Abstract
Myelodysplastic syndrome (MDS) and myeloproliferative disorders are rare in children; they are divided into low-grade MDS (refractory cytopenia of childhood [RCC]), advanced MDS (refractory anemia with excess blasts in transformation), and juvenile myelomonocytic leukemia (JMML), each with different characteristics and management strategies. Underlying genetic predisposition is recognized in an increasing number of patients. Germ line GATA2 mutation is found in 70% of adolescents with MDS and monosomy 7. It is challenging to distinguish RCC from aplastic anemia, inherited bone marrow failure, and reactive conditions. RCC is often hypoplastic and may respond to immunosuppressive therapy. In case of immunosuppressive therapy failure, hypercellular RCC, or RCC with monosomy 7, hematopoietic stem cell transplantation (HSCT) using reduced-intensity conditioning regimens is indicated. Almost all patients with refractory anemia with excess blasts are candidates for HSCT; children age 12 years or older have a higher risk of treatment-related death, and the conditioning regimens should be adjusted accordingly. Unraveling the genetics of JMML has demonstrated that JMML in patients with germ line PTPN11 and CBL mutations often regresses spontaneously, and therapy is seldom indicated. Conversely, patients with JMML and neurofibromatosis type 1, somatic PTPN11, KRAS, and most of those with NRAS mutations have a rapidly progressive disease, and early HSCT is indicated. The risk of relapse after HSCT is high, and prophylaxis for graft-versus-host disease and monitoring should be adapted to this risk.
Learning Objectives
To recognize the challenges in making a diagnosis of MDS in children
To know the different therapeutic strategies in low-grade and advanced MDS
To understand the genetics of JMML and how it may be used for treatment stratification
Myelodysplastic and myeloproliferative disorders are rare in children and have different morphologic features, cytogenetic findings, prognostic factors, and therapeutic aims than in adults. Therefore, there is a need for a pediatric approach to the classification of these disorders1 as integrated in the 2008 version of the World Health Organization classification2 that recognizes the specific features of diseases in children vs those in adults (Table 1).
Table 1.
Characteristics of MDS in children and adults
| Characteristic | Children | Adults |
|---|---|---|
| Annual incidence per million | 1-2 | 40 |
| Associated abnormalities | 1/3 | <5% |
| Morphologic groups | ||
| Refractory anemia with ringed sideroblasts | <2% | 25% |
| Hypoplastic MDS | Common | Rare |
| Abnormal cytogenetics | 60% | 40% |
| −7/del(7q) | 30%-40% | 10% |
| −5/del(5q) | 1%-2% | 20% |
| Hypermethylation | >50% | >50% |
| Spliceosomal gene aberration | <2% | Common |
| Aim of treatment | Curative | Palliative |
The diseases are divided into low-grade myelodysplastic syndrome (MDS), advanced MDS, and juvenile myelomonocytic leukemia (JMML). The myeloid leukemia of Down syndrome represents a specific entity that should not be included in series of MDSs and will not be discussed in this review. There is an increasing recognition that more children have an underlying genetic predisposition for the development of MDS or JMML. For a more thorough overview, the reader is referred to several reviews published recently.3-7
Low-grade MDS
MDS with less than 2% blasts in peripheral blood (PB) or less than 5% blasts in the bone marrow (BM) are classified as refractory cytopenia of childhood (RCC) or low-grade MDS.1,2 The majority of the children with RCC have a hypoplastic BM, which resembles aplastic anemia. Experienced hematopathologists can differentiate RCC from aplastic anemia with a high degree of interobserver reproducibility.8
Advanced MDS
MDS with ≥2% blasts in PB or ≥5% but below 20% blasts in the BM are classified as refractory anemia with excess of blasts (RAEB).1,2 The subgroup RAEB in transformation (RAEB-T) with PB or BM having 20% to 29% blasts was kept in the pediatric classification but it should be emphasized that the blast count is not sufficient for differentiating acute myeloblastic leukemia (AML) from MDS. Diagnostics must include a comprehensive assessment of clinical features, progression rate, morphology, immunophenotype, and cytogenetics.
Disease in patients with a blast count above the 30% (or 20%, as suggested by the World Health Organization) threshold is conventionally defined as AML; however, patients who progress from MDS may retain the biological characteristics of MDS and may be classified as MDS-related AML (MDR-AML).
Primary vs secondary MDS
MDS often arises in a previously healthy child and is conformingly called de novo or primary, or MDS may develop in a child with a known predisposing condition and is then referred to as secondary. Patients with inherited BM failure disorders who have received chemotherapy or irradiation or after acquired aplastic anemia are at risk of secondary MDS. Children with so-called primary MDS may have an underlying yet unknown genetic defect predisposing them to MDS at a young age. Therefore, the distinction between primary and secondary disease may become arbitrary.
