Abstract
Conventional treatment of thalassemia, namely regular blood transfusion and iron chelation, improves perspectives and quality of life; however, successful treatment leads to more time in which long-term complications such as bone disease can develop. Thalassemia bone disease (TBD) is unique: all aspects, from bone anatomy and bone quality to mineral density, may be affected, with important morbidity including osteoporosis, fractures, spinal deformities, nerve compression, and pain. Clinical presentations include growth impairment, rickets-like features, back pain, spinal deformities, any sign of nerve compression, severe osteoporosis, and fragility fractures. Age, history, physical examination, and diagnostic tests support orientation on risk factors. These include bone marrow expansion, toxicity from iron overload and iron chelation, endocrine dysfunctions (hypogonadism, hypohyperparathyroidism, hypothyroidism, growth hormone deficiency, diabetes), and vitamin (D, C, K) and zinc deficiencies. Several of these may coexist in an individual for a long time and at different degrees, making clarification of the relative contribution and selection of the best therapeutic options a challenge. Milestones for prevention of TBD are early and full inhibition of bone marrow hyperplasia and iron toxicity. Empowering patients’ positive resources is key for achieving long-term healthy habits with regard to diet, physical activity, sunlight exposure, and lifestyle. Pain, related or unrelated to bone disease, is frequent in thalassemia. The most important targets for the hematologist include having an expert orientation on disease-related causes of pain, driving differential diagnosis, providing effective pain relief and, where feasible, removing the cause.
Learning Objectives
Become familiarized with the various causes of bone disease in thalassemia as well as its management
Become familiarized with disease-related causes of pain in thalassemia
Bone disease in thalassemia
Untreated thalassemia is associated with anemia, erythroid marrow hyperplasia, and skeletal deformities. Conventional treatment comprising blood transfusion and iron chelation, where applied regularly, improves perspectives and quality of life. More time also means more room for long-term complications. For example, osteoporosis is common in adult patients, with chronic anemia and iron overload as obvious predisposing factors; however, multiple risk factors may coexist in the same patient making it hard to quantify the relative contribution of each and to establish adequate management.
Thalassemia bone disease (TBD) is unique: all aspects, from bone anatomy and bone quality to mineral density, may be affected, with important morbidity including osteoporosis, fractures, spinal deformities, nerve compression, and pain.1-3
Many factors may contribute to TBD, including bone marrow expansion, increased bone turnover, endocrine and vitamin deficiencies, toxicity from iron overload, and iron chelation. In a thalassemic patient, several of these factors may coexist for a long time and at different degrees. This makes clarification of the relative contribution to TBD and selection of the best therapeutic options difficult.
Factors predisposing to TBD
Bone marrow expansion
Abnormal proliferation of bone marrow cells, independent of hematopoietic lineage, is associated with bone loss.1 In severe thalassemia, ineffective erythropoiesis causes a bone marrow expansion by a factor of up to 30 times, which is not fully cancelled even with an optimal transfusion regimen. Medullary trabeculae are destroyed with cortical thinning.4 The skull may have a “hair-on-end” appearance with bossing of facial bones, malocclusion, and the typical thalassemic facies. Long bones, mainly humeri, may loose the concave profile and be short, with signs of growth arrest and recovery like transverse epiphyseal radio-dense lines.
Iron overload
Iron metabolism may independently contribute to bone homeostasis.1 Reduced hepcidin levels negatively affect bone homeostasis by lowering bone formation and increasing bone resorption.5 In vitro studies demonstrate that iron inhibits human osteoblastic activity and favors osteoclast differentiation and bone resorption,6 through an elevated receptor activator of NF-κB ligand (RANKL)-to-osteoprotegerin (OPG) ratio.7 In humans with hemochromatosis or secondary iron overload, the causative relationship with osteoporosis is clouded by the copresence of hypogonadism and other endocrinopathies, but a strong trend to osteoporosis and fractures is also documented in young eugonadal patients, as well as improved iron clearing.8
Some findings indicate that in thalassemia intermedia and thalassemia major, bone disease differs at least in 1 aspect, low-vs-high bone turnover, respectively, with implications for treatment.9 The origin of such a difference may reside in different levels of iron overload and iron turnover.
