Abstract
Despite improvement in survival in diffuse large B-cell lymphoma (DLBCL) with the introduction of rituximab, central nervous system (CNS) relapse continues to represent a clinical challenge. A number of studies have evaluated clinical risk factors in an attempt to identify high-risk patients to direct CNS staging investigations and consider prophylaxis strategies. The CNS International Prognostic Index is a robust and reproducible risk model that can identity patients at high risk of CNS relapse, but its specificity remains limited. Studies are emerging of biomarkers that predict CNS relapse that can be integrated with clinical risk models to better identify high-risk patients for CNS-directed prophylaxis strategies. Because CNS parenchymal disease is the predominant compartment, prophylaxis should include deeply penetrant drugs such as high-dose methotrexate. However, this has been associated with toxicity and has limited use in older patients. Novel therapies are being tested in primary CNS lymphoma with encouraging results and may represent rational strategies to be further explored in the prophylaxis setting.
Learning Objectives
Identify clinical and biomarker risk factors associated with an increased risk of CNS relapse
Review current standard CNS prophylaxis strategies, including limitations
Review emerging novel treatment strategies under investigation that may lower the risk of CNS relapse
Introduction
The development of central nervous system (CNS) involvement, or “secondary CNS” relapse, poses a significant clinical challenge in the management of aggressive lymphomas. Lymphoblastic lymphoma/leukemia and Burkitt lymphoma have long been recognized to be associated with a very high risk of CNS relapse, and as such, treatment protocols uniformly incorporate CNS prophylaxis strategies. Further, retrospective studies evaluating dual translocation or “double hit” [DHIT] lymphomas, which harbor MYC and BCL2 translocations (with or without BCL6 translocation aka THIT), also report a high risk of CNS relapse, and similarly, CNS prophylaxis is also recommended, along with intensified chemotherapy protocols.1,2
In diffuse large B-cell lymphoma (DLBCL), the incidence of CNS relapse is only ∼5% in unselected cohorts.3 However, in certain high-risk groups, such as those with adrenal/kidney involvement, estimates as high as 40% have been reported.4 Although large-scale studies can demonstrate a reduction in the risk of CNS relapse with the introduction of rituximab for the treatment of DLBCL, the impact is small, likely reflecting the poor CNS penetration of rituximab.5,6 With the exception of primary testicular DLBCL, the time to CNS relapse is typically within the first 6 to 9 months of diagnosis, which may indicate the presence of occult disease at diagnosis. However, patients are not always routinely screened for CNS disease, and in the case of cerebrospinal fluid (CSF) evaluation, diagnostic sensitivity is low. Regardless, the overall consequence of CNS relapse is often devastating, and for most patients, the median overall survival is typically only a few months, highlighting the need to accurately identify at-risk patients, screen for CNS disease, and develop safe and effective treatment/prophylaxis strategies.
There has been considerable interest in defining high-risk patients to target prophylactic therapies. The CNS International Prognostic Index (IPI) is the most valid, robust model developed to date in the rituximab era. It is composed of the standard 5 IPI risk factors (age >60 years, elevated lactate dehydrogenase (LDH), performance status ≥2, >1 extranodal [EN] site, and stage 3/4) as well as kidney/adrenal involvement, for a total of 6 risk factors. It can effectively stratify patients into low-, intermediate-, and high-risk groups, the latter having a ≥10% risk of CNS relapse.7 This new benchmark can be used to evaluate both the impact of new treatment approaches that incorporate CNS penetrant agents and the utility of CNS risk biomarkers.
Herein, this review will focus on clinical and emerging biologic factors associated with a high risk of CNS relapse in DLBCL in the modern treatment era and explore current prophylaxis strategies.
Who is at risk of CNS relapse?
CNS relapse in the rituximab era
With the introduction of rituximab into the standard management of DLBCL, numerous studies have investigated whether this has impacted the frequency of CNS relapse. Collectively, evidence suggests a reduction in the frequency of CNS relapse with rituximab, but it is modest at best and only captured in adequately powered studies. The best evidence comes from the RICOVER-60 trial, which evaluated 1112 patients with aggressive B-cell lymphoma (primarily DLBCL [81.6%]) and reported a 2-year incidence of CNS disease of 6.9% using cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) administered every 2 weeks compared with 4.1% using rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP).5 Similar findings were reported from the British Columbia Cancer Agency (BCCA), with the main benefit seen in those who achieve a complete remission to primary therapy, which may reflect the improved systemic disease control with rituximab.8 A recent meta-analysis of 4859 patients treated with R-CHOP(-like) on 7 prospective trials demonstrated an overall CNS recurrence risk of ∼5% in rituximab-treated patients.3
Clinical risk factors for CNS relapse
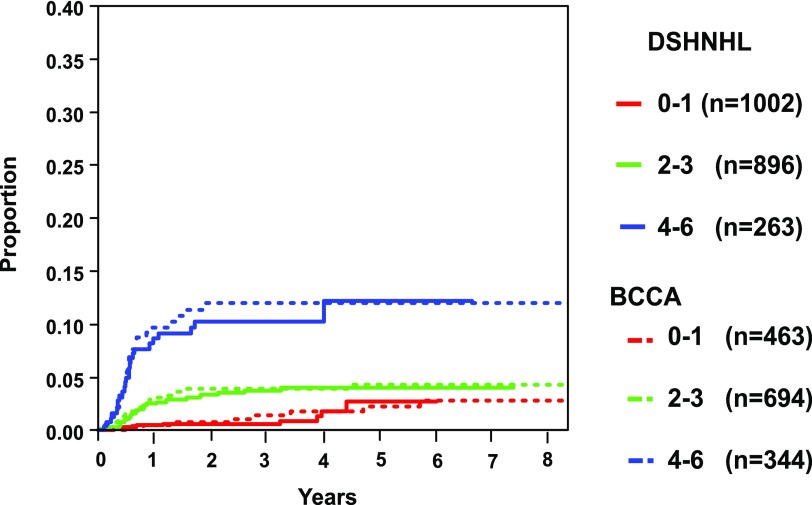
