Abstract
Therapy-related myeloid neoplasms (t-MN) combine t-MDS and therapy related acute myeloid leukemia (t-AML) patients in one entity because of their similar pathogenesis, rapid progression from t-MDS to t-AML, and their equally poor prognosis. Treatment with epipodophyllotoxins like etoposide has been associated with a short interval between treatment and development of t-AML, with fusion oncogenes like KMT2A/MLL-MLLT3 and a better prognosis. In contrast, treatment with alkylating agents has been associated with a longer latency, an initial MDS phase, adverse cytogenetics, and a poor prognosis. The pathogenesis of t-MN can be explained by direct induction of an oncogene through chromosomal translocations, induction of genetic instability, or selection of a preexisting treatment-resistant hematopoietic stem cell clone. Recent evidence has highlighted the importance of the last mechanism and explains the high frequency of TP53 mutations in patients with t-MN. After previous cytotoxic therapy, patients present with specific vulnerabilities, especially evident from the high nonrelapse mortality in patients with t-MN after allogeneic hematopoietic cell transplantation. Here, the prognostic impact of currently known risk factors and the therapeutic options in different patient subgroups will be discussed.
Learning Objectives
Therapy-related myeloid neoplasms (t-MN) are a subgroup of the WHO 2016 classification of AML comprising t-MDS and t-AML
Seven percent of adult patients with AML have t-AML
Fifteen percent of patients with t-AML present with favorable risk fusion genes (CBF, PML-RARA), 50% have adverse cytogenetics, and the most frequent molecular aberration in t-AML and t-MDS affects TP53 (33%)
Patients are treated according their genetic risk profile, and minimal residual disease assessment helps to guide allogeneic transplantation for patients with favorable risk
Epidemiology of therapy-related myeloid neoplasms
Classification
Therapy-related myeloid neoplasms (t-MN) are a subgroup of acute myeloid leukemia (AML) in the revised 2016 World Health Organization classification comprising myelodysplastic syndrome (t-MDS) and acute myeloid leukemia (t-AML) patients who were exposed to cytotoxic or radiation therapy for an unrelated malignancy or autoimmune disease (eg, multiple sclerosis or rheumatologic disease).1 t-MDS and t-AML are combined in the group of t-MN because no major differences in outcome between these 2 categories were noted.2 The revised 2016 classification recommends that the associated cytogenetic abnormality should be identified in the final diagnosis, and the family history should be considered, especially regarding cancer susceptibility.1 t-MN should be distinguished from AML with myelodysplasia-related changes (often called secondary AML), which is diagnosed if 50% or more of the bone marrow cells are dysplastic in at least 2 lineages, if the patient had a previous diagnosis of MDS or MDS/MPN, or if myelodysplasia-associated cytogenetic aberrations are present.1
Population-based incidence rates and prevalence
Large series of population-based AML registries consistently report a t-AML frequency of ∼7%.3-5 In a Swedish population-based study, the median age of patients with t-AML was comparable with de novo AML patients (both 70 years).5 Women predominate in t-AML, because the most frequent primary malignancy is breast cancer, followed by non-Hodgkin lymphoma.3 Based on the Surveillance, Epidemiology and End Results (SEER) database, the risk for t-AML after chemotherapy treatment of the first primary malignancy is 4.7-fold higher than the risk for AML in the general population.6 SEER data show that 10 years after the start of chemotherapy, the excess absolute risk of developing AML is 2.15 cases in 1000 women with breast cancer and 5.8 cases in 1000 patients with Hodgkin lymphoma compared with the general population.6 The cumulative risk of developing t-AML after 6 years was 0.9% in patients of the German Hodgkin Study Group.7 The t-AML risk has increased during the last 3 decades for non-Hodgkin lymphoma, declined for ovarian cancer and multiple myeloma, and remained constant for breast cancer and Hodgkin lymphoma,6 possibly reflecting changes in the use of cytotoxic regimens for these diseases over time.
A large cooperative study investigated epidemiology and outcome of 1837 t-MDS patients.8 Median age was 68 years and cytogenetic risk according to the revised International Prognostic Scoring System (IPSS-R) was 2% very good, 36% good, 17% intermediate, 15% poor, and 31% very poor. IPSS-R was very low in 8%, low in 20%, intermediate in 17%, high in 23%, and very high in 32%. The most frequent primary diseases were non-Hodgkin lymphoma (28%), breast cancer (16%), multiple myeloma (6%), and prostate cancer (6%). The median time for progression from t-MDS to overt AML is 4 to 7 months.9
Distinct mechanisms may lead to therapy-related myeloid neoplasms
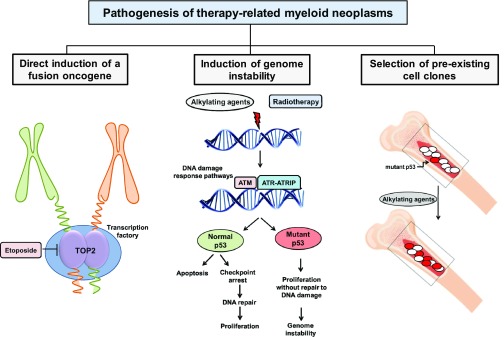
Several classes of cytotoxic agents like topoisomerase II (TOP2) inhibitors, alkylating agents, antimetabolites, and antitubulin agents in addition to radiotherapy have been associated with t-AML. AML cases that develop after treatment with TOP2 inhibitors, such as etoposide or the group of anthracyclines, have a short latency of 2 to 3 years after initial treatment, are associated with KMT2A/MLL gene rearrangements, and usually are not preceded by an MDS phase. In contrast, t-AMLs that develop after treatment with alkylating agents or radiotherapy have a longer latency of 5 to 10 years, typically have unbalanced aberrations of chromosomes 5 and 7 and/or a complex karyotype, and are often preceded by MDS.10 Different models have been proposed that may lead to t-MN: (1) direct induction of an oncogene, (2) induction of genetic instability allowing multiple and possibly complex aberrations to occur, and (3) selection of a preexisting clone that is treatment resistant and permissive to genetic instability (Figure 1). Inherited cancer susceptibility may be a fourth mechanism that explains why patients develop more than one type of malignancy.
Figure 1.
Mechanisms of t-MN pathogenesis.
