Abstract
Sickle cell disease (SCD) is an inheritable hemoglobinopathy characterized by polymerization of hemoglobin S in red blood cells resulting in chronic hemolytic anemia, vaso-occlusive painful crisis, and multiorgan damage. In SCD, an increased reactive oxygen species (ROS) generation occurs both inside the red blood cells and inside the vascular lumen, which augment hemolysis and cellular adhesion. This review discusses the evolving body of literature on the role of ROS in the pathophysiology of SCD as well as some emerging therapeutic approaches to SCD with a focus on the reduction of ROS.
Learning Objectives
Review the contribution of reactive oxygen species in red blood cells to the pathophysiology of sickle cell disease
Explain therapeutic approaches for sickle cell disease
Introduction
Sickle cell disease (SCD) manifests in infancy as sickle hemoglobin S (HbS) replaces fetal hemoglobin (HbF). HbS is distinguished from normal adult hemoglobin by a single-point mutation in the β-globin gene. Individuals with SCD suffer from chronic hemolytic anemia, painful crises, multisystem organ damage, and a shorter lifespan. Polymerization of deoxy HbS induces impairment in red blood cell (RBC) membrane structure, viscosity, and biochemical properties, resulting in early removal by the reticuloendothelial system or trapping in microvessels, and is considered a critical event for SCD complications.1 Sickled RBCs as well as circulating reticulocytes initiate adhesion with endothelial cells, polymorphonuclear neutrophils (PMNs), and platelets, which leads to a cascade of endothelial cell damage, a dysfunctional vascular system, ischemia, reactive oxygen species (ROS), and ultimately, damage to multiple vital organs. A growing body of literature indicates that ROS augments known pathways of hemolysis and adhesion and also plays an independent role in SCD pathophysiology. Both vaso-occlusion and hemolysis generate ROS and are caused by ROS, thus creating a malicious cycle. This review focuses on the role of oxidative stress on sickle cell pathophysiology. We acknowledge that this review does not in its scope give nearly the equivalent attention to the vast literature on HbS polymerization, hemolysis, and cellular adhesion.
The role of ROS in SCD pathophysiology
Sources and mechanism of ROS accumulation inside the SCDs RBCs
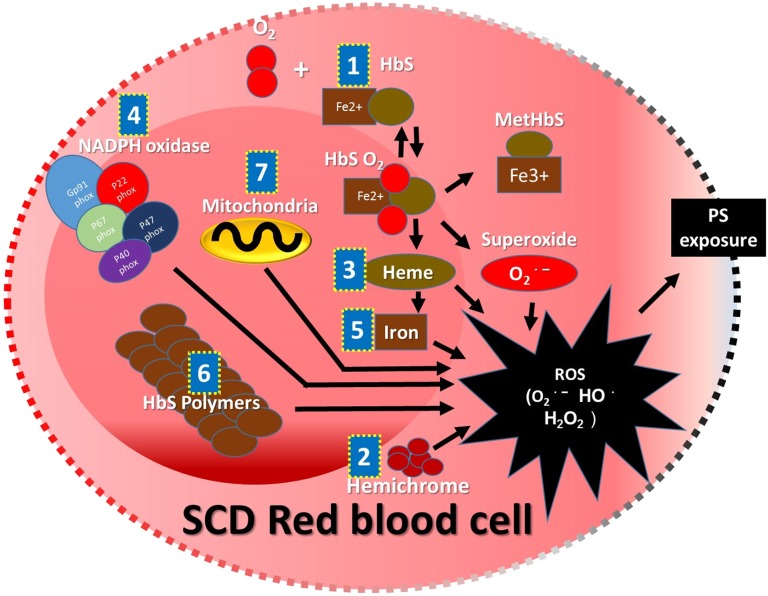
In addition to the damage that is done to SCD RBC membranes by HbS polymerization, SCD RBC membrane damage is caused by both HbS polymerization and ROS, leading to hemolysis.2 Hemolysis releases intracellular ROS into the vasculature. The ROS, primarily superoxide, hydrogen peroxide (H2O2), hydroxyl radical (OH•), and lipid oxidation products, are generated endogenously in healthy RBCs. However, the self-sustaining antioxidant defense mechanism in healthy RBCs effectively neutralizes ROS. In SCD, HbS-containing RBCs not only generate significantly higher levels of ROS compared with adult hemoglobin– or HbF-containing RBCs but also, tend to accumulate ROS because of reduced availability of endogenous antioxidants, including selenium, Peroxiredoxin-II (Prx-II), and activity of Glutathione peroxidase-1 (GPx-1).3-6 There are several mechanisms responsible for the higher levels of ROS in SCD RBCs. (1) Auto-oxidative unstable HbS induces an increased rate of methemoglobin formation and superoxide generation.7 (2) Sickle erythrocyte membrane-bound hemichrome facilitates superoxide- and peroxide-driven OH• generation at a higher rate compared with normal erythrocyte membranes.8 (3) Increased activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases causes overproduction of ROS.9 (4) The reduced level of Prx-II in SCD RBCs causes reduced interaction of Prx-II and hemoglobin, resulting in susceptibility to denaturation of hemoglobin by H2O2. Denaturation of hemoglobin in SCD leads to dissociation of heme from hemoglobin followed by free iron release from heme, which causes accelerated endogenous ROS production.5 (5) H2O2 accumulated in RBCs oxidizes Fe2+ into Fe3+ and also, generates the highly reactive OH• by the Fenton chemical reaction. OH• generated in SCD RBCs tends to react with biomolecules to produce a series of new free radicals.8 (6) Increased ROS formation is directly related to adenosine triphosphate utilization. It is speculated that the adenosine triphosphate is being used to maintain a proper reduction state and in the polymerization of sickle hemoglobin.10 (7) The presence of RBCs with abnormally retained mitochondria has suggested mitochondria as a candidate source of ROS in SCD RBCs11 (Figure 1).