There have been a number of studies that have focused on the evaluation of clinical risk factors associated with CNS relapse in DLBCL. However, many were small or developed in the pre-rituximab era, included heterogeneous aggressive lymphomas subtypes, and lacked a validation cohort. In an effort to develop a robust clinical model to identify patients at high risk of CNS relapse in the rituximab era, the German High-Grade Lymphoma Study Group (DSHNHL) used a training set of 2164 patients with aggressive B-cell lymphoma (80% of whom had DLBCL) who were treated with R-CHOP or rituximab plus cyclophosphamide, doxorubicin, vincristine, etoposide, and prednisone (R-CHOEP) on prospective trials.7 The IPI risk factors, in addition to other risk factors (ie, sex, albumin, bulky disease, B symptoms) and specific EN sites, were first evaluated in univariate analysis. The total IPI was the strongest risk factor, and in multivariate analysis, all factors except >1 EN site were significant. The latter likely reflects the tight association between EN sites and stage 4, evaluation of a clinical trial population, and the importance of increasing risk with cumulative EN site involvement as outlined below. Regardless, given that this has been a strong risk factor in other studies, it was carried forward to the final model. Evaluation of specific EN sites in multivariate analysis along with the 5 IPI factors yielded kidney/adrenal as critical sites for CNS relapse, and thus, the final model included 6 risk factors and was termed the CNS-IPI. Grouping the risk categories into low risk (0-1 factors), intermediate risk (2-3 factors), and high risk (≥ 4 factors) resulted in a 2-year risk of CNS recurrence of 0.6%, 3.4%, and 10.2%, respectively (Table 1 and Figure 1).7 The high-risk group represented 12.3% of all patients, and importantly, those with 5 and 6 risk factors had a risk of CNS recurrence that was further elevated at 15.0% and 32.5%, respectively. However, this specificity refinement is at the expense of sensitivity, and only 62 (∼3%) and 15 (<1%) patients fall into these extreme risk categories, respectively.7 Remarkably, applying this model to a validation set of 1597 patients from a retrospective population-based database at the BCCA with DLBCL treated with R-CHOP demonstrated similar 2-year CNS relapse risk estimates (low risk, 0.8%; intermediate risk, 3.9%; and high risk, 12.0%; Figure 1 and Table 1), the latter representing 23% of all patients, reflecting a more “real world” population estimate. Further, a separate study of 1532 of positron emission tomography–staged DLBCL patients treated with R-CHOP(-like) demonstrated similar CNS relapse estimates by risk group (Table 1).9 CNS recurrence in the high-risk patients occurred <6 to 9 months from diagnosis in all of these cohorts, supporting the notion that occult disease may have been present at the time of the original diagnosis.
Table 1.
Risk of CNS relapse in DLBCL treated with R-CHOP(-like) chemotherapy using the CNS-IPI
| Study cohort | Number of patients | 2-y risk of CNS relapse | |||
|---|---|---|---|---|---|
| Overall (all DLBCL) | Low (0-1 factors) | Intermediate (2-3 factors) | High (4-5 factors) | ||
| DSHNHL (7) | 2164* | 4% | 0.6% | 3.4% | 10.2% |
| BCCA (7) | 1597 | 4% | 0.8% | 3.9% | 12% |
| Danish (9) | 1532 | 4% (3 y) | 0.4% (3 y) | 3% (3 y) | 11% (3 y) |
*DLBCL n = 1735 (80%); sensitivity analysis produced similar results.
Figure 1.
Risk of CNS relapse according to the number of CNS-IPI risk factors. Adapted and reprinted with permission. © 2017 American Society of Clinical Oncology. All rights reserved. Originally published by the American Society of Clinical Oncology. Schmitz et al. J Clin Oncol. 2016;34(24):3150-3156.
The CNS-IPI represents the first robust model to estimate the risk of CNS relapse and serves as a useful benchmark to evaluate the relevance of other risk factors, including biomarkers. In addition, it can be used to evaluate whether novel treatment approaches that include CNS-penetrant agents may impact the frequency of CNS relapse (see below). However, it does not capture the full spectrum of those at high risk, and the high-risk group collectively has only a 10% to 12% risk of CNS relapse. Notably, 40% of CNS relapses do occur in the intermediate-risk category.7 Clinical risk factors beyond this model have also been described in other studies. The standard IPI and CNS-IPI incorporate EN >1 as a risk factor; however, it is intuitive that the greater the number of EN sites, the greater the CNS risk, which has been supported in earlier studies.10 This was established in a study by El-Galaly and colleagues, where >2 EN sites was associated with a 2-year CNS risk of 15.2%, but again this was at the expense of sensitivity (35% vs 55.7% CNS-IPI).10 The revised National Comprehensive Cancer Network (NCCN) IPI suggested that LDH >3× the upper limit normal, indicative of high tumor burden, may enhance prognostication in DLBCL.11 A separate study evaluating this as a CNS risk factor evaluated 581 patients with DLBCL and found that 6.7% had a LDH of this magnitude and had a heightened CNS risk (8 of 39 patients [20.5%]).11
Specific EN sites in DLBCL have been associated with an elevated risk of CNS relapse.12 Prior to the development of the CNS-IPI, kidney and adrenal involvement were noted to impart a significant risk of CNS recurrence in DLBCL (Table 2).4,13 In the German DSHNHL training cohort, skin was also noted to be associated with increased risk but was not included due to rarity and limited information. In multivariate analysis of the BCCA validation cohort, pericardial, orbit, bone marrow, and testes involvement emerged as CNS risk factors.
Table 2.