Iron chelation
A few studies specifically addressed the role of iron chelation therapy in the prevention or control of iron overload–associated bone disease. Both positive and negative effects of iron chelation have been reported.
Deferoxamine has been associated with bone dysplasia, especially with early start, high doses, and reduced iron stores. Long bones show metaphyseal changes with rickets-like widening and sclerotic lesions. Severe epiphyseal dysplasia may cause genu varum or valgum and require surgery. Vertebral body changes, mainly platyspondyly at the thoracolumbar tract, result in a short and kyphotic trunk.10
These changes have not been observed with the oral chelators. Deferiprone may induce arthropathy with synovial and cartilage alterations and subchondral bone flattening. Deferasirox has been associated both with an increase in bone mineral density (BMD),11,12 and with hypercalciuria and nephrocalcinosis.13,14
Hypogonadism
Free estrogen and testosterone are key in increasing OPG messenger RNA and decreasing RANKL. Lack of sex steroids with delayed puberty from any cause contributes to failure of achieving optimal peak bone mass and predisposing to severe osteoporosis.15 In thalassemia, early pituitary damage from iron toxicity is responsible for hypogonadotrophic hypogonadism and delayed/incomplete puberty. This condition is very common even today in patients treated according to the best standards.16
Parathyroid dysfunction
Hypoparathyroidism is a late but common complication of iron overload/toxicity with poor or no chelation. Although its relationship with hypercalciuria and nephrolithiasis is clear, it is not so for osteoporosis, with contrasting findings in different papers. In optimally treated patients, hypoparathyroidism is uncommon, whereas secondary hyperparathyroidism due to vitamin D deficiency is receiving more attention, with increasing diagnostic challenges in the individual patient.3,17
Hypothyroidism
Both hyperthyroidism and hypothyroidism are associated with osteoporosis and increased risk of fractures.18 In thalassemia, hypothyroidism is frequent and easily corrected with thyroid hormone replacement. In contrast, the time on hormone replacement is inversely related with BMD and increases the risk of fractures even in the presence of euthyroidism. This suggests that caution should be used in prescribing long-term thyroxin treatment in any thalassemic patient with borderline thyroid function.
Growth hormone deficiency
During childhood and adolescence, growth hormone (GH) plays a key role in linear growth and attainment of appropriate height and peak bone mass. GH interacts directly with GH receptors on osteoblasts and via locally produced insulin-like growth factor 1 (IGF1). GH deficiency with low IGF1 and the corresponding binding protein is frequent both in pediatric and adult patients and has been related to iron toxicity.19 Treatment with GH increases vertebral and femoral BMD over time.20
Diabetes
Insulin-dependent diabetes type 2 in thalassemia is a long-term complication from iron toxicity on pancreatic cells. It is more frequent in adult patients with poor control of iron overload.
Liver disease
Hepatitis C virus is now disappearing where direct-acting antiviral agents have been applied,21 but long exposure to viral and iron load may make chronic liver disease and cirrhosis irreversible. This may favor bone disease throughout different pathogenic mediators, like fibronectin, IGF1, and vitamin D metabolism.
