Abstract
Patients with inherited bone marrow failure syndromes (IBMFSs) classically present with specific patterns of cytopenias along with congenital anomalies and/or other physical features that are often recognizable early in life. However, increasing application of genomic sequencing and clinical awareness of subtle disease presentations have led to the recognition of IBMFS in pediatric and adult populations more frequently than previously realized, such as those with early onset myelodysplastic syndrome (MDS). Given the well-defined differences in clinical management needs and outcomes for aplastic anemia, acute myeloid leukemia, and MDS in patients with an IBMFS vs those occurring sporadically, as well as nonhematologic comorbidities in patients with IBMFSs, it is critical for hematologists to understand how to approach screening for the currently known IBMFSs. This review presents a practical approach for the clinical hematologist that outlines when to suspect an IBMFS and how to use various diagnostic tools, from physical examination to screening laboratory tests and genomics, for the diagnosis of the most frequent IBMFSs: Fanconi anemia, telomere biology disorders, Diamond-Blackfan anemia, GATA2 deficiency syndrome, Shwachman-Diamond syndrome, and severe congenital neutropenia.
Learning Objectives
Recognize classical as well as subtle presentations of inherited bone marrow failure syndromes in children and adults
Apply a targeted physical examination and history as well as traditional screening tests and modern genomic sequencing to the diagnosis of inherited bone marrow failure syndromes in clinical practice
Introduction
Bone marrow failure (BMF), defined as the inability of hematopoiesis to meet physiologic demands for the production of a sufficient number of functional blood cells, can be classified into 3 main categories based on presumed etiology: inherited, secondary, or idiopathic. As their name implies, the inherited causes of BMF, herein called the inherited BMF syndromes (IBMFSs), classically present with specific patterns of cytopenias in a syndromic setting featuring congenital anomalies and/or other physical features (Table 1) often recognizable early in life. As a result, the IBMFSs have been traditionally thought of as diagnoses mainly relevant to pediatric hematologists. However, it is increasingly recognized that patients with an IBMFS can present with BMF alone without any syndromic features and, conversely, with typical physical features without BMF. In both scenarios, the underlying IBMFS diagnosis is often significantly delayed, even well into adulthood, when additional signs such as excess toxicity during stem cell transplantation (SCT) or treatment of an early-onset cancer prompt an IBMFS workup.
Table 1.
The most common physical features of the IBMFSs organized by typical first presenting cytopenia
| Physical feature | Macrocytosis | ||||||
|---|---|---|---|---|---|---|---|
| Thrombocytopenia | Anemia | Neutropenia | |||||
| TAR | TBD* | FA* | DBA | SDS | GATA2 deficiency | SCN* | |
| Head and neck | Oral leukoplakia,† early gray hair, ophthalmic anomalies | Microcephaly, hearing loss/ear anomalies, ophthalmic anomalies/small eyes | Craniofacial defects, short/webbed neck | Hearing loss | Hearing loss, dental carries, gingivitis | ||
| Pulmonary | Pulmonary fibrosis, emphysema | Pulmonary dysfunction, PAP | |||||
| Cardiovascular | Septal defects | Congenital heart defects | |||||
| Gastrointestinal | Liver cirrhosis, hepatopulmonary syndrome, esophageal strictures | Tracheoesophageal fistula, esophageal atresia | Pancreatic dysfunction | ||||
| Genitourinary/ gynecologic | Urethral strictures | Renal/GU tract anomalies, infertility | Renal/GU tract anomalies | GU tract anomalies, preterm labor | |||
| Musculoskeletal | Absent radii with thumbs present‡ | Short stature, avascular necrosis, osteoporosis | Short stature, thumb/radii anomalies‡ | Short stature, thumb anomalies | Short stature, metaphyseal dysostosis | Bone mineral loss | |
| Dermatologic | Lacy reticulated skin pigmentation,* dysplastic nails* | Cafe au lait spots, hypo-/hyperpigmentation | Eczema | HPV-related warts, panniculitis | Eczema | ||
| Vascular | Arteriovascular malformations | Lymphedema, DVT/PE | |||||
| Immunologic | Variable immunodeficiency | Recurrent infections | Mycobacterial, fungal, viral infections, autoimmune disease | Recurrent infections | |||
| CNS/neurologic | Developmental delay, cerebellar hypoplasia | Developmental delay, learning disabilities, hypopituitarism | Developmental delay | Developmental delay, learning disabilities | Behavioral disorders | Developmental delay, epilepsy | |
| Malignancy | MDS/AML | MDS/AML, SCC anogenital region, SCC head and neck, SCC and basal cell skin cancer | MDS/AML, SCC anogenital region, SCC head and neck, SCC and basal cell skin cancer | MDS/AML, GI tract malignancies, osteogenic sarcoma | MDS/AML | MDS/AML, SCC anogenital region, SCC and basal cell skin cancer | MDS/AML |
This table provides an overview of the most common features (in bold); see detailed reviews for additional features.4,5,7,8,18,20,27-29,31,32,36,37,41,49
DBA, Diamond-Blackfan anemia; DVT/PE, deep vein thrombosis/pulmonary embolism; FA, Fanconi anemia; GI, gastrointestinal; GU, genitourinary; HPV, human papillomavirus; MDS/AML, myelodysplastic syndrome/acute myeloid leukemia; PAP, pulmonary alveolar proteinosis; SCC, squamous cell carcinoma; SCN, severe congenital neutropenia; SDS, Shwachman-Diamond syndrome; TAR, thrombocytopenia absent radii; TBD, telomere biology disorder.