GATA2 mutations
GATA2 germ line mutation has been identified as the cause of a wide spectrum of diseases, including monocytopenia, congenital deafness, lymphedema (MonoMAC or Emberger syndrome),9 or cytopenia complicated by systemic infections and an increased risk of developing MDS or AML.10 A study of more than 600 children and adolescents with MDS enrolled in the European Working Group of Myelodysplastic Syndromes in Childhood studies identified 57 patients with germ line GATA2 mutations.11 Germ line GATA2 mutations were found in 15% of advanced and 7% of all patients with primary MDS but were absent in MDS secondary to therapy or acquired aplastic anemia. Mutation carriers were older (age 12 vs 10 years; no child with GATA2 mutation was younger than age 4 years) at diagnosis and were more likely to present with monosomy 7 (70% vs 11%) and advanced disease (46% vs 18%) as compared with wild-type patients. GATA2 mutation was very common among patients with monosomy 7 (37% in patients of all ages) peaking in adolescence (72% of all monosomy 7 patients). Unexpectedly, monocytosis was more frequent in GATA2-mutated patients and was associated with monosomy 7; mutational status had no effect on the hematologic phenotype. The overall survival and the outcome from hematopoietic stem cell transplantation (HSCT) performed in 50 GATA2-mutated patients was independent of genotype status.11 This study shows that GATA2 deficiency is the most common germ line predisposition for pediatric MDS with very high prevalence in adolescents with monosomy 7. GATA2 mutations do not confer poor prognosis in childhood MDS. However, the high risk for progression to advanced disease might guide decision-making toward timely HSCT thus avoiding noncurative immunosuppressive therapies (ISTs). The ideal time point for HSCT in GATA2 disease seems to be during the hypocellular phase of MDS and before manifestation of severe complications (ie, invasive infections), underscoring the need for close monitoring.11,12 Studies of GATA2 highlight the contribution of de novo germ line mutations with delayed-latency oncogenic effect in what has so far been considered primary MDS.
Inherited BM failure
Children with inherited BM failure syndromes have an increased but variable risk of MDS or AML. The risk is highest in Fanconi anemia, dyskeratosis congenita, and severe congenital neutropenia (SCN). Myeloid neoplasia develops in a large fraction of patients with Fanconi anemia during childhood or early adult life. The risk varies according to genetic subgroup and associated abnormalities.13 The traits of Fanconi anemia may not be recognized, and the diagnosis should always be considered, even in adults.
The cumulated risk of MDS in SCN is 15% after 15 years, according to the International SCN Register.14 There is no direct cause-and-effect relationship between the occurrence of MDS and therapy with granulocyte colony-stimulating factor, but the highest risk of MDS is seen in patients with a poor response to granulocyte colony-stimulating factor.
MDS develops in 30% of those with Shwachman-Diamond syndrome and is often associated with chromosome 7 abnormalities in which isochromosome 7q is common and may be transient and not associated with progression.15
Pathophysiology
MDS is a clonal disease arising in a progenitor cell restricted to myelopoiesis, erythropoiesis, and megakaryopoiesis. The initiating events of MDS have remained obscure in children and in adults until now. Recent studies have greatly illuminated the genomic landscape of MDS and adults. The most common mutations found in MDS in adults occur in genes involved in RNA splicing (eg, SF3B1, SRSF2, U2AF1, and ZRSR2) and epigenetic modification (eg, TET2, ASXL1, and DNMT3A). The genomic landscape seems very different in children in whom identification of mutations in TET2 is very rare16 and in whom spliceosome mutations are very uncommon.17
Because of the heterogeneity of MDS, different mechanisms of initiation and progression of the disease are likely to exist. Genetic damage in a pluripotent hematopoietic progenitor cell may give rise to genetic instability with subsequent acquisition of numerous molecular abnormalities. About 30% of children with MDS have a known constitutional disorder. The recent finding that GATA2 mutation is present in a large proportion of childhood MDS may lead to speculations that an even higher proportion of the children have an inherited abnormality predisposing them to the acquisition of genetic changes. Subsequent events, such as mutations in proto-oncogenes like RAS, TP53, or WT1, and karyotypic changes such as monosomy 7 may be part of a final common pathway of disease progression. Methylation studies in children with RAEB or RAEB-T have demonstrated that at least half the patients had hypermethylation of the p15 gene or CALCA and CDKN2B genes,18 a frequency similar to that in adult MDS.
Clinical and laboratory features
The presenting features in virtually all cases of MDS are those of pancytopenia. Single-lineage cytopenia or macrocytosis may occasionally be the presenting characteristic. In a few patients, the cytopenia is an incidental finding during a routine workup. A few patients have been diagnosed during evaluation as possible sibling stem cell donors. Fetal hemoglobin (HbF) is frequently moderately elevated whereas white blood cell (WBC) count is low to normal. Leukocytosis is generally not a feature of MDS, and in the case of increased WBC count, the diagnosis should be reconsidered. Some patients present with moderate hepatosplenomegaly but most have no organomegaly.
