function. Figure 1 provides an overview of the sites of action of the commonly used anticoagulants. Figure 2 represents the degree of renal and hepatic dependency of several of the anticoagulants discussed in this chapter.
Table 1.
Evidence for use of anticoagulant class according to renal function
| eGFR (mL/min) | UFH | LMWHs | Warfarin | Direct oral anticoagulants |
|---|---|---|---|---|
| >90 | Yes | Yes | Yes | Yes |
| 60-89 | Yes | Yes | Yes | Yes |
| 30-59 | Yes | Yes | Yes | Rivaroxaban dose adjustment |
| 15-29 | Yes | Dose adjustments may be needed; bioaccumulation possible | Yes | Rivaroxaban and dabigatran contraindicated |
| Enoxaparin use with caution | Apixaban use with caution | |||
| <15 | Yes | Use contraindicated outside selected patients with appropriate monitoring | Yes | Rivaroxaban and dabigatran contraindicated; see text for discussion of apixaban |
Yes indicates there is evidence for use without dose adjustment.
Figure 1.
Commonly used anticoagulants and their site of action.
Figure 2.
Renal and hepatic dependency of drugs discussed.
UFH and LMWHs
Renal disease
UFH exerts its anticoagulant effect by inhibiting factor Xa and thrombin equally, with an onset of action of 3 to 5 minutes and a half-life between 0.5 and 2 hours.12,35,36 UFH clearance is dependent on hepatic and vascular endothelial heparin enzymes, and there is also nonspecific binding to the endothelium and plasma proteins.35 UFH is a highly charged molecule, so it can also bind to circulating and surface-bound plasma proteins, as well as plastic tubing and dialysis membrane surfaces, making its half-life unpredictable.35 To maintain circuit patency, the short and unpredictable half-life of UFH mandates a bolus of UFH at the start of hemodialysis, with additional doses to maintain the anticoagulant effect. Additional disadvantages of UFH include the need for monitoring if used at therapeutic doses, variable pharmacodynamics, potential for heparin-induced thrombocytopenia or osteoporosis with repeated exposure, lack of prefilled syringes (posing a safety risk), and need for an infusion pump. Cost (pharmacy, nursing, and more recently, for drug acquisition) has also become an issue, as the price of UFH has increased dramatically during the last decade in many jurisdictions.
There are several forms of LMWH that are produced via cleavage of UFH (Table 2). LMWHs preferentially inhibit factor Xa with less thrombin inhibition, providing a biologic rationale for reduced bleeding risk compared with UFH.35,36 Of the available LMWHs, tinzaparin has the shortest half-life (5 hours) and the greatest degree of extrarenal metabolism.35,36 Although anti-Xa heparin levels can be used to monitor the anticoagulant effect of LMWH, such levels are used infrequently in clinical practice. These levels do, however, provide information regarding bioaccumulation, which remains a concern in patients with renal disease.37 There are concerns regarding potential bioaccumulation of LMWH in patients with ESRD,38 and drug costs generally continue to exceed those of UFH.21 However, recent evidence has shown that selected LMWHs can be safely used, at a prophylactic or therapeutic dose, in patients with impaired renal function.39-41
Table 2.