Direct induction of a fusion oncogene
In this model, the oncogene is induced in a susceptible target cell during cytotoxic therapy leading to clonal outgrowth of the transformed cell. This mechanism is mediated by TOP2 inhibitors and leads to fusion oncogenes upon chromosomal translocation. TOP2 induces a double-strand break during DNA replication and can link 2 DNA strands together after replication. However, TOP2 inhibitors stabilize the double-strand break and delay the ligation of the free DNA ends. The free DNA end can thus more easily recombine with DNA from another chromosome. Why specific gene fusions like KMT2A/MLL-MLLT3, RUNX1-RUNX1T1, or CBFB-MYH11 are so frequently found in AML, and specifically t-AML, is a major question in this field. The breakpoint hotspot of the KMT2A gene coincides with a peak of DNase I hypersensitivity and CCCTC-binding factor and thus is a highly accessible chromatin site that is preferentially targeted by TOP2.10 It is further suggested that frequently translocated chromosomes are colocated in so-called “transcription factories,” where the transcription complex is organized in a specific nuclear compartment. Here, different chromosomes enter the vicinity and may be fused to each other.10 Finally, only very potent oncogenic fusion genes will provide a selective advantage and thus lead to outgrowth of leukemia. KMT2A fusion genes are well known as one of the most powerful oncogenes in leukemogenesis.11 Recent experimental evidence confirmed that de novo fusion of KMT2A and MLLT3 in human cells is sufficient to transform hematopoietic stem cells.12
Induction of genetic instability
Another mechanism could be that cytotoxic therapy induces aberrations, which lead to genetic instability and later to the acquisition of leukemogenic aberrations (Figure 1). This might explain the long latency of t-MNs and high frequency of complex cytogenetic aberrations found in patients with previous alkylating chemotherapy or radiotherapy, but there are little experimental data to support this hypothesis. In support of increased genotoxicity of previous chemotherapy, Itzhar and colleagues evaluated copy number alterations (CNA) by array comparative genomic hybridization and found on average 3.46 CNAs in t-AML compared with 1.9 CNAs in de novo AML.13 However, Wong and colleagues found a similar number of single nucleotide variants, indels, and transversions upon genome-wide sequencing in t-AML compared with de novo AML.14 An alternative mechanism has therefore been proposed, which suggests selection of preexisting transformed and treatment-resistant hematopoietic clones during chemotherapy.
Selection of preexisting hematopoietic cell clones
Strong evidence for this third mechanism of clonal selection in the pathogenesis of t-MN has been provided by Wong and colleagues.14 They found a significantly higher frequency of mutations in TP53 and ABC transporters in t-AML and t-MDS compared with de novo AML, as also reported in other patient series.15,16 Most importantly, the specific TP53 mutation was also found in hematopoietic cells 3 to 6 years before the onset of t-MN at low frequency (0.003%-0.7%) in 2 patients even before the application of chemotherapy. Moreover, in 9 of 19 older cancer-free individuals (68-89 years old), TP53 mutations were found in peripheral blood with a low variant allele frequency of 0.01% to 0.37%. In a mouse model of both wild-type and heterozygous Tp53+/− hematopoietic stem/progenitor cells, the Tp53+/− cells preferentially expanded after exposure to chemotherapy.14
Clonal hematopoiesis of indeterminate potential as a risk factor for t-MN.
This observation has been corroborated by recent data showing an age-dependent increase of clonal hematopoiesis in healthy individuals that is most likely driven by leukemia-associated mutations in DNMT3A, TET2, ASXL1, JAK2, PPM1D, TP53, SF3B1, BCORL1, and others.17-19 Clonal hematopoiesis without cell dysplasia and blast increase (ie, not fulfilling the criteria for MDS or AML) has been termed clonal hematopoiesis of indeterminate potential (CHIP).20 Individuals with CHIP have a 13-fold increased risk of developing a hematologic malignancy, and the data by Wong et al suggest that this risk is increased in the context of cytotoxic therapy, at least if a TP53 mutation is present.14 Interestingly, somatic mutations in PPM1D have been found in CHIP and also in peripheral blood of patients with breast, ovarian, and lung cancer (in ∼1% of patients).21-23 PPM1D is a serine/threonine phosphatase that negatively regulates p53.24 Truncating PPM1D mutations are considered gain-of-function mutations that suppress p53 activity, impair the p53-dependent G1 checkpoint, and thus may lead to chemotherapy resistance and clonal outgrowth under chemotherapy.25 In patients with ovarian cancer, PPM1D mutations were not found before treatment, but were present after treatment in 0.37% of cases compared with a frequency of 0.03% in controls.26 In another study of ovarian cancer, the frequency of somatic mosaic PPM1D mutations in peripheral blood mononuclear cells was significantly associated with prior chemotherapy, and the variant allele frequency increased in 85% of the patients during chemotherapy.22 In the same study, TP53 mutations developed in peripheral blood cells from 2 of 15 patients of whom sequential samples during chemotherapy were available.
In summary, recent evidence suggests that cells harboring somatically acquired preexisting mutations in the p53 pathway accumulate under the selective pressure of chemotherapy and give rise to clonal hematopoiesis that may evolve into MDS or AML after additional genetic events (Figure 1). The incidence of CHIP under chemotherapy and the rate of transformation should be studied in the future.
Inherited cancer susceptibility
A few years ago, the German AML Study Group (AMLSG) published data on the latency from diagnosis of the primary malignancy to t-AML.3 Seven percent of a cohort of 2835 patients with AML developed t-AML after chemotherapy and/or radiotherapy for the primary malignancy, with a median latency of 4.04 years. However, another 3% developed AML after a diagnosis of an independent malignancy that had never been treated with chemotherapy or radiotherapy. Compared with t-AML patients, these patients more often had prostate cancer (23% vs 9%), bladder cancer (9% vs 1%), and renal cell carcinoma (9% vs 2%), but less often had breast cancer (10% vs 52%).3 AML developed in these patients with no history of chemotherapy or radiotherapy, with a median latency of 5 years, which is similar to that of patients with t-AML. Thus, a number of t-AMLs may not be induced or selected by the previous treatment but may actually be caused by an inherited cancer susceptibility. Germline variants in the DNA damage response pathway have been associated with an increased risk of t-MN (genes like BRCA1, BRCA2, BARD1, TP53,27,28 RAD51, and HLX1,29 Fanconi genes,30 and the anti-apoptotic gene BCL2L10),31 supporting an effect of cancer susceptibility to AML risk in some patients. Although the patient numbers are small in the aforementioned studies, the available data suggest that genetic cancer susceptibility contributes to the risk of myeloid neoplasms, especially under cytotoxic therapy.