Clinical and emerging biomarker factors associated with an increased risk of CNS relapse in DLBCL patients treated with R-CHOP(-like) chemotherapy
| Frequency | 2 y risk CNS relapse | Comment | |
|---|---|---|---|
| Clinical risk factors | |||
| High-risk CNS-IPI ≥4 | 12%-23% | 10%-12% | Robust CNS risk model; low specificity |
| Extranodal sites >2 | 9.5% | 15.3% | Greater specificity but lower sensitivity |
| High LDH >3× ULN | 6.7% | ∼30% | Estimated from Kaplan-Meier curve |
| Kidney/adrenal | 2% | ∼40% | Very high CNS risk with concurrent testicular involvement |
| Testicular | 5% | 10% (limited);24% (advanced) | Rituximab is not protective of CNS relapse |
| Uterine | 2% | 44% (4 y) | Independent risk factor; ovarian involvement is not a risk factor |
| Biomarkers | |||
| MYC+ BCL2+ DHIT | ∼5% | 13%-50% | Estimates highly variable depending on selection criteria |
| MYC+ BCL2+ DEs | ∼30% | All, 9.3%; CNS-IPI high, 22.7%; CNS-IPI intermediate, 11% | Independent risk factor of CNS-IPI |
| ABC DLBCL | All ∼30%-40% MYC+BCL2+(DE) MYC−BCL2−(non-DE) |
9.1% 15.3% 2.2% |
Similar results with non-GCB DLBCL; impact mostly in DEs (MYC+BCL2+) |
| CD5+ DLBCL | 5%-10% | 12.7%* | Multivariate analysis not performed; only observed in a Japanese series |
| IgM paraprotein | 12.5% | 41%* | Multivariate analysis not performed |
DEs, dual expressors.
Reported as frequency of CNS relapse.
Testicular involvement in particular has long been recognized to be uniquely associated with a heightened risk of CNS relapse.14 Since the majority of patients with testicular involvement have limited-stage disease, they fall predominantly in the low- and intermediate-risk CNS-IPI groups and thus are not captured well in this risk model. Notably, a recent study suggested that rituximab has not had an impact on the reduction of CNS relapse in testicular DLBCL in either limited-stage (5-year risk of CNS relapse, 10%) or advanced-stage patients (5-year risk of CNS relapse, 24%).15 Further, having 2 high-risk EN sites (testes and adrenal/kidney involvement) was associated with an extremely high risk, with 6 out of 8 patients (75%) ultimately developing a CNS recurrence.15 The CNS relapse risk was lower (5-year risk, 6%) in an International Extranodal Lymphoma Study Group study evaluating R-CHOP with intrathecal CNS prophylaxis in limited-stage testicular DLBCL, which may reflect patient selection.16 Other studies maintain that the testes is a relevant site in the rituximab era, and taken together, this remains an important high-risk EN site regardless of the CNS-IPI risk score.17
Uterine (but not ovarian) involvement is another notable EN site, associated with a very high risk of CNS relapse independent of other risk factors (Table 2).18 More inconsistent results have been reported for breast, epidural, or bone involvement. Historically, sinus involvement has been associated with an increased CNS relapse risk.19 However, rituximab has eliminated this risk, and routine CNS prophylaxis is no longer endorsed.20
Biomarkers associated with CNS relapse
The low positive predictive value of the CNS-IPI and other clinical risk factors/models highlight the need for more objective biomarkers to identify high-risk patients. The presence of a MYC translocation, in particular a MYC translocation occurring with a BCL2 translocation (“classic” DHIT) or triple-hit (THIT) lymphoma (MYC/BCL2 with a BCL6 translocation), has been associated with an increased risk of CNS relapse in retrospective series. DHIT DLBCL is rare, occurring in ∼5% of DLBCL, and occurs exclusively in germinal center B-cell–like (GCB) DLBCL, where it is found in ∼10% to 20% of all GCB DLBCL.21,22 Historically, it has been associated with a high risk of CNS involvement of up to 50%; however, estimates vary widely across studies (Table 2). In many series, fluorescence in situ hybridization studies were undertaken specifically in patients with high-risk clinical or pathological features,23 and some studies have combined all high grade B-cell histologies with variable treatment regimens.23,24 Of interest, a recent retrospective study suggests a lower incidence of CNS (4.5% at 2 years) when an unselected population of DLBCL treated with R-CHOP is evaluated for the presence of DHIT or THIT disease; thus, there may be other biological factors at play.25 Nevertheless, at this time, it is recommended that all patients with DHIT/THIT lymphoma, including those with DLBCL, should have CNS-directed staging investigations, and primary treatment should include a dose-intensive regimen and CNS prophylaxis.1
Dual expression of MYC and BCL2 protein in DLBCL has been associated with a poor prognosis in multiple studies,21,22 and unlike classic DHIT, it is more common, occurring in approximately one-third of cases, and is predominantly found in activated B-cell (ABC)/non-GCB DLBCL, occurring in up to three-quarters of all cases.21,22,26,27 The impact of dual expression of MYC and BCL2 (“dual expressers” [DEs]) (using cutoffs of MYC ≥40% and BCL2 ≥50%) and risk of CNS relapse was evaluated in R-CHOP–treated DLBCL patients. Notably, MYC+BCL2+ DE DLBCL was associated with a higher 2-year risk of CNS relapse compared with non-DEs (9.7% vs 2.2%, P = .02).25 In addition, ABC-type (defined by Lymph2Cx)28 or non-GCB DLBCL (defined by the Hans algorithm)29 was also associated with a high risk of CNS relapse; however, only MYC+BCL2+ DE status and the CNS-IPI were associated with CNS relapse in multivariate analysis.30 Further, within ABC/non-GCB DLBCL, MYC+BCL2+ DEs defined those at elevated risk of CNS relapse (2-year risk ∼15% [DEs] vs ∼2% to 3% [non-DEs], P < .05). Importantly, within the high-risk CNS-IPI group (≥4 risk factors), cases that are MYC+BCL2+ have a 2-year risk of CNS relapse of 22.7% compared with only 2.3% for those that were non-DEs (P = .02). Further, within the CNS-IPI intermediate-risk group, MYC+BCL2+ DEs were also identified as having a high risk of CNS relapse (11% vs 3.2%, P = .049).25 Although this needs to be validated, including defining optimal protein cutoffs, this study highlights the complementary role of clinical and biomarker risk factors.