Comparison of unfractionated and low-molecular-weight heparins
| Heparin | Tinzaparin | Dalteparin | Enoxaparin | |
|---|---|---|---|---|
| Prophylaxis VTE | 5000 units subcutaneously twice to three times daily | 3500-4500 units subcutaneously once daily | 5000 units subcutaneously once daily | 30 mg subcutaneously every 12 h |
| Treatment VTE | Intravenously, based on aPTT (2.5-3.5x baseline 60-120 s) | 175 units/kg subcutaneously once daily | 200 units/kg subcutaneously once daily | 1.5 mg/kg subcutaneously once daily or 1 mg/kg twice daily |
| Average molecular weight (daltons) | 15 000 | 6500 | 6000 | 4500 |
| Anti Xa: IIa ratio | 1:1 | 1.9:1 | 2.2:1 | 2.7-4/1:1 |
| Monitoring | aPTT | Anti-Xa heparin level | Anti-Xa heparin level | Anti-Xa heparin level |
| Peak onset | SC: 2-4 h intravenously: immediate | 4-6 h | 4 h | 3 h |
| Plasma half-life | 1.5 h (1-2 h) | 1.4 h | 2-2.3 h | 3.5-4.2 hr |
| Antidote | Protamine | Protamine (88% neutralized) | Protamine (74% neutralized) | Protamine (54% neutralized) |
| Elimination | Hepatic/renal | Hepatic/renal | Renal | Renal |
| Adjust dose for kidney failure | No | No | Yes (CrCl < 10-30 mL/min) | Yes |
Liver disease
Unlike the case with renal dysfunction, there is no single widely accepted test of hepatic function. Thus, it is difficult or impossible to predict in an individual patient how their hepatic dysfunction will affect anticoagulation. In general, as noted, drugs that required monitoring using indirect tests, such as the INR or aPTT, are avoided in patients with hepatic dysfunction severe enough to cause a baseline coagulopathy. More specific tests such as the anti-Xa heparin level may be used; however, such tests have not been validated in these patients. Commercially available whole-blood viscoelastic tests such as thromboelastography (TEG) have been studied in patients with acute, chronic liver disease, as well as in liver transplantation. TEG testing provides information on the kinetics of clot formation and clot quality; however, to our knowledge, these tests have not been evaluated to monitor anticoagulants in this setting, but they have been used to guide transfusion strategies with hemostatic agents in patients with severe bleeding.42
When choosing particular anticoagulant agents in patients with liver disease, LMWHs offer the advantage of fixed, weight-adjusted dosing without monitoring, but have the disadvantage of requiring subcutaneous administration, potential for exacerbating bleeding, and (generally) higher costs. Although standard dosages of LMWHs can be used in patients with cirrhosis, renal function is often overestimated in patients with decompensated cirrhosis, allowing the potential for bioaccumulation. Close clinical monitoring of patients with cirrhosis is recommended; Xa heparin level monitoring may be considered in patients receiving longer-term treatment.
Oral anticoagulants
Vitamin K antagonist
Warfarin.
Warfarin has been the most widely prescribed anticoagulant for the last 60 years.30 Warfarin is primarily eliminated via the liver, with dose adjustments according to the INR.
Renal disease.
Warfarin metabolism is influenced by coincident drugs, foods, and other factors. Thus, its pharmacodynamics profile is unpredictable at the best of times, and particularly unreliable in patients with kidney or renal disease. Despite this, longstanding experience has led to warfarin being the long-term anticoagulant of choice in patients with renal disease. Recent evidence of dysfunctional calcium handling, perhaps contributing to accelerated vascular disease, is contributing to a reanalysis of the risks and benefits of warfarin in patients with renal disease.30 Particularly for chronic indications (such as atrial fibrillation), the net benefit of warfarin in such patients is unclear when the risk reduction in stroke and systemic embolization is weighed against other complications such as bleeding and the potential for accelerated vascular disease that might be precipitated by warfarin. At present, warfarin continues to be used widely in patients with ESRD at risk for stroke and systemic embolization because of atrial fibrillation; however, this treatment practice may change on the basis of new evidence or better understanding of the risk/benefit ratio of anticoagulation.
Liver disease.
Warfarin use in patients with severe hepatic disease may be complicated if baseline coagulation testing is abnormal, as it makes measurement of the anticoagulation effect difficult or impossible. In patients with normal baseline coagulation testing, warfarin use can be considered, taking into account the coincident increase in risk of bleeding in such patients while acknowledging that even with normal INR and aPTT results, there may be significant imbalances in the levels of pro- and anticoagulant factors. An INR of 2.0 to 3.0 should be targeted for most patients with normal baseline coagulation testing. In patients with abnormal coagulation testing, an alternate anticoagulant should be strongly considered, as even “functional assays” such as prothrombin levels may be influenced by hepatic synthetic impairment.