BM findings
The BM may be hypo-, normo-, or hypercellular. Decreased cell content is more common in children compared with adults. Both the PB and BM display characteristic dysplastic features with macrocytic erythropoiesis, small or unusually large megakaryocytes, and dysgranulopoiesis.8 The presence of the characteristic dysplastic features is suggestive of MDS but is not diagnostic. There is interobserver variation in the evaluation of dysplasia, so centralized review is recommended and it may increase diagnostic accuracy.8 Children who meet the criteria for refractory cytopenia with multilineage dysplasia should be considered as having RCC until the prognostic significance of a multilineage dysplasia is clarified.3,6
Cytogenetics
An abnormal karyotype is found in 55% of children with advanced primary MDS and in 76% with secondary advanced MDS.19 Monosomy 7 is the most common cytogenetic abnormality in childhood MDS and is seen in 25% of the patients.19 Acquired trisomy 8 and trisomy 21 are the most common numerical abnormalities after monosomy 7. Constitutional 8 mosaicism may be clinically silent and should be tested for when trisomy 8 is found in the BM.20
Monosomy 7 is a poor prognostic factor for adults with MDS. The outcome for children with monosomy 7 is similar to that of other children with MDS but very poor for the few with monosomy 7 combined with structural abnormalities.19 Favorable cytogenetic aberrations identified in adults [ie, −Y, del(20q), and del(5q)] are so infrequent in children that their prognostic importance cannot be evaluated. Structural complex abnormalities defined as ≥3 chromosomal aberrations including at least 1 structural aberration are associated with a very poor outcome.19
Separating MDS from AML
There are significant differences in clinical features, cytogenetics, and response to therapy between MDS and AML, the major differential diagnosis of advanced MDS. There are fundamental biological differences between MDS and AML. The morphologically based classification is only a surrogate marker for the distinction between biological entities, and the blast count in a single specimen is insufficient for differentiating MDS from AML.7 Biological features rather than any arbitrary cutoff in blast count may be more important in distinguishing MDS from AML.
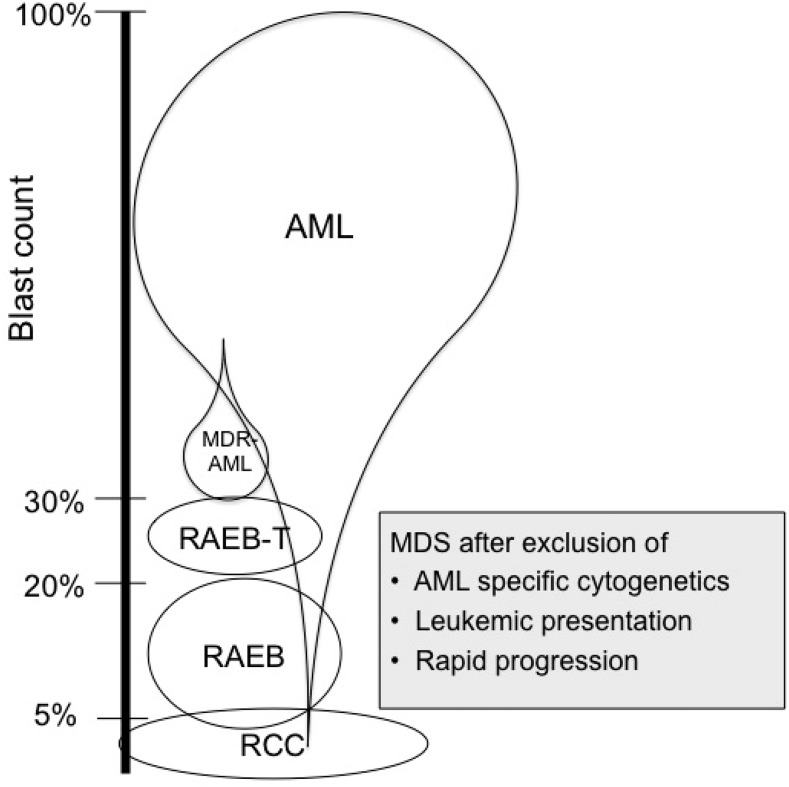
In borderline cases with BM blasts of 20% to 30% and with no cytogenetic clues regarding the diagnosis, it is recommended to repeat the BM examination after 2 weeks. If the blast count has increased to more than 30%, the diagnosis should be regarded as AML. Significant organomegaly and especially increased WBC counts are suggestive of AML. Most children with myeloid malignancies have clear-cut AML, some have MDS with low blast count, and only a few have borderline features. The major diagnostic pitfall is possibly undue haste in starting therapy. Figure 1 shows the relative distribution of AML and MDS and illustrates that AML may be diagnosed at any blast level.
Figure 1.
Blast count and relative distribution of AML and MDS illustrates that AML can be diagnosed at any blast level. Adapted from Hasle and Niemeyer.7
Treatment of low-grade MDS
IST with anti-thymocyte globulin (ATG) and cyclosporine in children with hypocellular RCC and karyotypes other than monosomy 7 or in those with 3 or more chromosomal abnormalities resulted in a complete or partial response in 75% after 6 months. The failure-free survival at 3 years was only 57%, but because of a high salvage rate with HSCT, the overall survival was 88%.21
A comparison of responses after IST with horse ATG (lymphoglobulin) and rabbit ATG (thymoglobulin) shows a superior overall response at 6 months for horse ATG (74% vs 53%), which translates into superior transplantation-free and failure-free survival.22 The presence of a minor paroxysmal nocturnal hemoglobinuria clone predicts a more favorable response to IST than that for patients with a paroxysmal nocturnal hemoglobinuria clone <0.1%.23
With the improved outcomes currently seen in unrelated donor HSCT in pediatric RCC, it is unclear whether newly diagnosed children who lack a matched sibling donor should receive IST with horse ATG or proceed directly to transplantation from an unrelated donor. With the disappointing results seen after treatment with rabbit ATG, there has been a move toward first-line matched unrelated donor HSCT. Compared with IST, transplantation offers a more complete restoration of hematopoiesis and lower relapse rates. The major potential drawbacks of first-line matched unrelated donor transplantation are the difficulties in finding donors, time from diagnosis to HSCT, graft-versus-host disease (GvHD) or graft rejection, and treatment-related mortality.24 Given the low risk of relapse, HSCT with reduced-intensity conditioning may be an attractive alternative.25
Treatment of advanced MDS
MDS is a clonal early stem cell disorder with limited residual nonclonal stem cells. Myeloablative therapy is therefore the only realistic treatment option with a curative potential. Therapy strategies such as hematopoietic growth factors, differentiating agents, antiangiogenic drugs, low-dose cytotoxic drugs, or experimental agents have been investigated in adults and elderly patients who are not candidates for HSCT. None of these approaches have been documented to prolong survival, and they are generally not indicated in children and adolescents in whom the aim of treatment is cure. Children with MDS are at high risk of cytopenia-related complications, and optimal supportive care should be the primary focus during all phases of the disease.