There are very few other published studies evaluating biological or pathological factors that are associated with CNS relapse. CD5+ DLBCL has been associated with a poor prognosis and may be risk factor for CNS recurrence.30,31 In a study of 337 Japanese patients, the 2-year risk of CNS relapse was high, and rituximab was not protective (11.6% for chemotherapy vs 12.7% for rituximab chemotherapy).30 However, in a separate study from Taiwan, CNS risk was not elevated in CD5+ DLBCL.32
The presence of an immunoglobulin M paraprotein in DLBCL has also been identified as a risk factor for CNS relapse. In a study of 151 patients, 17 (11.3%) had a serum monoclonal immunoglobulin M, almost all of which were non-GCB subtype and had a very poor prognosis (3-year progression-free survival, 23.5%). Further, 2 patients had CNS disease at the time of diagnosis, and 5 additional patients developed CNS recurrence (29%).33 Validation of this and other objective biomarkers would be of great value in defining high-risk patients.
Site and timing of CNS relapse to guide CNS-directed staging investigations
Since the introduction of rituximab, several studies have evaluated the timing of CNS relapse and the compartment in which it occurs. This information can guide both CNS staging investigations and prophylaxis strategies (see below). In a study of 1732 R-CHOP–treated DLBCL patients, 56% patients had an isolated CNS relapse, 44% had concurrent systemic disease, and 73% of CNS relapses involved the CNS parenchyma (in 61% of CNS relapses, it was the sole CNS site).34 By the CNS-IPI, all risk groups had CNS parenchyma as the predominant site, and, in addition, in the high-risk group, the leptomeninges was also involved in almost half of patients. CNS events occurred early, with the exception of rare CNS relapse in the low-risk group, where the median time to relapse was almost 2 years, which was driven in part by the occurrence of limited-stage testicular DLBCL.15,34 The importance of the parenchyma as a risk site in the rituximab era was also observed in a NCCN prospective cohort study of 979 of R-CHOP–treated DLBCL patients, where 65% of CNS relapses were observed to involve the parenchyma.35 Further, a combined analysis of 3 prospective trials (including the NCCN cohort) reported an incidence of parenchymal involvement of 57% in R-CHOP–treated DLBCL.17
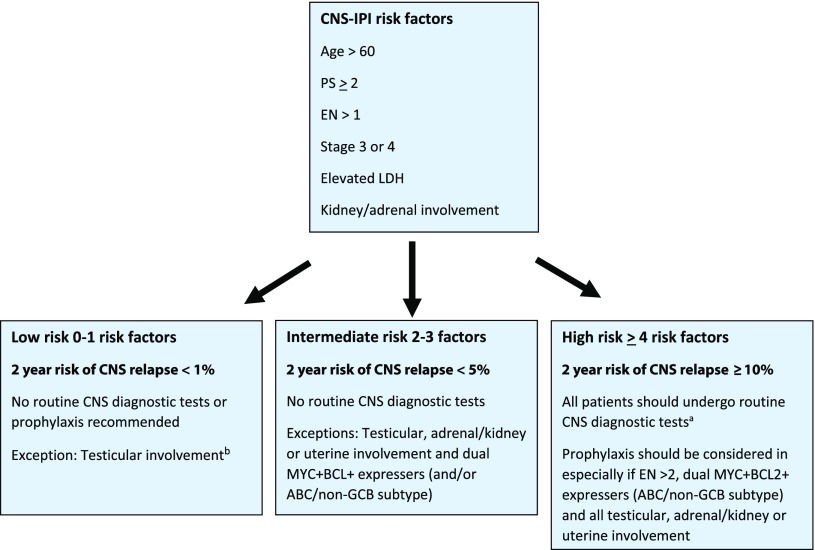
A suggested algorithm for CNS staging workup and prophylaxis is shown in Figure 2. In addition to classic DHIT DLBCL, all patients with a high CNS-IPI score and/or testicular, adrenal/kidney or uterine involvement should undergo CNS-directed staging investigations. If MYC+BCL2+ and COO information is available, then those that are MYC+BCL2+ DE and ABC/non-GCB should also be considered for CNS diagnostic procedures, even those in the intermediate-risk category. CNS staging tests should include magnetic resonance imaging of the head and, if unavailable, a computed tomography scan of head and consideration should be giving to repeating it with re-staging investigations given that disease may develop during or shortly after the completion of R-CHOP. In addition, analysis of the CSF should be performed. As cytomorphology is associated with a high false-negative rate, flow cytometry of the CSF fluid should be performed, as it is a more sensitive tool to detect occult CNS disease.36,37 Recently, next-generation sequencing of cell-free DNA from the CSF has been evaluated in solid tumors with known or suspected CNS involvement and may in the future better identify at-risk individuals.38
Figure 2.
A suggested algorithm for the selection of patients for CNS-directed staging investigations and criteria for use of CNS prophylaxis. aCNS diagnostic tests: magnetic resonance imaging (preferred) of the head or computed tomography scan of the head; CSF for cytology and flow cytometry. A CNS evaluation should also be performed with response assessment. bKidney/adrenal or uterine involvement and only low-risk CNS-IPI is exceedingly rare. If present, CNS diagnostics are recommended. Note: for patients with MYC+BCL2+ DHIT or THIT DLBCL, all patients should undergo CNS diagnostic tests and receive prophylaxis.
It is anticipated that more uniform and aggressive CNS-directed staging will reveal a higher incidence of CNS involvement at diagnosis and may shift historical risk estimates. Further, patients identified as having CNS disease at diagnosis should receive a more CNS-directed treatment approach.39
What is the optimal CNS prophylaxis strategy?