Direct oral anticoagulants
The direct oral anticoagulants (DOACs) have been used in stroke prevention in patients with atrial fibrillation, as well as in prophylaxis and treatment of VTE in the general population. Unlike vitamin K antagonists, they are typically administered at fixed doses and do not require routine monitoring and subsequent dose adjustment. The DOACs are composed of dabigatran (direct thrombin inhibitor), rivaroxaban, apixaban, and edoxaban (factor Xa inhibitors). Argatroban is a parenteral direct thrombin inhibitor, and fondaparinux is a factor Xa inhibitor that is administered subcutaneously.
A large fraction of patients with indications for chronic oral anticoagulants do not receive them as a result of concerns about bleeding and lack of reversal agents. Drugs such as idarucizumab or andexanet, which can rapidly reverse anticoagulant effect in the setting of emergencies such as major (life-threatening) bleeding or urgent surgery, may provide reassurance to patients and clinicians about the net clinical benefit of anticoagulant threrapy.43
Renal disease
The metabolism and elimination of DOACs decreases with worsening renal function. Patients with impaired renal function are at particularly high risk for deterioration of renal function via acute kidney injury. In these circumstances, drug bioaccumulation may lead to an increased risk of bleeding. Further experience in using DOACs in patients with moderate to severe renal impairment and in those at risk for acute injury are required. The degree of renal elimination also varies with DOACs. For example, renal elimination of dabigatran is approximately 80%, and thus dabigatran should be avoided in patients with moderate to severe renal impairment. Although the US product monograph for apixaban does not prohibit its use in patients across the spectrum of renal function pending further study, apixaban should be used with caution in patients with severe renal insufficiency. If its use is chosen, its dose should be guided by the product monograph.44
Liver disease
The DOACs have not been studied in patients with hepatic dysfunction. Their effect may be altered by changes in serum proteins, resulting in an altered free fraction compared with patients with normal hepatic function; further, their half-life may be increased as a result of reduced clearance, particularly for those products with higher hepatic clearance. If a DOAC is strongly desired, dabigatran etexilate may be preferred over rivaroxaban and apixaban because of its reduced protein binding and relative renal clearance. DOACs offer the advantages of LMWH (similar pharmacokinetic profile, absent need for monitoring) at reduced cost and without the need for injection; however, they have not been studied in these patients, and should be used with extreme caution.
Direct thrombin inhibitors
Dabigatran
Dabigatran is a prodrug that is converted to an active form after absorption from the gastrointestinal tract. Dabigatran directly and reversibly inhibits thrombin. Although unfractionated heparin primarily binds to free thrombin, dabigatran binds fibrin and clot-bound thrombin in addition to free thrombin.45 The inhibition of thrombin interferes with the pleotropic effects of thrombin, including preventing conversion of fibrinogen to fibrin and limiting thrombin-mediated platelet aggregation.
Dosing should be based on the appropriate product monograph recommendations; in general, however, dabigatran should be used with caution in patients with creatinine clearances of less than 50 mL/min.
Liver disease.
No dosage adjustments in the setting of liver disease are indicated in the Canadian product monograph, and there was no change in exposure seen in a study of patients with moderate impairment (Child Pugh class B). Use is not recommended in patients with severe impairment (Child-Pugh class C), acute liver disease, or increased liver enzymes 2 or more times the upper limit of normal.
Argatroban
Renal disease.
Argatroban is a parenteral direct thrombin inhibitor. It is cleared independent of renal function and can be used across the range of renal function. aPTT monitoring is generally used with a target aPTT in the range used in most institutions for unfractionated heparin. Argatroban has a short half-life, and thus its effect is quickly lost when the infusion is stopped. The dose required to achieve therapeutic anticoagulation is usually between 1 and 2 μg/kg/min. Once the desired aPTT is achieved, dose variation is small. Most patients can be monitored every 24 hours. Idarucizumab will not reverse the effect of argatroban.
Liver disease
Argatroban is exquisitely sensitive to hepatic function. It should not be used in patients with significant liver dysfunction because of its persistent and nonreversible effect. In patients with evidence of mild hepatic dysfunction, the drug can be started at lower than normal infusion rates, with frequency monitoring and with attention to bleeding complications. Inadvertent administration to patients with significant liver dysfunction can result in long-lasting anticoagulation.
