Abstract
β-Thalassemias are characterized by reduced production of β-globin chain, resulting in α/β-chain unbalance and precipitation of α-globin–heme complexes and determining ineffective erythropoiesis. Ineffective erythropoiesis, chronic hemolytic anemia, and compensatory hematopoietic expansion are the disease hallmarks, and they are related to the severity of the chain unbalance. Several clinical forms of β-thalassemia, including the coinheritance of β-thalassemia with hemoglobin E resulting in hemoglobin E/β-thalassemia, have been described. Clinically, β-thalassemias can be classified as transfusion-dependent thalassemia (TDT) and non–transfusion-dependent thalassemia (NTDT) according to the severity of the phenotype, which is caused by a wide spectrum of mutations in a homozygous or compound heterozygous state. Current treatment of TDT consists of regular transfusions that lead to iron overload, requiring iron chelation to prevent iron-related organ toxicity. NTDT patients do not require transfusions or only occasionally require them; however, they develop iron overload as well because of increased intestinal iron absorption caused by chronic anemia. Hematopoietic stem cell allogenic transplant is the only approved cure for β-thalassemia; however, it is still limited by clinical conditions and the availability of matched donors as well as by potential graft-versus-host disease (GVHD). Gene therapy could avoid the GVHD risk, although hematopoietic stem cells must be genetically modified ex vivo. Epigenetic manipulation and genomic editing are novel experimental approaches. An increased understanding of the pathophysiology that controls the disease process prompted us to explore alternative therapeutic approaches that address the underlying chain unbalance, ineffective erythropoiesis, and iron dysregulation. Molecules, such as JAK2 inhibitors and the activin-receptor ligand trap that target ineffective erythropoiesis, are already in clinical trials with promising results. Other agents aimed to generate iron-restricted erythropoiesis are also under experimental evaluation.
Learning Objectives
Understand new therapeutic approaches to treat transfusion-dependent and non–transfusion-dependent thalassemia patients
Learn gene therapy and genome editing approaches
Understand fetal hemoglobin induction by molecular approaches
Describe preliminary results of sotatercept and luspatercept phase 2 clinical trials
Introduction
Hemoglobinopathies are the most common inherited monogenic disorders, and β-thalassemias and sickle cell disease are the most clinically relevant. More than 300 000 babies are born every year with a hemoglobinopathy.1 World Health Organization statistics show that, in the Mediterranean, Eastern European, and Middle Eastern regions, frequencies range from 0.1 to 4.9/1000 live births. However, because of the migrations in the recent years, hemoglobinopathies are no longer confined to these high-incidence regions. β-Thalassemias result from >300 different mutations of the β-globin gene, determining reduced production of β-globin chains.2 This results in unbalance between α- and β-globin chains, intracellular accumulation of free α-chains, and precipitation of α-globin–heme complexes on red cell membranes.3 Subsequent decrease in late-stage erythroid differentiation and hemolysis of erythrocytes determine ineffective erythropoiesis (IE), the hallmark of β-thalassemia. Anemia, hypoxia, and subsequent elevated erythropoietin levels lead to erythroid hyperplasia in bone marrow and spleen, dysregulated iron homeostasis, and increased levels of reactive oxygen species in erythroid cells.4
Clinically, β-thalassemia can be classified in transfusion-dependent thalassemia (TDT) and non–transfusion-dependent thalassemia (NTDT) according to the severity of phenotype caused by the α:β-globin unbalance ratio deriving from a wide spectrum of mutations in a homozygous or compound heterozygous state.5,6
Current treatment of TDT consists of regular transfusions to maintain hemoglobin (Hb) levels >9 to 10 g/dL.5 Chronic transfusions lead to iron overload and thus, eventual multiorgan damage.5 NTDT patients spontaneously maintain Hb levels >7 g/dL and occasionally require transfusions for surgical procedures, for infections, or during pregnancy.6 They also develop iron overload, especially in the liver, because of increased intestinal iron absorption caused by chronic anemia.6 Iron chelation therapy is mandatory to remove iron in excess, but adherence to treatment is variable. Splenectomy, extensively performed in the past decades, is now limited to selected cases, because it has been shown to independently increase the risk of thrombosis, pulmonary hypertension, leg ulcers, and cholelithiasis.7
Hematopoietic stem cell transplant (HSCT) is the only approved curative option. However, the procedure is still limited by the availability of matched donors and the risk of graft-versus-host disease. Allo-HSCT shows better results in young patients (<18 years old) with an HLA identical sibling.8 New approaches from alternative donors showed promising results. Moreover, the HSCT conditioning regimen has been progressively improved.9 Gene transfer therapy could overcome the graft-versus-host disease risk by using the patient’s hematopoietic stem cells genetically modified ex vivo. Clinical trials of gene therapy are in progress with encouraging results10,11 (Figure 1A; Table 1). Additional studies of gene therapy approaches are focused on improving transduction efficiency and conditioning regimens.12
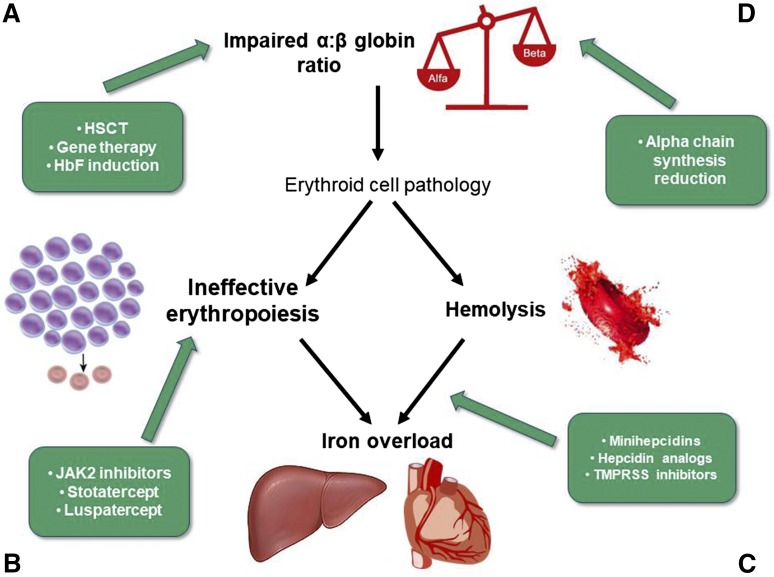
Figure 1.
New therapeutic targets in β-thalassemias: (A,D) impaired α:β-globin ratio, (B) ineffective erythropoiesis, and (C) iron metabolism and hemolysis. TMPRSS6, transmembrane protease serine 6.
Table 1.
Currently active gene therapy trials for β-thalassemias
| Protocol no. (study name) and status | Phase | Gene | Vector | Location | Sponsor | Condition | Conditioning regimen | Intervention | Start date |
|---|---|---|---|---|---|---|---|---|---|
| NCT02453477 (TIGET-BTHAL) recruiting | 1/2 | β-globin | GLOBE | Italy | IRCCS San Raffaele | β-TM | Myeloablative | Transplantation of HSCs transduced ex vivo with an LV (intrabone injection) | May 2015 |
| NCT01745120 (HGB-204) active, not recruiting | 1/2 | βA-T87Q-globin | BB305 | United States, Thailand, Australia | Bluebird Bio | β-TM | Myeloablative | Transplantation of HSCs transduced ex vivo with an LV | August 2013 |
| NCT01745120 (HGB-205) active, not recruiting | 1/2 | βA-T87Q-globin | BB305 | France | Bluebird Bio | β-TM and severe sickle cell anemia | Myeloablative | Transplantation of HSCs transduced ex vivo with an LV | July 2013 |
| NCT02906202 recruiting | 3 | βA-T87Q-globin | BB305 | France, Germany, Greece, Italy, Thailand, United States | Bluebird Bio | Myeloablative | Transplantation of HSCs transduced ex vivo with an LV | August 2016 | |
| NCT01639690 active, not recruiting | 1 | β-globin | TNS9.3.55 | United States | Memorial Sloan-Kettering Cancer Center | β-TM | Partial cytoreduction (Bu 8 mg/kg) for 3 patients, myeloablative (Bu 14 mg/kg) for 1 patient | Transplantation of HSCs transduced ex vivo with an LV | July 2012 |
Bu, busulfan; HSC, hematopoietic stem cell; LV, lentiviral vector; TM, thalassemia major.
This review will mainly focus on the novel pharmacological treatments for thalassemia that are in preclinical and clinical development.
Pharmacological therapies for thalassemias
Induction of fetal Hb
Since the discovery that patients with hereditary persistence of fetal hemoglobin (HbF) have a less severe phenotype, researchers looked toward strategies to increase HbF levels (Figure 1A).13 Hydroxyurea (HU), the only approved drug to induce HbF in hemoglobinopathies, is extensively used for sickle cell anemia, whereas its use in thalassemia is limited, and there are no randomized, controlled trials showing that HU has any effect on blood transfusion need. Because of the increase knowledge of the molecular mechanisms controlling the γ-gene expression during the last decade, the following new approaches are now in experimental phases.
Modulation of γ-globin gene regulators by knockdown of BCL11A, a strong silencer of the γ-globin gene, induces γ-globin synthesis. Reducing BCL11A expression by RNA interference14 and acting more specifically on BCL11A on erythroid lineage via genome editing technique, such as zinc finger nucleases or CRISP-cas9, are very interesting molecular approaches.15,16 Other transcription factors that could be involved in HbF regulation include KLF117 and MYB.18
Forced chromatin looping redirecting the locus control region, the major transcriptional enhancer of the β-globin locus, to the γ-globin promoter by zinc finger–mediated tethering of the “looping factor” Ldb1 has been shown to induce HbF in vitro.16,19
Forkhead-box-class-O3 is a critical transcription factor during erythropoiesis.20 It has been identified as a potential inducer of HbF; however, its role in hemoglobinopathies remains to be elucidates. Metformin, an approved drug for diabetes type 2, is a forkhead-box-class-O3 inducer.21 An ongoing phase 1 clinical trial is evaluating the use of metformin as an HbF inducer in patients with sickle cell anemia and NTDT (NCT02981329). These technologies are in development in preclinical studies, and their safety profile in humans must be carefully evaluated.22
Targeting IE
IE is the hallmark of thalassemias, responsible for the clinical expression of the disease. β-Thalassemia mouse models have been extensively used to study IE and have been used to identify new drugs or strategies to correct IE.23
Janus kinase 2 inhibitors.
Janus kinase 2 (JAK2) is an important kinase in the signaling pathway of the erythropoietin receptor (Figure 1B). Its central role in the process of erythropoiesis through the JAK2/STAT5 pathway led to the hypothesis that the administration of JAK2 inhibitors might reduce IE and eventually, splenomegaly.23 Ruxolitinib, a JAK1/JAK2 oral inhibitor, is approved for the treatment of adult patients with polycythemia vera who are resistant or intolerant to HU and for the treatment of disease-related splenomegaly or symptoms in adult patients with myelofibrosis.24 Ruxolitinib has been tested in a phase 2 single-arm, multicenter study to explore the efficacy and safety in regularly transfused patients with β-thalassemia (the TRUTH Study; NCT02049450) (Table 2). The primary end point was the percentage change of red blood cells transfusions between weeks 6 and 30 vs baseline period; the secondary end point was the change of spleen volume from baseline evaluated by magnetic resonance imaging/computed tomography at weeks 12 and 30. A slight improvement in pretransfusion Hb and a trend in reduction of transfusions were observed in treated thalassemia patients. More significant was the reduction in spleen volume over time. Particularly, the mean spleen volume reductions from baseline at weeks 12 and 30 were −19.7% and −26.8%, respectively.25 Based on these results, JAK2 inhibitors could be suitable to reduce massive splenomegaly as an alternative to splenectomy or in preparation to splenectomy (Figure 1B).
Table 2.
Clinical trials with new drugs for β-thalassemias
| Drug trial name | Trial no. | Phase | Purpose | Disease | No. of patients | Status |
|---|---|---|---|---|---|---|
| Luspatercept | NCT01749540/Extension NCT02268409 | 2 | Open-label, ascending dose study to evaluate the effects | TDT and NTDT | 64 (30 TDT, 34 NTDT) | Completed/active, not recruiting |
| Luspatercept BELIEVE Study | NCT02604433 | 3 | Double-blind, randomized, placebo-controlled, multicenter study to determine the efficacy and safety plus BSC vs placebo plus BSC | TDT | 300 | Active, not recruiting |
| Sotatercept (ACE-011) | NCT01571635 | 2A | Dose-finding study to determine safety and tolerability | TDT and NTDT | 46 | Active, not recruiting |
| Ruxolitinib TRUTH Study | NCT02049450 | 2 | Study of efficacy and safety | TDT | 30 | Completed |
BSC, best supportive care.
Activin receptor trap ligand.
Members of the transforming growth factor-β (TGF-β) family, including TGF-βs, activins, growth differentiation factors (GDFs), and bone morphogenetic proteins, are key regulators of human hematopoiesis, modulating proliferation, differentiation, migration, and apoptosis. Activin receptors (ActRs) are shared by multiple TGF-βs, including BMPs, myostatin, and GDF11 (Figure 1B). After activated by ligands, these receptors phosphorylate the receptor-regulated SMAD proteins 2, 3, and 4, which translocate to the nucleus to regulate gene expression in concert with other nuclear factors.26 GDF11 that is overexpressed in thalassemia erythroblasts has been suggested as the target for 2 compounds: luspatercept (ACE-536) and sotatercept (ACE-011). Luspatercept and sotatercept prevent activins binding to ActR and subsequent activation of SMAD signaling pathway, improving erythroid maturation and red cell production.27 It has been shown in mice models that decreased levels of GDF11 promote terminal erythropoiesis by inducing apoptosis through the Fas-FasL pathway and reducing reactive oxygen species and α-globin aggregates. Additional investigations are ongoing in animals and tissue cultures to better elucidate this mechanism.27,28 Luspatercept and sotatercept are the ligand trap that consists of the extracellular domains of ActRIIB and ActRIIA, respectively, linked to the human immunoglobulin G1 Fc domain. They were originally developed to treat postmenopausal osteoporosis; because they unexpectedly increased Hb and hematocrit,29 their use has been proposed for anemias of various etiologies, such as thalassemias and myelodysplastic syndromes. Both ACE-536 and ACE-011 have been tested in separated phase 2 clinical trials (NCT01749540/Extension NCT02268409 and NCT015716359, respectively) (Table 2) in patients affected by TDT and NTDT with extremely encouraging results. The drug is administered subcutaneously every 3 weeks. In the multicenter, open-label, dose-finding study to evaluate ACE-536, 83% of TDT patients had a reduction in transfusion burden ≥33%, with 67% of the patients showing a reduction ≥50% over any 12-week period during the study compared with baseline. Among the NTDT patients, 78% and 56% had an increase in Hb ≥ 1.0 and ≥1.5 g/dL, respectively, over any 12-week period compared with baseline. In both TDT and NTDT patients, a parallel reduction of liver iron concentration was observed. Both drugs were generally well tolerated, with no related serious adverse event. Adverse events were mostly mild to moderate, including bone pain, myalgia, arthralgia, headache, asthenia, and musculoskeletal pain.30,31
Currently, a phase 3, double-blind, randomized, placebo-controlled, multicenter study (the BELIEVE Study) (Table 2) to determine the efficacy and safety of luspatercept plus best supportive care vs placebo plus best supportive care in adults who require regular red blood cell transfusion because of β-thalassemia (NCT02604433) is ongoing in 19 countries.32
Iron restriction erythropoiesis.
Iron is an essential element for erythropoiesis, and its distribution is strictly controlled by hepcidin, a hormone produced in the liver (Figure 1C).33 Patients with β-thalassemias have an inappropriately low level of hepcidin; as a consequence, iron absorption and iron release from macrophages are increased, leading to iron overload in parenchymal organs. Increasing hepcidin in β-thalassemia mice has been associated with improvement of anemia and iron overload.34 The mechanism suggested is to generate a systemic iron deficiency or an erythroid-targeted iron restriction through minihepcidins or hepcidin mimetics, which produce a redistribution of iron from the liver to the spleen and restrict the iron supply to erythropoiesis.35 As a consequence, a reduction of IE and an amelioration of erythroid cells survival have been observed in mice.35 These observations have led to the development of hepcidin agonists, which are now in preclinical and phase 1 clinical studies.34 Other possible approaches to modulate iron metabolism to impact on erythropoiesis could be the inhibition of its negative regulators, such as bone morphogenic protein BMP6. The deletion of matriptase-2/TMPRSS6 in a mouse model of β-thalassemia has been shown to increase hepcidin expression, improve IE, and reduce iron load.36 Increase in hepcidin synthesis can also be achieved by the use of exogenous transferrin through the downregulation of TfR1, which results in an increase of erythroid precursors enucleation and amelioration of terminal erythroid differentiation and maturation in β-thalassemia mice37 (Table 3). All of these findings are still in preclinical phase or very early clinical development and require additional data before being considered for therapy in thalassemias.
Table 3.
New therapeutic approaches in preclinical development for thalassemias
| Drug | Mechanisms | Material | Reference |
|---|---|---|---|
| Minihepcidins | Hepcidin upregulation | Th3/+ mice | 34,35 |
| TMPRSS6-LRx | TMPRSS6 disruption | Th3/+ mice, monkeys | 36 |
| Exogenous transferrin | TfR1 downregulation | Th3/+ mice | 37 |
| ERFE downregulation | Hepcidin upregulation | Th3/+ mice | 33,36 |
| IOX1 | Selective silencing of α-globin | Erythroid cultures | 39 |
α-Globin synthesis reduction.
The accumulation of free α-globin in excess in erythroid precursors, subsequent to the decreased production of β-globins, is the key pathophysiological mechanism leading to IE (Figure 1D).38 Numerous clinical observations support that a natural reduction of α-chain (α-thalassemia) coinherited with β-thalassemia ameliorates the phenotype expression of the disease. Thus, the downregulation of α-chain production could improve chain unbalance and reduce IE. Recently, a panhistone demethylase inhibitor (IOX1), which selectively downregulates α-globin expression without perturbing erythroid differentiation or general gene expression, has been selected in a targeted small molecules screen by Mettananda et al.39 In a serum free erythroid differentiation system starting from primary human CD34+ cells, they showed that the silencing of α-globin expression in erythroid cells is feasible. This could be considered as a new alternative treatment of thalassemia. Potential other approaches following the same principle of silencing α-chain expression may include posttranscriptional silencing through RNA interference, epigenetic drugs altering the chromatin environment, or genome editing to disrupt some of the α-globin genes.
Other molecules.
Several agents have been tested in β-thalassemia mice that prove different mechanisms of action on reducing anemia and hemolysis, especially showing antioxidant effect, hemichromes reduction, and free heme reduction. These findings are quite promising in animal models or in vitro culture systems; however, the interpretation requires caution before translating it into human studies.40,41
Conclusions
The wide heterogeneity of β-thalassemia genotypes and phenotypes, which include the TDT and NTDT forms, has led to the consideration that treatment has to be tailored patient by patient. Recent studies in animal models have shown that IE can be targeted with different approaches, and some of them are in clinical phase. Phase 2 and phase 3 clinical trials are ongoing in patients with TDT or NTDT with molecules, such as ActR ligand trap (sotatercept and luspatercep). The results are very promising, and they may have a significant impact for the treatment of β-thalassemias in the next few years. Phase 1 studies using hepcidin mimetics or minihepcidin peptides are also ongoing to evaluate if iron-restricted erythropoiesis may reduce IE, anemia, and hemolysis. HbF reactivation through pharmacological agents still remains an appealing approach; however, HU, which is currently the only HbF inducer licensed for treatment of sickle cell disease, has much less efficacy in β-thalassemias. New molecular fascinating approaches controlling the switch from HbF to HbA have been considered for therapeutic purposes. Targeting BCL11A as a transcriptional factor that play a major role in controlling the Hb switch is a very attractive option to increase the synthesis of HbF.
Allogenic HSCT is the only cure for β-thalassemias since 1982, with results that improved dramatically through better conditioning regimens. However, it is not applicable to all patients because of the availability of fully matched donors. Data from the ongoing gene therapy clinical trials (Table 1) will be crucial to further improve this curative option.
A new era for the treatment of β-thalassemias just started. If the molecules now in clinical trials are approved, a tailored therapy, according to patient phenotype and complications, will be possible. Moreover, a combination of different strategies could be considered. New promising therapeutic approaches might revolutionize the management of β-thalassemia patients, with good chances of improving the quality of life and survival of many patients.
References
- 1.Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010;115(22):4331-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2(4):245-255. [DOI] [PubMed] [Google Scholar]
- 3.Tavazzi D, Duca L, Graziadei G, Comino A, Fiorelli G, Cappellini MD. Membrane-bound iron contributes to oxidative damage of beta-thalassaemia intermedia erythrocytes. Br J Haematol. 2001;112(1):48-50. [DOI] [PubMed] [Google Scholar]
- 4.Gardenghi S, Marongiu MF, Ramos P, et al. . Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109(11):5027-5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cappellini MD, Cohen A, Porter J, et al. Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT). 3rd ed. Nicosia, Cyprus: Thalassaemia International Federation; 2014. [PubMed] [Google Scholar]
- 6.Taher A, Vichinsky E, Musallam K, et al. Guidelines for the Management of Non Transfusion Dependent Thalassaemia (NTDT). Nicosia, Cyprus: Thalassaemia International Federation; 2013. [PubMed] [Google Scholar]
- 7.Taher AT, Musallam KM, Karimi M, et al. . Overview on practices in thalassemia intermedia management aiming for lowering complication rates across a region of endemicity: the OPTIMAL CARE study. Blood. 2010;115(10):1886-1892. [DOI] [PubMed] [Google Scholar]
- 8.Angelucci E, Pilo F, Coates TD. Transplantation in thalassemia: revisiting the Pesaro risk factors 25 years later. Am J Hematol. 2017;92(5):411-413. [DOI] [PubMed] [Google Scholar]
- 9.Locatelli F, Merli P, Strocchio L. Transplantation for thalassemia major: alternative donors. Curr Opin Hematol. 2016;23(6):515-523. [DOI] [PubMed] [Google Scholar]
- 10.Walters MC, Rasko J, Hongeng S, et al. . Update of results from the Northstar Study (HGB-204): a phase 1/2 study of gene therapy for beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta AT87Q-globin vector (LentiGlobin BB305 drug product) [abstract]. Blood. 2015;126(23): Abstract 201. [Google Scholar]
- 11.Cavazzana M, Antoniani C, Miccio A. Gene therapy for β-hemoglobinopathies. Mol Ther. 2017;25(5):1142-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liddonici MR, Aprile A, Frittoli MC, et al. . Plerixaform and G-CSF combination mobilizes hematopoietic stem and progenitors cells with a distinct transcriptional profile and a reduced in vivo homing capacity compared to plerixaform alone. Haematologica. 2017;102(4):e120-e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musallam KM, Taher AT, Cappellini MD, Sankaran VG. Clinical experience with fetal hemoglobin induction therapy in patients with β-thalassemia. Blood. 2013;121(12):2199-2212. [DOI] [PubMed] [Google Scholar]
- 14.Guda S, Brendel C, Renella R, et al. . miRNA-embedded shRNAs for lineage-specific BCL11A knockdown and hemoglobin F induction. Mol Ther. 2015;23(9):1465-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vierstra J, Reik A, Chang KH, et al. . Functional footprinting of regulatory DNA. Nat Methods. 2015;12(10):927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breda L, Motta I, Lourenco S, et al. . Forced chromatin looping raises fetal hemoglobin in adult sickle cells to higher levels than pharmacologic inducers. Blood. 2016;128(8):1139-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg J, Papadopoulos P, Georgitsi M, et al. . Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat Genet. 2010;42(9):801-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stadhouders R, Aktuna S, Thongjuea S, et al. . HBS1L-MYB intergenic variants modulate fetal hemoglobin via long-range MYB enhancers. J Clin Invest. 2014;124(4):1699-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W, Rupon JW, Krivega I, et al. . Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell. 2014;158(4):849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang R, Ghaffari S. Advances in understanding the mechanisms of erythropoiesis in homeostasis and disease. Br J Haematol. 2016;174(5):661-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Weiss M, Sumazin P, Sheehan VA. Metformin induces FOXO3-dependent fetal hemoglobin production in primary erythroid cells [abstract]. Blood. 2016;128(22): Abstract 322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thein SL. Molecular basis of β-thalassemia and potential therapeutic targets [published online ahead of print 20 June 2017]. Blood Cells Mol Dis. [DOI] [PMC free article] [PubMed]
- 23.Rivella S. β-Thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica. 2015;100(4):418-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison CN, Schaap N, Vannucchi AM, et al. . Janus kinase-2 inhibitor fedratinib in patients with myelofibrosis previously treated with ruxolitinib (JAKARTA-2): a single-arm, open-label, non-randomised, phase 2, multicentre study. Lancet Haematol. 2017;4(7):e317-e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aydinok Y, Karakas Z, Cassinerio E, et al. . Efficacy and safety of ruxolitinib in regularly transfused patients with thalassemia: results from single-arm, multicenter, phase 2a truth study [abstract]. Blood. 2016;128(22); Abstract 852. [Google Scholar]
- 26.Suragani RN, Cawley SM, Li R, et al. . Modified activin receptor IIB ligand trap mitigates ineffective erythropoiesis and disease complications in murine β-thalassemia. Blood. 2014;123(25):3864-3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suragani RN, Cadena SM, Cawley SM, et al. . Transforming growth factor-β superfamily ligand trap ACE-536 corrects anemia by promoting late-stage erythropoiesis. Nat Med. 2014;20(4):408-414. [DOI] [PubMed] [Google Scholar]
- 28.Oikonomidou PR, La P, Gupta R, et al. . Genetic investigation of the role of GDF11 in the treatment of β-thalassemia and MDS [abstract]. Blood. 2016;128(22); Abstract 2439. [Google Scholar]
- 29.Ruckle J, Jacobs M, Kramer W, et al. . Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res. 2009;24(4):744-752. [DOI] [PubMed] [Google Scholar]
- 30.Piga AG, Tartaglione I, Gamberini R, et al. . Luspatercept increases hemoglobin, decreases transfusion burden and improves iron overload in adults with beta-thalassemia [abstract]. Blood. 2016;128(22): Abstract 851. [Google Scholar]
- 31.Cappellini MD, Porter J, Origa R, et al. . Interim results from a phase 2A, open-label, dose-finding study of sotatercept (ACE-011) in adult patients (PTS) with beta-thalssemia [abstract]. Haematologica. 2015;100(suppl 1):17-18. Abstract S137.25552679 [Google Scholar]
- 32.National Institutes of Health. An efficacy and safety study of luspatercept (ACE-536) versus placebo in adults who require regular red blood cell transfusions due to beta (β) thalassemia (BELIEVE). https://clinicaltrials.gov/ct2/show/NCT02604433. Accessed 27 August 2017.
- 33.Camaschella C, Pagani A, Nai A, Silvestri L. The mutual control of iron and erythropoiesis. Int J Lab Hematol. 2016;38(suppl 1):20-26. [DOI] [PubMed] [Google Scholar]
- 34.Vyoral D, Jiri Petrak. Therapeutic potential of hepcidin - the master regulator of iron metabolism. Pharmacol Res. 2017;115:242-254. [DOI] [PubMed] [Google Scholar]
- 35.Casu C, Oikonomidou PR, Chen H, et al. . Minihepcidin peptides as disease modifiers in mice affected by β-thalassemia and polycythemia vera. Blood. 2016;128(2):265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nai A, Rubio A, Campanella A, et al. . Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood. 2016;127(19):2327-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Rybicki AC, Suzuka SM, et al. . Transferrin therapy ameliorates disease in beta-thalassemic mice. Nat Med. 2010;16(2):177-182. [DOI] [PubMed] [Google Scholar]
- 38.Higgs DR, Engel JD, Stamatoyannopoulos G. Thalassaemia. Lancet. 2012;379(9813):373-383. [DOI] [PubMed] [Google Scholar]
- 39.Mettananda S, Fisher CA, Sloane-Stanley JA, et al. . Selective silencing of α-globin by the histone demethylase inhibitor IOX1: a potentially new pathway for treatment of β-thalassemia. Haematologica. 2017;102(3):e80-e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makis A, Hatzimichael E, Papassotiriou I, Voskaridou E. 2017 Clinical trials update in new treatments of β-thalassemia. Am J Hematol. 2016;91(11):1135-1145. [DOI] [PubMed] [Google Scholar]
- 41.Motta I, Scaramellini N, Cappellini MD. Investigational drugs in phase I and phase II clinical trials for thalassemia. Expert Opin Investig Drugs. 2017;26(7):793-802. [DOI] [PubMed] [Google Scholar]