With imperfect risk assessment and a lack of definitive evidence that CNS prophylaxis is protective, there are varied CNS prophylaxis strategies. The most widely used CNS prophylaxis practice is intrathecal (IT) methotrexate (MTX) (and IT arabinoside [araC]), with rationale extrapolated from successful use in the treatment of acute lymphoblastic leukemia. However, in acute lymphoblastic leukemia, CNS recurrences tend to involve the leptomeninges, and with restricted CNS penetration, IT prophylaxis has limited value in DLBCL, which is dominated by CNS parenchymal involvement. The majority of studies do not support that IT prophylaxis is protective. The NCCN evaluated 989 patients with DLBCL, of which 117 high-risk patients had received CNS prophylaxis (primarily IT therapy [71.8%]).35 This cohort represents a younger good risk population where CNS staging investigations are aggressive, and as such, the CNS recurrence rate was lower than prior reports (2.5%) and occurred later. Of interest, CNS recurrence was marginally higher in those who received prophylaxis than in those who did not (5.4% vs 1.4%, P = .08). This may reflect both the enrichment of high-risk patients selected for CNS prophylaxis and the limitations of IT chemotherapy. In contrast, a study evaluating the role of IT chemotherapy in DLBCL with flow cytometry–positive CSF demonstrated a high degree of CSF complete remission, suggesting there may be a role for IT therapy in the treatment of patients with established involvement in the absence of concurrent parenchymal disease.37
Interestingly, IT prophylaxis is the only type of CNS protection integrated into dose-adjusted (DA)-EPOCHR (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), which is used with high efficacy in the treatment of Burkitt lymphoma. A similar protocol was used in a study of patients with MYC+ aggressive B-cell lymphoma (primarily DLBCL), some of whom had classic DHIT disease, and the overall outcome was excellent. Whether this represents selection of a lower risk population entered into a clinical trial or a truly protective effective is unknown, and publication of the final study results is awaited. Further, large-scale analyses evaluating the risk of CNS relapse in DA-EPOCHR patients have not yet been performed.
Given the high rate of parenchymal involvement, successful prophylactic strategies should ideally integrate agents that deeply penetrate all CNS compartments. The most widely explored systemic treatment is high-dose methotrexate (HD-MTX). This approach was first supported from an earlier Groupe d’Etudes des Lymphomes de l’Adulte/Lymphoma Study Association (GELA/LYSA) study in the pre-rituximab era comparing ACVBP, which incorporates 2 cycles of HD-MTX (3 g/m2) into standard CHOP chemotherapy. The dose-intensive regimen was associated with fewer CNS recurrences (0.8% vs 2.7%, P = .002) (Table 3).40 Two other phase 2 studies support the integration of CNS-penetrating systemic agents with a reduction of CNS events observed, but these studies also used dose-intensive regimens and/or high-dose araC, which may also contribute to the risk reduction (Table 3). The Nordic group evaluated dose-intensive R-CHOEP followed by 1 cycle of araC and 1 cycle of HD-MTX (3 g/m2) in DLBCL patients with an age-adjusted IPI 2-3, and the risk of CNS relapse was 4.4%.41 CNS relapse occurred early (<6 months), suggesting that prophylaxis should ideally be given earlier, during primary chemotherapy. Recently, the United Kingdom National Cancer Research Institute evaluated modified R-CODOX-M-R-IVAC (cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate [CODOX-M]/ifosfamide, etoposide, and high-dose cytarabine [IVAC]) in a phase 2 study of 108 patients with DLBCL, IPI ≥ 3, including 8 patients with CNS disease at diagnosis, and overall, 27% had kidney/adrenal involvement. Considering those without CNS disease at diagnosis, the 2-year risk of CNS relapse for all patients was 4.6%, and for intermediate- and high-risk CNS-IPI risk groups, it was 0% and 6.2% (3 out of 55 patients), respectively, suggesting that this approach may be protective (Table 3).42 Interestingly, of the 8 patients with CNS involvement at baseline, only 2 developed CNS relapse.
Table 3.
Studies evaluating HD-MTX for CNS prophylaxis in DLBCL
| Study | Study type | Lymphoma type/risk | Primary treatment | Systemic CNS prophylaxis (# cycles) | CNS relapse |
|---|---|---|---|---|---|
| GELA/LYSA, Tilly et al40 | Prospective phase 3 | All aggressive 80% DLBCL |
1. ACVBP 2. CHOP |
1. HDMTX 3 g/m2 (2) 2. None |
0.8%* 2.7%* P = .002 |
| Nordic, Holte et al41 | Prospective phase 2 | DLBCL (74%) FL 3A aaIPI 2-3 |
DI R-CHOEP14 |
AraC 3 g/m2 (1) HDMTX 3 g/m2 (1) |
4.4%* |
| UK NCRI/Bloodwise, Phillips et al42 | Prospective phase 2 | DLBCL IPI ≥3 | R-CODOX-M-R-IVAC | HDMTX 3 g/m2 (+IT) Ifosfamide, AraC (+IT) |
All 4.6% (2 y) 0 intermediate-risk CNS-IPI 6.2% high-risk CNS-IPI |
| US-MGH, Abramson et al43 | Retrospective | DLBCL High CNS risk† |
R-CHOP (97%) |
HDMTX 3-3.5 g/m2 (3) |
3%* |
| Australia, Cheah et al44 | Retrospective | DLBCL High CNS risk‡ |
1. CHOP(-like) ± R§ 2. CHOP(-like) ± R 3. Dose-intense |
1. None (IT alone) 2. HDMTX 1-3 g/m2 (2) 3. HDMTX 1-3 g/m2 (2) (+IT) |
1. 18.4% (3 y) 2. 6.9% (3 y) 3. 2.3% (3 y) P = .009 |
| Italy, Ferreri et al45 | Retrospective | DLBCL High CNS risk¶ |
R-CHOP |
None HDMTX 3 g/m2 ± IT |
12%* 0 |
ACVBP, doxorubicin, cyclophosphamide, vindesine, bleomycin, prednisone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; DI, dose intensive; GELA, Groupe d’Etudes des Lymphomes de l’Adulte; LYSA, Lymphoma Study Association; R-CODOX-M-R-IVAC, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate (CODOX-M)/ifosfamide, etoposide, and high-dose cytarabine (IVAC).