The DNA methyltransferase inhibitors azacitidine and decitabine have shown clinical efficacy in randomized studies in high-risk MDS in adults. Azacitidine increased overall survival compared with conventional care.26 Preliminary data from pediatric MDS show stable disease or response in 50% of the patients and thus these treatments may be a bridge to HSCT.27
AML-type chemotherapy
Conventional AML-type intensive chemotherapy without HSCT is unlikely to eradicate the primitive pluripotent cells involved in MDS, which renders the therapy noncurative in most patients. Induction chemotherapy results in complete remission of less than 60% and overall survival of less than 30%.7 Patients with advanced MDS (MDR-AML) who have more than 30% blasts may benefit from intensive chemotherapy before HSCT.28
HSCT
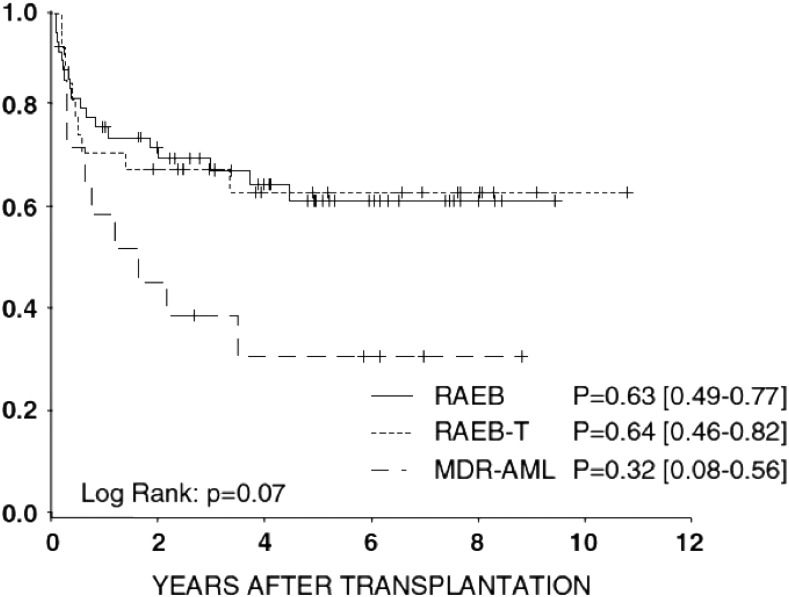
HSCT is the therapy of choice for virtually all forms of advanced MDS in childhood with the best available donor. Myeloablative therapy with busulfan, cyclophosphamide, and melphalan has cured more than half of children with MDS after both matched family donor and matched unrelated donor HSCT.28 Total body irradiation can generally be omitted because it has no superior antileukemic efficacy and is associated with more long-term adverse effects in children. Outcome after HSCT is similar in patients with RAEB or RAEB-T but significantly lower in patients with MDR-AML (Figure 2).28 Patients age 12 years or older have an increased risk of treatment-related mortality; thus, a conditioning regimen with thiotepa, treosulfan, and fludarabine is recommended.
Figure 2.
Probability of 5-year overall survival in advanced MDS according to subgroup. Adapted from Strahm et al.28
Whether AML-type induction chemotherapy before HSCT for advanced MDS can reduce relapse and improve outcome is controversial. Small series of patients who have received transplants as first-line therapy, which spares them from toxicity related to induction chemotherapy, have shown survival of 65% to 70%, similar to that of patients receiving chemotherapy before HSCT.7
The experience within the European Working Group of Myelodysplastic Syndromes in Childhood of using intensive chemotherapy before HSCT did not show any difference in event-free or overall survival, relapse, or treatment-related mortality; however, children with MDR-AML had a significantly decreased risk of relapse if they had received prior intensive chemotherapy.28
Relapse after HSCT is associated with a very grave outcome. Successful withdrawal of immunosuppressive therapy and donor leukocyte infusions in early relapse have occasionally been reported. Close monitoring of chimerism status after HSCT may allow initiation of pre-emptive immunotherapy. Second HSCT for relapse or graft failure after HSCT has shown survival rates of 50%.29
JMML
JMML is a unique pediatric disorder, different from chronic myelomonocytic leukemia in adults. For a detailed description of JMML, recently published reviews are available.5,6 The incidence of JMML is close to 1 per million children per year.30 The median age at presentation is 1.8 years, 35% are younger than age 1 year at presentation, and only 4% are older than age 5 years. JMML has a male predominance with a male:female ratio of 2:1.31
Clinical and laboratory features
Patients present with pallor, fever, infection, bleeding, or symptoms from organomegaly. Hepato- or splenomegaly, lymphadenopathy, or skin rash may be the first signs of JMML. Elevated WBC count with absolute monocytosis, anemia, and thrombocytopenia are almost universal. WBC count at presentation exceeds 50 × 109/L in 30% and is above 100 × 109/L in 7% of patients. Blood film appearance is characteristic and often more helpful in diagnosing than BM morphology in which monocytosis is often much more discrete.