Factor Xa inhibitors
Fondaparinux
Renal disease.
Fondaparinux is a parenteral antithrombin dependent inhibitor of factor Xa. Fondaparinux is very dependent on renal function for clearance; it should not be used in patients with creatinine clearances (CrCls) of less than 30mL/min and should be used in others with abnormal renal function with care. As it is not neutralizable, bleeding complications in patients with significant renal function abnormalities are likely to be more severe and prolonged. Monitoring is possible, using the anti-Xa level calibrated against standard curves calibrated for fondaparinux. Andexanet, the reversal agent for the direct Xa inhibitors that is currently under development, reverses the effect of fondaparinux.
Liver disease.
Fondaparinux is dependent on antithrombin for its activity. Severe reductions in synthesis or loss resulting from nephrotic syndrome might, therefore, reduce the activity of fondaparinux and heparins; however, such an effect is unlikely to be clinically important. Fondaparinux has no hepatic clearance, and thus it could be used in the setting of hepatic dysfunction; however, its lack of neutralizability makes it a less attractive option in many circumstances, as many patients with significant hepatic dysfunction also have a high risk of bleeding.
Rivaroxaban
Renal disease.
Rivaroxaban has variable pharmacokinetic clearance directly related to the degree of renal impairment. The risk of bleeding increases as the renal function decreases as a result of increasing rivaroxaban plasma concentrations. Caution should be exercised in using rivaroxaban in patients with moderate renal impairment (CrCl, 30-49 mL/min), with consideration of a dose reduction to 15 mg daily if used. Patients with severe renal impairment (CrCl < 30 mL/min) were excluded from clinical trials of rivaroxaban. Therefore, rivaroxaban should not be used in patients with severe renal impairment (CrCl < 30 mL/min).
Liver disease.
Safety and efficacy data for rivaroxaban are lacking in patients with significant hepatic disease (eg, acute clinical hepatitis, chronic active hepatitis, liver cirrhosis), as these patients were excluded from clinical trials. Rivaroxaban is contraindicated in patients with hepatic disease (including Child-Pugh class B and C), with evidence of coagulopathy and clinically relevant bleeding risk. The limited data available for patients with mild hepatic impairment (Child-Pugh class A) without coagulopathy indicate there is no difference in pharmacodynamic response or pharmacokinetics compared with healthy subjects.46
Apixaban
Renal disease.
Apixaban is mainly dependent on hepatic metabolism for clearance. In clinical studies, patients with CrCl 50 to 80 mL/min had fewer major bleeding episodes with apixaban than comparator drugs, including aspirin, warfarin, and enoxaparin.47 In patients with CrCl < 50 mL/min, no statistical difference in bleeding risk was observed, although in the ARISTOTLE atrial fibrillation trial, there were numerically fewer bleeds with apixaban than with warfarin, with the effect being most prominent in patients with creatinine clearances of 30 mL/min.48 However, this is not necessarily evidence of safety of apixaban in patients with CrCl of 30 to 50 mL/min. Patients with lower GFR are at increased risk for acute kidney injury, which can lead to increased plasma concentrations of apixaban, and potentially increased bleeding risk. In our opinion, further study is needed in patients with CrCl lower than 30 mL/min, as there are limited clinical data to support safety in this population. However, the product monograph in the United States does not prohibit use in patients with CrCl of lower than 30 mL/min, and there is increasing use of apixaban in such patients. In all patients, if apixaban is chosen, long-term dosing should be based on package insert recommendations. The widespread use of the 2.5-mg dose, rather than the 5-mg dose, is probably inappropriate for many patients.
Liver disease.
Use of apixaban is contraindicated in patients with significant hepatic disease, given its dependence on hepatic clearance. It should be used with caution in patients with less severely impaired hepatic function.
Edoxaban
Renal disease.
Edoxaban is a direct factor Xa inhibitor that is approved for use in the United States.