Renal disease
Differently from the past, kidney dysfunction has growing importance in thalassemia, due to prolonged survival and the rising frequency of renal hyperfiltration, hypercalciuria, kidney stones, and tubular dysfunction.22
Vitamin D deficiency
The prevalence of vitamin D deficiency is high in thalassemic patients, even for those living in regions with high sunshine exposure and in those with normal diet. The diagnosis of deficiency is elusive. Signs and symptoms of vitamin D deficiency are nonspecific and common in thalassemia: muscle weakness, bone pain, osteopenia/osteoporosis. The presence of radiological and magnetic resonance imaging (MRI) changes in the long-bone metaphyses, periosteal reaction, and adjacent soft-tissue edema have diagnostic value, but their absence does not exclude vitamin D deficiency. The assessment of 25-hydroxy vitamin D serum levels is key for vitamin D deficiency, but may overestimate it, due to preanalytical (season, skin color) and analytical (different assay variability) reasons.23
Secondary hyperparathyroidism is strongly associated with vitamin D deficiency. These 2 conditions, throughout calcium channel regulation, may be the bridge to cardiac iron load and dysfunction.
Vitamin C deficiency
In subjects with a normal diet, vitamin C deficiency is possible in the presence of hyperconsumption, like chronic iron toxicity.24 In thalassemic patients with high iron overload and poor or no chelation, ascorbate deficiency may contribute to TBD, by impairing chondrocyte and osteoblast function, with impaired long bones growth, subperiosteal hemorrhage, and fractures.25
Vitamin K deficiency
Even though the effect of vitamin K2 on bone mass density is limited, its deficiency may contribute to osteoporotic fractures, whereas vitamin K2 administration improves osteoblastic function and inhibits osteoclastic function. In thalassemia, vitamin K2, combined with calcitriol, improves lumbar spine BMD.26
Zinc deficiency
Many other deficiencies that may potentially impact on bone health and growth in thalassemia have been reported, but for a few of them a clinical relevance has been demonstrated (Table 1).
Table 1.
Schematic summary of factors that may contribute to TBD and their management
| Factor | Key mechanism | Treatment | Prevention |
|---|---|---|---|
| Bone marrow expansion | Ineffective erythropoiesis | Transfusion at optimal Hb levels | Transfusion at optimal Hb levels |
| Iron overload | Iron toxicity | Optimal iron chelation | Early and regular iron chelation |
| Iron chelation | Overchelation; drug toxicity | Tune chelation intensity on iron overload; avoid high doses | Tune chelation intensity on iron overload |
| Hypogonadism | Iron toxicity | Replacement therapy | Early and regular iron chelation |
| Hypoparathyroidism | Iron toxicity | Replacement therapy | Early and regular iron chelation |
| Hyperparathyroidism | Vitamin D deficiency | Vitamin D2 or D3 | Vitamin D2 or D3 |
| Hypothyroidism | Iron toxicity | Replacement therapy | Early and regular iron chelation |
| GH | Iron toxicity | Replacement therapy | Early and regular iron chelation |
| Diabetes | Iron toxicity | Replacement therapy | Regular iron chelation; lifestyle |
| Liver disease | Viral hepatitis; iron toxicity | Antiviral therapy; regular iron chelation | Safe blood; regular iron chelation |
| Vitamin D deficiency | Iron toxicity | Vitamin D2 or D3 | ? |
| Renal disease | Hypercalciuria | Correct causes | ? |
| Vitamin C deficiency | Iron toxicity | Vitamin C–rich diet | Optimal iron chelation |
Hb, hemoglobin TBD, thalassemia bone disease.
Low serum zinc levels are frequently observed in thalassemia and associated with hemolysis, oxidative damage, and the effect of iron chelators.27 Benefits for osteoporosis and height growth are based on uncontrolled studies.28
Clinical aspects and management of TBD
Bone disease in thalassemia may be asymptomatic for years. The starting point in the individual patient is to assess the relative contribution of the many potential risk factors. Age, history, physical examination, and diagnostic tests are helpful in orienting the diagnosis. Clinical presentations include growth impairment, rickets-like features, back pain, spinal deformities, any sign of nerve compression, severe osteoporosis, and fragility fractures. Often, osteoporosis-related fractures, including vertebral ones, are unappreciated. In adults, height loss from serial height measurements is a useful tool in detecting patients with vertebral fractures and lowering future fracture risk.29
In very young patients, any sign of bone marrow expansion and bone enlargement must be assessed and followed. In the absence of any validated method to quantify them, changes at serial checks are useful in predicting progression. Imaging findings on radiography and MRI studies and radiographs are useful, as well as serial pictures of the patient; at diagnosis, even asking parents to share previous pictures may help.
