Abstract
Allogenic hematopoietic stem cell recipients may have preformed antibodies directed against foreign HLA antigens. The use of partially HLA-mismatched allogeneic hematopoietic stem cell donors allows for the possibility of the presence of circulating HLA donor-specific antibodies (DSAs) in the recipient. The presence of DSAs at the time of stem cell infusion increases the risk of primary graft failure. More recently developed technology using solid phase immunoassays (SPIs) with fluorochrome-conjugated beads has greatly improved the ability to detect and classify DSAs. When used in combination with the classic lymphocytotoxic complement-dependent and flow cytometric crossmatch tests, SPIs help provide DSA strength assessment. Parous females frequently harbor DSAs. DSAs tend to be of higher intensity when directed against haploidentical first-degree relatives. DSA assessment requires frequent monitoring as their relative strength can change over time. Although the criteria that constitutes a prohibitive DSA is unknown, desensitization techniques can result in engraftment rates as experienced in fully HLA-matched allogeneic blood or marrow transplantation recipients.
Learning Objectives
To recognize that HLA DSAs may cause primary graft failure in HLA-mismatched allografts
To illustrate the importance of incorporating classic crossmatch testing assessment with SPIs in assessing DSA strength
To recognize that the presence of DSAs is not an absolute barrier to hematopoietic stem cell transplantation
Introduction
Allogeneic blood or marrow transplantation (alloBMT) remains the definitive curative treatment of many patients with relapsed and refractory hematologic malignancies. Historic barriers to alloBMT use included preparative regimen toxicity, graft-versus-host disease (GVHD), and the lack of availability of suitable HLA-matched donors, with each of these barriers augmented in older patients.1 However, the advent of reduced-intensity conditioning (RIC) has significantly reduced the preparative toxicity, and older patients with comorbidities or heavily pretreated individuals can now undertake alloBMT. Concurrently, GVHD prevention, with posttransplant cyclophosphamide, has expanded access to alloBMT by reducing the incidence of GVHD from alternative donors to that seen with matched donors.2 Lastly, the use of alternative donor pools from related and unrelated partially HLA-mismatched donors and cord blood have greatly expanded patient access to alloBMT.3
Notably, during the past several years, there has been a steady increase in the use of alternative or HLA-mismatched alloBMT performed. Over 95% of patients, regardless of ethnicity, have readily available, haploidentical donors from among their parents, siblings, children, or first-degree relatives. In 2009, mismatched alloBMT represented 5% of the alloBMTs performed in the Unites States for acute myeloid leukemia,4 but represented 65% of all alloBMTs performed at the Sidney Kimmel Comprehensive Cancer Center (SKCCC) at Johns Hopkins. Since that time, the percentage of mismatched alloBMTs at the SKCCC has increased: 76% in 2011, and 81% in 2015 with >85% being haploidentical transplants. Moreover, in the United States, haploidentical donors are the only donor type that has increased in number since 2015, with all other donor types showing decline or stability in use. However, the use of partially HLA-mismatched donors has revealed a new hurdle, HLA donor-specific antibodies (DSAs).
HLA DSAs are preformed antibodies in the recipient directed against the candidate donor’s class I and/or class II HLA antigens. The class I antigens, HLA-A, -B, and -C, are expressed on most cells, and the class II antigens, HLA-DR, -DQ, and -DP, are restricted primarily to antigen presenting cells. The use of partially HLA-mismatched donors allows for the possibility of DSAs. Importantly, the classic “10 out of 10” HLA-matched alloBMTs are HLA-matched with regard to HLA-A, -B, -C, DRB1, and DQB1, whereas HLA-DPB1, DRB3, DRB4, and DRB5 are not necessarily matched. Consequently, mismatching occurs in more than half of the “10 out of 10” HLA-matched unrelated donor alloBMTs.6 Patients can form antibodies to foreign HLA antigens after exposure to foreign cells or tissue. Common exposures include pregnancy, blood product transfusion, and previous organ or blood transplantation. Importantly, HLA antibodies are dynamic. After inflammatory events, such as infection or tissue trauma, reactivation of dormant HLA-specific memory B cells may result in the production of DSAs without re-exposure to foreign tissue.7 Therefore, HLA antibody evaluation requires reassessment over time.