Molecular genetics of t-MN
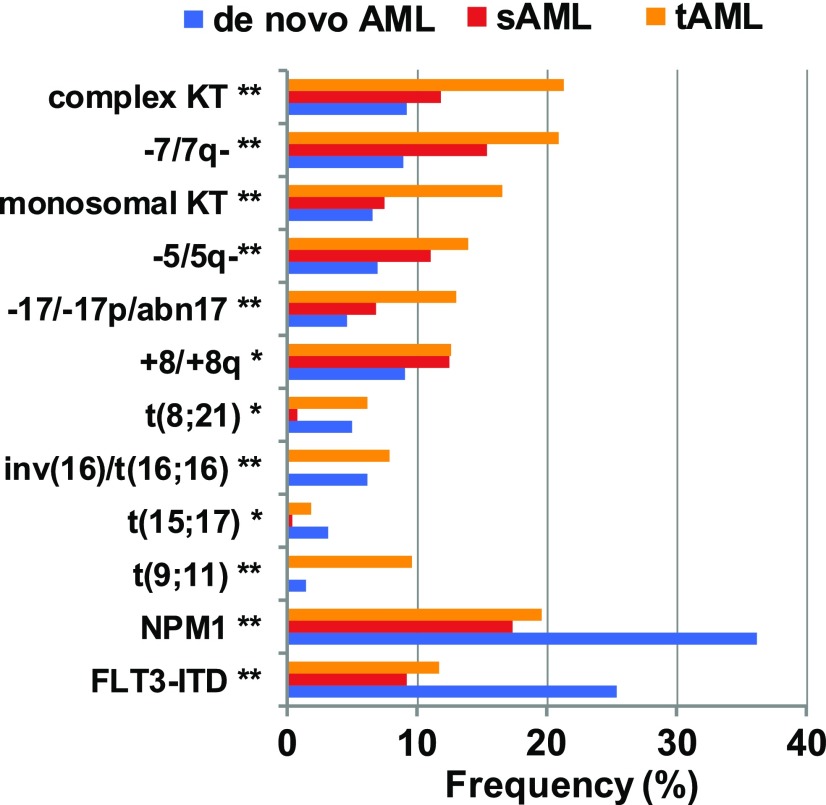
In our updated series of the German AML study group of 230 patients with t-AML, 280 patients with secondary AML (sAML) after MDS and 3144 patients with de novo AML who were all treated with intensive chemotherapy, the most frequent cytogenetic abnormalities were complex karyotype, monosomy 5 and 7, and abnormalities of chromosome 17 (Figure 2), which are all associated with an unfavorable prognosis. These aberrations were most frequent in t-AML, less frequent in sAML, and least frequent in de novo AML. Comparison of the relative mutation frequencies in t-AML, sAML, and de novo AML showed that the fusion genes RUNX1-RUNXT1, CBFB-MYH11, and PML-RARA, which are all associated with a favorable prognosis, had a similar frequency in t-AML and de novo AML, but were less frequent in sAML. The frequency of AML-defining mutations in NPM1 and FLT3, which are associated with a favorable and unfavorable prognosis, respectively, were highest in de novo AML and similarly reduced in t-AML and sAML. The KMT2A-MLLT3 fusion was more frequent in t-AML than in de novo or sAML (Figure 2).
Figure 2.
Frequency of cytogenetic aberrations in t-AML, secondary, and de novo AML (AMLSG registry data).
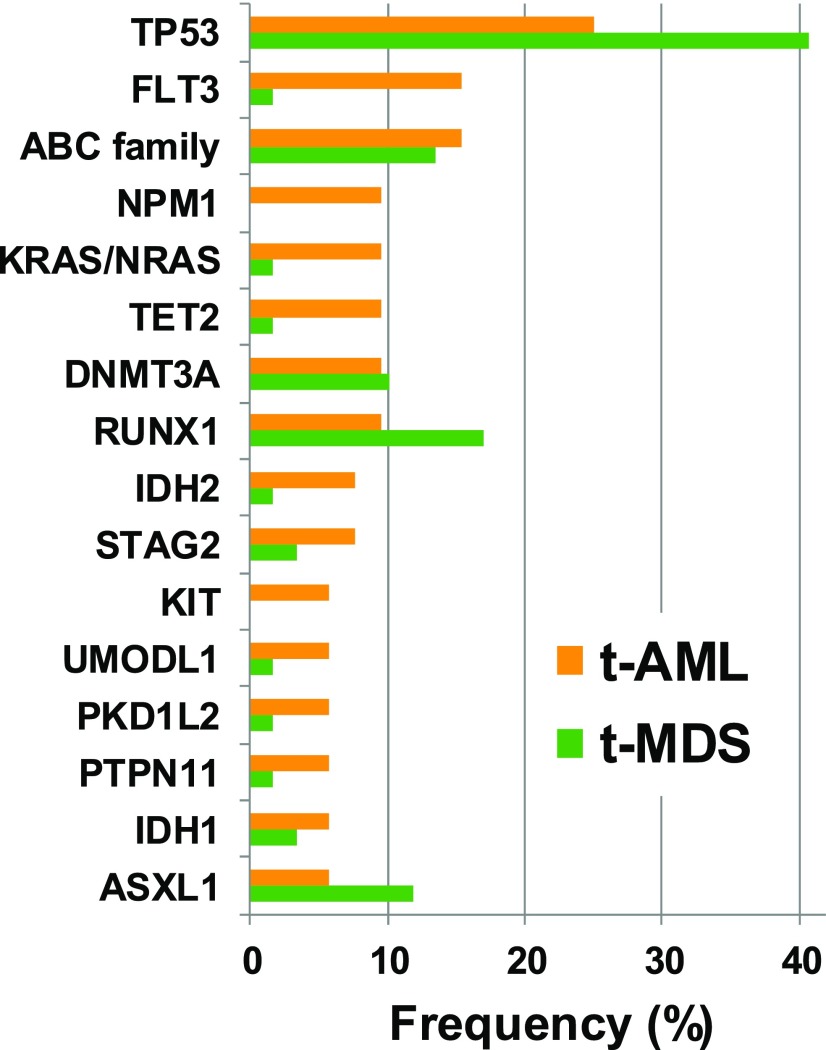
Whole-genome and targeted-sequencing studies showed that TP53 is the most frequently mutated gene in patients with t-MN.14-16 Approximately one third of t-AML and t-MDS patients present with a TP53 mutation (Figure 3).14 Other genes with a mutation frequency of >5% in t-AML include NPM1, FLT3-ITD, ABC transporter genes, NRAS, KRAS and PTPN11, TET2, DNMT3A, IDH1 and IDH2, STAG2, RUNX1, and ASXL1. In t-MDS, RUNX1 and ASXL1 are more often mutated compared with t-AML; ABC transporter genes and DNMT3A have a similar mutation frequency in t-MDS and t-AML, and the other genes are less frequently mutated in patients with t-MDS than in those with t-AML.14 Targeted sequencing with an extended gene panel did not identify any molecular aberration in 9% of the patients (n = 10). However, 8 of these 10 patients had at least 1 cytogenetic aberration, leaving only a small proportion without a detectable genetic abnormality.14 In summary, fusion genes with favorable prognosis are found in ∼15% of patients with t-AML, and cytogenetic aberrations with unfavorable prognosis including t(9;11) are detected in ∼50% of patients with t-AML.3 Christiansen et al found a high frequency of mutations in the RAS/RAF pathway in patients with t-AML (41%), but a rather low frequency in patients with t-MDS (8%).32 Interestingly, 3 of 5 patients with t(9;11) and the KMT2A-MLLT3 fusion had the BRAF V600E mutation.32
Figure 3.
Frequency of molecular aberrations in t-AML (n = 52) and t-MDS (n = 59) patients based on data by Wong et al.14 Samples from 22 patients were sequenced by whole-genome sequencing and samples from 89 patients were sequenced with a gene panel covering 149 genes.