Syndromic features and their severity vary by the specific gene involved.
Diagnostic triad seen in the classical TBD presentations; these features may be variably present or even absent in patients with known TBD. Adult onset forms often feature pulmonary fibrosis and cytopenias as predominant features.
In FA, if the radius is absent, the thumb is absent, whereas in TAR, bilateral radii are often absent with thumbs present.
Modern advances in our understanding of the IBMFSs are attributed to the increasing clinical awareness of these disorders in both children and adults as well as the expanding use of genomic sequencing. Two recent investigations that used gene panel–based or whole-exome sequencing have shed light on the potential yield of a broad-based genetic testing approach in patients with clinically diagnosed IBMFSs.1,2 Among the 75 Canadian and 365 Japanese patients sequenced, genetic diagnoses were identified in 27% to 59% of cases and confirmed the clinical diagnosis in 85% to 93% of the cases, suggesting that clinical features and laboratory tests provide accurate diagnoses for most patients. The remaining 7% to 15% of patients had an alternative IBMFS diagnosed by genetic testing, likely reflecting the significant overlap in their clinical features. Even with this technology, a genetic diagnosis remained elusive for a substantial subset of patients, emphasizing the continued need for clinical diagnoses based on history and laboratory findings.
Similar genomic investigations have also been performed in patients with apparently sporadic aplastic anemia (AA) and myelodysplastic syndrome (MDS) to determine how often IBMFSs are overlooked in clinical diagnosis. Keel et al3 found damaging germline IBMFS variants in 2 (2%) of 96 and 12 (11%) of 106 children and young adults with presumed sporadic AA or MDS, respectively, undergoing SCT at their institution. Similarly, Lindsley et al4 identified likely genetic diagnoses of Shwachman-Diamond syndrome (SDS) in 5 (2%) of 239 adults younger than age 40 years undergoing hematopoietic SCT for MDS as part of an international registry. Wlodarski et al5 identified germline GATA2 mutations in 28 (7%) of 426 children age 18 years or younger with sporadic MDS in Germany.
Given the clear differences in clinical management needs and outcomes for AA and MDS in patients with IBMFSs vs those occurring sporadically, it is critical for hematologists to understand when and how to screen for the currently known IBMFSs in these patients. These diagnoses should also be considered for those who present with chronic, unexplained cytopenias. In this review, we present a practical outline for when to suspect IBMFSs and how the practicing hematologist can use all of the available diagnostic tools, from physical examination to screening laboratory tests and genomics, for diagnosing IBMFSs. We use cases to illustrate real-world scenarios for putting this framework into practice and refer the reader to excellent disease-specific reviews for detailed descriptions of each syndrome.
Case 1
A 20-year-old healthy male presents with chest pain and night sweats. A complete blood count (CBC) shows pancytopenia with a white blood cell count of 2,700/μL, hemoglobin 8.9 g/dL, mean corpuscular volume 101 fL, and platelet count 5,000/μL. A bone marrow biopsy shows a hypocellular marrow with refractory anemia with excess blasts. Cytogenetics are notable for loss of 7q in all cells examined. Should an IBMFS diagnosis be considered for this patient?