Increased HbF for age is a main characteristic of JMML with the notable exception of those with monosomy 7, almost all of whom have normal HbF. A macular-papular skin rash is seen in 35% of the patients. Monosomy 7 (mostly as the sole abnormality) is present in 25% to 30% of patients with JMML, 10% have other cytogenetic aberrations, and 60% show a normal karyotype.31
Differential diagnoses
JMML may mimic infections and immunodeficiency, so delays in making the correct diagnosis are common. Conversely, infections, inborn errors of metabolism, and immunodeficiency may cause monocytosis and organomegaly thus representing diagnostic pitfalls. A diagnosis of JMML, especially in infants, should be made with caution.32 A period of observation is recommended for patients without clear-cut features or confirmatory molecular genetics. Viral infections such as Epstein-Barr virus, cytomegalovirus, herpesvirus 6, and parvovirus may mimic JMML. And various types of immunodeficiency—Wiskott-Aldrich syndrome, leukocyte adhesion defect, and osteopetrosis—may mimic JMML.33 The international consensus on current diagnostic criteria for JMML includes molecular genetics as a mandatory part of the workup (Table 2).5
Table 2.
Diagnostic criteria of JMML
| I. Clinical and hematologic features (all 4 features mandatory) |
| Peripheral blood monocyte count > 1 × 109/L |
| Blast percentage in peripheral blood and bone marrow <20% |
| Splenomegaly (not always apparent at diagnosis) |
| Absence of Philadelphia chromosome (BCR/ABL rearrangement) |
| II. Oncogenetic studies (1 finding is sufficient) |
| Somatic mutation in PTPN11, KRAS, or NRAS |
| Clinical diagnosis of NF1 or germ line NF1 mutation |
| Germ line CBL mutation and loss of heterozygosity of CBL |
| III. For patients with the clinical and hematologic features under (I) but without an oncogenetic criterion (10%), at least 2 of the following criteria must be fulfilled: |
| Monosomy 7 or any other chromosomal abnormality |
| HbF increased for age |
| Myeloid precursors in peripheral blood |
| Spontaneous growth or granulocyte-macrophage colony-stimulating factor hypersensitivity in colony assay |
| Hyperphosphorylation of STAT5 |
Table 3.
Characteristics of JMML-like NS/MPD in children
| Characteristic | JMML-like | JMML |
|---|---|---|
| NS status | NS | No NS |
| Age at onset | <2 mo | Median, 1.8 y |
| Leukocytosis | Present | Present |
| Monocytosis | Present | Present |
| Hepatosplenomegaly | Present | Present |
| Myelopoiesis | Polyclonal | Clonal |
| Cytogenetics | Normal | Abnormal in 35% |
| PTPN11 mutation | 90% (germ line) | 35% (somatic) |
| Most common substitution | T73I | E76K |
| Biological effect | Moderate gain of function | Strong gain of function |
| Outcome | Spontaneous regression | Fatal without transplantation |
Noonan syndrome
Infants with Noonan syndrome (NS) may show a JMML-like myeloproliferative disorder (NS/MPD) with spontaneous regression (Table 2). NS/MPD is diagnosed during the first few months of life, often during the first weeks in contrast to the median age of 1.8 years for diagnosis of non-NS JMML. In the majority of patients, the hematologic abnormalities gradually resolve, but normalization may take several months or even years; this is especially true for the monocytosis and splenomegaly, which may persist for several years.
NS/MPD has striking parallels with the transient leukemia/transient abnormal myelopoiesis of newborns with Down syndrome. Unlike the GATA1 mutation in transient abnormal myelopoiesis, NS/MPD has no somatic molecular marker, and there is no documented effective therapy in patients with NS with an aggressive course.
After the identification of PTPN11 germ line mutation in 50% of patients with NS, studies in non-NS JMML showed somatic PTPN11 mutations in 35%.34 The PTPN11 mutations found in JMML have a stronger SHP-2 activation than the mutations in NS, whereas the mutations in NS/MPD have an intermediate gain of function effect.35 It is presumed that the strong activation resulting from the PTPN11 mutation in JMML is incompatible with life when occurring as a germ line mutation.