There is a warning for edoxaban, regarding reduced efficacy in patients with nonvalvular atrial fibrillation with CrCl higher than 95 mL/min with evidence of increased ischemic stroke risk. There is limited experience with use of edoxaban in patients with impaired renal function. Pending additional studies, it should be used with caution in patients with CrCl of less than 50 mL/min.
Liver disease.
Edoxaban is not recommended for use in patients with moderate or severe hepatic impairment (Child-Pugh class B or C disease). There are limited data in mild liver dysfunction, which suggests dose adjustment is not required in patients with Child-Pugh class A liver disease.
Conclusion
Patients with significant renal or hepatic disease present many challenges to the hematologist. They have an increased risk for both “typical” and “atypical” thrombosis, and treating thrombosis can be complex because of a high risk of bleeding and the effect of these diseases on the pharmacokinetics and pharmacodynamics of anticoagulant drugs. Despite the existing classification of anticoagulant agents according to their mechanism of action, individual agents within a group still differ in safety and efficacy in patients with varying degrees of renal or liver dysfunction. Not only should individual drug characteristics be considered in the choice of anticoagulant, but the degree of renal and liver metabolism must also be weighed. Further study of all anticoagulant agents is needed in patients with varying degrees of kidney or liver disease to better appreciate the pharmacologic properties in these unique and challenging populations. The morbidity and mortality associated with thrombosis and the risk for significant hemorrhage when anticoagulants are used at therapeutic dosages supports the use of thromboprophylaxis to reduce numbers of clots, and thus reduce need for therapeutic anticoagulation. During the next 5 years, we envision an expansion of indications for the newer oral anticoagulant drugs; however, use in patients with renal or live disease will remain limited, pending completion of rigorous studies designed to establish both their safety and efficacy.
References
- 1.Monreal M, Falgá C, Valle R, et al. ; RIETE Investigators. Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med. 2006;119(12):1073-1079. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho AC. Bleeding in a uremia--a clinical challenge. N Engl J Med. 1983;308(1):38-39. [DOI] [PubMed] [Google Scholar]
- 3.Castaldi PA, Rozenberg MC, Stewart JH. The bleeding disorder of uraemia. A qualitative platelet defect. Lancet. 1966;2(7454):66-69. [DOI] [PubMed] [Google Scholar]
- 4.Eknoyan G, Wacksman SJ, Glueck HI, Will JJ. Platelet function in renal failure. N Engl J Med. 1969;280(13):677-681. [DOI] [PubMed] [Google Scholar]
- 5.Ocak G, van Stralen KJ, Rosendaal FR, et al. . Mortality due to pulmonary embolism, myocardial infarction, and stroke among incident dialysis patients. J Thromb Haemost. 2012;10(12):2484-2493. [DOI] [PubMed] [Google Scholar]
- 6.Tveit DP, Hypolite IO, Hshieh P, et al. . Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. 2002;39(5):1011-1017. [DOI] [PubMed] [Google Scholar]
- 7.Wattanakit K, Cushman M, Stehman-Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19(1):135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo DS, Rabbat CG, Clase CM. Thromboembolism and anticoagulant management in hemodialysis patients: a practical guide to clinical management. Thromb Res. 2006;118(3):385-395. [DOI] [PubMed] [Google Scholar]
- 9.Ambühl PM, Wüthrich RP, Korte W, Schmid L, Krapf R. Plasma hypercoagulability in haemodialysis patients: impact of dialysis and anticoagulation. Nephrol Dial Transplant. 1997;12(11):2355-2364. [DOI] [PubMed] [Google Scholar]