Prognosis and treatment of t-MN
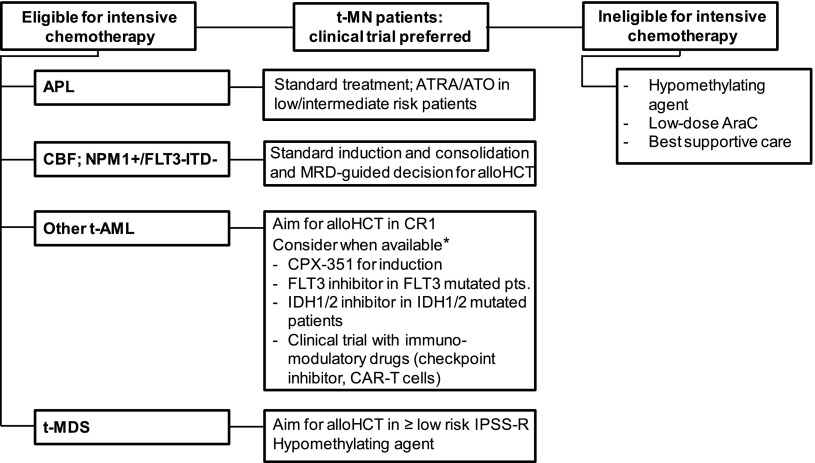
The prognosis of therapy-related acute promyelocytic leukemia (t-APL) and core binding factor (CBF) leukemia (t(8;21) and inv(16) or t(16;16)) has been considered comparable with their cytogenetic de novo AML counterpart.33 t-AML with adverse cytogenetic risk has as dismal an outcome as de novo AML with adverse cytogenetics. Therefore, it was suggested to treat patients with t-AML as those with de novo AML according to their cytogenetic risk profile.34,35 Several factors may complicate the treatment of patients with t-AML, such as organ dysfunction from prior therapy, depletion of normal hematopoietic stem cells, damage to marrow stroma, chronic immunosuppression, and refractoriness to transfusion support.35 The prognosis and therapy of patients with t-AML will be discussed by genetic subgroup in the following section (Figure 4).
Figure 4.
Algorithm for the treatment of t-MN patients. *None of the mentioned treatments are currently approved for AML. alloHCT, allogeneic hematopoietic stem cell transplantation; CAR, chimeric antigen receptor; CBF, core-binding factor leukemia (ie, AML with t(8;21), inv(16), or t(16;16)); CR1, first complete remission; DA, daunorubicine and cytarabine; ITD, internal tandem duplication.
Therapy-related acute promyelocytic leukemia
Excellent response rates, low relapse rates, and exceptional overall survival (OS) were recently reported for patients with APL treated with ATRA and arsenic trioxide.36,37 In a series of 29 t-APL patients, 19 were treated with ATRA and arsenic trioxide and 10 were treated with ATRA and chemotherapy.38 The complete response (CR) rate in patients with t-APL treated with ATRA and arsenic was 89% compared with 70% in the ATRA and chemotherapy group (P = not significant). The CR rate in the ATRA and arsenic group was comparable with a cohort of 85 patients with de novo APL (CR 94%).38 Three-year OS was 65% in both groups (ATRA with arsenic and ATRA with chemotherapy groups). Therefore, ATRA and arsenic trioxide should be considered the standard of care in low- and intermediate-risk APL patients, independent of the etiology, and high-risk t-APL patients should be treated according to the local standard for high-risk de novo APL patients.
t-AML with t(8;21);RUNX1-RUNX1T1
t-AML patients with t(8;21) have a high response rate after intensive induction therapy, but these patients have a shorter overall survival (OS) compared with de novo AML with t(8;21) in 2 of 3 studies. Comparison of the outcome of 13 t-AML patients with t(8;21) to 38 de novo AML patients with t(8;21) by Gustafson et al revealed that the t-AML group achieved CR at similar frequency (91% vs 95%), but relapsed more often (70% vs 39%) and had decreased median OS (19 months vs >37 months).39 Krauth et al observed 2-year survival rates of 46.8% for 16 t-AML and 76.4% for 95 de novo AML patients with t(8;21).40 However, comparable outcomes were observed for 9 t-AML and 128 de novo AML patients with t(8;21) in the AMLSG series.3
t-AML patients with t(8;21) should be treated with standard induction and consolidation treatment, and response should be monitored by molecular minimal residual disease (MRD) assessment. If a positive MRD result indicates treatment failure, allogeneic hematopoietic cell transplantation (HCT) should be discussed with the patient. MRD studies for t-AML patients with t(8;21) have not been published. However, for de novo AML patients with t(8;21) who achieved CR, a <3-log reduction of transcript levels after consolidation 241 identified high-risk patients with OS after conventional chemotherapy of only 27%, whereas allogeneic HCT increased OS to 72% in these high-risk patients.41
In contrast, in low-risk patients with a >3-log reduction of transcript levels after the second consolidation cycle had an OS of 100% after conventional chemotherapy, and allogeneic HCT resulted in an OS of 76%.41 Because the risk of nonrelapse mortality after allogeneic HCT is high in t-AML, and a negative MRD result indicates disease control by chemotherapy, allogeneic HCT should be withheld in t-AML patients with negative MRD for RUNX1-RUNX1T1.
t-AML with inv16/t(16;16); CBFB/MYH11
t-AML with inv(16) or t(16;16) had a high response rate but poor survival in 2 independent studies. Borthakur et al assessed the outcome of 17 t-AML patients with CBF leukemia (13 with inv(16), 4 with t(8;21)) and 171 de novo CBF AML patients.42 Although the CR rate was 92% in the total cohort and the relapse rate was comparable in t-AML patients (33%) and de novo AML patients (36%), median OS was only 1.9 years in t-AML patients but longer than 5 years in de novo AML patients. In the study by Kayser et al, comparison of 15 t-AML patients with inv(16) or t(16;16) with 142 de novo AML patients with inv(16) or t(16;16) revealed t-AML as a significant adverse prognostic factor with a hazard ratio of 2.35.3
As for patients with t(8;21)-positive t-AML, t-AML patients with inv(16) or t(16;16) should be treated with standard induction and consolidation therapy and monitored by MRD assessment. Allogeneic HCT should be discussed with the patient if a positive MRD result indicates treatment failure. A negative MRD result indicates disease control by chemotherapy and allogeneic HCT should be withheld. In a study with 115 CBFB/MYH11 positive de novo and secondary AML patients in complete remission, CBFB/MYH11 copy numbers >10 in peripheral blood or >50 in bone marrow (per 105 ABL copies) after the end of consolidation were associated with an estimated relapse of nearly 100% and an estimated 5-year survival of 57% (if >10 copies in peripheral blood) and 25% (if >50 copies in bone marrow).43 The efficacy of allogeneic HCT in these MRD-positive patients has not been reported yet.
t-AML with t(9;11); KMT2A-MLLT3
Adult patients with this translocation are specifically enriched in the group of t-AML patients and account for 11% of t-AML patients in the series of the AMLSG.3 A meta-analysis based on individual patient data of younger patients with 11q23 translocations evaluated the prognostic impact in 180 AML patients.44 Sixteen percent of the patients had t-AML, and 42% of these patients had t(9;11). Seventy-one percent of the patients achieved CR and the median OS was 19.6 months (4-year OS was 29%). Secondary AML (including t-AML and AML after MDS) was an independent negative risk factor for OS. In a donor/no donor analysis of 65 patients with t(9;11), of whom 51% had secondary or t-AML, patients with an available donor in first remission had improved OS (5-year OS without donor 21%, with donor 51%).45 Based on these data, allogeneic HCT in first CR should be recommended for eligible t-AML patients with t(9;11).