Reported as frequency of CNS relapse.
High CNS risk = 1, high-risk EN sites (bone marrow, sinus, testes, epidural disease, liver, kidney/adrenal/orbit; 2, >2 EN sites and elevated LDH; 3, high risk by Hollender criteria.
High CNS risk = 1, high-risk EN sites (bone marrow, breast, testis, kidney/adrenal, sinus, nasopharynx, liver, paravertebral; 2, any two of the following: multiple EN sites, elevated LDH, or B symptoms.
Three treatment groups are listed: (1) 1991-2003 CHOP(-like) CHOP and MACOP-B (HD-MTX <1 g/m2) with IT MTX; (2) >2003 R-CHOP and HD-MTX (1-3 g/m2) following R-CHOP completion; and (3) <65 y, age-adjusted IPI ≥2, hyper-CVAD or CODOX-M-IVAC ± rituximab (when available), and IT MTX.
High CNS risk = 1, high-risk EN sites (testis, spine, skull, sinus, orbit, nasopharynx, kidney/adrenal, breast); 2, advanced stage and increased LDH.
A retrospective study from Massachusetts General Hospital evaluated R-CHOP with midcycle (days 10-15) HD-MTX (3-3.5 g/m2) for up to 3 cycles in high-risk DLBCL and demonstrated a CNS relapse rate of only 3%.43 Further, a multicenter retrospective study evaluating era-specific recommendations from Australia also supported a reduced risk of CNS relapse with R-CHOP and HD-MTX, and there may be added value using a more dose-intensive chemotherapy regimen (Table 3).44 Similarly, an Italian retrospective study in DLBCL evaluated the impact of a policy recommending HD-MTX (±IT) for high-risk patients and reported no CNS recurrences.45 None of these studies are definitive, but they do provide a rationale for this approach.
From the available evidence, HD-MTX for 2 to 4 cycles is the preferred prophylaxis with early integration if feasible, typically on days 10 to 15 of R-CHOP, at a dose of 3 to 3.5 g/m2 in younger patients with good renal function. Renal toxicity is typically the main limiting factor affecting feasibility of this approach, especially in older patients. In addition, rare hepatotoxicity can occur with HD-MTX, and it is contraindicated in those with effusions, as this can be a drug reservoir, thereby enhancing toxicity. Further, given that HD-MTX can also cause neutropenia, growth factor support should be considered if HD-MTX is to be integrated with R-CHOP. Given the high frequency of leptomeningeal disease in high-risk CNS-IPI patients, IT chemotherapy may be an alternative if HD-MTX is contraindicated.
Outside of DHIT DLBCL, where CNS prophylaxis is standard, it should be considered in those with a high-risk CNS-IPI (≥4 risk factors) particularly in cases involving >2 EN sites and/or those accompanied by dual expression of MYC and BCL2. Patients with testicular, kidney/adrenal, and likely also uterine involvement should receive prophylaxis regardless of their CNS-IPI (Figure 2). Of note, CNS relapse in limited-stage testicular DLBCL typically occurs late; thus, HD-MTX may be administered at the conclusion of R-CHOP to minimize toxicity. The International Extranodal Lymphoma Study Group 30 phase 2 study is currently evaluating IT liposomal araC and a lower dose of HD-MTX (1.5 g/m2) in primary testicular DLBCL to spare patients the toxicity of HD-MTX.
A similar approach is endorsed by the NCCN guidelines, with CNS prophylaxis recommended in those with a high-risk CNS-IPI, testicular involvement, or DHIT DLBCL. However, given the uncertainty, either IT prophylaxis (MTX and/or cytarabine) or HD-MTX is endorsed. The European Society of Medical Oncology guidelines stipulate HD-MTX as the preferred type of CNS prophylaxis in high-intermediate/high-risk IPI (especially for those with >1 EN site or elevated LDH) and for those with testicular or adrenal/renal involvement.
Emerging novel therapy approaches in preventing CNS relapse
Major progress has been made identifying patients at high risk of CNS relapse, but the development of effective and safe prophylactic strategies has lagged behind. Given the limitations of IT therapy and the toxicity of HD-MTX, there is great interest to explore other strategies.
In the last decade, there has been a number of novel therapies under investigation in DLBCL in an effort to improve outcome, particularly in the poor-risk ABC/non-GCB subgroup. Early studies of ibrutinib and lenalidomide support selectivity in ABC/non-GCB leading to trials integrating these agents in the up-front setting. Further, emerging data support the idea that these agents are active in primary CNS lymphoma, in keeping with predominantly ABC subtype, with penetration of all CNS compartments.46,47 Evidence for a role for these agents in the prevention of CNS relapse is highlighted from a recent pooled analysis of 136 patients enrolled in 2 phase 2 studies evaluating R-CHOP and lenalidomide (R2-CHOP) in DLBCL, 18% of whom had a high-risk CNS-IPI score.48 Although not specifically confined to ABC/non-GCB DLBCL, only 1 patient (0.7% of all study patients) developed CNS relapse.43 Given the recent observation that non-GCB/ABC DLBCL is associated with an increased risk of CNS relapse,25 integration of these agents into primary therapy may have a significant impact on CNS, and the results of phase 3 studies (R-CHOP ± lenalidomide #NCT01856192; #NCT02285062; R-CHOP ± ibrutinib #NCT01855750) are eagerly awaited.
More recently, frequent PDL1/2 copy-number alterations and increased PDL1/2 protein expression were demonstrated in primary CNS lymphoma and testicular DLBCL, leading to a strong rationale for PD1 inhibitors in these immune sanctuary sites.49,50 A case series of 5 patients with relapsed/refractory primary CNS lymphoma (n = 4) or testicular DLBCL (n = 1) treated with nivolumab demonstrated responses in all patients, 3 of whom have remained in a durable remission for over 1 year.51 A phase 2 study evaluating nivolumab is ongoing in these disease settings (#NCT02857426).