- 10.Janssen MJ, van der Meulen J. The bleeding risk in chronic haemodialysis: preventive strategies in high-risk patients. Neth J Med. 1996;48(5):198-207. [DOI] [PubMed] [Google Scholar]
- 11.Decousus H, Tapson VF, Bergmann JF, et al. ; IMPROVE Investigators. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. [DOI] [PubMed] [Google Scholar]
- 12.Davenport A. Low-molecular-weight heparin for routine hemodialysis. Hemodial Int. 2008;12(suppl 2):S34-S37. [DOI] [PubMed] [Google Scholar]
- 13.Suranyi M, Chow JS. Review: anticoagulation for haemodialysis. Nephrology (Carlton). 2010;15(4):386-392. [DOI] [PubMed] [Google Scholar]
- 14.Crowther MA, Clase CM, Margetts PJ, et al. . Low-intensity warfarin is ineffective for the prevention of PTFE graft failure in patients on hemodialysis: a randomized controlled trial. J Am Soc Nephrol. 2002;13(9):2331-2337. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai H, Uezono S, Kawano H, Ikeda N, Kodama K, Akiyama H. Factors affecting outcome of intracerebral hemorrhage in patients undergoing chronic hemodialysis. Ren Fail. 2010;32(8):923-927. [DOI] [PubMed] [Google Scholar]
- 16.Phelan PJ, O’Kelly P, Holian J, et al. . Warfarin use in hemodialysis patients: what is the risk? Clin Nephrol. 2011;75(3):204-211. [DOI] [PubMed] [Google Scholar]
- 17.Holden RM, Harman GJ, Wang M, Holland D, Day AG. Major bleeding in hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(1):105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malek-Marín T, Arenas D, Gil T, et al. . Spontaneous retroperitoneal hemorrhage in dialysis: a presentation of 5 cases and review of the literature. Clin Nephrol. 2010;74(3):229-244. [DOI] [PubMed] [Google Scholar]
- 19.Awobusuyi JO, Mapayi FA, Adedolapo A. Blood loss during vascular access cannulation: quantification using the weighed gauze and drape method. Hemodial Int. 2008;12(1):90-93. [DOI] [PubMed] [Google Scholar]
- 20.Cohen AT, Tapson VF, Bergmann JF, et al. ; ENDORSE Investigators. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet. 2008;371(9610):387-394. [DOI] [PubMed] [Google Scholar]
- 21.Pettigrew M, Soltys GI, Bell RZ, et al. . Tinzaparin reduces health care resource use for anticoagulation in hemodialysis. Hemodial Int. 2011;15(2):273-279. [DOI] [PubMed] [Google Scholar]
- 22.Falgá C, Capdevila JA, Soler S, et al. ; RIETE Investigators. Clinical outcome of patients with venous thromboembolism and renal insufficiency. Findings from the RIETE registry. Thromb Haemost. 2007;98(4):771-776. [PubMed] [Google Scholar]
- 23.Rodriguez-Castro KI, Simioni P, Burra P, Senzolo M. Anticoagulation for the treatment of thrombotic complications in patients with cirrhosis. Liver Int. 2012;32(10):1465-1476. [DOI] [PubMed] [Google Scholar]
- 24.Qi X, Ren W, Guo X, Fan D. Epidemiology of venous thromboembolism in patients with liver diseases: a systematic review and meta-analysis. Intern Emerg Med. 2015;10(2):205-217. [DOI] [PubMed] [Google Scholar]
- 25.Smith CB, Hurdle AC, Kemp LO, Sands C, Twilla JD. Evaluation of venous thromboembolism prophylaxis in patients with chronic liver disease. J Hosp Med. 2013;8(10):569-573. [DOI] [PubMed] [Google Scholar]
- 26.Tripodi A, Anstee QM, Sogaard KK, Primignani M, Valla DC. Hypercoagulability in cirrhosis: causes and consequences. J Thromb Haemost. 2011;9(9):1713-1723. [DOI] [PubMed] [Google Scholar]
- 27.Tripodi A, Primignani M, Lemma L, Chantarangkul V, Mannucci PM. Evidence that low protein C contributes to the procoagulant imbalance in cirrhosis. J Hepatol. 2013;59(2):265-270. [DOI] [PubMed] [Google Scholar]
- 28.Fontana RJ. Prophylactic anticoagulation in cirrhotics: a paradox for prime time? Gastroenterology. 2012;143(5):1138-1141. [DOI] [PubMed] [Google Scholar]