Molecular genetics
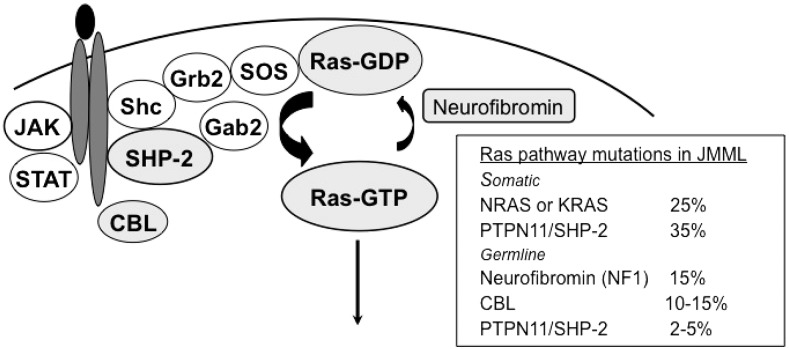
JMML is a clonal disorder that arises from a pluripotent stem cell. The mononuclear cells of PB and BM showed spontaneous proliferation when cultured in semisolid systems, and granulocyte-macrophage colony-stimulating factor hypersensitivity provided clues to the diagnosis, but it has been replaced by molecular studies. The granulocyte-macrophage colony-stimulating factor signal transduction pathway plays a major role in the pathogenesis of JMML, and mutations are found in the Ras signal transduction pathway downstream of the receptor in about 90% of patients (Figure 3); thus, JMML belongs to the group of diseases called RASopathies. Members of the Ras family of signaling proteins regulate cellular proliferation by cycling between an active guanosine triphosphate (GTP) –bound state (Ras-GTP) and an inactive guanosine diphosphate (Ras-GDP) –bound state. Ras activation is a crucial component of the proliferative response to growth factors. Point mutations in NRAS or KRAS that cause high constitutive Ras-GTP levels are noted in 25% of patients (Figure 3).
Figure 3.
Aberrations in the Ras signaling pathway that lead to excessive proliferation.
Children with neurofibromatosis type 1 (NF1) have an increased risk of malignant myeloid disorders, especially JMML. About 15% of children with JMML carry the clinical diagnosis of NF1.31 The NF1 gene functions as a tumor-suppressor gene, and loss of the normal NF1 allele occurs in the leukemic cells of NF1 patients. As expected, leukemic cells showed an elevated percentage of Ras in the GTP-bound state (Figure 3). NF1 and Ras mutations have been considered mutually exclusive, indicating that one abnormality is sufficient to activate Ras; however, the presence of a network of mutations in a large fraction of JMML patients has challenged the concept.36 In patients who have NF1 along with JMML and who are slightly older than others who have JMML, the clinical features of NF1 are often obvious, and genetic testing for NF1 is not necessary for JMML diagnostics.
CBL mutations are the genetic subset of JMML most recently described and found in 40% of those without NF1, RAS, or PTPN11 mutations, corresponding to 10% to 15% of patients with JMML overall. CBL mutations are mostly autosomal dominant germ line events, and patients with CBL mutations and JMML frequently display loss of heterozygosity for the CBL locus in the leukemic cells. Children with CBL mutations may be at risk for vascular disease later in life and some may show developmental delays.37
Natural course and prognostic factors.
The natural course of JMML is highly dependent on the underlying genetic abnormalities. Patients with germ line CBL and PTPN11 (NS) and some patients with somatic NRAS mutations have a slowly progressive disease that in most cases shows spontaneous remission. In contrast, patients with NF1, somatic PTPN11, KRAS, and most with NRAS mutations have JMML, which is rapidly fatal if left untreated. Low platelet count (<33 × 109/L), age older than 4 years, and high HbF (>15%) are strongly predictive of poor survival in multivariate analysis. Blastic transformation is infrequent, and most untreated patients die as a result of organ failure from infiltration of the leukemic cells. The most common cytogenetic aberration, monosomy 7, has in most series not been associated with outcome. The number of recurrent somatic mutations in genes involved in signal transduction, splicing, Polycomb repressive complex 2, and transcription at diagnosis seem to be the major determinants of outcome.38 Hypermethylation, gene expression profile, and mutation of SETBP1 all add significant prognostic information.38,39
Treatment.
Intensive chemotherapy is mostly unsuccessful in JMML because of an increased risk of treatment-related death, a low rate of true remissions, and long-term survival less than 10%. Low-dose chemotherapy with 6-mercaptopurine alone or combined with cytarabine may be indicated in patients with complications from hyperleukocytosis, organomegaly, or pulmonary infiltrates and may show some effective responses.40
Demethylating therapy with azacitidine in a retrospective series of 12 patients showed partial response in 50%.41 The potential benefit of azacitidine is being tested prospectively. The identification of JAK3 mutation in about 5% of patients with JMML38 raises the possibility of therapy with inhibitors for the subset of patients with these mutations.
Evaluating the efficacy of JMML therapy is difficult and had not been standardized until the recent publication of uniform criteria of response, including WBC count, platelet count, precursors and blasts in PB, BM blast percentage, spleen size, and extramedullary disease.42 The complex response evaluation helps describe the heterogeneous picture of response to therapy.