t-AML with NPM1 mutation
Specific data for the prognostic impact of NPM1 mutations in t-AML are currently not available. Although the frequency of NPM1 mutations is lower in t-AML than in de novo AML, it is still one of the most frequent mutations in this AML cohort. NPM1-mutated AML with normal cytogenetics and wild-type FLT3 belongs to the European LeukemiaNet (ELN) favorable risk group. Within the German-Austrian study group, we treat these patients with intensive induction and consolidation therapy without a strict upper age limit. Because the treatment response can be monitored in the majority of NPM1-mutated patients by MRD, this approach is also feasible for t-AML patients with mutant NPM1. The cutoff of >200 NPM1 copies per 104 ABL copies after completion of consolidation therapy has been established by the AMLSG as a strong risk factor for relapse.46 Ivey and colleagues suggested an alternative cutoff to discriminate patients at high risk of relapse (ie, positive NPM1 MRD in peripheral blood after 2 cycles of intensive chemotherapy).47 Allogeneic HCT should be considered in MRD-positive patients after completion of consolidation. In a retrospective analysis of the ALFA0702 trial presented at the ASH meeting in 2015, allogeneic HCT significantly improved OS of NPM1 mutated nonfavorable ELN patients with insufficient MRD reduction, but not in patients with >4-log MRD reduction.48 Patients with concomitant FLT3-ITD and/or adverse cytogenetic aberrations should be advised to undergo allogeneic HCT in first CR.
Other adult t-AML patients
Adult AML patients with ELN risk classification higher than favorable have a poor outcome when treated with chemotherapy alone. It would be desirable to identify chemotherapy-responsive patients from this large patient group and especially in t-AML patients. With this aim, Lindsley and colleagues defined a set of 8 genes (SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2) that identified secondary AML patients with >95% specificity if at least one of these genes was mutated.49 This algorithm was then applied to a cohort of 101 t-AML patients, of whom 33% harbored secondary AML-type mutations, 23% had TP53 mutations, and 47% had mutations common in de novo AML (like NPM1, FLT3, DNMT3A, etc.). t-AML patients with secondary-type mutations had similar patient characteristics as de novo AML patients with secondary-type mutations, confirming that t-AML patients represent a heterogeneous group of patients, with one third being similar to AML cases that evolved from MDS. Although CR rates were comparable between the 3 genetically defined t-AML groups, patients with secondary-type and TP53 mutations significantly more often required 2 induction courses to achieve CR (55% of patients) compared with patients with de novo AML mutations (7% of patients), suggesting relative chemotherapy resistance. Whether this translates into higher relapse rates and shorter survival remain to be shown.49 This molecular classification refines the cytogenetic classification of t-AML and should be further evaluated as a prognostic and predictive marker in t-AML patients.
Currently, all eligible t-AML patients with an ELN score higher than favorable should be considered as candidates for allogeneic HCT in CR1, if a suitable related or unrelated donor is available, and their inclusion in clinical trials should be encouraged.
Is there a better induction regimen for t-AML patients than 7+3? This question has not been specifically addressed, but induction outcomes were reported for patients with unfavorable cytogenetics that are frequently found in t-AML patients. High-dose daunorubicin as used in the E1900 trial resulted in a median OS of 10.6 months in patients with unfavorable cytogenetics, which was comparable with the median OS of 10.2 months in the standard dose arm.50 In a randomized trial of the Polish Adult Leukaemia Group, the addition of cladribine to daunorubicin and cytarabine resulted in significantly higher CR rates after 2 cycles of induction (67.5% vs 56%) and median 3-year OS of 36% vs 20% compared with daunorubicin and cytarabine in patients with unfavorable cytogenetics.51 Because cladribine is not licensed for AML and has not been tested in a large number of t-AML patients, 7+3 induction should be considered standard in t-AML patients eligible for intensive chemotherapy.
Older AML patients who are unlikely to benefit from standard induction chemotherapy
These patients constitute the majority of AML patients and are very difficult to treat. t-AML patients are on average older than de novo AML patients and are frequently not eligible for intensive treatment. These patients should be primarily treated in clinical trials. Outside of trials, hypomethylating agents, low-dose cytarabine, and best supportive care are currently available treatment options. Several studies reported the effect of hypomethylating agents in t-MDS and t-AML patients and found similar efficacy in t-MN patients compared with de novo MDS or AML patients.52-54 Overall response rates with azacitidine or decitabine were 38% to 42%, with a CR rate of 14% to 21%, similar to the 45% overall response rate in de novo AML patients.52 In the study by Bally et al, 71% of t-MN patients had a complex karyotype compared with 43% in de novo AML. OS was shorter in t-MN patients, with 14% at 2 years compared with 34% in de novo AML; however, multivariate analysis revealed that only cytogenetics and age, but not etiology, were independent predictors of survival.52
Low-dose cytarabine was compared with hydroxyurea in a randomized trial including 121 de novo AML, 53 sAML, and 28 MDS patients.55 However, the number of sAML patients with t-AML was not specified. In the entire population, low-dose cytarabine resulted in a higher CR rate (18% vs 1%) and better OS (hazard ratio, 0.6; 95% confidence interval, 0.44-0.81). A subgroup analysis revealed no benefit in patients with adverse cytogenetics, sAML, and MDS.
In summary, hypomethylating agents seem to have a similar activity in t-MN patients as in de novo AML and can be applied, if appropriate clinical trials are not available. Low-dose cytarabine may be effective in some patients with t-AML, but no effect should be expected in patients with adverse cytogenetics.
Treatment-related MDS
In the aforementioned large cooperative study of 1837 t-MDS patients, the median OS was 16 months, and allogeneic HCT was performed in 16% of patients who had a median survival of 24 months.8 The discriminatory power of the IPSS-R was inferior in t-MDS compared with de novo MDS patients and a revised prognostic model was proposed.8 Another study compared the IPSS-R score in t-MN patients to de novo MDS patients and found that the IPSS-R score can distinguish the 5 prognostic subgroups, but OS was shorter in t-MN patients than in de novo MDS patients, particularly in the very-low-risk and low-risk groups.56 The median survival in t-MDS patients with very-low-, low-, and intermediate-risk IPSS-R was 56.5, 21.7, and 15.8 months, respectively, whereas de novo MDS patients had median survival of 105.6, 63.6, and 36 months.56 Thus, the survival of t-MDS patients with low-risk IPSS-R was shorter than the survival of de novo MDS patients with intermediate-risk IPSS-R. t-MDS patients should be treated in clinical trials whenever possible, and allogeneic HCT should be offered to eligible patients with IPSS-R low or higher if a suitable donor is available.