- 29.Lisman T, Kamphuisen PW, Northup PG, Porte RJ. Established and new-generation antithrombotic drugs in patients with cirrhosis - possibilities and caveats. J Hepatol. 2013;59(2):358-366. [DOI] [PubMed] [Google Scholar]
- 30.Ansell J, et al. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(suppl 6):160s-198s. [DOI] [PubMed] [Google Scholar]
- 31.Bellomo R, Atkins RC. Membranous nephropathy and thromboembolism: is prophylactic anticoagulation warranted? Nephron. 1993;63(3):249-254. [DOI] [PubMed] [Google Scholar]
- 32.Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18(8):2221-2225. [DOI] [PubMed] [Google Scholar]
- 33.Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118(3):397-407. [DOI] [PubMed] [Google Scholar]
- 34.Wilkieson TJ, Ingram AJ, Crowther MA, et al. . Low-intensity adjusted-dose warfarin for the prevention of hemodialysis catheter failure: a randomized, controlled trial. Clin J Am Soc Nephrol. 2011;6(5):1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davenport A. What are the anticoagulation options for intermittent hemodialysis? Nat Rev Nephrol. 2011;7(9):499-508. [DOI] [PubMed] [Google Scholar]
- 36.Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006;144(9):673-684. [DOI] [PubMed] [Google Scholar]
- 37.Ribic C, Lim W, Cook D, Crowther M. Low-molecular-weight heparin thromboprophylaxis in medical-surgical critically ill patients: a systematic review. J Crit Care. 2009;24(2):197-205. [DOI] [PubMed] [Google Scholar]
- 38.Rabbat CG, Thorpe KE, Russell JD, Churchill DN. Comparison of mortality risk for dialysis patients and cadaveric first renal transplant recipients in Ontario, Canada. J Am Soc Nephrol. 2000;11(5):917-922. [DOI] [PubMed] [Google Scholar]
- 39.PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group; Cook D, Meade M, Guyatt G, et al. . Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305-1314. [DOI] [PubMed] [Google Scholar]
- 40.Douketis J, Cook D, Meade M, et al. ; Canadian Critical Care Trials Group. Prophylaxis against deep vein thrombosis in critically ill patients with severe renal insufficiency with the low-molecular-weight heparin dalteparin: an assessment of safety and pharmacodynamics: the DIRECT study. Arch Intern Med. 2008;168(16):1805-1812. [DOI] [PubMed] [Google Scholar]
- 41.Park D, Southern W, Calvo M, et al. . Treatment with Dalteparin is Associated with a Lower Risk of Bleeding Compared to Treatment with Unfractionated Heparin in Patients with Renal Insufficiency. J Gen Intern Med. 2016;31(2):182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mallett SV. Clinical Utility of Viscoelastic Tests of Coagulation (TEG/ROTEM) in Patients with Liver Disease and during Liver Transplantation. Semin Thromb Hemost. 2015;41(5):527-537. [DOI] [PubMed] [Google Scholar]
- 43.Siegal DM, Curnutte JT, Connolly SJ, et al. . Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity. N Engl J Med. 2015;373(25):2413-2424. [DOI] [PubMed] [Google Scholar]
- 44.Apixaban full prescribing information. Princeton, NJ: Pfizer; 2016. [accessed 2016 Oct 1]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/202155s012lbl.pdf.
- 45.Harenberg J, Hentschel VA, Du S, et al. . Anticoagulation in patients with impaired renal function and with haemodialysis. Anticoagulant effects, efficacy, safety, therapeutic options. Hamostaseologie. 2015;35(1):77-83. [DOI] [PubMed] [Google Scholar]
- 46.Intagliata NM, Maitland H, Caldwell SH. Direct Oral Anticoagulants in Cirrhosis. Curr Treat Options Gastroenterol. 2016;14(2):247-256. [DOI] [PubMed] [Google Scholar]
- 47.Pathak R, Pandit A, Karmacharya P, et al. . Meta-analysis on risk of bleeding with apixaban in patients with renal impairment. Am J Cardiol. 2015;115(3):323-327. [DOI] [PubMed] [Google Scholar]
- 48.Hohnloser SH, Hijazi Z, Thomas L, et al. . Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33(22):2821-2830. [DOI] [PubMed] [Google Scholar]