Allogeneic HSCT is the only curative approach for JMML in patients with NF1, somatic PTPN11, KRAS, and most with NRAS. The overall survival with a conditioning regimen of busulfan, cyclophosphamide, and melphalan in a cohort of 100 patients was 64%.43 Conditioning with total body irradiation should be avoided because of a poorer antileukemic effect and a higher risk of late effects. The risk of relapse is high after using family or unrelated donor HSCT or cord blood transplantation. Younger age at HSCT, male sex, low HbF, and low blast percentage in BM predict for improved survival, whereas spleen size and monosomy 7 are not prognostic.43 Disease recurrence remains the major cause of treatment failure. Reduced intensity and duration of GvHD prophylaxis may significantly contribute to successful leukemia control, and both acute and chronic GvHD are associated with a lower risk of relapse.43 Because of the high risk of relapse and the impact of graft-versus-leukemia disease, it is recommended to give low-intensity GvHD prophylaxis that should be discontinued 60 to 90 days after HSCT. An exception may be patients with KRAS mutations who have a low risk of relapse; for them, a high-intensity GvHD prophylaxis is indicated.5
Relapse occurs in up to 40% of patients and often at a median of only 2 to 4 months from HSCT. Early detection of donor cells by increasing mixed chimerism may be successfully eradicated by discontinuing ongoing IST. Donor lymphocyte infusion in JMML relapse is largely unsuccessful. If there is no response to withdrawal of IST, a second transplantation with the same or an alternative donor should be offered as soon as possible and may cure about one-third of the patients.5
Conclusion and future directions
MDS in children is a heterogeneous disease, which should be reflected in the management of the patients. The increasing knowledge of germ line and somatic genetic aberrations may help us better understand the disease and refine recommendations for management.
The prognosis of JMML is strongly associated with genetic mutations. HSCT is the treatment of choice for most patients, but for some patients a watch-and-wait strategy is recommended. Further studies may identify targets for potential therapy for this subgroup of patients.
References
- 1.Hasle H, Niemeyer CM, Chessells JM, et al. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;17(2):277-282. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Harris NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008 [Google Scholar]
- 3.Hasegawa D. The current perspective of low-grade myelodysplastic syndrome in children. Int J Hematol. 2016;103(4):360-364. [DOI] [PubMed] [Google Scholar]
- 4.Hofmann I. Myeloproliferative Neoplasms in Children. J Hematop. 2015;8(3):143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locatelli F, Niemeyer CM. How I treat juvenile myelomonocytic leukemia. Blood. 2015;125(7):1083-1090. [DOI] [PubMed] [Google Scholar]
- 6.Chang TY, Dvorak CC, Loh ML. Bedside to bench in juvenile myelomonocytic leukemia: insights into leukemogenesis from a rare pediatric leukemia. Blood. 2014;124(16):2487-2497. [DOI] [PubMed] [Google Scholar]
- 7.Hasle H, Niemeyer CM. Advances in the prognostication and management of advanced MDS in children. Br J Haematol. 2011;154(2):185-195. [DOI] [PubMed] [Google Scholar]
- 8.Baumann I, Führer M, Behrendt S, et al. Morphological differentiation of severe aplastic anaemia from hypocellular refractory cytopenia of childhood: reproducibility of histopathological diagnostic criteria. Histopathology. 2012;61(1):10-17. [DOI] [PubMed] [Google Scholar]
- 9.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nat Genet. 2011;43(10):929-931. [DOI] [PubMed] [Google Scholar]
- 10.Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol. 2015;169(2):173-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wlodarski MW, Hirabayashi S, Pastor V, et al. ; EWOG-MDS. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127(11):1387-1397. [DOI] [PubMed] [Google Scholar]
- 12.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biol Blood Marrow Transplant. 2014;20(12):1940-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cioc AM, Wagner JE, MacMillan ML, DeFor T, Hirsch B. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with fanconi anemia: morphologic and cytogenetic characteristics. Am J Clin Pathol. 2010;133(1):92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg PS, Zeidler C, Bolyard AA, et al. Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br J Haematol. 2010;150(2):196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadieu J, Fenneteau O, Beaupain B, et al. ; Associated investigators of the French Severe Chronic Neutropenia Registry*. Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica. 2012;97(9):1312-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutinho DF, Monte-Mór BC, Vianna DT, et al. TET2 expression level and 5-hydroxymethylcytosine are decreased in refractory cytopenia of childhood. Leuk Res. 2015;39(10):1103-1108. [DOI] [PubMed] [Google Scholar]
- 17.Hirabayashi S, Flotho C, Moetter J, et al. ; European Working Group of MDS in Childhood. Spliceosomal gene aberrations are rare, coexist with oncogenic mutations, and are unlikely to exert a driver effect in childhood MDS and JMML. Blood. 2012;119(11):e96-e99. [DOI] [PubMed] [Google Scholar]
- 18.Vidal DO, Paixão VA, Brait M, et al. Aberrant methylation in pediatric myelodysplastic syndrome. Leuk Res. 2007;31(2):175-181. [DOI] [PubMed] [Google Scholar]