The question of whether MDS patients should be treated with cytoreductive agents before allogeneic HCT was addressed in retrospective studies. MDS patients who received azacitidine before transplantation or who were transplanted up-front without cytoreduction had similar outcomes after 3 years.57 Similarly, treatment of MDS patients with azacitidine or intensive chemotherapy before allogeneic HCT resulted in comparable outcome after 3 years (OS 55% vs 48%, relapse 40% vs 37%, and nonrelapse mortality (NRM) 19% vs 20% for azacitidine vs intensive chemotherapy, respectively).58 Therefore, allogeneic HCT should be planned as quickly as possible. If a delay in transplantation is expected, hypomethylating agents or intensive chemotherapy may be considered.58
Allogeneic HCT for t-MN patients
Risk factors for overall survival
Allogeneic HCT is often the only curative approach for t-MN patients. Several studies reported the outcomes of t-MN patients after allogeneic HCT, each including >250 patients.59-61 Overall survival of t-MN patients at 3 to 5 years after allogeneic HCT was 22% to 35%.60,61 Five adverse risk factors for OS were identified: age >35 years, poor-risk cytogenetics, t-AML not in remission or advanced t-MDS, and donor other than an HLA-identical sibling or a partially or well-matched unrelated donor.61 Patients with none of these risk factors had a predicted 5-year OS of 50%, whereas patients with 3 risk factors had a predicted survival of 10% at 5 years.
High NRM in t-MN patients undergoing allogeneic HCT
The cumulative incidence of relapse at 3 to 5 years was 31% to 36%. These studies noted high NRM of 32% to 41% at 1 year and up to 61% at 5 years. The analysis of the CIBMTR identified age >35 years, a lower Karnofsky performance score, and t-MDS before transplantation as unfavorable risk factors for NRM.61 An obvious approach to reduce the high NRM would be reduced-intensity conditioning. However, reduced-intensity conditioning resulted in similar NRM rates as myeloablative conditioning in the CIBMTR and EBMT series.60,61 However, the study by Kröger et al compared NRM rates before 1998 and since 1998 and found a significantly reduced NRM in more recent years.60
When outcome of allogeneic HCT in t-MN patients was compared with de novo MDS/AML patients, no differences were noted for relapse and NRM after adjustment for disease category, age, and cytogenetics.59 Relapse rates were similar within low- and high-risk cytogenetic groups (according to IPSS), whereas patients with intermediate-risk cytogenetics and t-MN had a higher relapse rate compared with de novo MDS/AML patients.59 Armand and colleagues compared the outcome after allogeneic HCT in 80 t-MDS/t-AML patients with 476 de novo MDS/AML patients who were stratified into 3 cytogenetic risk groups. Disease etiology did not affect OS, incidence of relapse, or NRM, emphasizing that genetic risk rather than previous treatment affects the outcome after allogeneic HCT.62 Thus, allogeneic HCT is an important treatment option in t-MN patients, and age and cytogenetics are the most important prognostic factors. Novel strategies are required to reduce NRM without compromising antileukemic activity.
Novel treatment approaches for t-MN
Targeted therapies should be evaluated in t-MN patients as in de novo AML patients. Promising data have been reported for multi-kinase and FLT3 inhibitors (midostaurin, sorafenib, quizartinib) and for IDH1 and IDH2 inhibitors. A large randomized phase 3 trial of midostaurin or placebo with intensive induction and consolidation therapy was presented at the ASH meeting in 2015 and showed a significantly improved OS in midostaurin-treated de novo AML patients.63 Midostaurin use in t-AML patients should be studied in the future. The DOT1L inhibitor EPZ-5676 inhibits the interaction of DOT1L with KMT2A and therefore may be of specific relevance to t-AML with t(9;11). Initial results of a phase 1 clinical trial with EPZ-5676 presented at the ASH 2015 meeting indicated an acceptable safety profile and some clinical activity as demonstrated by a marrow response and resolution of leukemia cutis.64
CPX-351 is a liposomal formulation of cytarabine and daunorubicin packaged in liposomes at a 5:1 molar ratio, which improved survival compared with the same drugs administered conventionally in animal models of leukemia.65
In a randomized phase 3 trial in newly diagnosed older secondary AML patients including 20% t-AML patients, CPX-351 was compared with 7+3 induction.66 The CR/CRi rate was higher in the CPX-351 compared with the 7+3 cohort (47.7% vs 33.3%, P = .016). OS was significantly improved with CPX-351, resulting in a median OS of 9.6 vs 6 months in the 7+3 group (P = .005), and 60-day mortality was in favor of CPX-351. Thus, CPX-351 may become a new treatment option in sAML and specifically in t-MN patients.
Because of the high incidence of mutations in the RAS pathway, inhibitors of RAS signaling are of special interest in t-MN patients. A recent phase 1/2 trial of trametinib, an inhibitor of mitogen-activated protein kinase 1 (MEK1) and MEK2, showed an overall response rate of 20% in relapsed/refractory AML and MDS patients with NRAS or KRAS mutations, whereas only 3% of RAS wild-type patients responded.67
t-MN with mutated TP53 or complex chromosome aberrations are unlikely to be cured with targeted agents or with chemotherapy, because the checkpoint for apoptosis and cell-cycle arrest is defective, thus allowing the aberrant cell to escape the selective pressure of specific pathway inhibitors. Conceptually, an immunologic approach should be more successful because the immune attack is independent of genetic aberrations. It was suggested that cancer cells with multiple aberrations are more immunogenic than cells with one or few aberrations, and thus novel immunologic therapies like chimeric antigen receptor T cells, bispecific antibodies, and checkpoint inhibitors should be evaluated in t-MN patients with a high-risk genetic profile.
Summary and outlook
Significant progress has been made in the understanding of the pathogenesis of therapy-related myeloid neoplasms. The direct induction of fusion oncogenes by epipodophyllotoxins is likely a result of both stochastic and deterministic processes in the cell. In addition, strong evidence was recently obtained indicating that a significant number of t-MNs develop through clonal selection of preexisting, treatment-resistant clones, which exhibit a high frequency of p53 pathway mutations (Figure 1).
Therapy-related acute promyelocytic leukemia has a high remission rate with ATRA and arsenic trioxide therapy and should be treated as de novo APL. Patients with therapy-related CBF leukemias seem to have a worse prognosis than their de novo AML counterparts. Nevertheless, we recommend initial intensive chemotherapy in these patients and the decision for allogeneic HCT should be based on the results of MRD assessment (Figure 4). In most other patients, allogeneic HCT in CR1 is the preferred treatment option for eligible patients, whereas treatment in clinical trials and with hypomethylating agents are preferred options for the remaining or relapsed/refractory patients.
Patients with t-MN often have complex cytogenetics and TP53 mutations and we know that chemotherapy can hardly overcome the underlying genetic defects. Therefore, it is hoped that novel immunologic approaches can attack these aberrant cells and eventually lead to improved treatment results in this underserved patient population.
Acknowledgments
The author thanks Arnold Ganser, Hartmut Döhner, Felicitas Thol, Nicolaus Kröger, and Michael Morgan for their review and helpful discussions and Michelle Maria Araujo Cruz for her help with figure 1.
Footnotes
This review was supported by an ERC grant under the European Union’s Horizon 2020 research and innovation program (No. 638035). M.H. is a Heisenberg chair of the DFG (HE 5240/6-1).