General approach
Whom to evaluate for IBMFS
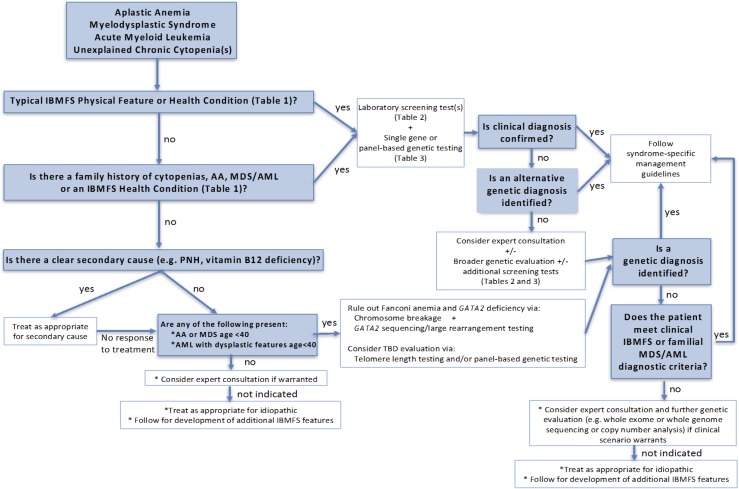
An evaluation for IBMFS should be considered for all patients presenting with AA, MDS, acute myeloid leukemia (AML), and chronic unexplained cytopenias. This assessment is likely most critical for patients who are younger than age 40 years at presentation, but given the observed diagnoses of some patients with IBMFSs into their 50s or older, a strict age cutoff is difficult. Patients with excess hematologic or other toxicities during treatment of an early-onset solid tumor characteristic of the IBMFSs also warrant an evaluation (Figure 1).
Figure 1.
An approach to evaluating patients presenting with AA, MDS, AML, or unexplained chronic cytopenias for the presence of an IBMFS.
Critical components of the IBMFS evaluation
Each of the IBMFSs often present with a typical pattern of initial cytopenias, which can help guide the clinician in paying particular attention to specific features of the physical examination and medical history (Table 1). For example, in a patient first presenting with macrocytosis and thrombocytopenia, careful attention to physical examination and prior imaging may reveal abnormally small thumbs and a horseshoe kidney suspicious for Fanconi anemia (FA). Conversely, these same hematologic findings in a patient with prior imaging showing basilar pulmonary fibrosis and dysplastic nails on physical examination would be suspicious for a telomere biology disorder (TBD). The peripheral blood smear and bone marrow findings may also provide additional clues to the underlying IBMFS diagnosis (Table 2). Patients should also be asked about a family history of similar cytopenias, AA, MDS/AML, or clinical stigmata of the IBMFSs. Sporadic causes of the presenting hematologic abnormalities, such as paroxysmal nocturnal hemoglobinuria or vitamin deficiencies should be ruled out, while recognizing that these sporadic events may also occur concurrently with an IBMFS.6 If treatment of the secondary cause does not improve hematologic parameters, evaluation for IBMFSs should be considered (Figure 1).
Table 2.
Laboratory screening tests and hematologic features of the IBMFSs organized by typical first presenting cytopenia
| Macrocytosis | |||||||
|---|---|---|---|---|---|---|---|
| Thrombocytopenia | Anemia | Neutropenia | |||||
| TAR | TBD | FA | DBA | SDS | GATA2 deficiency | SCN | |
| Screening test | Arm X-ray | Decreased lymphocyte telomere lengths | Spontaneous and DEB/MMC-induced chromosome breaks and radial configurations | Elevated red cell adenosine deaminase | Decreased pancreatic isoamylase and/or trypsinogen | Increased FLT3 ligand | None |
| Peripheral blood | Normal-size platelets | Normal-size platelets, elevated hemoglobin F | Normal-size platelets, elevated hemoglobin F | Reticulocytopenia, elevated hemoglobin F | B- and T-cell defects, elevated hemoglobin F, neutrophil chemotaxis defects | Monocytopenia B and NK cell deficits, CD4:CD8 ratio <1 | |
| Pre-MDS/AML bone marrow findings | Decreased megakaryocytes | Hypocellular, evolving unilineage to trilineage dysplasia | Hypocellular, erythroid dysplasia, cytogenetics: +1q, +3q* | Normocellular, then hypocellular with age; decreased erythroid precursors | Hypocellular, myeloid dysplasia, cytogenetics: del(20)(q 11), i(7)(q10) | Hypocellular, megakaryocytic atypia, fibrosis | Promyelocyte maturation arrest |
| MDS/AML subtype | NA | NA | RCMD, RAEB, RARS, MDS-NOS | NA | Varied | RCC or RAEB (children); RCMD, RAEB, CMML (adults) | Varied |
| MDS/AML cytogenetics | NA | NA | Unbalanced +1q, −7/–7q, +3q* | NA | Abnormal chromosome 7 | Monosomy 7, trisomy 8, trisomy 21 | Varied |
| MDS/AML acquired genetic features | NA | NA | RUNX1* by large genomic events | NA | TP53 | ASXL1 | CSF3R, RUNX1 |
This table provides an overview of the most common laboratory screening tests and hematologic features of the IBMFSs (in bold); see detailed reviews for additional features.4,5,7,8,18,20,27,28,31,32,36,37,41,49-51
CMML, chronic myelomonocytic leukemia; DEB, diepoxybutane; MMC, mitomycin C; NA, not available; NK, natural killer; NOS, not otherwise specified; RAEB, refractory anemia with excess blasts; RARS, refractory anemia with ringed sideroblasts; RCC, refractory cytopenia of childhood; RCMD, refractory cytopenia with multilineage dysplasia.
Best detected by fluorescence in situ hybridization, array comparative genomic hybridization, or next-generation sequencing techniques because size is not usually detectable at the gross karyotypic level.
Case 1 (continued).
The patient recalls being told that his platelet count was low during an injury-related medical visit at age 18, but he neglected to follow-up for this finding. He denies any significant family history. Physical examination was normal, aside from hypoplastic thumbs. With the combination of abnormal thumbs, prior thrombocytopenia, macrocytosis, and early-onset hypocellular MDS, a clinical diagnosis of an IBMFS was considered. Which IBMFS is most likely and how should his workup proceed?
FA.
Classical presentation.
FA classically presents in childhood with a combination of congenital anomalies and BMF (Table 1). The most common physical features include short stature, thumb and/or radial ray anomalies, and café au lait or hypopigmented skin macules.7 Thrombocytopenia with or without macrocytosis or anemia is the most common peripheral blood abnormality (Table 2). Bone marrow biopsy may demonstrate a hypocellular marrow with prominent erythroid or multilineage dysplasia; isolated clonal abnormalities such as +1q, +3q, and −7q have been reported.8,9
Nonclassical presentation.
Twenty-five to 40% of patients may lack congenital anomalies.10 In addition, patients without congenital anomalies may present with early onset, sometimes multiple, cancers as their first indication of FA.11 Common malignancies include AML and squamous cell cancer (SCC) of the head and neck and the vulva with observed/expected ratios of 831, 311, and 2098, respectively.11 Physicians should have a high index of suspicion for FA in patients with early-onset SCC, MDS, AML, or AA before implementing standard radiation, chemotherapy, or bone marrow transplant conditioning regimens for the treatment of these conditions to avoid devastating toxicity resulting from their DNA repair deficit.12,13 Conversely, patients with severe, disproportionate toxicities with chemotherapy or radiation should be considered for FA evaluation, along with other DNA repair deficits or pharmacogenomic explanations.
Functional and genetic testing.
Screening for FA begins with peripheral blood lymphocyte exposure to mitomycin C (MMC) or diepoxybutane (DEB) in culture to evaluate for increased chromosome breaks and the characteristic radial configurations (Table 2). False negatives can occur in FA patients who have had a somatic hematopoietic reversion event, in which a hematopoietic stem or progenitor cell and thus its progeny have corrected the genetic defect on 1 allele, resulting in hematopoietic mosaicism.14,15 If this reversion event occurs in a pluripotent stem cell rather than in a more lineage-restricted cell, such as a lymphocyte precursor, a slow improvement in blood counts may be observed, but risk for BMF and/or MDS/AML development remains.16,17 Chromosome breakage testing by MMC and DEB can also be difficult to interpret in the setting of MDS/AML or recent chemotherapy.8 In these cases, MMC or DEB testing of cultured skin fibroblasts can demonstrate the characteristic findings and help make the clinical diagnosis.
Although not required for a clinical diagnosis, genetic testing of genes implicated in FA (Table 3) may be performed either sequentially or all at once via a panel-based approach that includes large rearrangement analysis either in silico or via alternative methods. Two mutations, 1 on each of the 2 alleles of a specific FA gene, are the most common finding and account for the autosomal recessive inheritance pattern seen in most FA patients (Table 3). Autosomal dominant (RAD51) and X-linked recessive (FANCB) patterns are also possible. Identification of the specific causative FA gene mutation(s) for a patient can (1) inform gene-specific disease features and management (eg, higher cancer risks for patients with FANCD1/BRCA2 mutations and defined cancer risks and prevention methods for heterozygous mutation carriers in the family), (2) allow site-specific genetic testing for family members to identify those who are heterozygous mutation carriers, (3) aid in the diagnosis of those with FA if chromosome breakage testing is not definitive, and (4) aid in future childbearing decisions. FANCA is the most commonly mutated gene in Europe and North America, accounting for 60% to 70% of diagnoses.7
Table 3.
IBMFS genetics and inheritance patterns
| Syndrome | Defect | Gene | Inheritance pattern |
|---|---|---|---|
| Fanconi anemia7 | FA/BRCA pathway double-strand break DNA repair and maintenance | FANCA | AR |
| FANCB | XLR | ||
| FANCC | AR | ||
| FANCD1/BRCA2 | AR | ||
| FAND2 | AR | ||
| FANCE | AR | ||
| FANCF | AR | ||
| FANCG/XRCC9 | AR | ||
| FANCI/KIAA1794 | AR | ||
| FANCJ/BRIP1/BACH1 | AR | ||
| FANCL | AR | ||
| FANCM | AR | ||
| FANCN/PALB2 | AR | ||
| FANCO/RAD51C | AR | ||
| FANCP/SLX4 | AR | ||
| FANCQ/ERCC4 | AR | ||
| FANCR/RAD51 | AD | ||
| FANCS/BRCA1 | AR | ||
| FANCT/UBE2T | AR | ||
| FANCU/XRCC2 | AR | ||
| FANCV/REV7/MAD2L2 | AR | ||
| Telomere biology disorders18,52 | Telomere maintenance | ACD | AD or AR |
| CTC1 | AR | ||
| DKC1 | XLR | ||
| NAF1 | AD | ||
| NHP2 | AD | ||
| NOP10 | AD | ||
| PARN | AD or AR | ||
| POT1 | AR | ||
| RTEL1 | AD or AR | ||
| TERC | AD | ||
| TERT | AD or AR | ||
| TINF2 | AD | ||
| WRAP53 | AR | ||
| STN1/OBFC1 | AR | ||
| Diamond-Blackfan anemia28,53 | Ribosome biogenesis | GATA1 | XLR |
| RPL5 | AD | ||
| RPL11 | AD | ||
| RPL15 | AD | ||
| RPL18 | AD | ||
| RPL26 | AD | ||
| RPL27 | AD | ||
| RPL31 | AD | ||
| RPL35 | AD | ||
| RPL35A | AD | ||
| RPS7 | AD | ||
| RPS10 | AD | ||
| RPS17 | AD | ||
| RPS19 | AD | ||
| RPS24 | AD | ||
| RPS26 | AD | ||
| RPS27 | AD | ||
| RPS28 | AD | ||
| RPS29 | AD | ||
| TSR2 | XLR | ||
| GATA2 deficiency syndrome32 | Hematopoietic differentiation and stem cell pool maintenance | GATA2 | AD |
| Shwachman-Diamond syndrome37-40 | Ribosome maturation | DNAJC21/HSP40 | AR |
| EFL1 | AR | ||
| SBDS | AR | ||
| Severe congenital neutropenia43,44 | Decreased production or premature apoptosis of neutrophils or their precursors | CSF3R | AR |
| ELANE | AD | ||
| G6PC3 | AR | ||
| GFI1 | AD | ||
| HAX1 | AR | ||
| JAGN1 | AR | ||
| VPS45 | AR | ||
| WAS | XLR | ||
| Thrombocytopenia absent radii54 | Deficiency of exon-junction complex Y14 | RBM8A | AR |
Genes are listed in alphabetical order and those in bold are the most common cause(s) of each syndrome. In the telomere biology disorder section, TERT is bolded as the most common cause of adult onset pulmonary fibrosis predominant presentations. Genes in this table are updated from the specific citations listed for each syndrome. In some cases the variant gene has been identified in a limited number of individuals. Other resources include https://www.ncbi.nlm.nih.gov/gene and https://www.omim.
TBDs.
Classical presentation.
The classic TBD presentation includes the mucocutaneous triad of dystrophic nails, oral leukoplakia, and abnormal skin pigmentation (Table 1).18 These mucocutaneous findings may not appear until late childhood or early adulthood. The most common presenting hematologic abnormalities are thrombocytopenia or macrocytosis with or without anemia and a hypocellular bone marrow.18
Nonclassical presentation.
The phenotypic spectrum of TBDs is quite broad, with the most severe presentations featuring disabling neurologic findings in childhood and the mildest forms being completely asymptomatic.18 Other features, often developing in adulthood, include early gray hair, liver cirrhosis, pulmonary fibrosis or emphysema, and predisposition to SCC of the head and neck and of the anogenital tract.10,19,20 The combination of pulmonary fibrosis and BMF co-occurring in an individual or a family is highly predictive of a TBD.19 The diagnosis of a TBD can be complicated by the phenomenon of anticipation, which results in young affected family members presenting before members of older generations.18 TBD patients may also be identified at the time of treatment for BMF or MDS/AML because of excess organ toxicity with traditional conditioning regimens, including pulmonary, hepatic, and veno-occlusive disease or when familial donors fail to mobilize adequate stem cells.12,21
Screening and genetic testing.
Evaluation of peripheral blood lymphocyte telomere lengths by flow fluorescence in situ hybridization can be performed as an initial screening test in a patient suspected of having a TBD.22 Lymphocyte telomere lengths below the first percentile for age are both sensitive and specific (97% and 91%, respectively) and may correlate with disease severity.23 However, telomere lengths between the first and tenth percentile have been observed in carriers of deleterious TBD mutations.24,25 Thus, telomere length less than the first percentile is not an absolute requirement for a TBD diagnosis. In addition, in rare patients with other IBMFS, such as FA and SDS, telomere lengths may be slightly below the first percentile.24 Thus, panel-based sequencing of the known TBD-associated genes (Table 3) can be helpful to establish the diagnosis and allows gene-specific management. Even with a panel-based genetic testing approach, only about 70% of patients with a clinical TBD diagnosis carry pathogenic variants in currently known genes, suggesting that others remain to be identified.24 Inheritance patterns of the known TBD-associated genetic variants may be x-linked recessive (XLR), autosomal recessive (AR), or autosomal dominant (AD) (Table 3).18
Diamond-Blackfan anemia.
Classical presentation.
The classic physical phenotype of a patient with Diamond-Blackfan anemia (DBA) includes short stature, thumb anomalies, and congenital heart disease.26 Fifty percent of affected patients have 1 congenital anomaly, and 25% have more than 1 anomaly.27,28 Peripheral blood shows macrocytic anemia and reticulocytopenia in 90% of individuals before 1 year of age, and bone marrow biopsy demonstrates a normocellular marrow with lack of erythrocyte precursors.28
Nonclassical presentation.
Patients with cryptic presentations may go unnoticed because of a lack of the above-mentioned physical findings or because of the occurrence of spontaneous hematologic remission, which occurs in approximately 25% of patients.10 Patients with DBA also have an increased risk of malignancy with an observed-to-expected ratio of 4.75 and a median age of onset of solid tumor malignancy of 35 years.26,29 The most common malignancies are gastrointestinal cancers, osteogenic sarcoma, and MDS.29
Screening and genetic testing.
Elevated serum adenosine deaminase levels are both sensitive (84%) and specific (95%) for DBA,30 but prior transfusion may affect testing results.26 Genetic testing can be performed by single-gene sequencing and large rearrangement testing, beginning with RPS19, which accounts for 25% of identified pathogenic variants, or via a multigene sequencing panel with large rearrangement analysis that includes all of the known genes implicated in DBA. DBA is most often inherited in an AD pattern, but an XLR pattern is also possible, depending on the specific causative gene (Table 3).28
Case 1 (continued).
In the setting of MDS requiring treatment, a skin biopsy was performed. Chromosome breakage analysis and panel-based sequencing and large rearrangement testing of all FA, DBA, and TBD genes, along with genes involved in hereditary thrombocytopenia with predisposition to MDS/AML and GATA2, were simultaneously performed on the cultured fibroblasts. DEB testing demonstrated increased breaks and radial configurations, and genetic testing revealed biallelic pathogenic variants in FANCA, which allowed initiation of modified MDS treatment and SCT planning in this patient with FA.
Case 2.
A 30-year-old male presented for stem cell transplant donor evaluation for his 33-year-old sister who was recently diagnosed with AML. His only significant history was recurrent sinus infections in childhood for which he underwent sinus surgery as a teenager. His predonation CBC was notable for a borderline white blood cell count of 3,400/μL and monocytopenia.
GATA2 deficiency syndrome.
Classical presentation.
Germline mutations in GATA2 cause an autosomal dominant heterogeneous IBMFS characterized by susceptibility to infection, pulmonary and vascular/lymphatic dysfunction, autoimmunity, and malignancy (Table 1).31,32 GATA2 deficiency is implicated in up to 7% of childhood and adolescent cases of MDS and has also been identified in unexplained chronic neutropenia cases.5,33 Neutropenia and monocytopenia are common peripheral blood findings, and the bone marrow is often hypocellular with fibrosis and megakaryocytic atypia.31,32 Lymphocyte subset analysis shows low B-cell and natural killer–cell levels. The significant overlap of clinical features and BMF presentation of GATA2 deficiency with other traditional IBMFSs make it a necessary addition to the IBMFS workup (Figure 1; Tables 1 and 2).
Nonclassical presentation.
Delayed diagnosis may occur as a result of normal hematologic parameters in childhood and lack of physical features or severe infectious complications until adulthood.31,34 Although GATA2 deficiency appears most commonly in childhood and adolescent MDS, adult-onset MDS and chronic myelomonocytic leukemia have occurred in affected adults even into their 50s.31
Genetic testing.
Sequencing and large rearrangement testing of all coding exons of GATA2 as well as the intron 5 enhancer region allow identification of the heterozygous pathogenic variant in most cases (Table 3).32 Genotype-phenotype correlation is observed with GATA2 null mutations and occurrence of lymphedema and/or severe viral infections.31
SDS.
Classical presentation.
SDS is classically characterized by childhood onset of exocrine pancreatic dysfunction and BMF (Table 1).35 Patients may also present with growth failure, metaphyseal dysostosis and osteopenia, hepatomegaly, and recurrent infections disproportionate to the level of neutropenia. The reported median age of diagnosis is 0.6 years; however, teenage and adult diagnoses are reported.4,35 The most common CBC abnormality is neutropenia, which may be variable or persistent (Table 2) and can be seen in combination with anemia and thrombocytopenia.36 Bone marrow biopsy demonstrates hypocellularity for age with dysgranulopoietic features.35,37
Nonclassical presentation.
Less than 50% of patients present with the classic combination of clinically symptomatic exocrine pancreatic insufficiency and BMF.37 In addition, pancreatic exocrine dysfunction may range from severe diarrhea and malnutrition to subclinical presentation and often resolves in childhood, which may lead to missed diagnoses.4,36
Screening and genetic testing.
Screening for deficiency of trypsinogen and pancreatic isoamylase may reveal the characteristic pancreatic deficit.37 Of note, for children younger than age 3 years, trypsinogen is the preferred test because pancreatic isoamylase production may not reach normal levels until age 3 years, even in healthy young children without SDS. Conversely, trypsinogen levels may normalize with age in patients with SDS, making pancreatic isoamylase the preferred test in those older than age 3 years. Molecular diagnosis of this autosomal recessive syndrome is made by sequencing and large rearrangement testing of the SBDS gene, which identifies the causative biallelic pathogenic variants in 90% of patients with a clinical SDS diagnosis (Table 3).36 Recently, biallelic or homozygous germline pathogenic variants in EFL1 and DNAJC21 have been identified in patients with SDS-like syndromes, suggesting that these genes should be considered in the workup of patients with a clinical diagnosis of SDS in whom pathogenic variants in SBDS are absent.38-40
Severe congenital neutropenia.
Classical presentation.
Severe congenital neutropenia (SCN) is a chronic state of severe neutropenia associated with a neutrophil count less than 500/µL lasting longer than 3 months, often presenting in the first year of life.41 Patients suffer from recurrent bacterial and invasive fungal infections, which can be ameliorated by the use of granulocyte colony-stimulating factor (G-CSF). Sepsis or MDS/AML are the major causes of death with hazard rates of 0.81% and 2.3% per year, respectively, in those receiving long-term G-CSF; higher hazards of either outcome are observed in patients requiring higher baseline doses of G-CSF to maintain adequate neutrophil counts than those with lower baseline requirements.42 Other findings may include chronic gingivitis or dental caries and decreased bone density resulting in an increased propensity to fracture (Table 1).41 Additional syndromic features vary by the specific causative SCN germline mutation, such as developmental delay in SCN resulting from HAX1 mutations.43 The CBC typically shows neutropenia with the bone marrow demonstrating the characteristic feature of myeloid maturation arrest.41
Nonclassical presentation.
The diagnosis of SCN can be complicated by varying clinical phenotypes in patients with the same genetic variants, leading to a spectrum from cyclic neutropenia to SCN as well as heterogeneity resulting from the specific causative gene.41
Genetic testing.
Comprehensive testing may be performed either sequentially or via a panel-based genetic test that evaluates the genes implicated in SCN. Patterns of inheritance include AD, AR, or XLR according to the specific causative gene (Table 3).43,44
Case 2 (continued).
Lymphocyte subsets from the donor revealed B-cell lymphopenia. His bone marrow biopsy was hypocellular with fibrosis and atypical megakaryocytes. The sister’s AML featured an acquired ASXL1 mutation. Genetic testing from skin fibroblasts confirmed the clinical diagnosis of a GATA2 deficiency syndrome in the donor and his sister.
Challenges in the clinical diagnosis of IBMFSs
As illustrated above, the IBMFSs are a heterogeneous group of diseases with variable clinical phenotypes, ages of presentation, and patterns of inheritance. Patients can present without syndromic features or without BMF, both of which challenge even the most astute clinicians. In addition, in those patients who do demonstrate a BMF phenotype, fluctuation in cytopenias due to spontaneous improvement or somatic reversion may occur.10,17 This phenomenon can make functional or screening tests falsely negative as may be observed in FA patients with spontaneous somatic reversion who demonstrate normal levels of chromosomal breakage in DEB testing of peripheral blood.14
Genetic testing is an indispensable tool in the diagnostic evaluation of IBMFSs that complements traditional clinical history, examination, and laboratory evaluation, especially in the setting of overlapping or adult presentations. However, clinical use of this powerful tool is currently limited by cost or access in most places. In addition, even when genetic testing is available, it may fail to provide the correct diagnosis. First, in the event of somatic reversion, the genetic variant or variants that cause a patient’s IBMFS may not be detectable in peripheral blood cells. In this instance, alternative tissue such as skin should be biopsied and sent to a Clinical Laboratory Improvement Amendments–approved laboratory for in vitro fibroblast culture, DNA extraction, and genetic testing. An alternative tissue source should also be considered in a patient with a diagnosis of MDS/AML because of the presence of malignant clones that accumulate additional genetic variants, which may complicate the interpretation of any genetic findings, and in those who have already underwent an allogeneic hematopoietic SCT. Second, the currently known IBMFS genes do not explain the clinically diagnosed IBMFSs in all patients. Thus, a subset of patients will remain without a genetic diagnosis. They will have to rely on a carefully made clinical diagnosis based on findings in the patient and the patient’s family history to guide management until new genes are discovered. Expert consultation may be helpful in this scenario. In the future, clinical or research-based whole-exome and/or whole-genome sequencing and copy number analysis will undoubtedly characterize additional genetic variants that cause the known as well as novel IBMFSs. Third, many rare genetic variants identified in known IBMFS genes cannot be classified as disease-causing or benign, leading to their classification as so-called variants of uncertain significance (VUS). VUSs are especially common in patients from ethnic minority populations in whom less genetic sequencing overall has been performed, resulting in a lack of knowledge about common variants unique to those subpopulations. In such cases, a careful clinical diagnosis can still be made if enough features are present, and the strength of the clinical presentation along with laboratory and/or other family-based investigations may help prove the pathogenicity of the VUS. Finally, there is additional overlap between the IBMFSs outlined here and many other inherited causes of single or multiple cytopenias, MDS/AML, and DNA repair defects. Consultation with experts in IBMFS presentation can help in difficult cases.