Figure 1.
Schematic representation of sources and mechanism of ROS accumulation inside the SCD RBCs. Auto-oxidative unstable HbS (1), membrane-bound hemichrome (2), dissociated heme (3), NADPH oxidase (4), heme free iron (5), excessive adenosine triphosphate utilization with higher rate of polymerization of HbS (6), and abnormally retained mitochondria (7) are the major intracellular sources and mechanisms reported for the excessive ROS accumulation that may cause shorter lifespan because of PS exposure followed by removal by reticuloendothelial system or caspase-mediated intravascular hemolysis.
Sources and mechanisms of ROS amplification in the intravascular lumen
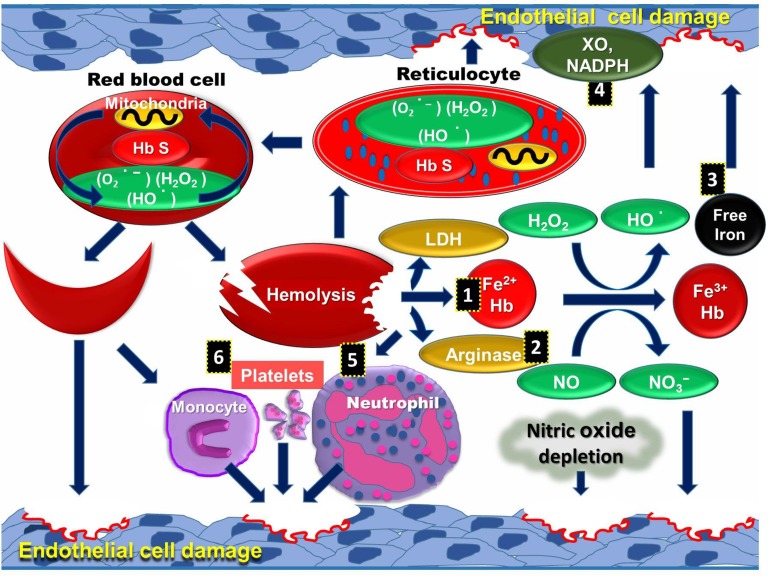
Multiple interlinked factors are responsible for the amplification of intravascular ROS and systemic oxidative stress in SCD patients. (1) Cell-free plasma hemoglobin scavenges nitric oxide (NO), which leads to vascular dysfunction, ROS production caused by hypoxia, and reperfusion injury.12 (2) Arginase released from hemolysis causes depletion of endothelial l-arginine followed by uncoupling of NO synthase activity in endothelial cells. (3) Frequent blood transfusion and unstable hemoglobin can cause iron overload, generating ROS through catalyzing chemical reactions.13 (4) Superoxide is produced from increased activities of endothelial xanthine oxidase14 and NADPH oxidases.15 It is speculated that superoxide released into plasma interacts with NO to deplete intravascular NO known to cause dysfunctional endothelium. The reaction of superoxide with NO also potentially generates more potent peroxynitrite.16 (5) Cell-free hemoglobin reacts with NO and also activates PMNs and platelets to induce ROS.17 (6) Activated platelets produce ROS in SCD through dysfunctional mitochondria18 (Figure 2).
Figure 2.
Schematic representation of sources of intravascular ROS in SCD pathophysiology. Mitochondria retention along with other causative factors in RBCs and reticulocytes can oxidize the membrane proteins and lipids, leading to intravascular hemolysis. Hemolytic products released to the vascular lumen further generate highly reactive ROS and create a cycle in SCD. Hemolytic products, including cell-free hemoglobin (1), arginase (2), free iron (3), and lactate dehydrogenase, are released into plasma, and increased activities of XO and NADPH (4) cause ROS mediated endothelial cell damage. These products activate PMNs (5), platelets, and monocytes (6), which further increase endothelial cell damage.