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. . The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-2405; Advance online publication. [DOI] [PubMed] [Google Scholar]
- 2.Arber DA, Vardiman JW, Brunning RD, et al. . Acute myeloid leukaemia and related precursor neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al., eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed. Geneva, Switzerland: WHO PRESS; 2008:109-148 [Google Scholar]
- 3.Kayser S, Döhner K, Krauter J, et al. ; German-Austrian AMLSG. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137-2145. [DOI] [PubMed] [Google Scholar]
- 4.Granfeldt Østgård LS, Medeiros BC, Sengeløv H, et al. . Epidemiology and Clinical Significance of Secondary and Therapy-Related Acute Myeloid Leukemia: A National Population-Based Cohort Study. J Clin Oncol. 2015;33(31):3641-3649. [DOI] [PubMed] [Google Scholar]
- 5.Hulegårdh E, Nilsson C, Lazarevic V, et al. . Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90(3):208-214. [DOI] [PubMed] [Google Scholar]
- 6.Morton LM, Dores GM, Tucker MA, et al. . Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121(15):2996-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichenauer DA, Thielen I, Haverkamp H, et al. . Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood. 2014;123(11):1658-1664. [DOI] [PubMed] [Google Scholar]
- 8.Kuendgen A, Tuechler H, Nomdedeu M, et al. . An analysis of prognostic kmarkers and the performance of scoring systems in 1837 patients with therapy-related myelodysplastic syndrome—a study of the International Working Group (IWG-PM) for Myelodysplastic Syndromes (MDS). ASH Annual Meeting. 2015;2015; Abstract 609. [Google Scholar]
- 9.Godley L, Larson RA. The Syndrome of Therapy-Related Myelodysplasia and Myeloid Leukemia. In: Bennett JM, ed. The Myelodysplastic Syndromes. New York, Basel: Marcel Dekker Inc.; 2002:139-176 [Google Scholar]
- 10.Cowell IG, Austin CA. Mechanism of generation of therapy related leukemia in response to anti-topoisomerase II agents. Int J Environ Res Public Health. 2012;9(6):2075-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barabé F, Kennedy JA, Hope KJ, Dick JE. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316(5824):600-604. [DOI] [PubMed] [Google Scholar]
- 12.Reimer J, Knoess S, Labuhn M, Charpentier EM, Klusmann J-H, Heckl D. Crispr-Cas9 induced MLL-rearrangements cause clonal outgrowth in CD34+ hematopoietic stem cells. ASH Annual Meeting. 2015;2015; Abstract 165. [Google Scholar]
- 13.Itzhar N, Dessen P, Toujani S, et al. . Chromosomal minimal critical regions in therapy-related leukemia appear different from those of de novo leukemia by high-resolution aCGH. PLoS One. 2011;6(2):e16623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong TN, Ramsingh G, Young AL, et al. . Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518(7540):552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shih AH, Chung SS, Dolezal EK, et al. . Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia. Haematologica. 2013;98(6):908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ok CY, Patel KP, Garcia-Manero G, et al. . Mutational profiling of therapy-related myelodysplastic syndromes and acute myeloid leukemia by next generation sequencing, a comparison with de novo diseases. Leuk Res. 2015;39(3):348-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal S, Fontanillas P, Flannick J, et al. . Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genovese G, Kähler AK, Handsaker RE, et al. . Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371(26):2477-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie M, Lu C, Wang J, et al. . Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steensma DP, Bejar R, Jaiswal S, et al. . Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126(1):9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruark E, Snape K, Humburg P, et al. ; Breast and Ovarian Cancer Susceptibility Collaboration; Wellcome Trust Case Control Consortium. Mosaic PPM1D mutations are associated with predisposition to breast and ovarian cancer. Nature. 2013;493(7432):406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swisher EM, Harrell MI, Norquist BM, et al. . Somatic Mosaic Mutations in PPM1D and TP53 in the Blood of Women With Ovarian Carcinoma. JAMA Oncol. 2016;2(3):370-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zajkowicz A, Butkiewicz D, Drosik A, Giglok M, Suwiński R, Rusin M. Truncating mutations of PPM1D are found in blood DNA samples of lung cancer patients. Br J Cancer. 2015;112(6):1114-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Guezennec X, Bulavin DV. WIP1 phosphatase at the crossroads of cancer and aging. Trends Biochem Sci. 2010;35(2):109-114. [DOI] [PubMed] [Google Scholar]
- 25.Kleiblova P, Shaltiel IA, Benada J, et al. . Gain-of-function mutations of PPM1D/Wip1 impair the p53-dependent G1 checkpoint. J Cell Biol. 2013;201(4):511-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pharoah PD, Song H, Dicks E, et al. ; Australian Ovarian Cancer Study Group; Ovarian Cancer Association Consortium. PPM1D Mosaic Truncating Variants in Ovarian Cancer Cases May Be Treatment-Related Somatic Mutations. J Natl Cancer Inst. 2016;108(3):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulz E, Valentin A, Ulz P, et al. . Germline mutations in the DNA damage response genes BRCA1, BRCA2, BARD1 and TP53 in patients with therapy related myeloid neoplasms. J Med Genet. 2012;49(7):422-428. [DOI] [PubMed] [Google Scholar]
- 28.Churpek JE, Marquez R, Neistadt B, et al. . Inherited mutations in cancer susceptibility genes are common among survivors of breast cancer who develop therapy-related leukemia. Cancer. 2016;122(2):304-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jawad M, Seedhouse CH, Russell N, Plumb M. Polymorphisms in human homeobox HLX1 and DNA repair RAD51 genes increase the risk of therapy-related acute myeloid leukemia. Blood. 2006;108(12):3916-3918. [DOI] [PubMed] [Google Scholar]
- 30.Voso MT, Fabiani E, Zang Z, et al. . Fanconi anemia gene variants in therapy-related myeloid neoplasms. Blood Cancer J. 2015;5:e323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fabiani E, Fianchi L, Falconi G, et al. . The BCL2L10 Leu21Arg variant and risk of therapy-related myeloid neoplasms and de novo myelodysplastic syndromes. Leuk Lymphoma. 2014;55(7):1538-1543. [DOI] [PubMed] [Google Scholar]
- 32.Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2005;19(12):2232-2240. [DOI] [PubMed] [Google Scholar]
- 33.Klimek VM, Tray NJ. Therapy-related myeloid neoplasms: what’s in a name? Curr Opin Hematol. 2016;23(2):161-166. [DOI] [PubMed] [Google Scholar]
- 34.Churpek JE, Larson RA. The evolving challenge of therapy-related myeloid neoplasms. Best Pract Res Clin Haematol. 2013;26(4):309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. [DOI] [PubMed] [Google Scholar]
- 36.Lo-Coco F, Avvisati G, Vignetti M, et al. ; Gruppo Italiano Malattie Ematologiche dell’Adulto; German-Austrian Acute Myeloid Leukemia Study Group; Study Alliance Leukemia. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111-121. [DOI] [PubMed] [Google Scholar]
- 37.Burnett AK, Russell NH, Hills RK, et al. ; UK National Cancer Research Institute Acute Myeloid Leukaemia Working Group. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295-1305. [DOI] [PubMed] [Google Scholar]
- 38.Dayyani F, Kantarjian H, O’Brien S, et al. . Outcome of therapy-related acute promyelocytic leukemia with or without arsenic trioxide as a component of frontline therapy. Cancer. 2011;117(1):110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafson SA, Lin P, Chen SS, et al. . Therapy-related acute myeloid leukemia with t(8;21) (q22;q22) shares many features with de novo acute myeloid leukemia with t(8;21)(q22;q22) but does not have a favorable outcome. Am J Clin Pathol. 2009;131(5):647-655. [DOI] [PubMed] [Google Scholar]
- 40.Krauth MT, Eder C, Alpermann T, et al. . High number of additional genetic lesions in acute myeloid leukemia with t(8;21)/RUNX1-RUNX1T1: frequency and impact on clinical outcome. Leukemia. 2014;28(7):1449-1458. [DOI] [PubMed] [Google Scholar]
- 41.Zhu HH, Zhang XH, Qin YZ, et al. . MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013;121(20):4056-4062. [DOI] [PubMed] [Google Scholar]
- 42.Borthakur G, Lin E, Jain N, et al. . Survival is poorer in patients with secondary core-binding factor acute myelogenous leukemia compared with de novo core-binding factor leukemia. Cancer. 2009;115(14):3217-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin JA, O’Brien MA, Hills RK, Daly SB, Wheatley K, Burnett AK. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012;120(14):2826-2835. [DOI] [PubMed] [Google Scholar]
- 44.Krauter J, Wagner K, Schäfer I, et al. . Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27(18):3000-3006. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Kantarjian H, Pierce S, et al. . Prognostic significance of 11q23 aberrations in adult acute myeloid leukemia and the role of allogeneic stem cell transplantation. Leukemia. 2013;27(4):836-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krönke J, Schlenk RF, Jensen KO, et al. . Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: a study from the German-Austrian acute myeloid leukemia study group. J Clin Oncol. 2011;29(19):2709-2716. [DOI] [PubMed] [Google Scholar]
- 47.Ivey A, Hills RK, Simpson MA, et al. ; UK National Cancer Research Institute AML Working Group. Assessment of Minimal Residual Disease in Standard-Risk AML. N Engl J Med. 2016;374(5):422-433. [DOI] [PubMed] [Google Scholar]
- 48.Balsat M, Renneville A, Thomas X, et al. . NPM1 minimal residual disease as prognostic and predictive factor in young adults with acute myeloid leukemia: a study by the French ALFA Group. Blood. 2015:126:2581. [Google Scholar]
- 49.Lindsley RC, Mar BG, Mazzola E, et al. . Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125(9):1367-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luskin MR, Lee JW, Fernandez HF, et al. . Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood. 2016;127(12):1551-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holowiecki J, Grosicki S, Giebel S, et al. . Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30(20):2441-2448. [DOI] [PubMed] [Google Scholar]
- 52.Bally C, Thépot S, Quesnel B, et al. . Azacitidine in the treatment of therapy related myelodysplastic syndrome and acute myeloid leukemia (tMDS/AML): a report on 54 patients by the Groupe Francophone Des Myelodysplasies (GFM). Leuk Res. 2013;37(6):637-640. [DOI] [PubMed] [Google Scholar]
- 53.Fianchi L, Criscuolo M, Lunghi M, et al. . Outcome of therapy-related myeloid neoplasms treated with azacitidine. J Hematol Oncol. 2012;5:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klimek VM, Dolezal EK, Tees MT, et al. . Efficacy of hypomethylating agents in therapy-related myelodysplastic syndromes. Leuk Res. 2012;36(9):1093-1097. [DOI] [PubMed] [Google Scholar]
- 55.Burnett AK, Milligan D, Prentice AG, et al. . A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109(6):1114-1124. [DOI] [PubMed] [Google Scholar]
- 56.Ok CY, Hasserjian RP, Fox PS, et al. . Application of the international prognostic scoring system-revised in therapy-related myelodysplastic syndromes and oligoblastic acute myeloid leukemia. Leukemia. 2014;28(1):185-189. [DOI] [PubMed] [Google Scholar]
- 57.Damaj G, Mohty M, Robin M, et al. . Upfront allogeneic stem cell transplantation after reduced-intensity/nonmyeloablative conditioning for patients with myelodysplastic syndrome: a study by the Société Française de Greffe de Moelle et de Thérapie Cellulaire. Biol Blood Marrow Transplant. 2014;20(9):1349-1355. [DOI] [PubMed] [Google Scholar]
- 58.Damaj G, Duhamel A, Robin M, et al. . Impact of azacitidine before allogeneic stem-cell transplantation for myelodysplastic syndromes: a study by the Société Française de Greffe de Moelle et de Thérapie-Cellulaire and the Groupe-Francophone des Myélodysplasies. J Clin Oncol. 2012;30(36):4533-4540. [DOI] [PubMed] [Google Scholar]
- 59.Chang C, Storer BE, Scott BL, et al. . Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110(4):1379-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kröger N, Brand R, van Biezen A, et al. ; Myelodysplastic Syndromes Subcommittee of The Chronic Leukaemia Working Party of European Group for Blood and Marrow Transplantation (EBMT). Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94(4):542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litzow MR, Tarima S, Pérez WS, et al. . Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115(9):1850-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armand P, Kim HT, DeAngelo DJ, et al. . Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13(6):655-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stone RM, Mandrekar S, Sanford BL, et al. . The multi-kinase inhibitor midostaurin prolongs survival sompared with placebo in combination with daunorubicin/cytarabine induction, high-dose C consolidation, and as maintenance therapy in newly diagnosed acute myeloid leukemia (AML) patients age 18-60 with FLT3 mutations: an international prospective randomized placebo-controlled double-blind trial (CALGB 10603/RATIFY [Alliance]). ASH Annual Meeting. 2015;2015 Abstract 6. [Google Scholar]
- 64.Stein EM, Garcia-Manero G, Rizzieri DA, et al. . A phase 1 study of the DOT1L inhibitor, pinometostat (EPZ-5676) in adults with relapsed or refractory leukemia: safety. clinical activity, exposure and target inhibition [abstract]. Blood. 2015;126(23). Abstract 2547. [Google Scholar]
- 65.Tardi P, Johnstone S, Harasym N, et al. . In vivo maintenance of synergistic cytarabine:daunorubicin ratios greatly enhances therapeutic efficacy. Leuk Res. 2009;33(1):129-139. [DOI] [PubMed] [Google Scholar]
- 66.Lancet JE, Uy GL, Cortes JE, et al. . Final results of a phase III randomized trial of CPX-351 versus 7+3 in older patients with newly diagnosed high risk (secondary) AML. ASCO Annual Meeting. 2016: Abstract 7000. [Google Scholar]
- 67.Borthakur G, Popplewell L, Boyiadzis M, et al. . Activity of the oral mitogen-activated protein kinase kinase inhibitor trametinib in RAS-mutant relapsed or refractory myeloid malignancies. Cancer. 2016;122(12):1871-1879; Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]