Conclusions
Significant progress has been made identifying patients at high risk of CNS relapse. The CNS-IPI represents a robust risk model and serves as a useful benchmark to evaluate the impact of novel treatment approaches. Further study into CNS biomarkers like ABC/non-GCB and MYC+ BCL2+ DEs may further define high-risk patients and be used in conjunction with the clinical risk model. More aggressive CNS staging in high-risk patients and using state-of-the-art diagnostic techniques will identify patients with CNS disease at diagnosis and appropriately direct them to CNS-specific therapies. The exploration of novel agents that cross the blood–brain barrier or, in the case of PD1 inhibitors, target the mechanism of immune evasion require further study in high-risk patients.
References
- 1.Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer. 2014;120(24):3884-3895. [DOI] [PubMed] [Google Scholar]
- 2.Mead GM, Sydes MR, Walewski J, et al. ; UKLG LY06 collaborators. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol. 2002;13(8):1264-1274. [DOI] [PubMed] [Google Scholar]
- 3.Ghose A, Elias HK, Guha G, Yellu M, Kundu R, Latif T. Influence of rituximab on central nervous system relapse in diffuse large B-cell lymphoma and role of prophylaxis: a systematic review of prospective studies. Clin Lymphoma Myeloma Leuk. 2015;15(8):451-457. [DOI] [PubMed] [Google Scholar]
- 4.Villa D, Connors JM, Sehn LH, Gascoyne RD, Savage KJ. Diffuse large B-cell lymphoma with involvement of the kidney: outcome and risk of central nervous system relapse. Haematologica. 2011;96(7):1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: an analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Blood. 2009;113(17):3896-3902. [DOI] [PubMed] [Google Scholar]
- 6.Rubenstein JL, Combs D, Rosenberg J, et al. . Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101(2):466-468. [DOI] [PubMed] [Google Scholar]
- 7.Schmitz N, Zeynalova S, Nickelsen M, et al. . CNS international prognostic index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol. 2016;34(26):3150-3156. [DOI] [PubMed] [Google Scholar]
- 8.Villa D, Connors JM, Shenkier TN, Gascoyne RD, Sehn LH, Savage KJ. Incidence and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: the impact of the addition of rituximab to CHOP chemotherapy. Ann Oncol. 2010;21(5):1046-1052. [DOI] [PubMed] [Google Scholar]
- 9.El-Galaly TC, Villa D, Michaelsen TY, et al. . The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: an international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer. 2017;75:195-203. [DOI] [PubMed] [Google Scholar]
- 10.Boehme V, Zeynalova S, Kloess M, et al. ; German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Incidence and risk factors of central nervous system recurrence in aggressive lymphoma: a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol. 2007;18(1):149-157. [eng.] [DOI] [PubMed] [Google Scholar]
- 11.Kim SJ, Hong JS, Chang MH, et al. . Highly elevated serum lactate dehydrogenase is associated with central nervous system relapse in patients with diffuse large B-cell lymphoma: Results of a multicenter prospective cohort study. Oncotarget. 2016;7(44):72033-72043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreri AJ. Risk of CNS dissemination in extranodal lymphomas. Lancet Oncol. 2014;15(4):e159-e169. [DOI] [PubMed] [Google Scholar]
- 13.Tomita N, Yokoyama M, Yamamoto W, et al. . Central nervous system event in patients with diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 2012;103(2):245-251. [DOI] [PubMed] [Google Scholar]
- 14.Zucca E, Conconi A, Mughal TI, et al. ; International Extranodal Lymphoma Study Group. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21(1):20-27. [DOI] [PubMed] [Google Scholar]
- 15.Kridel R, Telio D, Villa D, et al. . Diffuse large B-cell lymphoma with testicular involvement: outcome and risk of CNS relapse in the rituximab era. Br J Haematol. 2017;176(2):210-221. [DOI] [PubMed] [Google Scholar]
- 16.Vitolo U, Chiappella A, Ferreri AJ, et al. . First-line treatment for primary testicular diffuse large B-cell lymphoma with rituximab-CHOP, CNS prophylaxis, and contralateral testis irradiation: final results of an international phase II trial. J Clin Oncol. 2011;29(20):2766-2772. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Chen B, Xu X. Impact of rituximab on incidence of and risk factors for central nervous system relapse in patients with diffuse large B-cell lymphoma: a systematic review and meta-analysis. Leuk Lymphoma. 2014;55(3):509-514. [DOI] [PubMed] [Google Scholar]
- 18.El-Galaly TC, Cheah CY, Hutchings M, et al. . Uterine, but not ovarian, female reproductive organ involvement at presentation by diffuse large B-cell lymphoma is associated with poor outcomes and a high frequency of secondary CNS involvement. Br J Haematol. 2016;175(5):876-883. [DOI] [PubMed] [Google Scholar]
- 19.Laskin JJ, Savage KJ, Voss N, Gascoyne RD, Connors JM. Primary paranasal sinus lymphoma: natural history and improved outcome with central nervous system chemoprophylaxis. Leuk Lymphoma. 2005;46(12):1721-1727. [DOI] [PubMed] [Google Scholar]
- 20.Murawski N, Held G, Ziepert M, et al. . The role of radiotherapy and intrathecal CNS prophylaxis in extralymphatic craniofacial aggressive B-cell lymphomas. Blood. 2014;124(5):720-728. [DOI] [PubMed] [Google Scholar]
- 21.Green TM, Young KH, Visco C, et al. . Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3460-3467. [DOI] [PubMed] [Google Scholar]
- 22.Johnson NA, Slack GW, Savage KJ, et al. . Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheah CY, Oki Y, Westin JR, Turturro F. A clinician’s guide to double hit lymphomas. Br J Haematol. 2015;168(6):784-795. [DOI] [PubMed] [Google Scholar]
- 24.Oki Y, Noorani M, Lin P, et al. . Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891-901. [DOI] [PubMed] [Google Scholar]