- 19.Göhring G, Michalova K, Beverloo HB, et al. Complex karyotype newly defined: the strongest prognostic factor in advanced childhood myelodysplastic syndrome. Blood. 2010;116(19):3766-3769. [DOI] [PubMed] [Google Scholar]
- 20.Hasle H, Clausen N, Pedersen B, Bendix-Hansen K. Myelodysplastic syndrome in a child with constitutional trisomy 8 mosaicism and normal phenotype. Cancer Genet Cytogenet. 1995;79(1):79-81. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimi A, Baumann I, Führer M, et al. Immunosuppressive therapy with anti-thymocyte globulin and cyclosporine A in selected children with hypoplastic refractory cytopenia. Haematologica. 2007;92(3):397-400. [DOI] [PubMed] [Google Scholar]
- 22.Yoshimi A, van den Heuvel-Eibrink MM, Baumann I, et al. Comparison of horse and rabbit antithymocyte globulin in immunosuppressive therapy for refractory cytopenia of childhood. Haematologica. 2014;99(4):656-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aalbers AM, van der Velden VH, Yoshimi A, et al. The clinical relevance of minor paroxysmal nocturnal hemoglobinuria clones in refractory cytopenia of childhood: a prospective study by EWOG-MDS. Leukemia. 2014;28(1):189-192. [DOI] [PubMed] [Google Scholar]
- 24.Samarasinghe S, Marsh J, Dufour C. Immune suppression for childhood acquired aplastic anemia and myelodysplastic syndrome: where next? Haematologica. 2014;99(4):597-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strahm B, Locatelli F, Bader P, et al. Reduced intensity conditioning in unrelated donor transplantation for refractory cytopenia in childhood. Bone Marrow Transplant. 2007;40(4):329-333. [DOI] [PubMed] [Google Scholar]
- 26.Gore SD, Fenaux P, Santini V, et al. A multivariate analysis of the relationship between response and survival among patients with higher-risk myelodysplastic syndromes treated within azacitidine or conventional care regimens in the randomized AZA-001 trial. Haematologica. 2013;98(7):1067-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cseh AM, Niemeyer CM, Yoshimi A, et al. Therapy with low-dose azacitidine for MDS in children and young adults: a retrospective analysis of the EWOG-MDS study group. Br J Haematol. 2016;172(6):930-936. [DOI] [PubMed] [Google Scholar]
- 28.Strahm B, Nöllke P, Zecca M, et al. ; EWOG-MDS study group. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG-MDS 98 study. Leukemia. 2011;25(3):455-462. [DOI] [PubMed] [Google Scholar]
- 29.Kato M, Yoshida N, Inagaki J, et al. Salvage allogeneic stem cell transplantation in patients with pediatric myelodysplastic syndrome and myeloproliferative neoplasms. Pediatr Blood Cancer. 2014;61(10):1860-1866. [DOI] [PubMed] [Google Scholar]
- 30.Hasle H, Wadsworth LD, Massing BG, McBride M, Schultz KR. A population-based study of childhood myelodysplastic syndrome in British Columbia, Canada. Br J Haematol. 1999;106(4):1027-1032. [DOI] [PubMed] [Google Scholar]
- 31.Niemeyer CM, Arico M, Basso G, et al. ; European Working Group on Myelodysplastic Syndromes in Childhood (EWOG-MDS). Chronic myelomonocytic leukemia in childhood: a retrospective analysis of 110 cases. Blood. 1997;89(10):3534-3543. [PubMed] [Google Scholar]
- 32.Karow A, Baumann I, Niemeyer CM.. Morphologic differential diagnosis of juvenile myelomonocytic leukemia–pitfalls apart from viral infection. J Pediatr Hematol Oncol. 2009;31(5):380. [DOI] [PubMed] [Google Scholar]
- 33.Strauss A, Furlan I, Steinmann S, et al. Unmistakable Morphology? Infantile Malignant Osteopetrosis Resembling Juvenile Myelomonocytic Leukemia in Infants. J Pediatr. 2015;167(2):486-488. [DOI] [PubMed] [Google Scholar]
- 34.Tartaglia M, Niemeyer CM, Fragale A, et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148-150. [DOI] [PubMed] [Google Scholar]
- 35.Kratz CP, Schubbert S, Bollag G, Niemeyer CM, Shannon KM, Zenker M. Germline mutations in components of the Ras signaling pathway in Noonan syndrome and related disorders. Cell Cycle. 2006;5:1607-1611. [DOI] [PubMed] [Google Scholar]
- 36.Caye A, Strullu M, Guidez F, et al. Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network. Nat Genet. 2015;47(11):1334-1340. [DOI] [PubMed] [Google Scholar]
- 37.Niemeyer CM, Kang MW, Shin DH, et al. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42(9):794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stieglitz E, Taylor-Weiner AN, Chang TY, et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat Genet. 2015;47(11):1326-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakaguchi H, Muramatsu H, Okuno Y, et al. Aberrant DNA Methylation Is Associated with a Poor Outcome in Juvenile Myelomonocytic Leukemia. PLoS One. 2015;10(12):e0145394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gustafsson B, Hellebostad M, Ifversen M, Sander B, Hasle H. Acute respiratory failure in 3 children with juvenile myelomonocytic leukemia. J Pediatr Hematol Oncol. 2011;33(8):e363-e367. [DOI] [PubMed] [Google Scholar]
- 41.Cseh A, Niemeyer CM, Yoshimi A, et al. Bridging to transplant with azacitidine in juvenile myelomonocytic leukemia: a retrospective analysis of the EWOG-MDS study group. Blood. 2015;125(14):2311-2313. [DOI] [PubMed] [Google Scholar]
- 42.Niemeyer CM, Loh ML, Cseh A, et al. Criteria for evaluating response and outcome in clinical trials for children with juvenile myelomonocytic leukemia. Haematologica. 2015;100(1):17-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Locatelli F, Nöllke P, Zecca M, et al. ; European Working Group on Childhood MDS; European Blood and Marrow Transplantation Group. Hematopoietic stem cell transplantation (HSCT) in children with juvenile myelomonocytic leukemia (JMML): results of the EWOG-MDS/EBMT trial. Blood. 2005;105(1):410-419. [DOI] [PubMed] [Google Scholar]