Measuring DSAs
HLA antibody testing methods have been previously reviewed, and a brief discussion follows.8-10 Two types of assays are frequently used to monitor patients’ circulating HLA antibodies: crossmatch and solid-phase immunoassays (SPIs). Crossmatch assays require donor tissue: the patient’s serum is incubated with the donor’s T and B lymphocytes, and in this manner, crossmatch assays directly assess antibody reactivity with the donor’s cell surface antigens. In SPIs, the patient’s serum is incubated with soluble HLA antigens bound to a solid matrix. These tests are used to assess the presence and relative strength of HLA alloantigen-specific antibodies. A meaningful evaluation of specificity and the relative strength of DSAs requires the use of SPIs in conjunction with crossmatch assays. SPIs indicate specificity and sensitivity, and crossmatch assays aid in antibody strength assessment.
In its basic version, the complement-dependent cytotoxicity crossmatch (CDCXM) detects complement-activating antibodies. In the more sensitive flow cytometric crossmatch (FCXM), fluorophore-conjugated anti-human immunoglobulin G (IgG) is used to detect all cell-bound antibodies. The degree of cytotoxicity or fluorescent signal is a measure of the relative strength of donor-reactive antibodies. The FCXM is more sensitive than the CDCXM and is less subjective and less labor intensive. Crossmatch assays provide a direct measure of the relative strength of donor-reactive antibodies, accounting for variability of antigen expression by the donor cells and for the combined strength of multiple DSAs. However, non-HLA antibodies, such as autoantibodies and therapeutic antibodies, may confound the assay interpretation.
HLA antibody screening and characterization is currently done by SPIs. SPIs utilize HLA molecules bound to a solid matrix. The solid support can be a microtiter plate for the enzyme-linked immunosorbent assay or polystyrene bead arrays to be analyzed on a conventional flow cytometer or a particle flow analyzer. The most frequently used are Luminex-based SPIs. The main advantages of these methods are the high specificity and sensitivity, semiquantitation, and multiplexing capability. The targets are suspensions of a maximum of 100 different fluorescently labeled microbead populations coated with HLA antigens, with the HLA proteins derived from cell membranes or obtained from transfected cell lines expressing a single recombinant HLA protein. After incubation with serum, anti-IgG, conjugated with a reporter fluorochrome, is added, and HLA-bound antibodies are detected by analyzing the beads on a Luminex platform, a dual-laser particle flow analyzer. The mean fluorescence intensity (MFI) of the reporter signal is proportional to the amount of antibody bound to each bead and is used semiquantitatively for estimation of antibody levels.
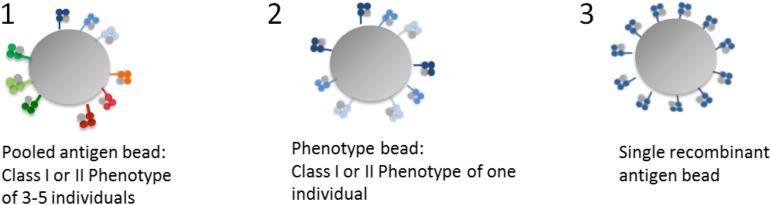
Kits come in 3 formats according to their targets: screening assays with antigens pooled from multiple cell lines, phenotype panels in which each bead population carries either class I or class II proteins of a cell line derived from a single individual and single antigen bead (SAB), and panels of HLA class I or class II antigens in which each bead population is coated with multiple copies of a single recombinant antigen (Figures 1 and 2). Screening assays are used for detecting HLA class I or II antibody presence or absence and for monitoring changes in antibody level. Phenotype and SAB beads are used for assessing antibody specificity and level. SAB arrays cover the most common (and some rare) alleles of all polymorphic loci: HLA-A, -B, and -C, DRB1, DRB3, DRB4, DRB5, DQA1, DQB1, DPA1, and DPB1. They are instrumental for the definition of antibody specificities in complex sera, the identification of epitope specificity, and the detection of HLA-Cw, DQA, DPA, and DPB antibodies. Although the SAB assays are the most sensitive and specific, recombinant protein denaturation can lead to the creation or exposure of new or “cryptic” epitopes that bind non-HLA antibodies (false positive). Furthermore, antibody strength is difficult to assess when several DSAs are present because MFIs are not additive. Phenotype panels are less prone to artifacts due to denaturation and allow a better estimation of antibody strength because antigen density is more representative of HLA density found on the cell surface. The use of a combination of both assays renders more accurate results, with SABs providing specificity and phenotype beads aiding in strength assessment and in ruling out false positives due to SAB protein denaturation. More recently, SAB assays have been modified to detect complement-fixing antibodies (eg, C1q, a fragment generated at the beginning of the classical pathway) in an effort to characterize antibody functionality.