The start of regular transfusion allows bone expansion to freeze or even reverse. Mean pretransfusional hemoglobin levels must be adequate (9-9.5 g/L in β-thalassemia major). Full reversal is possible only during the first years of life. Plastic surgery is rarely applied to thalassemia, whereas orthodontic interventions are effective but require light forces and special care.30
Reducing a severe iron overload must always be a priority in treating or preventing TBD, due to the deleterious effect of iron toxicity.6 Choice of chelators, doses, and iron chelation scheme should be tailored to fit individual needs. Strict monitoring may prevent overchelation that, at least for deferoxamine, is associated with bone alterations.10
Hypogonadotrophic hypogonadism and GH deficiency remain frequent even in the modern era of iron chelation, as the cause is early and irreversible damage from iron toxicity to sensitive pituitary cells. It is difficult to prevent this toxicity, at least in thalassemia major, as it is difficult to handle iron chelation in infanthood at low iron overload. The impact of hypogonadism and GH deficiency on TBD is very important, as both hinder progression to normal peak bone mass during childhood and puberty, severely predisposing patients to osteoporosis in adulthood. The diagnosis of hypogonadism is clinical, based on the absence of pubertal development or delay or incomplete maturation of secondary sex characteristics, confirmed by low luteinizing hormone, follicle-stimulating hormone, and estradiol/testosterone levels.
To prevent hypogonadism and GH deficiency, iron chelation should start as early as possible. It is likely that the recommendation of most guidelines, to start iron chelation after 10 to 20 transfusions or when serum ferritin is above 1000, is inadequate.31
Throughout childhood and adolescence, biannual growth assessment, including standing and sitting height, bone age, and pubertal staging, is functional to well-timed diagnosis and treatment. In deficient patients, replacement therapy with GH or sexual hormones has been proven effective in thalassemia.31 Zinc supplementation may be considered in individual patients with low serum levels.
The decision to start supplementing to prevent vitamin D deficiency is based on regular monitoring of serum levels of 25-OH vitamin D. A calcium-rich diet and cholecalciferol should be preferred to oral calcium and active metabolites in a condition like thalassemia, where independent factors contribute to hypercalciuria and the risk of kidney stones and nephrocalcinosis. The presence of hypoparathyroidism requires a more aggressive approach.32
Management of osteoporosis in thalassemia follows general principles. Treatment should always be considered if osteoporosis is present or there is a history of fragility fracture, or in the setting of osteopenia with a high risk for fracture. There is evidence that bisphosphonates in thalassemia improve BMD but data on long-term effects are lacking.32 Oral alendronate, IV zoledronate, neridronate, and clodronate have been tested in randomized controlled trials. Even if head-to-head trials have not been done, zoledronate and neridronate should be considered as first-line agents in the management of thalassemia-associated osteoporosis.32 Prescribers should be aware that in thalassemia, side effects like atypical fractures of the femur have also been found.32,33