ROS augments sickle pathophysiology pathways: hemolysis and adhesion
The presence of oxidized HbS and deoxygenated polymerization of HbS in the RBC creates a cycle between ROS, hemolysis, and RBC adhesion. A complete discussion of the mechanism of SCD RBC adhesion was recently reviewed and is beyond the scope of this article.19 The membrane damage to SCD RBCs and reticulocytes by ROS causes vascular endothelial damage20 through abnormal cellular adhesion and the release of intravascular hemolytic products as discussed above. SCD reticulocytes have increased adherence to endothelial cells. ROS generated by hemolysis activates monocytes and PMNs in the vascular lumen, resulting in inflammation, which damages vascular endothelial cells. The abnormal adhesion of SCD RBCs to the endothelium causes oxidative stress and inflammatory responses. There are increased thiobarbituric acid–reactive substance and activation of the transcription factor NF-κB, which are known cellular oxidant stress markers.21 One possibility for increased adhesion is the premature externalization of phosphatidylserine (PS), which arises on sickle RBC surfaces caused by internal oxidation of scramblase by ROS. PS is a known adhesive mediator between sickle RBCs and endothelial cells. An association between PS externalization level and phospholipid scramblase activity in sickle RBCs under basal conditions has been reported.22 In addition to PS externalization on RBCs, oxidative stress increases the surface expression of a subset of cellular adhesion molecules, Intercellular Adhesion Molecule 1, E-selectin, and Vascular cell adhesion molecule-1, in endothelial cells and facilitates the transendothelial migration of monocytes and an increase in platelet-endothelial cell adhesion molecule-1 phosphorylation.21 Damage to vascular endothelial cell leads to vascular narrowing, causing ischemia, reperfusion, infarction, and ultimately, multiple organ failure.
ROS reduction therapies in SCD
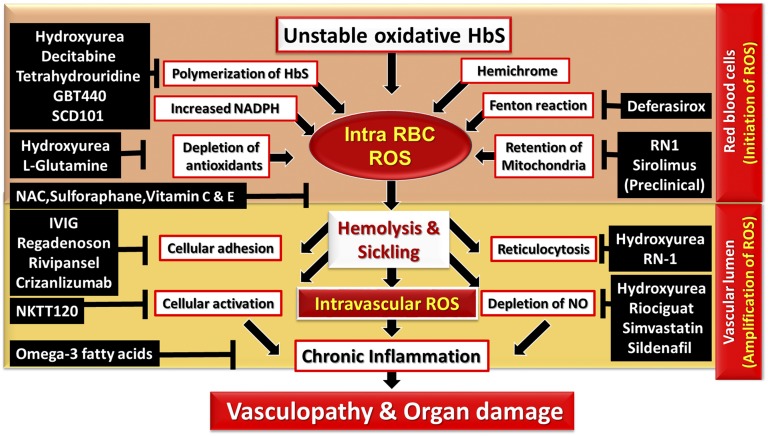
The constant hemolytic process and development of the chronic systemic oxidative stress deplete the antioxidant defense sources in SCD patients. Despite an increased understanding of ROS’s role in pathophysiology of SCD, the previously investigated ROS-reducing antioxidants have had mixed success.23 l-glutamine, a drug that specifically targets intracellular RBC ROS, has been recently approved by the Food and Drug Administration for SCD pain crisis. In addition, hydroxyurea (HU), which has been Food and Drug Administration approved for SCD for decades, has several ROS reduction mechanisms (Figure 3).
Figure 3.
Schema of ROS-mediated pathophysiology and potential mechanism of drug targets in SCD. (1) Intracellular ROS in the RBC. Auto-oxidative unstable HbS, HbS polymerization, membrane-bound hemichrome, activity of NADPH oxidases, depleted antioxidants, iron-mediated Fenton reaction, and mitochondrial retention facilitate excessive ROS generation in the RBCs. (2) ROS in the intravascular lumen. RBC intravascular hemolysis abnormally activates body immune and inflammatory responses that further amplify ROS and magnify the impact on endothelial cells. Chronic inflammatory and immunological responses alter vascular dysfunction, leading to hypoxia and infarction-mediated irreversible damage in multiple organ systems. This network of ROS production can be disrupted or inhibited by the treatment of various agents. IVIG; immunoglobin IV; NAC, N-acetylcysteine.
Clinical
HU (hydroxycarbamide).
The disease-modifying effect by HU was initially considered to be elicited through the single mechanism of HbF induction (Table 1). However, it has been increasingly recognized that HU has other additional mechanisms, such as elevating the levels of NO and activities of GPx-1, Glutathione, and catalase.4,24 Decreased inflammation may result from its ability to act as an NO donor. HU inhibits vaso-occlusive crisis (VOC) through disrupting the process of neutrophil-mediated sickle erythrocyte PS exposure and reducing the expression of several adhesion molecules, including P-selectin, E-selectin, Intercellular Adhesion Molecule, and Vascular cell adhesion molecule.
Table 1.
Novel ROS reducing agents studied in human SCD patients
| Source | Agent | Mechanism | Stage | Result |
|---|---|---|---|---|
| Ref. 25 and NCT01179217 | l-glutamine | Changes in NADPH, NO, and nicotinamide adenine dinucleotide redox | Phases 2 and 3 | Phase 2: significant changes in both the NADH level and nicotinamide adenine dinucleotide redox potential; phase 3: in progress |
| Ref. 54 | l-arginine | NO production | Phase 2 | Reduces pain intensity |
| NCT01849016 and ref. 55 | N-acetylcysteine | Increases blood glutathione and decreases PS, AGEs, and cell-free hemoglobin | Phase 2 | Patients experienced no painful crises or other significant SCD-related complications during the study period |
| NCT00508027 and refs. 32 and 56 | Simvastatin | Increases NO metabolites and reduces C-reactive protein and interleukin-6 | Phase 2 | Significant levels of protection have been reported |
| NCT00110697 | Deferasirox | Iron chelation | Phase 2 | Reduces vaso-occlusive pain crisis |
| NCT02947100, NCT02525107, NCT02604368, and ref. 57 | Ω-3 fatty acids | ROS reduction | Phases 1/2/3 | Reduces frequency of pain episodes |
| NCT01715480 and ref. 36 | Broccoli sprout homogenate, which contains sulforaphane | Nrf2 induction | Phase 1 | Induces NRF2 target heme oxiginase-1 expression |
AGE, advanced glycation end-product.
l-glutamine.
l-glutamine can significantly increase the nicotinamide adenine dinucleotide redox potential in sickle RBC.25 l-glutamine depletion in SCD patients is associated with pulmonary hypertension and hemolysis. Oral l-glutamine administration to SCD patients resulted in a significant reduction in their resting energy expenditure.26 In a phase 3 study, SCD patients > 18 years old had a lower rate of sickle cell crises per 48 weeks in the l-glutamine arm relative to placebo with a ratio of 0.64 (0.45 to 0.89).27
Riociguat.
Riociguat is a specific soluble guanylate cyclase stimulator. On binding of NO, soluble guanylate cyclase catalyzes the synthesis of the second messenger guanosine 3′,5′-cyclic monophosphate, which promotes vasodilation, inhibits smooth muscle proliferation, promotes leukocyte recruitment, promotes platelet aggregation, and promotes vascular remodeling.28 A multicenter, phase 2 study to assess the safety, tolerability, and efficacy of riociguat is recruiting subjects (NCT02633397).
N-acetylcysteine.
N-acetylcysteine is an antioxidant drug that scavenges ROS. It decreased the frequency of VOC in phase 2 clinical trials (NCT01849016) in SCD patients.29
Deferasirox.
Deferasirox has been approved for iron chelation in SCD patients. In addition, it has shown a significant reduction of vaso-occlusive pain crisis.30
Simvastatin.
Simvastatin is a statin molecule widely used for its anticholesterolemic drug. It also has an NO production mechanism that prevented endothelial damages in obesity patients and increases antioxidants enzymes, including superoxide dismutase, GPx, and catalase.31 In SCD patients, simvastatin has been shown to increase NO metabolites and reduce C-reactive protein and interleukin-6 (NCT00508027).32 Atorvastatin, a drug of similar class, has shown weaker evidence of efficacy.33 This class of drugs has not been further tested or proven in a larger trial.
Ω-3 fatty acids.
A previous single-center study with 140 patients found that Ω-3 fatty acids docosahexaenoic acid and eicosapentaenoic acid significantly reduced the median rates of clinical vaso-occlusive events (0 compared with 1.0 per year, P < .0001), severe anemia (3.2% compared with 16.4%; P < .05), and blood transfusion (4.5% compared with 16.4%; P < .05).34 Phase 1 and 2 studies using a new formulation of Ω-3 fatty acids Omegatex to reduce inflammatory pain in children and young adults with SCD are recruiting patients (NCT02947100). Fish oil administration decreased pain rates but not interleukin-6 levels in a study conducted by Okpala et al.35 In addition, phase 3 studies of Ω-3 fatty acids encapsulated in a soft gelatin capsule using advanced Lipid Technologies (ALT) are under investigation to prevent pain crisis in patients with SCD (NCT02525107 and NCT02604368). The results of this study will provide evidence about the potential therapeutic effect of Ω-3 fatty acids in SCD.
Sulforaphane.
Preclinical studies showed that activation of nuclear factor erythroid 2–related factor 2 (NRF2) with sulforaphane in erythroid progenitors significantly increased the expression of NRF2 targets heme oxiginase-1, NAD(P)H quinone dehydrogenase-1, and hemoglobin subunit γ 1. A phase 1 study of the administration of broccoli sprout homogenate, which contains sulforaphane, in adults with SCD showed a significant induction of NRF2 target heme oxiginase-1 expression. The study suggested that more potent NRF2 inducers may offer clinical benefits to SCD patients (NCT01715480).36
Vitamin C and vitamin E.
Previous studies showed that levels of vitamins C and E in SCD patients were depleted, particularly during the disease severity.6 However, the supplementation of vitamin C and vitamin E for SCD patients has shown limited success.17
Preclinical (tested in SCD mouse model)
NRF2 inducers (monomethyl fumarate; 2-cyano-3,12 dioxooleana-1,9 diene-28-imidazolide).
Recent studies in the SCD mouse model have shown that activation of the master regulator of antioxidant cell defense transcription factor Nrf2 ameliorates inflammation and multiple organ damages (Table 2). Monomethyl fumarate reduced oxidative stress and reduced sickle retinopathy–related pathology in SCD moice.37 SCD mice treated with 2-cyano-3,12 dioxooleana-1,9 diene-28-imidazolide showed reduced inflammation and improved organ function.38
Table 2.
Potential ROS-reducing agents studied in preclinical models
| References | Agent | Mechanism | Stage | Result |
|---|---|---|---|---|
| Ref. 37 | Monomethyl fumorate | Ntf2 induction and HbF induction | SCD mice | Reduces sickle retinopathy |
| Ref. 38 | 2-Cyano-3,12 dioxooleana-1,9 diene-28-imidazolide (Nrf2 inducer) | Nrf2 induction | SCD mice | Reduces inflammation and organ damages |
| Ref. 40 | INK128 and sirolimus | Mammalian target of rapamycin inhibition | SCD mice | Reduces organ damages |
| Ref. 39 | Sildenafil | Promotes endothelial NO synthase and inhibits NADPH oxidation | SCD mice | Protects vascular endothelium |
| Ref. 11 | RN-1 (LSD-1 inhibitor) | Mitophagy induction and reduces SCD-RBC ROS | SCD mice | Increases SCD RBCs lifespan |
| Ref. 11 | Sirolimus (mammalian target of rapamycin inhibitor) | Mitophagy induction and reduces SCD-RBC ROS | SCD mice | Increases SCD RBCs lifespan |
PDE5 inhibitor (sildenafil).
Sildenafil (100 mg/kg per day) restored endothelial NO synthase interaction with HSP90, increased AKT‐mediated phosphorylation of endothelial NO synthase, and prevented of oxidative stress generated by NADPH oxidase in SCD mice.39
Mammalian target of rapamycin inhibitors (INK128, sirolimus).
SCD mice treated with INK128 or sirolimus significantly increased hemoglobin level and reduced reticulocyte counts. Sirolimus also prolonged the lifespan of sickle RBCs, reduced spleen size, and reduced organ damage in an SCD mouse model.40 In another study in the SCD mouse model, sirolimus reduced mitochondrial retention and ROS accumulation in RBCs.11
Inhibition of HbS polymerization in SCD
HbF expression
HU (hydroxycarbamide).
HbF inhibits the polymerization of HbS and is associated with less severe illness and longer survival. HbF can be elevated through treatment with HU, a ribonuclease reductase. Several studies have shown that HU has multiple benefits for sickle cell patients: decreased VOC, decreased hospitalization, fewer episodes of acute chest syndrome, and increased lifespan.
Decitabine and tetrahydrouridine.
To develop more potent drugs for HbF induction, research has focused on drugs that inhibit enzyme-catalyzing repressive epigenetic modification of DNA and histones. These enzymes are components of corepressor complexes recruited by the known transrepressors of γ-globin. Biochemical purification of a multiprotein repressive complex, direct repeat erythroid definitive, that recruited to the DR elements within the γ-globin gene promoter identified testicular orphan nuclear receptor 2 and 4, DNA methyltransferase-1 (DNMT-1), and lysine-specific demethylase-1 (LSD-1) as core elements and other proteins, including histone deacetylases and corepressor of RE1-silencing transcription factor, to be components of the multiprotein direct repeat erythroid definitive corepressor complex. BCL11A also recruits a multiprotein repressive complex that includes histone deacetylases, DNMT-1, and LSD-1 to repress γ-globin expression.41 Decitabine is a DNMT-1 inhibitor. Oral administration of decitabine is limited by its rapid inactivation by cytidine deaminase in vivo. Therefore, a recent phase 1 (NCT01685515) trial combined decitabine with cytidine deaminase inhibitor tetrahydrouridine for oral administration in patients with severe, symptomatic SCD who had not benefited from HU. The combination produced increased HbF and HbF-containing RBCs in patients who had previously not responded to HU. Decitabine was shown as safe and well tolerated by the SCD patients in combination with tetrahydrouridine.42
RN-1.
RN-1 is a promising preclinical drug candidate. RN-1 is an LSD-1 inhibitor, which increased HbF to high levels and was safely administered for >260 days in the baboon (Papio anubis).43,44 It has been shown that RN-1 can reduce organ damage in SCD animal models.45 In addition, RN-1 reduces ROS accumulation in RBCs. The ROS reduction was associated with erythroid-specific mitophagy gene expression and elimination of mitochondria in the RBCs.11
Inhibition of HbS polymerization by HbF-independent approaches
Apart from HbF induction, there are four other approaches for blocking HbS polymerization, and they are currently at different drug development stages: (1) blocking intramolecular contacts in sickle cell fibers; (2) increasing oxygen affinity; (3) reducing the concentration of 2,3 diphosphoglycerate; and (4) reducing intracellular hemoglobin concentrations.46
GBT440.
This drug increases the affinity of hemoglobin for oxygen and therefore, inhibits HbS polymerization when subjected to hypoxic conditions. In a prospective phase 1/2 study, SCD patients showed a reduction in intravascular hemolysis and increased hemoglobin. This drug is currently in phase 3 clinical trials (NCT03036813).
SCD-101
SCD-101 inhibits in vitro sickling by increasing both solubility and delay time in solutions of deoxyhemoglobin. In a phase 1b study of 26 individuals with HbSS and HbS/β0 thalassemia, this drug was well tolerated and relieved chronic pain and fatigue at higher doses. However, no impact on hemoglobin level or hemolysis was observed (NCT02380079).
Blocking cellular adhesions in SCD
Immunoglobulin IV
A previous preclinical study reported that immunoglobulin IV prevents VOC by inhibiting adhesion.47 In a phase 1 clinical trial, low-dose immunoglobulin IV at 200 to 400 mg/kg decreased Mac-1. A phase 2 study is recruiting patients (NCT01757418).
Regadenoson
Regadenoson is an adenosine A2a receptor agonist. Adenosine increases blood flow and decreases inflammation. A2a receptors are ubiquitously expressed on invariant natural killer T cells, neutrophils, macrophages, and leukocytes. A multicenter, phase 2 study using Regadenoson for acute pain crisis and acute chest syndrome is recruiting patients (NCT01788631).
NKTT120
NKTT120 is an anti-invariant natural killer T-cell monoclonal antibody. Twenty-one SCD patients enrolled in an open label, multicenter, and single–ascending dose trial received seven doses from 0.001 to 1.0 mg/kg. NKTT120 caused invariant natural killer T-cell depletion after 2 to 5 months of treatment in the absence of toxicity or serious adverse events (NCT01783691).48
Rivipansel (GMI-1070)
Rivipansel (GMI-1070) is a panselectin agonist. A phase 2 clinical trial (NCT00911495) enrolled 76 patients ages between 12 and 60 years old from 22 locations. This drug showed no increase in adverse events compared with placebo. Mean cumulative use of IV opioids was reduced by 83% with GMI-1070 vs placebo. The median time from time to VOC resolution showed a trend down 28% vs 48%, but it was not statistically significant. A phase 3 clinical study for the treatment of VOC in hospitalized subjects using Rivipansel is recruiting patients (NCT002187003).
Crizanlizumab (SelG1)
Crizanlizumab is a humanized monoclonal antibody that binds selectively to P-selectin and blocks interaction with its P-selectin glycoprotein ligand. A recent multicenter phase 2 Study to Assess Safety and Impact of SelG1 With or Without Hydroxyurea Therapy in Sickle Cell Disease Patients with Pain Crises (SUSTAIN) reported that Crizanlizumab therapy reduced the rate of pain crisis to 45.3% in SCD patients compared with those in placebo group. Consistent with its antiadhesion mechanism, this study showed no significant changes in hemolytic markers.49
Transfusion Therapy in SCD
The goals of transfusion therapy are to improve oxygen carrying capacity, reduce hemolysis, and suppress the production of circulating reticulocytes. The risks of transfusion are red cell alloimmunization, hyperhemolysis, hyperviscosity, and iron overload. In SCD, the use of simple transfusion is recommended for symptomatic anemia, aplastic crisis, splenic sequestration with severe anemia, and prevention of ACS caused by anesthesia during surgery. Exchange or simple transfusion is recommended for use in the setting of hepatic sequestration, intrahepatic cholestasis, and multisystem organ failure. Exchange transfusion is recommended for acute stroke. Chronic transfusion patients receive the transfusion in a standard interval, usually to achieve a goal of HbS <30%. Chronic transfusion is currently recommended for prevention of stroke in patients with prior stroke and elevated transcranial Doppler. Many treatment centers are using chronic transfusions to decrease the frequencies of VOC and acute chest syndrome and to slow the progression of pulmonary hypertension.50 Chronic transfusion reduces neural cell adhesion molecule, ROS-containing reticulocytes, and HbS-containing RBCs. In the current setting of improvement in RBC phenotyping and iron chelation therapy, evaluation on chronic transfusion in sickle cell patients is warranted.51-53
Conclusion
In summary, although SCD is caused by a single gene mutation, there are multiple mechanisms involved in the pathophysiology. The generation of ROS augments hemolysis, endothelial damage, and cell adhesion. Understanding all mechanisms of SCD progression is important to create treatments that are effective in all patients with SCD. It is most likely that, given the complex nature of this disease, a multimodal therapy approach will be necessary.
References
- 1.Oder E, Safo MK, Abdulmalik O, Kato GJ. New developments in anti-sickling agents: can drugs directly prevent the polymerization of sickle haemoglobin in vivo? Br J Haematol. 2016;175(1):24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life. 2012;64(1):72-80. [DOI] [PubMed] [Google Scholar]
- 3.Claster S, Wood JC, Noetzli L, et al. . Nutritional deficiencies in iron overloaded patients with hemoglobinopathies. Am J Hematol. 2009;84(6):344-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho CS, Kato GJ, Yang SH, et al. . Hydroxyurea-induced expression of glutathione peroxidase 1 in red blood cells of individuals with sickle cell anemia. Antioxid Redox Signal. 2010;13(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han YH, Kim SU, Kwon TH, et al. . Peroxiredoxin II is essential for preventing hemolytic anemia from oxidative stress through maintaining hemoglobin stability. Biochem Biophys Res Commun. 2012;426(3):427-432. [DOI] [PubMed] [Google Scholar]
- 6.Gizi A, Papassotiriou I, Apostolakou F, et al. . Assessment of oxidative stress in patients with sickle cell disease: the glutathione system and the oxidant-antioxidant status. Blood Cells Mol Dis. 2011;46(3):220-225. [DOI] [PubMed] [Google Scholar]
- 7.Hebbel RP, Morgan WT, Eaton JW, Hedlund BE. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc Natl Acad Sci USA. 1988;85(1):237-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Repka T, Hebbel RP. Hydroxyl radical formation by sickle erythrocyte membranes: role of pathologic iron deposits and cytoplasmic reducing agents. Blood. 1991;78(10):2753-2758. [PubMed] [Google Scholar]
- 9.George A, Pushkaran S, Konstantinidis DG, et al. . Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood. 2013;121(11):2099-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee T, Kuypers FA. Reactive oxygen species and phosphatidylserine externalization in murine sickle red cells. Br J Haematol. 2004;124(3):391-402. [DOI] [PubMed] [Google Scholar]
- 11.Jagadeeswaran R, Vazquez BA, Thiruppathi M, et al. . Pharmacological inhibition of LSD1 and mTOR reduces mitochondrial retention and associated ROS levels in the red blood cells of sickle cell disease. Exp Hematol. 2017;50:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ. Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood. 2007;110(6):2166-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fung EB, Harmatz P, Milet M, et al. ; Multi-Center Study of Iron Overload Research Group. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: a report from the multi-center study of iron overload. Am J Hematol. 2007;82(4):255-265. [DOI] [PubMed] [Google Scholar]
- 14.Aslan M, Ryan TM, Adler B, et al. . Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci USA. 2001;98(26):15215-15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood KC, Hebbel RP, Granger DN. Endothelial cell NADPH oxidase mediates the cerebral microvascular dysfunction in sickle cell transgenic mice. FASEB J. 2005;19(8):989-991. [DOI] [PubMed] [Google Scholar]
- 16.Aslan M, Thornley-Brown D, Freeman BA. Reactive species in sickle cell disease. Ann N Y Acad Sci. 2000;899:375-391. [DOI] [PubMed] [Google Scholar]
- 17.Amer J, Ghoti H, Rachmilewitz E, Koren A, Levin C, Fibach E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br J Haematol. 2006;132(1):108-113. [DOI] [PubMed] [Google Scholar]
- 18.Cardenes N, Corey C, Geary L, et al. . Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood. 2014;123(18):2864-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Field JJ. Can selectin and iNKT cell therapies meet the needs of people with sickle cell disease? Hematology Am Soc Hematol Educ Program. 2015;2015:426-432. [DOI] [PubMed] [Google Scholar]
- 20.Gee BE, Platt OS. Sickle reticulocytes adhere to VCAM-1. Blood. 1995;85(1):268-274. [PubMed] [Google Scholar]
- 21.Sultana C, Shen Y, Rattan V, Johnson C, Kalra VK. Interaction of sickle erythrocytes with endothelial cells in the presence of endothelial cell conditioned medium induces oxidant stress leading to transendothelial migration of monocytes. Blood. 1998;92(10):3924-3935. [PubMed] [Google Scholar]
- 22.Barber LA, Palascak MB, Joiner CH, Franco RS. Aminophospholipid translocase and phospholipid scramblase activities in sickle erythrocyte subpopulations. Br J Haematol. 2009;146(4):447-455. [DOI] [PubMed] [Google Scholar]
- 23.Muskiet FA, Muskiet FD, Meiborg G, Schermer JG. Supplementation of patients with homozygous sickle cell disease with zinc, alpha-tocopherol, vitamin C, soybean oil, and fish oil. Am J Clin Nutr. 1991;54(4):736-744. [DOI] [PubMed] [Google Scholar]
- 24.Silva DG, Belini Junior E, Torres LS, et al. . Relationship between oxidative stress, glutathione S-transferase polymorphisms and hydroxyurea treatment in sickle cell anemia. Blood Cells Mol Dis. 2011;47(1):23-28. [DOI] [PubMed] [Google Scholar]
- 25.Niihara Y, Zerez CR, Akiyama DS, Tanaka KR. Oral L-glutamine therapy for sickle cell anemia, I: Subjective clinical improvement and favorable change in red cell NAD redox potential. Am J Hematol. 1998;58(2):117-121. [DOI] [PubMed] [Google Scholar]
- 26.Williams R, Olivi S, Li CS, et al. . Oral glutamine supplementation decreases resting energy expenditure in children and adolescents with sickle cell anemia. J Pediatr Hematol Oncol. 2004;26(10):619-625. [DOI] [PubMed] [Google Scholar]
- 27.Niihara Y, Viswanathan K, Miller ST, et al. . Phase 3 study of L-glutamine therapy in sickle cell anemia and sickle β0-thalassemia subgroup analyses show consistent clinical improvement. Blood. 2016;128(22):1318.27609540 [Google Scholar]
- 28.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol. 2013;218:279-313. [DOI] [PubMed] [Google Scholar]
- 29.Pace BS, Shartava A, Pack-Mabien A, Mulekar M, Ardia A, Goodman SR. Effects of N-acetylcysteine on dense cell formation in sickle cell disease. Am J Hematol. 2003;73(1):26-32. [DOI] [PubMed] [Google Scholar]
- 30.Jordan LB, Vekeman F, Sengupta A, Corral M, Guo A, Duh MS. Persistence and compliance of deferoxamine versus deferasirox in Medicaid patients with sickle-cell disease. J Clin Pharm Ther. 2012;37(2):173-181. [DOI] [PubMed] [Google Scholar]
- 31.Ungureanu D, Filip C, Artenie A, Artenie R. Evaluation of simvastatin antioxidant effects. Rev Med Chir Soc Med Nat Iasi. 2003;107(1):66-71. [PubMed] [Google Scholar]
- 32.Hoppe C, Kuypers F, Larkin S, Hagar W, Vichinsky E, Styles L. A pilot study of the short-term use of simvastatin in sickle cell disease: effects on markers of vascular dysfunction. Br J Haematol. 2011;153(5):655-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bereal-Williams C, Machado RF, McGowan V II, et al. . Atorvastatin reduces serum cholesterol and triglycerides with limited improvement in vascular function in adults with sickle cell anemia. Haematologica. 2012;97(11):1768-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daak AA, Ghebremeskel K, Hassan Z, et al. . Effect of omega-3 (n-3) fatty acid supplementation in patients with sickle cell anemia: randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2013;97(1):37-44. [DOI] [PubMed] [Google Scholar]
- 35.Okpala I, Ibegbulam O, Duru A, et al. . Pilot study of omega-3 fatty acid supplements in sickle cell disease. APMIS. 2011;119(7):442-448. [DOI] [PubMed] [Google Scholar]
- 36.Doss JF, Jonassaint JC, Garrett ME, Ashley-Koch AE, Telen MJ, Chi JT. Phase 1 study of a sulforaphane-containing broccoli sprout homogenate for sickle cell disease. PLoS One. 2016;11(4):e0152895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Promsote W, Powell FL, Veean S, et al. . Oral monomethyl fumarate therapy ameliorates retinopathy in a humanized mouse model of sickle cell disease. Antioxid Redox Signal. 2016;25(17):921-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keleku-Lukwete N, Suzuki M, Otsuki A, et al. . Amelioration of inflammation and tissue damage in sickle cell model mice by Nrf2 activation. Proc Natl Acad Sci USA. 2015;112(39):12169-12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musicki B, Bivalacqua TJ, Champion HC, Burnett AL. Sildenafil promotes eNOS activation and inhibits NADPH oxidase in the transgenic sickle cell mouse penis. J Sex Med. 2014;11(2):424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Tran J, Wang H, et al. . mTOR Inhibition improves anaemia and reduces organ damage in a murine model of sickle cell disease. Br J Haematol. 2016;174(3):461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blobel GA, Bodine D, Brand M, et al. . An international effort to cure a global health problem: a report on the 19th Hemoglobin Switching Conference. Exp Hematol. 2015;43(10):821-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molokie RE, Lavelle D, Gowhari M, et al. . Phase 1 evaluation of oral tetrahydrouridine-decitabine as non-cytotoxic epigenetic disease modification for sickle cell disease. Blood. 2016;128(22):124. [Google Scholar]
- 43.Rivers A, Vaitkus K, Ibanez V, et al. . The LSD1 inhibitor RN-1 recapitulates the fetal pattern of hemoglobin synthesis in baboons (P. anubis). Haematologica. 2016;101(6):688-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibanez V, Vaitkus K, Rivers A, et al. . Efficacy and safety of long-term RN-1 treatment to increase HbF in baboons. Blood. 2017;129(2):260-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui S, Lim KC, Shi L, et al. . The LSD1 inhibitor RN-1 induces fetal hemoglobin synthesis and reduces disease pathology in sickle cell mice. Blood. 2015;126(3):386-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eaton WA, Bunn HF. Treating sickle cell disease by targeting HbS polymerization. Blood. 2017;129(20):2719-2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turhan A, Jenab P, Bruhns P, Ravetch JV, Coller BS, Frenette PS. Intravenous immune globulin prevents venular vaso-occlusion in sickle cell mice by inhibiting leukocyte adhesion and the interactions between sickle erythrocytes and adherent leukocytes. Blood. 2004;103(6):2397-2400. [DOI] [PubMed] [Google Scholar]
- 48.Field JJ. Can selectin and iNKT cell therapies meet the needs of people with sickle cell disease? Hematology Am Soc Hematol Educ Program. 2015;2015:426-432. [DOI] [PubMed] [Google Scholar]
- 49.Ataga KI, Kutlar A, Kanter J, et al. . Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N Engl J Med. 2017;376(5):429-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalff A, Dowsing C, Grigg A. The impact of a regular erythrocytapheresis programme on the acute and chronic complications of sickle cell disease in adults. Br J Haematol. 2010;149(5):768-774. [DOI] [PubMed] [Google Scholar]
- 51.Driss F, Moh-Klaren J, Pela AM, Tertian G. Regular automated erythrocytapheresis in sickle cell patients. Br J Haematol. 2011;154(5):656-659. [DOI] [PubMed] [Google Scholar]
- 52.Michot JM, Driss F, Guitton C, et al. . Immunohematologic tolerance of chronic transfusion exchanges with erythrocytapheresis in sickle cell disease. Transfusion. 2015;55(2):357-363. [DOI] [PubMed] [Google Scholar]
- 53.Ballas SK. From total blood exchange to erythrocytapheresis and back to treat complications of sickle cell disease. Transfusion. 2017;57(9):2277-2280. [DOI] [PubMed] [Google Scholar]
- 54.Morris CR, Kuypers FA, Lavrisha L, et al. . A randomized, placebo-controlled trial of arginine therapy for the treatment of children with sickle cell disease hospitalized with vaso-occlusive pain episodes. Haematologica. 2013;98(9):1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nur E, Brandjes DP, Teerlink T, et al. ; CURAMA study group. N-acetylcysteine reduces oxidative stress in sickle cell patients. Ann Hematol. 2012;91(7):1097-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoppe C, Jacob E, Styles L, Kuypers F, Larkin S, Vichinsky E. Simvastatin reduces vaso-occlusive pain in sickle cell anaemia: a pilot efficacy trial. Br J Haematol. 2017;177(4):620-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tomer A, Kasey S, Connor WE, Clark S, Harker LA, Eckman JR. Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n-3 fatty acids. Thromb Haemost. 2001;85(6):966-974. [PubMed] [Google Scholar]