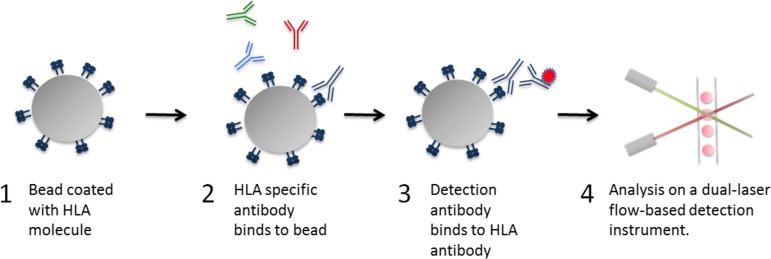
Figure 1.
Luminex HLA antibody assay principle. The principle of the Luminex-based HLA antibody assay is depicted. (1) Bead coated with HLA molecules. Up to 100 different sets of color-coded beads, each bearing 1 or several HLA types can be tested simultaneously. Each bead set is internally dyed with differing ratios of 2 fluorochromes resulting in a unique signal. (2) After incubation with the test serum, the HLA antibody, if present, binds to the appropriate HLA molecule. Non-HLA antibodies are discarded after washing. (3) The bound HLA antibody is detected by a phycoerythrin-conjugated secondary antibody specific for human IgG. (4) The beads are analyzed on a dual-laser flow-based detection instrument. The red laser classifies the bead and determines the HLA molecule that is being detected. The green laser determines the magnitude of the phycoerythrin-derived signal, which is proportional to the amount of HLA antibody bound.
Figure 2.
Luminex bead characteristics. The 3 formats of Luminex beads.
SPI result interpretation requires an understanding of HLA molecular polymorphism and serologic patterns of cross-reactivity. HLA antibodies recognize structural motifs (epitopes) that contain a small number of amino acids present in the donor and not in the recipient. Each HLA molecule contains several epitopes, and, at the same time, epitopes are shared by different HLA molecules. Epitope sharing is the reason why, as is often the case, an individual exposed to a single antigen can produce antibodies specific not only for the original antigen, but also for other antigens to which he or she has not been previously exposed. Identifying patterns of reactivity against shared epitopes allows accurate specificity assignment and assessment of antibody complexity. Pattern recognition is also essential for distinguishing true positive and true negative reactions. A single positive bead may be identified as a false positive if the expected epitope-sharing reactivity is not observed. At the other end of the spectrum, epitope reactivity patterns may continue below MFI thresholds commonly considered as positive, and false negatives could be assigned if only MFI values were used as the criterion for positivity.
It is important to note that SPIs are not quantitative assays. MFI values do not translate directly into the antibody level. MFI values taken out of context are meaningful neither to define antibody specificity nor to estimate the antibody level. MFI values are affected by lot-to-lot and interassay variability. Variability in the amount of protein coating different bead populations confounds the comparison of HLA antibody levels across specificities in the same array and of the same specificity across lots.8 For example, SAB beads carry a higher amount of HLA-Cw, DQ, and DP proteins, and therefore MFI values are inflated. The use of MFI values may lead to underestimating antibody levels when the epitope is distributed across multiple beads, as in the case of the public epitopes Bw4 and Bw6. Moreover, MFI values of SAB may underestimate the cumulative strength of multiple DSAs. Strong antibodies may saturate beads, and the actual antibody level may be revealed only after serum dilutions. In addition, some sera render increased MFIs on dilution due to the presence of interfering substances, such as IgM and complement. A variety of serum treatments may be used to remove interfering substances, such as hypotonic dialysis, EDTA, and molecular weight exclusion columns. A general increase in the level of MFI values is caused by serum treatment, and this effect differs according to the method used, which is important to take into account when comparing results obtained by different laboratories. Despite these shortcomings, MFI values can predict crossmatch results and assess immunological risk. The correlation of MFIs with CDCX and FCXM are being used to assess the clinical relevance of DSAs, and thresholds are tailored according to the immunological risk acceptable to each individual transplantation program. Furthermore, interlaboratory harmonization is possible, as proved by proficiency testing programs offered by professional societies such as the American Society for Histocompatibility and Immunogenetics and the College of American Pathologists. Accredited laboratories must fulfill proficiency testing requirements, in which samples are distributed among participating laboratories and results for crossmatch and SPI antibody assessment are compared.
The significance of DSAs
The presence of DSAs, permissible by the use of partially HLA-mismatched donors, raises a barrier previously limited to solid organ transplant recipients: antibody-mediated (graft) rejection. In fact, the presence of high levels of DSAs is generally considered a contraindication to solid organ transplantation.8 Over 25 years ago, Anasetti et al9 determined that DSA detected in a positive CDCXM presented a significant risk for primary graft failure for patients undergoing myeloablative conditioning. Likewise, using a CDCXM assay, Ottinger et al10 demonstrated that a positive crossmatch predicted inferior overall survival secondary to primary graft failure. More recently, DSA as detected via SPI was shown to increase the risk for primary graft failure,11 including anti-DPB1 DSAs.12 Overall, the presence of DSA predicts up to a 10-fold increase of primary engraftment failure,13-15 especially with an MFI >10 000.16 Ciurea et al17 reported a higher risk of graft failure in patients with DSA levels >5000 and complement-binding DSAs. Chang et al16 reported correlation of DSAs (MFI ≥ 10 000) to primary graft rejection and DSAs (MFI ≥ 2000) with primary poor graft function. As previously mentioned, comparing results obtained by different laboratories requires caution given the variability in MFI values due to variations in methodology, such as serum treatment.
It is unknown if an antibody to any particular class I or II HLA antigen is more highly associated with primary graft failure, and we are actively studying HLA antigen expression and density on different cellular elements within the allogeneic graft. The mechanism of DSA-mediated primary graft failure likely involves both complement-mediated lysis of stem cells and antibody-dependent cell-mediated cytotoxicity by FcR+ macrophages and natural killer cells.18
The incidence of DSAs among haploidentical alloBMT candidates
Between January 2006 and March 2011, 296 consecutive adult patients under evaluation for their first haploidentical alloBMT were screened for DSAs using SPIs.17 During this time, generally 5 donors were evaluated at initial screening. If <5 siblings were evaluable, partially HLA-mismatched donors (parents or children) were also evaluated. A total of 957 potential donors were evaluated, including 853 potential related donors and 104 potential unrelated donors. Of the potential related donors, 87% were HLA mismatched. The overall incidence of HLA-specific antibodies, whether specific for donor or third-party antigens, was 23.0%. Females were 4 times more likely to harbor an HLA-specific antibody (43%). There was a 52% antibody incidence in parous females compared with a 31% detection rate in nulliparous females. More importantly, DSAs to ≥1 potential donor was identified in 14.5% of our bone marrow transplant (BMT) candidates. However, DSAs were detected in only 4.9% of the male alloBMT candidates compared with 30.6% of our female candidates. With respect to DSAs, parous females had an incidence of 43%, whereas nulliparous females had an incidence of 12.5%.19 These results are similar to other published reports, where rates of sensitization range from 20% to 40%.3,12-14,16,20-22
The relative strength of DSA among haploidentical alloBMT candidates
Assessment of the presence or absence of circulating HLA antibodies and of their specificity and strength is an important step in the evaluation of immunological risk for donor selection. The presence of class I (HLA-A, -B, and -C) and/or class II (HLA-DR, -DRB3, -DRB4, -DRB5, -DQA, -DQB, -DPA, and -DPB) DSAs are considered potentially deleterious for primary graft failure during alloBMT. Evaluation of the relative strength of DSAs requires the use of SPIs in conjunction with crossmatch assays. Crossmatch assays are subject to variability; however, within a single laboratory, serial tests can provide consistent measures of relative antibody strength when test results are given as dilution titers for CDCXM and as ratios to control sera or median fluorescent channels for FCXM. Because of their much greater sensitivity, the SPIs are further subject to inherent test-to-test variation and are, at best, semiquantitative when using MFI values as an indication of relative antibody strength. Correlation of SPI results with crossmatch tests (virtual crossmatch) can be used to assess clinical relevance of DSAs. We have found better correlation of crossmatch results with sera tested on solid-phase phenotype panels than with the single-antigen panels.23 Using a threshold for positivity of 1000 MFI, we find that positive FCXM tests generally correlate with MFI ≥ 5000 on phenotype panels and ≥10 000 to 15 000 on single antigen panels. Positive CDCXM results are associated with ≥10 000 MFI on phenotype panels and with >10 000 MFI on single-antigen panels when tested at a 1:8 dilution.17,21 It is important to note that we treated all samples with hypotonic dialysis prior to SAB testing, which renders higher MFI values. This should be taken into account when comparing results from laboratories using other serum treatments. Because the antigen concentrations are enhanced on SAB for HLA-C, -DQ, and –DP, to improve their detection, higher thresholds for positivity (∼2×) are used for characterizing antibodies to these antigens.
We consider antibodies to have a weak to low level with phenotype panel MFI values from 1000 to 3000; moderate from 3000 to 5000; and strong when >5000. Based on the above correlations of relative antibody strength, we have found that DSAs directed against a haploidentical donor tend to be moderate to strong. Among the patients with detectable DSAs, antibodies to haploidentical donors were characterized as moderate to strong in ∼70% of cases. In contrast, DSAs were characterized as weak in half of the cases when they were directed against a mismatched unrelated donor.19 This difference is likely attributable to the maternal in utero exposure to her child’s HLA repertoire.
Even if the initial tests for DSAs against a potential donor are negative, because HLA antibodies are dynamic, multiple DSA analyses should be considered before the start of partially HLA-mismatched alloBMT conditioning. At the SKCCCs, monthly HLA antibody screening starts at the time of donor evaluation, and a final crossmatch is performed between days −30 and −14 of the intended alloBMT. Because transfusions or infections can evoke increases in HLA antibodies, sensitized patients are screened weekly for the development of any DSA.
Desensitization
The often urgent need to proceed to alloBMT, which precludes the time to find donors to whom the patient does not have DSAs, has led many transplant centers to develop means to lower DSA levels.24 In brief, antibody absorption techniques with Staphylococcus protein A columns (not available in the United States) and donor HLA-matched hyperplatelet transfusions have been reported. IVIg has been more frequently used. Direct antibody removal with therapeutic plasma exchange (TPE) has been used, and chemotherapeutics or monoclonal antibodies, such as bortezomib and rituximab, targeting antibody production have been used. Moreover, many of these approaches have been tried in combination and with varying success.
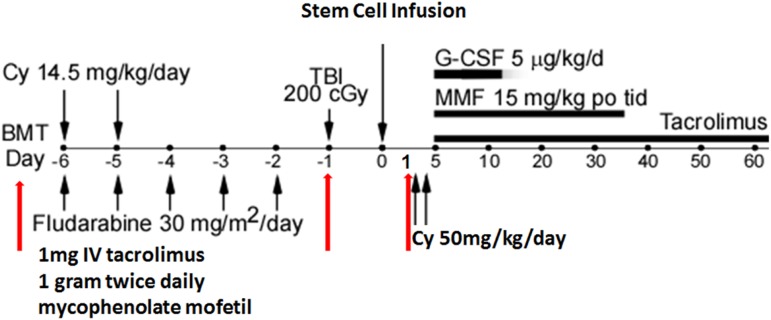
At the SKCCC, DSA desensitization for alloBMT uses a modified desensitization approach that has been highly successful in >200 sensitized renal transplant recipients.25 Our initial results were published in 2013 and updated in 2015.19,26 Our desensitization practice for mismatched alloBMT recipients is integrated into our RIC regimen, which consists of low fludarabine (30 mg/m2; days −6 through −2), cyclophosphamide (14.5 mg/kg; days −6 and −5), and total body irradiation (200 cGy; day −1) followed by unmanipulated stem cell infusion (day 0; target 4.0 × 108 nucleated cells/kg), posttransplant cyclophosphamide (50 mg/kg days +3 and +4), followed by tacrolimus or sirolimus with mycophenolate mofetil and filgrastim (day +5 and onward). The goal of desensitization is to reduce DSA to levels well below that consistent with a positive flow cytometric crossmatch result.
Candidates for partially HLA-mismatched haploidentical, unrelated registry donors or umbilical cord blood BMT are evaluated for desensitization based on DSA levels. Virtual crossmatches are used in conjunction with actual crossmatches to assess relative antibody strength. Virtual crossmatches are used as stand-alone only if actual crossmatches are uninterpretable (eg, poor cell viability, autologous, or therapeutic antibodies interference). In our current approach, patients with CDCXM-level DSAs are recommended to use other options. Patients with CDCXM-negative and FCXM-positive DSAs are considered to be candidates for desensitization. Patients with low levels of DSAs do not usually require desensitization.
The desensitization process starts 1 to 2 weeks before conditioning and consists of alternate-day, single plasma volume TPE using 5% albumin as the replacement fluid with post-TPE/IVIg (0.1 g/kg), tacrolimus (1 mg IV/d), mycophenolate mofetil (1 g twice daily), and 1 TPE/IVIg treatment on day −1 (Figure 3). On day −7 of desensitization, DSA levels are repeated to ensure a MFI reduction suggestive of a negative FCXM before conditioning commencement. Thereafter, TPE and IVIg are stopped, but the tacrolimus and mycophenolate mofetil are continued through day −1. The number of preconditioning TPE/IVIg treatments planned is based on starting DSA levels correlated to crossmatch tests. For patients with DSAs at a level of weak to moderately positive FCXM, the number of treatments is 3 to 4. This number is increased to 5 to 6 for patients with a strongly positive FCXM or a crossmatch due to the presence of class II antibodies because, in our experience, these are less amenable to reduction,25,27 or when additional risk factors are present, such as multiple DSAs, HLA mismatches repeated from a previous transplant, a child-to-mother transplant, and if the relative DSA level was increasing before the initiation of treatment. An additional DSA measurement is made on day −1. For those on day −1 mounting a DSA rebound, to the point where the predicted flow crossmatch would be positive, 1 to 2 additional TPE/IVIg treatments are prescribed on day +1 and potentially on day +2, and additional monitoring is performed on days +3 and +5 to determine if additional post-BMT treatments are needed. For patients with low levels of DSAs, 1 TPE/IVIg treatment on day −1 may be considered if additional risk factors (see above) are present.
Figure 3.
The number of exchanges was determined by the baseline strength of DSAs and rebound.
In our 2015 update, we reported the effects of desensitization in 15 alloBMT candidates using the previously described process. Twelve patients had FCXM-positive DSA levels, and 3 others were desensitized; 2 for low-level DSAs that were increasing before the initiation of conditioning; and 1 because of multiple low-level DSAs. Fourteen (93%) of these patients’ DSA levels fell below a level predicted to be flow crossmatch positive and underwent alloBMT. One patient’s transplant was cancelled because the desensitization goal was not met. Only 2 patients experienced any substantial DSA rebound during the condition period. All 14 patients engrafted by posttransplant day +60. Four patients experienced grade 1 GVHD with skin involvement. Despite initial engraftment, 7 patients suffered relapses of their underlying diseases from 3 to 12 months after transplantation. All of these patients subsequently succumbed to their relapsed disease. Two other patients died of infections, 1 from a non–transplant-related cause. Four patients remain disease free at a range of 1 to 3.9 years after BMT. The small number of patients and their disease heterogeneity preclude any comparison of these outcome data with our much larger group of patients who were transplanted without DSAs.