- 25.Savage KJ, Slack GW, Mottok A, et al. . Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182-2188. [DOI] [PubMed] [Google Scholar]
- 26.Horn H, Ziepert M, Becher C, et al. ; German High-Grade Non-Hodgkin Lymphoma Study Group. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood. 2013;121(12):2253-2263. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Xu-Monette ZY, Tzankov A, et al. . MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021-4031, quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott DW, Wright GW, Williams PM, et al. . Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123(8):1214-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hans CP, Weisenburger DD, Greiner TC, et al. . Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275-282. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki K, Yamaguchi M, Suzuki R, et al. . CD5-positive diffuse large B-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann Oncol. 2011;22(7):1601-1607. [DOI] [PubMed] [Google Scholar]
- 31.Xu-Monette ZY, Tu M, Jabbar KJ, et al. . Clinical and biological significance of de novo CD5+ diffuse large B-cell lymphoma in Western countries. Oncotarget. 2015;6(8):5615-5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chuang WY, Chang H, Shih LY, Wang PN, Chang YS, Lin TL, et al. . CD5 positivity is an independent adverse prognostic factor in elderly patients with diffuse large B cell lymphoma. Virchows Arch. 2015;467(5):571-582. [DOI] [PubMed]
- 33.Cox MC, Di Napoli A, Scarpino S, et al. . Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One. 2014;9(4):e93903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kansara R, Villa D, Gerrie AS, Klasa R, Shenkier T, Scott DW, et al. Site of central nervous system (CNS) relapse in patients with diffuse large B-cell lymphoma (DLBCL) by the CNS-IPI risk model [published online ahead of print 22 July 2016]. Br J Haematol. doi: 10.1111/bjh.14229. [DOI] [PubMed]
- 35.Kumar A, Vanderplas A, LaCasce AS, et al. . Lack of benefit of central nervous system prophylaxis for diffuse large B-cell lymphoma in the rituximab era: findings from a large national database. Cancer. 2012;118(11):2944-2951. [DOI] [PubMed] [Google Scholar]
- 36.de Graaf MT, de Jongste AH, Kraan J, Boonstra JG, Sillevis Smitt PA, Gratama JW. Flow cytometric characterization of cerebrospinal fluid cells. Cytometry B Clin Cytom. 2011;80(5):271-281. [DOI] [PubMed] [Google Scholar]
- 37.Wilson WH, Bromberg JE, Stetler-Stevenson M, et al. . Detection and outcome of occult leptomeningeal disease in diffuse large B-cell lymphoma and Burkitt lymphoma. Haematologica. 2014;99(7):1228-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pentsova EI, Shah RH, Tang J, et al. . Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreri AJ, Donadoni G, Cabras MG, et al. . High doses of antimetabolites followed by high-dose sequential chemoimmunotherapy and autologous stem-cell transplantation in patients with systemic B-cell lymphoma and secondary CNS involvement: final results of a multicenter phase II trial. J Clin Oncol. 2015;33(33):3903-3910. [DOI] [PubMed] [Google Scholar]
- 40.Tilly H, Lepage E, Coiffier B, et al. ; Groupe d’Etude des Lymphomes de l’Adulte. Intensive conventional chemotherapy (ACVBP regimen) compared with standard CHOP for poor-prognosis aggressive non-Hodgkin lymphoma. Blood. 2003;102(13):4284-4289. [DOI] [PubMed] [Google Scholar]
- 41.Holte H, Leppä S, Björkholm M, et al. . Dose-densified chemoimmunotherapy followed by systemic central nervous system prophylaxis for younger high-risk diffuse large B-cell/follicular grade 3 lymphoma patients: results of a phase II Nordic Lymphoma Group study. Ann Oncol. 2013;24(5):1385-1392. [DOI] [PubMed] [Google Scholar]
- 42.Phillips EH, Kirkwood AA, Lawrie A, et al. . Low rates of CNS relapse in high risk DLBCL patients treated with R-CODOX-M and R-IVAC: results from a phase 2 UK NCRI/Bloodwise trial. Blood. 2016;128(22):1855. [Google Scholar]
- 43.Abramson JS, Hellmann M, Barnes JA, et al. . Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer. 2010;116(18):4283-4290. [DOI] [PubMed] [Google Scholar]
- 44.Cheah CY, Herbert KE, O’Rourke K, et al. . A multicentre retrospective comparison of central nervous system prophylaxis strategies among patients with high-risk diffuse large B-cell lymphoma. Br J Cancer. 2014;111(6):1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferreri AJ, Bruno-Ventre M, Donadoni G, et al. . Risk-tailored CNS prophylaxis in a mono-institutional series of 200 patients with diffuse large B-cell lymphoma treated in the rituximab era. Br J Clin Oncol. 2015;168(5):654-662. [DOI] [PubMed] [Google Scholar]
- 46.Rubenstein JL, Fraser E, Formaker P, Chi-Chiang Lee J, Chen N, Kock M, et al. Phase I investigation of lenalidomide plus rituximab and outcomes of lenalidomide maintenance in recurrent CNS lymphoma [abstract]. J Clin Oncol. 2016;34(suppl):7502.
- 47.Dunleavy K, Lai CE, Roschewski M, et al. . Phase I study of dose-adjusted-Teddi-R with ibrutinib in untreated and relapsed/refractory primary CNS lymphoma. Blood. 2015;126(23):472. [Google Scholar]
- 48.Ayed AO, Chiappella A, Nowakowski GS, et al. . Lenalidomide plus R-CHOP (R2CHOP) in patients with DLBCL is associated with a lower risk of CNS relapse: combined analysis from two phase 2 studies. Blood. 2016;128(22):3033. [Google Scholar]
- 49.Twa DD, Mottok A, Chan FC, et al. . Recurrent genomic rearrangements in primary testicular lymphoma. J Pathol. 2015;236(2):136-141. [DOI] [PubMed] [Google Scholar]
- 50.Chapuy B, Roemer MG, Stewart C, et al. . Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood. 2016;127(7):869-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayak L, Iwamoto FM, LaCasce A, Mukundan S, Roemer MG, Chapuy B, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma [published online ahead of print 29 March 2017]. Blood. doi:10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed]