Abstract
Treatment with tyrosine kinase inhibitors (TKIs) results in remission and prolongation of survival in most chronic myeloid leukemia (CML) patients but fails to eliminate the leukemia stem cells (LSCs) responsible for disease development and propagation. This accounts for the clinical observation that TKI discontinuation leads to rapid leukemia relapse. Most patients require continued treatment to prevent relapse, with associated risk of relapse, toxicity, teratogenic effects, financial burden, and noncompliance. Understanding LSC resistance to TKI and development of strategies to increase the proportion of CML patients achieving treatment-free remissions is a critical area of investigation in CML. In addition, LSCs are the source of TKI resistance, relapse, or disease progression, which is another major area of need in CML treatment. It is now understood that BCR-ABL kinase-independent mechanisms are responsible for retention of LSC subpopulations. It is likely that both cell-intrinsic and microenvironmental mechanisms contribute to LSC maintenance. Here, we review the current understanding of mechanisms underlying persistence of CML LSCs during TKI treatment, recently described approaches to target these cells and emerging clinical trials, and the challenges impeding more rapid progress in achieving cures for a greater number of CML patients.
Learning Objectives
Identify the limitations of current treatment approaches to CML
Give examples of novel approaches to enhance treatment-free remissions in CML
Introduction
Chronic myeloid leukemia (CML) arises from stem cell transformation by the BCR-ABL gene. The development of BCR-ABL tyrosine kinase inhibitors (TKIs) has revolutionized therapy for CML. TKIs are highly effective in inducing remission, preventing disease progression, and prolonging survival of chronic phase (CP) CML patients. However, limitations of TKI treatment include (1) treatment failure in a subset of patients, related to inadequate response, disease progression, and drug toxicities precluding drug administration, and (2) persistence of leukemia stem cells (LSCs) in the broader patient population, such that only a small proportion of patients can maintain treatment-free remission (TFR) after discontinuing TKI treatment.1 Risks of prolonged TKI treatment include noncompliance, associated with risk of relapse and progression; toxicity, including serious vascular complications; and teratogenic effects, precluding pregnancy while on treatment. There is considerable evidence that CML LSCs are resistant to the effects of TKIs and persist in patients on long-term therapy.2 Although a subset of CP CML patients can maintain remission after stopping TKIs, most patients require continued treatment to prevent relapse. Importantly, the high costs of TKIs has a huge financial impact, compounded by increasing cost of drugs and the increasing prevalence of CML resulting from improved survival and the costs associated with continued, regular molecular monitoring of these patients. In addition to the societal burden, high copayment requirements impact individual patients and adversely affect adherence to treatment.3 Future availability of generic TKIs at reduced price could markedly impact the cost of care for CML. However, although a generic version of imatinib (IM) is now available, potential cost savings benefits are yet to be realized in the United States because of complex factors, including high price of generics, competition from second-generation TKIs, physician behavior, issues with copayments, and third-party payers. Persistent LSCs, in addition to being the reason for lack of cure, may also be the ultimate source of acquired TKI resistance, relapse, or disease progression, which remains the other unmet need in CML treatment. Understanding LSC resistance to TKIs and development of strategies to increase the proportion of patients that may be cured of their leukemia is an exceptionally important area of investigation in CML.
Resistance of CML LSCs to TKIs
TKIs have a strong antiproliferative effect on LSCs but induce only modest levels of apoptosis. Quiescent LSCs are especially resistant to TKI-induced apoptosis and elimination.4,5 Several studies have found that TKIs effectively inhibit kinase activity within LSCs and that LSC resistance is therefore BCR-ABL kinase independent.6,7 These findings have had a huge impact in defining the direction of CML research over the last 15 years, and there is wide agreement that approaches to increase treatment-free remissions are the major need in CML research.
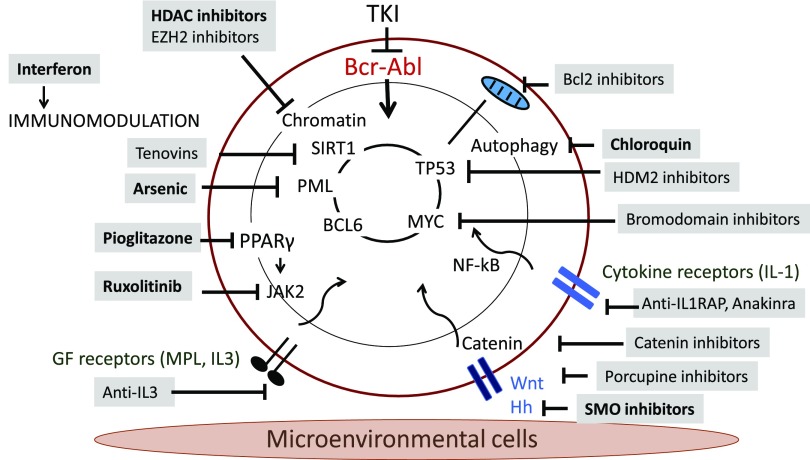
Diverse intracellular regulatory mechanisms contributing to CML LSC maintenance and drug resistance have been identified, including JAK/STAT, NF-κB, and β-catenin signaling and regulatory networks involving MYC, SIRT1, and P53, and form the basis for several of the novel therapeutic strategies described here (Figure 1; Table 1). In addition, the bone marrow microenvironment (BMM) is known to play an important role in regulating LSC maintenance and in protecting LSCs from antileukemia treatment. Leukemia-induced alterations in BMM function may also contribute to enhanced growth and maintenance of LSCs compared with normal hematopoietic stem cells (HSCs) and provide CML LSCs with a competitive advantage.8 Importantly, BMM abnormalities may persist after treatment-induced remission and contribute to LSC retention.
Figure 1.
Mechanisms of persistence and approaches to targeting CML leukemia stem cells.
Table 1.
Clinical trials with leukemia stem cell targeting agents in CML
| Agent | Target or survival factor pathway | Clinical trial |
|---|---|---|
| As2O3 | PML inhibition | NCT01397734 |
| Hydroxychloroquine | Autophagy inhibition | NCT01227135 |
| BMS-833923 | Hedgehog pathway inhibition | NCT01218477 |
| Interferon | Multiple mechanisms | NCT00219739, NCT02001818, NCT01872442 |
| LDE225 | Hedgehog pathway inhibition | NCT01456676 |
| Panobinostat | Histone deacetylase inhibition | NCT00451035 |
| Pioglitazone | PPARγ activation | NCT01751425, NCT02973711 |
| Ruxolitinib | JAK2 inhibition | NCT02889003 |
| Zileuton | ALOX5 inhibition | NCT02047149 |
Finally, there is increasing interest in the role of intercellular heterogeneity in tumor resistance and relapse. Even cell populations that are purified to phenotypic homogeneity demonstrate intercellular heterogeneity in molecular profiles and functional potential. LSC populations vary greatly in their capacity to generate leukemia, associated with variability in gene expression profiles and differential sensitivity to TKIs and other treatments.9,10 An important question is whether LSC retention on TKI reflects retention of preexisting populations vs. acquisition of adaptive resistance mechanisms.
Approaches to targeting leukemia stem cells enhancing TFR
Signaling inhibition
JAK2 kinase inhibition.
There has been considerable interest in identifying critical downstream signaling mechanisms that could be targeted to eliminate LSCs. The JAK kinases are intracellular nonreceptor kinases that mediate cytokine-mediated signaling via activation of STAT transcription factors. CML cells demonstrate increased STAT5 phosphorylation, nuclear translocation, and transcriptional activity, and STAT5 inactivation attenuates CML development in mouse models. Although BCR-ABL can directly activate STAT5 independently of the JAK2 kinase, inhibition of JAK2 activity by using ruxolitinib in combination with BCR-ABL TKIs results in the loss of LSCs both in vitro and in vivo. These findings implicate JAK2 as an upstream mediator of JAK/STAT signaling in CML LSCs, possibly related to cytokines expressed in the BMM.11 Expression of the thrombopoietin receptor MPL is elevated in CML LSCs with increased proliferative and regenerative capacity.9 Human and murine CML LSCs with high MPL expression showed enhanced JAK/STAT signaling and proliferation and increased leukemogenic capacity in vivo. CML LSCs with high MPL expression had reduced sensitivity to TKI treatment but increased sensitivity to JAK inhibitors. These studies suggest that high MPL-expressing CML LSCs are potential targets for therapy and provide a further rationale for JAK2 inhibitor therapy to deplete LSC in TKI-treated patients. In addition, reactivation of the serine-threonine phosphatase PP2A, which is inactivated in CML LSCs, leads to marked inhibition of STAT5 and β-catenin signaling and may represent an alternative strategy to target this pathway and deplete LSCs in CML when clinically applicable agents are available.12 At this time, several clinical trials are evaluating the effects of addition of ruxolitinib to TKI in CML patients with suboptimal response to TKI. The combination has been well tolerated and appears to represent a promising approach.
PPARγ activation.
Prost et al have reported that treatment with glitazones, activators of peroxisome proliferator-activated receptor-γ (PPARγ) approved for treatment of diabetes, can gradually deplete residual CML LSCs.13 PPARγ activation decreases expression of STAT5 and its downstream targets, HIF2α and CITED2, which appear to play an important role in maintaining quiescence and stemness of CML LSCs.13 These observations were tested in the Pioglityazone and Imatinib for CML Patients (ACTIM) phase 2 clinical trial, in which pioglitazone was added to IM treatment in CML patients who had received IM with achievement of major molecular response (MMR) but without achieving molecular response grade 4.5 (MR4.5).14 Twenty-four patients were included. The combination was well tolerated, and the cumulative incidence of MR4.5 was 56% by 12 months, compared with 23% with IM alone in a parallel cohort. These results are encouraging but need confirmation in a randomized clinical trial.
Identification and inhibition of key regulatory networks
SIRT1, p53, and MYC
The p53 protein is an important regulator of cell cycle and apoptosis. The NAD+ dependent deacetylase SIRT1 is an important p53 regulator and is overexpressed in human CML LSCs. Pharmacological inhibition of SIRT1 or SIRT1 knockdown increased apoptosis and depletes CP CML LSCs. SIRT1 effects were enhanced in combination with TKIs.15 SIRT1 inhibition increased p53 acetylation and transcriptional activity in CML progenitors, and the inhibitory effects of SIRT1 targeting on CML cells depended on p53 expression and acetylation. Therefore, SIRT1 inhibition may represent a potential approach to activate p53 to target CML LSCs. An alternative approach to p53 activation is via inhibition of interactions between p53 and its negative regulator HDM2. A small-molecule inhibitor of this interaction, MI-219, reduced CML but not normal primitive progenitors both in vitro and in vivo.16 These data suggest that a p53-activating agent may be effective in targeting CML LSCs.
Holyoake and colleagues used proteomics, transcriptomics, and network analyses to show that p53 and c-MYC represent critical, connected nodes that regulate aberrant protein expression in human CML LSCs.17 The combination of MDM2 and BET inhibitors was used to upregulate the p53 apoptotic pathway and downregulate c-MYC, respectively, and resulted in synergistic and selective killing—and near elimination—of transplantable human LSCs in mice, while sparing normal HSCs. These studies demonstrate the utility of an unbiased systems approach to identify critical targets for LSC targeting.
FOS and DUSP1
Using gene expression analysis, Azam and colleagues found that BCR-ABL kinase and growth factor signaling led to convergent enhancement of FBJ osteosarcoma oncogene (c-FOS) and dual-specificity phosphatase 1 (DUSP1) expression. These genes played a critical role in driving tumor growth in a BCR-ABL mouse model of CML.18 Pharmacological inhibition of c-FOS and DUSP1 eradicated minimal residual disease (MRD) in multiple in vivo models, including mice xenotransplanted with human CML cells. They concluded that c-FOS and DUSP1 represent important targets for therapy to eradicate LSCs. Interestingly, this mechanism was also active in other types of kinase-driven leukemias.
Autophagy inhibition
TKI treatment induces autophagy in CML cells, associated with endoplasmic reticulum stress and intracellular Ca2+ flux. Suppression of autophagy using either pharmacological inhibitors or RNA interference enhanced TKI-induced cell death in primary CML cells and elimination of CML stem cells.19 These intriguing observations have resulted in a trial of hydroxychloroquine with IM for chronic myeloid leukemia (CHOICES) to enhance the therapeutic effects of TKIs. This trial has accrued and results are currently pending.
Epigenetic targeting
Histone deacetylase inhibition
Zhang et al investigated the ability of histone deacetylase inhibitors (HDACis) to target CML stem cells.20 The HDACi LBH589 (panobinostat) given in combination with IM was effective in inducing apoptosis in quiescent CML progenitors and eliminating CML LSCs capable of engrafting immunodeficient mice. In vivo administration of HDACis with IM markedly depleted LSCs in a genetic mouse model of CML. The HDACi and IM combination inhibited expression of genes regulating HSC maintenance and survival. These studies suggesting that HDACi treatment may effectively target LSCs in CML patients receiving TKI led to a phase 1 clinical trial to determine the safety and tolerability of LBH589 given in combination with IM in CML patients. CP CML patients treated with IM 400 mg/d with major or complete cytogenetic response and residual disease on quantitative PCR were eligible. Nine patients were enrolled, and no dose-limiting toxicity was observed with amended intermittent schedule. Reduction in BCR-ABL levels (>1 log) was seen in 4 of 9 patients. The study reached the 3rd dose level but was discontinued because of slow accrual.
EZH2 methyltransferase inhibition
Vetrie and colleagues evaluated chromatin modifications and showed that polycomb repressive complex 2 (PRC2) is dysregulated in CP CML LSCs, with extensive reprogramming of H3K27me3 targets.21 Treatment with an EZH2 inhibitor (EZH2i) sensitized LSCs to apoptosis but did not impair normal HSC survival. Treatment of primary CML cells with either EZH2i or TKI alone caused significant upregulation of H3K27me3 targets, and combined treatment further potentiated these effects and enhanced depletion of LSCs compared with TKI alone. These findings indicate that EZH2 and H3K27me3 reprogramming is important for LSC survival but renders them sensitive to combined EZH2i and TKI treatment. The Orkin group similarly found EZH2 overexpression in CML LSCs and identified EZH2 as a selective vulnerability for CML LSCs, regardless of BCR-ABL1 mutational status.22 These observations support EZH2 inhibition as a promising therapeutic strategy to more effectively target LSCs in patients with CML receiving TKIs that is likely to be evaluated in future trials.
Protein arginine methyltransferase inhibition
Pan and colleagues reported protein arginine methyltransferase 5 (PRMT5) overexpression in CML cells.23 PRMT5 knockdown or inhibition with the small-molecule inhibitor PJ-68 reduced human CML LSC survival and self-renewal, prolonged survival in a murine model of CML, and inhibited long-term engraftment of human CML CD34+ cells in a xenograft model. PRMT5 effects may be related to inhibition of the Wnt/β-catenin pathway. These results suggest that epigenetic modification on histone arginine methylation regulates self-renewal of CML LSCs, and that PRMT5 may represent a therapeutic target against LSCs, as clinically applicable inhibitors become available.
Targeting the microenvironment
Inflammatory signaling
There is considerable evidence that CML development is associated with altered function of the BMM, contributing to altered regulation of leukemic and normal stem cells.8 Decreased CXCL12 expression in CML BM contributes to altered interactions of CML LSCs with the BMM. Observations made in a genetic mouse model and validated using human CML samples indicate that leukemia development markedly alters inflammatory cytokine expression in the BM, selectively impairs normal long-term HSC growth, and provides a growth advantage to CML LSCs. TKI treatment only partially corrects these abnormalities.
Autocrine production of tumor necrosis factor-α (TNF-α) enhances survival of CML LSCs by promoting NF-κB/p65 activity and expression of the common β-chain receptor.24 Inhibition of autocrine TNF-α signaling via a small-molecule TNF-α inhibitor induced apoptosis in CML LSCs and, in combination with nilotinib, induced significantly more apoptosis and reduction in CML LSCs than either treatment alone, suggesting that TNF-α may be a potential therapeutic target.
Expression of the pivotal pro-inflammatory cytokine interleukin-1 (IL-1) is increased in CML BM. Moreover, the IL-1 receptors, IL-1 receptor accessory protein (IL1-RAP) and IL-1 receptor type 1, are upregulated on CML LSCs, which also demonstrate increased IL-1-induced signaling. Treatment with recombinant IL-1 receptor antagonist (IL-1RA) inhibited IL-1 signaling in CML LSCs and inhibited growth of CML LSCs. The combination of IL-1RA with TKI resulted in significantly greater inhibition of CML LSCs compared with TKI alone. These studies support a role for IL-1 signaling in maintenance of CML LSCs after TKI treatment.25 Ågerstam et al demonstrated therapeutic effects of in vivo administration of IL1RAP antibodies in mice xenotransplanted with CP and blast crisis (BC) CML cells.26 Targeting occurs via blockage of IL-1 signaling and engagement of effector cells. These studies support continued exploration of anti-IL-1 strategies to enhance LSC elimination. A humanized anti-IL1RAP antibody is expected to enter clinical trials soon.
Developmental factors
Wnt signaling from the BMM contributes to preservation of CML LSCs after TKI treatment. CML progenitors demonstrated enhanced sensitivity to Wnt stimulation, associated with increased expression of the FZD4 receptor.27 Secretion of Wnt ligands requires their modification by the O-acyl transferase Porcupine (PORCN). WNT974, a potent and selective PORCN inhibitor, antagonized Wnt signaling in human CML CD34+ cells and, in combination with the TKI nilotinib (NIL), significantly inhibited growth of CML LSCs in vitro and in vivo, compared with NIL alone. PORCN inhibitors are being tested clinically and represent a potential approach to inhibit Wnt secretion and signaling and enhance selective targeting of CML stem cells.
Hedgehog binding to the Patched receptor allows activation of SMO and the transcription factor GLI1. SMO deletion or pharmacological inhibition leads to loss of LSCs in mouse models of CML. This pathway is activated in CD34+ CP CML stem/progenitor cells through kinase-independent mechanisms. LDE225 (sonidegib), a small-molecule, clinically investigated SMO inhibitor, used alone and in combination with NIL, inhibited the Hedgehog pathway in CD34+ CP CML cells, reducing the number and self-renewal capacity of CML LSCs in vitro and their engraftment in NSG mice.28 The SMO inhibitor BMS-833923 has been tested in combination with dasatinib in CML patients with suboptimal TKI responses. However, despite observation of expected toxicities due to SMO inhibition and lack of effect on normal HSCs, no evidence of efficacy was shown, and further testing of this combination in CP CML was not supported.
Interferon-α
Interferon-α (IFNα) was previously frontline treatment of CML in patients ineligible for transplantation. Possible mechanisms of action, in addition to direct targeting of CML stem cells, include altered microenvironmental interactions and immune activation. Clinical trials testing combination therapy of IFNα plus IM indicate increased rate and depth of response compared with IM alone.29 However, IFNα has considerable side effects that make it challenging to use in this patient population. Further studies are required to clarify the role of IFNα in enhancing TFR.
Clinical trial design considerations
Despite the abundance of studies evaluating mechanisms of BCR-ABL-independent TKI resistance in CML LSCs and approaches to targeting CML LSC populations, translation of preclinical work on CML stem cell eradication to the clinical setting remains challenging. Studies to date have been hampered by difficulties in accrual. Given the outstanding prognosis with continued TKI treatment, one requirement for a successful intervention trial in this setting is that the additional agents be very well tolerated and easy to administer. Although several potentially useful targets for therapy have been identified, targeting of which results in reduced numbers of LSCs, potential toxicities to normal stem cells and nonhematological toxicities are a concern.
One potential trial design has been adopted by the French in a trial that will test candidate therapies in combination or sequentially with TKI in CP CML patients in CCR without achieving a deep molecular response: an adaptive trial based on a drop loser design (NCT0267063; Therapies in Combination or Sequentially With Tyrosine Kinase Inhibitors [TKIs] in Chronic Phase Chronic Myelogenous Leukemia Patients in CCR [ACTIW]). Patients are randomized in phase 2 trials to continue on the same TKI vs. one of the alternative treatment approaches with the objective to identify agents producing a 25% increase in MR4.5 compared with control. The trial will start with current available treatment options for the experimental arms, with new treatment options added as available. In addition, it would potentially be attractive to test such interventions with the intention of attempting TKI discontinuation, or in the setting of a failed attempt at discontinuation with requirement to restart TKI, where motivation would be high.
Resistant and advanced CML
Unfortunately, there has been rather limited progress in developing more effective treatment approaches for TKI-resistant and advanced-phase CML. Mutations in the BCR-ABL kinase domain are an established mechanism of TKI resistance and can usually be managed by using alternative BCR-ABL inhibitors. However, many cases of clinical TKI failure occur in the absence of resistant mutations, and despite adequate suppression of BCR-ABL kinase activity, through BCR-ABL kinase-independent mechanisms of resistance. Clearly improved understanding of the biology underlying progression and resistance is critical to the development of effective new approaches. The following approaches are promising, but yet to be tested for efficacy in clinical trials.
STAT3 inhibition
Deininger and colleagues reported that activation of STAT3 signaling by extrinsic or intrinsic mechanisms was a critical mechanism underlying BCR-ABL kinase-independent TKI resistance.30 A potent and selective STAT3 SH2 domain inhibitor, BP-5-087, reduced STAT3 phosphorylation and nuclear transactivation. BP-5-087 also restored TKI sensitivity in primary cells from CML patients with BCR-ABL kinase-independent TKI resistance.
Wnt inhibitors
It is recognized that enhanced Wnt/β-catenin activity is a characteristic of BC CML and drives expansion and enhanced self-renewal of LSCs. Carter and colleagues confirmed overexpression of β-catenin in BC CML stem cells and showed that combined inhibition of β-catenin and Bcr-Abl synergistically targeted TKI-resistant BC CML progenitors.31 A novel Wnt/β-catenin signaling modulator, C82, when combined with nilotinib, synergistically killed TKI-resistant primary BC CML cells with or without BCR-ABL kinase mutations and significantly prolonged survival of mice xenografted with primary BCR-ABLT315I/E255V BC CML cells. These studies support a potential benefit of β-catenin inhibition to overcome TKI resistance in BC CML.
BCL-2 inhibition
Carter et al demonstrated increased BCL-2 expression at the protein level in CML BM cells.32 Selective inhibition of BCL-2, aided by TKI-mediated MCL-1 and BCL-XL inhibition, markedly decreased LSC numbers and prolonged survival in a murine CML model. This combination also effectively eradicated LSCs from BC CML patient samples. These results support the role of BCL-2 as a key survival factor for CML LSCs and the utility of combined inhibition of BCL-2 and BCR-ABL to target resistant, advanced CML.
MEK inhibition
Green and colleagues performed a large-scale RNA interference screen to evaluate BCR-ABL-independent TKI-resistance mechanisms and identify TKI-sensitizing genes.33 They identified enhanced RAF/MEK/ERK signaling after IM treatment, related to upregulation of PRKCH, as a potential resistance mechanism.33 PRKCH was also upregulated in samples from CML patients with BCR-ABL-independent IM resistance. Combined treatment with IM and the MEK inhibitor trametinib prolonged survival in mouse models of BCR-ABL-independent IM-resistant CML but had negligible effect on normal HSCs. MEK inhibition may be a therapeutically targetable mechanism in resistant CML.
Future directions
Our ability to identify and characterize LSC populations that persist in patients in molecular remission after TKI treatment is currently limited because of the rarity of these cells (<1:1000 to 1:10 000) and lack of markers to selectively detect these cells. Although the presence of a low frequency of leukemic cells can be inferred using PCR or fluorescence in situ hybridization assays performed on purified HSC populations, these approaches do not allow isolation and characterization of these populations. In addition, in vivo functional analysis of CML LSC populations is difficult because of poor growth in current xenograft models. There has recently been a tremendous advance in single-cell analysis and cell tracking technologies, which may enhance the feasibility of identifying and characterizing residual LSCs in the future and increase the chances of successful translation into new treatments.
Leukemia relapse after TKI discontinuation may also reflect failure of host immune surveillance. Defects in immune regulation seen at diagnosis are improved in patients achieving deep remission, including increased NK cell and effector cytotoxic T cell function, reduced PD-1 expression on T cells, and reduced numbers of monocytic myeloid-derived suppressor cells, and these responses were retained in patients maintaining TFR after TKI discontinuation.34 These observations suggest development of immune therapy-based therapeutic approaches in the future.
References
- 1.Etienne G, Guilhot J, Rea D, et al. . Long-term follow-up of the French Stop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35(3):298-305. [DOI] [PubMed] [Google Scholar]
- 2.Chu S, McDonald T, Lin A, et al. . Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118(20):5565-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Experts in Chronic Myeloid Leukemia. The price of drugs for chronic myeloid leukemia (CML) is a reflection of the unsustainable prices of cancer drugs: From the perspective of a large group of CML experts. Blood. 2013;121(22):4439-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holtz MS, Slovak ML, Zhang F, Sawyers CL, Forman SJ, Bhatia R. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99(10):3792-3800. [DOI] [PubMed] [Google Scholar]
- 5.Graham SM, Jørgensen HG, Allan E, et al. . Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319-325. [DOI] [PubMed] [Google Scholar]
- 6.Chu S, Holtz M, Gupta M, Bhatia R. BCR/ABL kinase inhibition by imatinib mesylate enhances MAP kinase activity in chronic myelogenous leukemia CD34+ cells. Blood. 2004;103(8):3167-3174. [DOI] [PubMed] [Google Scholar]
- 7.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang B, Ho YW, Huang Q, et al. . Altered microenvironmental regulation of leukemic and normal stem cells in chronic myelogenous leukemia. Cancer Cell. 2012;21(4):577-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang B, Li L, Ho Y, et al. . Heterogeneity of leukemia-initiating capacity of chronic myelogenous leukemia stem cells. J Clin Invest. 2016;126(3):975-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giustacchini A, Thongjuea S, Barkas N, et al. . Single-cell transcriptomics uncovers distinct molecular signatures of stem cells in chronic myeloid leukemia. Nat Med. 2017;23(6):692-702. [DOI] [PubMed] [Google Scholar]
- 11.Gallipoli P, Cook A, Rhodes S, et al. . JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood. 2014;124(9):1492-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neviani P, Harb JG, Oaks JJ, et al. . PP2A-activating drugs selectively eradicate TKI-resistant chronic myeloid leukemic stem cells. J Clin Invest. 2013;123(10):4144-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prost S, Relouzat F, Spentchian M, et al. . Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature. 2015;525(7569):380-383. [DOI] [PubMed] [Google Scholar]
- 14.Rousselot P, Prost S, Guilhot J, et al. ; French CML Group. Pioglitazone together with imatinib in chronic myeloid leukemia: A proof of concept study. Cancer. 2017;123(10):1791-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Wang L, Li L, et al. . Activation of p53 by SIRT1 inhibition enhances elimination of CML leukemia stem cells in combination with imatinib. Cancer Cell. 2012;21(2):266-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peterson LF, Lo MC, Liu Y, et al. . Induction of p53 suppresses chronic myeloid leukemia. Leuk Lymphoma. 2017;58(9):1-14. [DOI] [PubMed] [Google Scholar]
- 17.Abraham SA, Hopcroft LE, Carrick E, et al. . Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells. Nature. 2016;534(7607):341-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesarwani M, Kincaid Z, Gomaa A, et al. . Targeting c-FOS and DUSP1 abrogates intrinsic resistance to tyrosine-kinase inhibitor therapy in BCR-ABL-induced leukemia. Nat Med. 2017;23(4):472-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellodi C, Lidonnici MR, Hamilton A, et al. . Targeting autophagy potentiates tyrosine kinase inhibitor-induced cell death in Philadelphia chromosome-positive cells, including primary CML stem cells. J Clin Invest. 2009;119(5):1109-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Strauss AC, Chu S, et al. . Effective targeting of quiescent chronic myelogenous leukemia stem cells by histone deacetylase inhibitors in combination with imatinib mesylate. Cancer Cell. 2010;17(5):427-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scott MT, Korfi K, Saffrey P, et al. . Epigenetic reprogramming sensitizes CML stem cells to combined EZH2 and tyrosine kinase inhibition. Cancer Discov. 2016;6(11):1248-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xie H, Peng C, Huang J, et al. . Chronic myelogenous leukemia-initiating cells require polycomb group protein EZH2. Cancer Discov. 2016;6(11):1237-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin Y, Zhou J, Xu F, et al. . Targeting methyltransferase PRMT5 eliminates leukemia stem cells in chronic myelogenous leukemia. J Clin Invest. 2016;126(10):3961-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallipoli P, Pellicano F, Morrison H, et al. . Autocrine TNF-α production supports CML stem and progenitor cell survival and enhances their proliferation. Blood. 2013;122(19):3335-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang B, Chu S, Agarwal P, et al. . Inhibition of interleukin-1 signaling enhances elimination of tyrosine kinase inhibitor-treated CML stem cells. Blood. 2016;128(23):2671-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ågerstam H, Hansen N, von Palffy S, et al. . IL1RAP antibodies block IL-1-induced expansion of candidate CML stem cells and mediate cell killing in xenograft models. Blood. 2016;128(23):2683-2693. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal P, Zhang B, Ho Y, et al. . Enhanced targeting of CML stem and progenitor cells by inhibition of porcupine acyltransferase in combination with TKI. Blood. 2017;129(8):1008-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irvine DA, Zhang B, Kinstrie R, et al. . Deregulated hedgehog pathway signaling is inhibited by the smoothened antagonist LDE225 (Sonidegib) in chronic phase chronic myeloid leukaemia. Sci Rep. 2016;6:25476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Preudhomme C, Guilhot J, Nicolini FE, et al. ; SPIRIT Investigators; France Intergroupe des Leucémies Myéloïdes Chroniques (Fi-LMC). Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363(26):2511-2521. [DOI] [PubMed] [Google Scholar]
- 30.Eiring AM, Page BDG, Kraft IL, et al. . Combined STAT3 and BCR-ABL1 inhibition induces synthetic lethality in therapy-resistant chronic myeloid leukemia. Leukemia. 2017;31(5):1253-1254. [DOI] [PubMed] [Google Scholar]
- 31.Zhou H, Mak PY, Mu H, et al. . Combined inhibition of β-catenin and Bcr-Abl synergistically targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo [published online ahead of print 18 April 2017]. Leukemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter BZ, Mak PY, Mu H, et al. . Combined targeting of BCL-2 and BCR-ABL tyrosine kinase eradicates chronic myeloid leukemia stem cells. Sci Transl Med. 2016;8(355):355ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Shan Y, Bai R, et al. . A therapeutically targetable mechanism of BCR-ABL-independent imatinib resistance in chronic myeloid leukemia. Sci Transl Med. 2014;6(252):252ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes A, Clarson J, Tang C, et al. . CML patients with deep molecular responses to TKI have restored immune effectors and decreased PD-1 and immune suppressors. Blood. 2017;129(9):1166-1176. [DOI] [PubMed] [Google Scholar]