Other interventions for TBD, such as denosumab, strontium ranelate, and teriparatide, have been published. All of them are interesting for the mechanism of action, but cannot be recommended due to the limited data available.16,32
Prevention of TBD
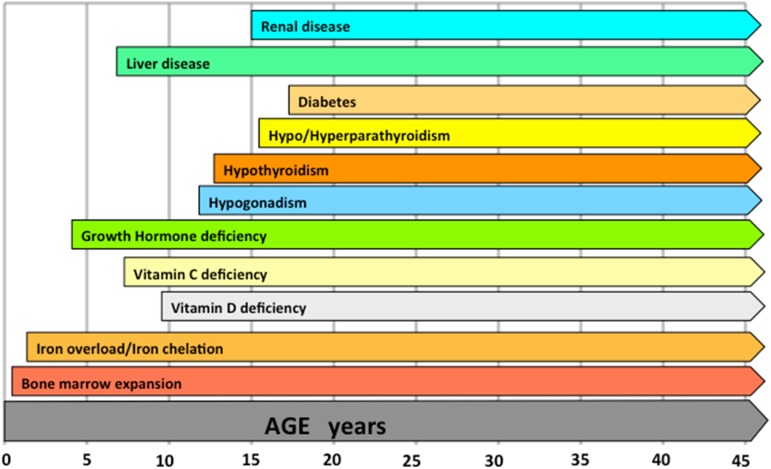
In any severe thalassemia phenotype, milestones for prevention of TBD are early and full inhibition of bone marrow hyperplasia and iron toxicity. This may be accomplished by the early start of regular transfusion and iron chelation. Excluding specific circumstances, leaving unbalanced severe anemia and iron overload and prescribing drugs for TBD should be considered medical nonsense and raise ethical concerns. An approximate timeline of the occurrence of complications or conditions relevant for bone disease if the treatment of thalassemia is not optimal is shown in Figure 1. Each thalassemic patient, regardless of transfusion dependence, should receive full information about risk factors and early sighs/symptoms of TBD. Follow-up should be personalized according to age and risk factors. Treating staff should be motivated in enpowering patients' positive resources, which are key for achieving long-term healthy habits with regards to diet, physical activity, sunlight exposure, and lifestyle.
Figure 1.
Approximate timeline of the occurrence of complications or conditions relevant for bone disease if the treatment of thalassemia is not optimal.
Pain in thalassemia
The most important target for the hematologist or any medical professional is to outline an expert orientation on disease-related causes of pain, to drive differential diagnosis, effective pain relief and, where feasible, removal of the cause.
Differently from sickle cell disease, pain is uncommon during the first years of life and becomes frequent in adult life.34 Most patients older than 35 years of age report chronic pain of moderate-severe intensity. Pain severity increases with age, but does not vary significantly with sex or thalassemia diagnosis. Quality of life due to pain in thalassemia declines greatly with age, compared with the general population.35 Chronic pain is more frequent in patients who started regular transfusions later and had a diagnosis of thalassemia intermedia; it is associated with a more expanded hypercellular bone marrow on MRI.36 Hydroxyurea treatment is able to induce MRI modifications and pain relief.36
The most common site of chronic pain is the lower back. Pain may be triggered by physical activity like prolonged standing and lifting of heavy objects, but the most frequent pain trigger is low hemoglobin level, with relief from transfusion, especially in patients with longer transfusion cycles.35,37,38 MRI imaging of the spine may reveal abnormal vertebral morphology, disc degeneration, and various degrees of osteoporosis. Changes are more extensive than in patients with back pain and no thalassemia.39 Collapse or crush fractures of the vertebral body are also frequently seen.
Patients with more sites of pain or more visits with pain showed higher symptoms of depression and anxiety.40
Diagnostic orientation by site
Headache
Headache characteristics such as onset, intensity, and recurrence, and the coexistence of fever or neurological signs, help orientation. Disease-related causes of headache include infections, mainly in patients with severe iron overload who have been splenectomized (cerebral abscess, meningitis). Otitis and sinusitis are more frequent in patients with thalassemic bone alterations. Occurrence of extramedullary erythropoiesis is possible but uncommon.
Chest pain
Onset, localization, and breath involvement help diagnostic orientation. In undertreated patients, rib displacement or fracture and osteochondritis are common. Splenectomized patients have a raised risk of pulmonary embolism. Onset of congestive heart failure may be slow and hidden with no edemas, mild weight gain, and isolated pain due to liver capsule distension; depending on irradiation, pain may involve the abdomen or back or chest. Aseptic pericarditis should be considered when severe iron overload goes along with no chelation. Acute coronary syndrome is uncommon in thalassemia. Masses of extramedullary erythropoiesis are typically asymptomatic, remain undiagnosed, and do not cause pain if not for nerve compression.
Abdominal pain
Onset and localization help diagnostic orientation. Gallstones, cholecystitis, cholangitis, and pancreatitis must always be considered. Kidney stones are frequent in thalassemia due to hypercalciuria. In patients on deferoxamine, pain may be the first sign of a life-threatening Yersinia infection. As for chest pain, abdominal pain may flag the onset of congestive heart failure. Splenectomized patients are at risk of portal vein thrombosis. Back or abdominal pain may also indicate a delayed hemolytic transfusion reaction. Masses of extramedullary erythropoiesis are typically asymptomatic and remain undiagnosed.41
Back pain
Osteoporosis may pass asymptomatic or cause compression signs and pain due to microfractures and vertebral bodies flattening.16 The same may happen with disk degeneration or in the presence of extramedullary erythropoiesis.
Kidney stones are frequent in thalassemia due to hypercalciuria. Also, gallstones with posterior irradiation should be considered in cases of back pain. Back pain, due to liver capsule distension, may herald congestive heart failure.
Back or abdominal pain may also indicate a delayed hemolytic transfusion reaction.
Foot pain
Foot stress fractures are associated with osteoporosis and TBD.
Joint pain
In thalassemia, arthropathy or arthritis may be associated with distinct factors: iron overload in the absence of iron chelation, hyperuricemia, and deferiprone.42
Conclusions
Conventional treatment of thalassemia improves perspectives of life, with more room for long-term complications such as bone disease and pain. Better knowledge of its many causes is key for optimal management and prevention.
References
- 1.Steer K, Stavnichuk M, Morris M, Komarova SV Bone health in patients with hematopoietic disorders of bone marrow origin: systematic review and meta-analysis. J Bone Miner Res. 2017;32(4):731-742. [DOI] [PubMed]
- 2.Baldini M, Marcon A, Ulivieri FM, et al. . Bone quality in beta-thalassemia intermedia: relationships with bone quantity and endocrine and hematologic variables. Ann Hematol. 2017;96(6):995-1003. [DOI] [PubMed] [Google Scholar]
- 3.Baldini M, Forti S, Orsatti A, et al. . Bone disease in adult patients with β-thalassaemia major: a case-control study. Intern Emerg Med. 2014;9(1):59-63. [DOI] [PubMed] [Google Scholar]
- 4.Tyler PA, Madani G, Chaudhuri R, Wilson LF, Dick EA. The radiological appearances of thalassaemia. Clin Radiol. 2006;61(1):40-52. [DOI] [PubMed] [Google Scholar]
- 5.Xu Z, Sun W, Li Y, et al. . The regulation of iron metabolism by hepcidin contributes to unloading-induced bone loss. Bone. 2017;94:152-161. [DOI] [PubMed] [Google Scholar]
- 6.Jeney V. Clinical impact and cellular mechanisms of iron overload-associated bone loss. Front Pharmacol. 2017;8:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morabito N, Gaudio A, Lasco A, et al. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: new pieces of the puzzle. J Bone Miner Res. 2004;19(5):722-727. [DOI] [PubMed]
- 8.Angelopoulos NG, Goula AK, Papanikolaou G, Tolis G Osteoporosis in HFE2 juvenile hemochromatosis: a case report and review of the literature. Osteoporos Int. 2006;17(1):150-155. [DOI] [PubMed]
- 9.Chatterjee R, Shah FT, Davis BA, et al. . Prospective study of histomorphometry, biochemical bone markers and bone densitometric response to pamidronate in β-thalassaemia presenting with osteopenia-osteoporosis syndrome. Br J Haematol. 2012;159(4):462-471. [DOI] [PubMed] [Google Scholar]
- 10.De Sanctis V, Pinamonti A, Di Palma A, et al. . Growth and development in thalassaemia major patients with severe bone lesions due to desferrioxamine. Eur J Pediatr. 1996;155(5):368-372. [DOI] [PubMed] [Google Scholar]
- 11.Poggi M, Sorrentino F, Pugliese P, et al. . Longitudinal changes of endocrine and bone disease in adults with β-thalassemia major receiving different iron chelators over 5 years. Ann Hematol. 2016;95(5):757-763. [DOI] [PubMed] [Google Scholar]
- 12.Casale M, Citarella S, Filosa A, et al. . Endocrine function and bone disease during long-term chelation therapy with deferasirox in patients with β-thalassemia major. Am J Hematol. 2014;89(12):1102-1106. [DOI] [PubMed] [Google Scholar]
- 13.Wong P, Polkinghorne K, Kerr PG, et al. . Deferasirox at therapeutic doses is associated with dose-dependent hypercalciuria. Bone. 2016;85:55-58. [DOI] [PubMed] [Google Scholar]
- 14.Efthimia V, Neokleous N, Agapidou A, et al. . Nephrolithiasis in beta thalassemia major patients treated with deferasirox: an advent or an adverse event? A single Greek center experience. Ann Hematol. 2013;92(2):263-265. [DOI] [PubMed] [Google Scholar]
- 15.Saki N, Abroun S, Salari F, Rahim F, Shahjahani M, Javad MA. Molecular aspects of bone resorption in β-thalassemia major. Cell J. 2015;17(2):193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong P, Fuller PJ, Gillespie MT, Milat F. Bone disease in thalassemia: a molecular and clinical overview. Endocr Rev. 2016;37(4):320-346. [DOI] [PubMed] [Google Scholar]
- 17.Soliman A, De Sanctis V, Yassin M. Vitamin D status in thalassemia major: an update. Mediterr J Hematol Infect Dis. 2013;5(1):e2013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mirza F, Canalis E. Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015;173(3):R131-R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skordis N, Kyriakou A. The multifactorial origin of growth failure in thalassaemia. Pediatr Endocrinol Rev. 2011;8(suppl 2):271-277. [PubMed] [Google Scholar]
- 20.Scacchi M, Danesi L, Cattaneo A, et al. . Bone turnover and mineral density in adult thalassemic patients: relationships with growth hormone secretory status and circulating somatomedins. Endocrine. 2016;53(2):551-557. [DOI] [PubMed] [Google Scholar]
- 21.Mangia A, Sarli R, Gamberini R, et al. . Randomised clinical trial: sofosbuvir and ledipasvir in patients with transfusion-dependent thalassaemia and HCV genotype 1 or 4 infection. Aliment Pharmacol Ther. 2017;46(4):424-431. [DOI] [PubMed] [Google Scholar]
- 22.Quinn CT, Johnson VL, Kim HY, et al. ; Thalassemia Clinical Research Network. Renal dysfunction in patients with thalassaemia. Br J Haematol. 2011;153(1):111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couchman L, Moniz CF. Analytical considerations for the biochemical assessment of vitamin D status. Ther Adv Musculoskelet Dis. 2017;9(4):97-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golriz F, Donnelly LF, Devaraj S, Krishnamurthy R. Modern American scurvy: experience with vitamin C deficiency at a large children’s hospital. Pediatr Radiol. 2017;47(2):214-220. [DOI] [PubMed] [Google Scholar]
- 25.Aghajanian P, Hall S, Wongworawat MD, Mohan S The roles and mechanisms of actions of vitamin C in bone: new developments. J Bone Miner Res. 2015;30(11):1945-1955. [DOI] [PMC free article] [PubMed]
- 26.Ozdemir MA, Yilmaz K, Abdulrezzak U, et al. . The efficacy of vitamin K2 and calcitriol combination on thalassemic osteopathy. J Pediatr Hematol Oncol. 2013;35(8):623-627. [DOI] [PubMed] [Google Scholar]
- 27.Ozturk Z, Genc GE, Gumuslu S Minerals in thalassaemia major patients: an overview. J Trace Elem Med Biol. 2017;41:1-9. [DOI] [PubMed]
- 28.Swe KM, Abas AB, Bhardwaj A, Barua A, Nair NS. Zinc supplements for treating thalassaemia and sickle cell disease. Cochrane Database Syst Rev. 2013;(6):CD009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikula AL, Hetzel SJ, Binkley N, Anderson PA Validity of height loss as a predictor for prevalent vertebral fractures, low bone mineral density, and vitamin D deficiency. Osteoporos Int. 2017;28(5):1659-1665. [DOI] [PubMed]
- 30.Einy S, Hazan-Molina H, Ben-Barak A, Aizenbud D. Orthodontic consideration in patients with beta-thalassemia major: case report and literature review. J Clin Pediatr Dent. 2016;40(3):241-246. [DOI] [PubMed] [Google Scholar]
- 31.Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V Guidelines for the Management of Transfusion Dependent Thalassaemia (TDT). Nicosia, Cyprus: Thalassemia International Federation; 2014. [PubMed] [Google Scholar]
- 32.Giusti A, Pinto V, Forni GL, Pilotto A. Management of beta-thalassemia-associated osteoporosis. Ann N Y Acad Sci. 2016;1368(1):73-81. [DOI] [PubMed] [Google Scholar]
- 33.Lampropoulou-Adamidou K, Tournis S, Triantafyllopoulos IK. Atypical femoral fracture in a beta-thalassemia major patient with previous bisphosphonate use: case report and a review of the literature. J Musculoskelet Neuronal Interact. 2016;16(1):75-78. [PMC free article] [PubMed] [Google Scholar]
- 34.Lal A. Assessment and treatment of pain in thalassemia. Ann N Y Acad Sci. 2016;1368(1):65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trachtenberg F, Foote D, Martin M, et al. ; Thalassemia Clinical Research Network. Pain as an emergent issue in thalassemia. Am J Hematol. 2010;85(5):367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angastiniotis M, Eleftheriou A. Thalassaemic bone disease: an overview. Pediatr Endocrinol Rev. 2008;6(suppl 1):73-80. [PubMed] [Google Scholar]
- 37.Green ST, Martin MB, Haines D, et al. ; Thalassaemia Clinical Research Network. Variance of pain prevalence and associated severity during the transfusion cycle of adult thalassaemia patients. Br J Haematol. 2014;166(5):797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogiatzi MG, Macklin EA, Fung EB, et al. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24(3):543-557. [DOI] [PMC free article] [PubMed]
- 39.Desigan S, Hall-Craggs MA, Ho CP, Eliahoo J, Porter JB. Degenerative disc disease as a cause of back pain in the thalassaemic population: a case-control study using MRI and plain radiographs. Skeletal Radiol. 2006;35(2):95-102. [DOI] [PubMed] [Google Scholar]
- 40.Oliveros O, Trachtenberg F, Haines D, et al. ; Thalassemia Clinical Research Network. Pain over time and its effects on life in thalassemia. Am J Hematol. 2013;88(11):939-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanner J, Malhotra S, El-Daly H, Godfrey EM. Case 243: extramedullary hematopoiesis in an adrenal myelolipoma. Radiology. 2017;284(1):292-296. [DOI] [PubMed] [Google Scholar]
- 42.Noureldine MH, Taher AT, Haydar AA, Berjawi A, Khamashta MA, Uthman I Rheumatological complications of beta-thalassaemia: an overview [published online ahead of print 22 March 2017]. Rheumatology. [DOI] [PubMed]