These results and other reports show that desensitization can reduce DSAs to levels permissible for donor engraftment.11,16,24,28-32 Factors associated with a poorer response to desensitization include initial DSA levels consistent with a positive complement-dependent cytotoxicity crossmatch and certain class II DSAs, because we have observed poorer responses with antibodies to HLA-DQ, -DR51, -DR52, and -DR53 antigens.25 Although clearly high levels of DSAs are associated with primary graft failure, it has yet to be defined if there is a “permissible” DSA level for alloBMT. Drawing any conclusions from published studies is confounded by differences in the definition of positive DSA levels. In 3 of 4 published studies using different MFI thresholds to define DSA positivity, the 3 reports with the highest MFI cut offs found the presence of DSAs to affect engraftment. Yoshihara et al,32 when comparing engraftment rates in haploidentical alloBMT patients based on a DSA MFI of ≥5000, found DSA-positive patients to have an engraftment rate of 62% vs 94% for DSA-negative patients. Ciurea et al,11 when comparing engraftment rates in haploidentical alloBMT patients based on a DSA MFI of ≥1500, found DSA-positive patients to have an engraftment rate of 25% compared with 95% for DSA-negative patients. Takanashi et al,15 comparing engraftment rates in unrelated cord blood transplantation based on a DSA MFI of ≥1000 found DSA-positive patients to have an engraftment rate of 32% compared with 83% for DSA-negative patients. However, Brunstein et al,20 when comparing engraftment rates in double umbilical cord transplantation based on a DSA MFI of ≥500, found DSA-positive patients to have an engraftment rate of 83% compared with 78% for DSA-negative patients and concluded that DSA presence does not impact engraftment.
Our unpublished data also support that weak and low-level DSA, as defined by an SAB MFI <5000 to HLA-A, -B, and -DR, do not affect engraftment. We analyzed 192 consecutive reduced-intensity transplantation alloBMT with mismatched donors between 2011 and 2014. We found 20 (10%) patients were DSA positive, and 172 (90%) patients were DSA negative. The rate of engraftment for DSA-positive patients was 86% compared with 89%, for DSA-negative patients, not clinically significant. However, comparisons of FCXM-negative, low MFI engraftment studies, which use different stem cell sources (peripheral blood vs bone marrow vs cord) that have large differences in product compassion and cellularity, should not be performed.
In conclusion, the expanding role of alternative donors as a hematopoietic stem cell source has greatly increased the availability of alloBMT. However, patients commonly can have preformed antibodies against HLA antigen mismatches with their intended donor, especially female patients directed against their children’s paternal HLA antigens. Because these DSAs are dynamic, their relative strength can change over time. Importantly, the presence of DSAs at the time of alloBMT can adversely affect engraftment rates. The development of SPIs has greatly increased the ability to detect DSAs. However, SPIs have inherent limits and do not represent a true quantitative or qualitative assay to assess DSA strength. The criteria that constitutes a prohibitive DSA is unknown; however, desensitization techniques can successfully lower DSA to ranges deemed safe and limit primary engraftment failure.
Acknowledgments
The authors thank the Johns Hopkins Immunogenetics Laboratory and the Hemapheresis and Transfusion Support (HATS) division and Hematologic Malignancies division at the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins for allowing us to represent their works.
References
- 1.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33(28):3152-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunstein CG, Fuchs EJ, Carter SL, et al. ; Blood and Marrow Transplant Clinical Trials Network. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, National Marrow Donor Program. Donor registry transplant data. Available at: http://bloodcell.transplant.hrsa.gov/RESEARCH/Transplant_Data/Registry_Tx_Data/index.html. Accessed 5 February 2011.
- 5.D'Souza A, Zhu X. Current uses and outcomes of hematopoietic cell transplantation (HCT): CIBMTR summary slides, 2016. Available at: https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/Pages/index.aspx. Accessed 7 June 2017.
- 6.Shaw BE, Mayor NP, Russell NH, et al. Diverging effects of HLA-DPB1 matching status on outcome following unrelated donor transplantation depending on disease stage and the degree of matching for other HLA alleles. Leukemia. 2010;24(1):58-65. [DOI] [PubMed] [Google Scholar]
- 7.Locke JE, Zachary AA, Warren DS, et al. Proinflammatory events are associated with significant increases in breadth and strength of HLA-specific antibody. Am J Transplant. 2009;9(9):2136-2139. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan HC, Liwski RS, Bray RA, Gebel HM. The road to HLA antibody evaluation: do not rely on MFI. Am J Transplant. 2017;17(6):1455-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320(4):197-204. [DOI] [PubMed] [Google Scholar]
- 10.Ottinger HD, Rebmann V, Pfeiffer KA, et al. Positive serum crossmatch as predictor for graft failure in HLA-mismatched allogeneic blood stem cell transplantation. Transplantation. 2002;73(8):1280-1285. [DOI] [PubMed] [Google Scholar]
- 11.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88(8):1019-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciurea SO, Thall PF, Wang X, et al. Donor-specific anti-HLA Abs and graft failure in matched unrelated donor hematopoietic stem cell transplantation. Blood. 2011;118(22):5957-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cutler C, Kim HT, Sun L, et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood. 2011;118(25):6691-6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takanashi M, Atsuta Y, Fujiwara K, et al. The impact of anti-HLA antibodies on unrelated cord blood transplantations. Blood. 2010;116(15):2839-2846. [DOI] [PubMed] [Google Scholar]
- 16.Chang YJ, Zhao XY, Xu LP, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciurea SO, Thall PF, Milton DR, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(8):1392-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor PA, Ehrhardt MJ, Roforth MM, et al. Preformed antibody, not primed T cells, is the initial and major barrier to bone marrow engraftment in allosensitized recipients. Blood. 2007;109(3):1307-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gladstone DE, Zachary AA, Fuchs EJ, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. 2013;19(4):647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunstein CG, Noreen H, DeFor TE, Maurer D, Miller JS, Wagner JE. Anti-HLA antibodies in double umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011;17(11):1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari M, Uppugunduri CR, Ferrari-Lacraz S, et al. The clinical relevance of pre-formed anti-HLA and anti-MICA antibodies after cord blood transplantation in children. PLoS One. 2013;8(8):e72141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruggeri A, Rocha V, Masson E, et al. Impact of donor-specific anti-HLA antibodies on graft failure and survival after reduced intensity conditioning-unrelated cord blood transplantation: a Eurocord, Société Francophone d’Histocompatibilité et d’Immunogénétique (SFHI) and Société Française de Greffe de Moelle et de Thérapie Cellulaire (SFGM-TC) analysis. Haematologica. 2013;98(7):1154-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zachary AA, Vega RM, Lucas DP, Leffell MS. HLA antibody detection and characterization by solid phase immunoassays: methods and pitfalls. Methods Mol Biol. 2012;882:289-308. [DOI] [PubMed] [Google Scholar]
- 24.Zachary AA, Leffell MS. Desensitization for solid organ and hematopoietic stem cell transplantation. Immunol Rev. 2014;258(1):183-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montgomery RA, Lonze BE, King KE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318-326. [DOI] [PubMed] [Google Scholar]
- 26.Leffell MS, Jones RJ, Gladstone DE. Donor HLA-specific Abs: to BMT or not to BMT? Bone Marrow Transplant. 2015;50(6):751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 2005;66(4):364-370. [DOI] [PubMed] [Google Scholar]
- 28.Costa LJ, Moussa O, Bray RA, Stuart RK. Overcoming HLA-DPB1 donor specific antibody-mediated haematopoietic graft failure. Br J Haematol. 2010;151(1):94-96. [DOI] [PubMed] [Google Scholar]
- 29.Nordlander A, Uhlin M, Ringdén O, Kumlien G, Hauzenberger D, Mattsson J. Immune modulation to prevent antibody-mediated rejection after allogeneic hematopoietic stem cell transplantation. Transpl Immunol. 2011;25(2-3):153-158. [DOI] [PubMed] [Google Scholar]
- 30.Ishiyama K, Anzai N, Tashima M, Hayashi K, Saji H. Rapid hematopoietic recovery with high levels of DSA in an unmanipulated haploidentical transplant patient. Transplantation. 2013;95(12):e76-e77. [DOI] [PubMed] [Google Scholar]
- 31.Gergis U, Mayer S, Gordon B, et al. A strategy to reduce donor-specific HLA Abs before allogeneic transplantation. Bone Marrow Transplant. 2014;49(5):722-724. [DOI] [PubMed] [Google Scholar]
- 32.Yoshihara S, Maruya E, Taniguchi K, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012;47(4):508-515. [DOI] [PubMed] [Google Scholar]