Conclusions
IBMFSs were once thought of as classical pediatric diagnoses, but advances in molecular diagnostics and increased clinical suspicion have led to a more dynamic understanding of them, including subtle clinical and adult presentations. The field of IBMFSs continues to grow, making it exciting and challenging for practicing hematologists and researchers alike. New genes causing BMF presentations that overlap with those presented above, including TCIRG1,45 SRP72,46 SAMD9,47 and SAMD9L,48 await incorporation into the IBMFS paradigm outlined here. Future research into the prevalence of each disorder in varied patient populations that present for hematologic care will improve our ability to recognize and diagnose individual patients and optimize their care. Now and likely well into the future, the sum of all available tools is greater than any alone, and a modern IBMFS workup should include a focused history and physical examination, screening tests, and genetic evaluation whenever possible.
Acknowledgments
The authors thank all of their patients and colleagues for contributing to the clinical and laboratory-based research efforts that are advancing this field and acknowledge the many individuals whose important work could not be cited because of space and reference number limitations.
This work is supported by grant HL129088 from the National Institutes of Health, National Heart, Lung, and Blood Institute (J.E.C.) and the Cancer Research Foundation (J.E.C.).
References
- 1.Muramatsu H, Okuno Y, Yoshida K, et al. . Clinical utility of next-generation sequencing for inherited bone marrow failure syndromes. Genet Med. 2017;19(7):796-802. [DOI] [PubMed] [Google Scholar]
- 2.Ghemlas I, Li H, Zlateska B, et al. . Improving diagnostic precision, care and syndrome definitions using comprehensive next-generation sequencing for the inherited bone marrow failure syndromes. J Med Genet. 2015;52(9):575-584. [DOI] [PubMed] [Google Scholar]
- 3.Keel SB, Scott A, Sanchez-Bonilla M, et al. . Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica. 2016;101(11):1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindsley RC, Saber W, Mar BG, et al. . Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wlodarski MW, Hirabayashi S, Pastor V, et al. ; EWOG-MDS. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127(11):1387-1397. [DOI] [PubMed] [Google Scholar]
- 6.Sreedharanunni S, Varma N, Sachdeva MUS, et al. . Paroxysmal nocturnal hemoglobinuria clones are not infrequent in patients with inherited bone marrow failure syndromes. Eur J Haematol. 2017;99(2):194-195. [DOI] [PubMed] [Google Scholar]
- 7.Mehta PA, Tolar J. Fanconi anemia. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2017. [updated 23 February 2017]. [Google Scholar]
- 8.Peffault de Latour R, Soulier J. How I treat MDS and AML in Fanconi anemia. Blood. 2016;127(24):2971-2979. [DOI] [PubMed] [Google Scholar]
- 9.Cioc AM, Wagner JE, MacMillan ML, DeFor T, Hirsch B. Diagnosis of myelodysplastic syndrome among a cohort of 119 patients with fanconi anemia: morphologic and cytogenetic characteristics. Am J Clin Pathol. 2010;133(1):92-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson DB, Link DC, Mason PJ, Bessler M. Inherited bone marrow failure syndromes in adolescents and young adults. Ann Med. 2014;46(6):353-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alter BP, Giri N, Savage SA, et al. . Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150(2):179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peffault de Latour R, Peters C, Gibson B, et al. ; Pediatric Working Party of the European Group for Blood and Marrow Transplantation; Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50(9):1168-1172. [DOI] [PubMed] [Google Scholar]
- 13.Hays L, Frohnmayer D, Frohnmayer L, Guinan E, Kennedy MA, Larsen K, eds; Fanconi Anemia: Guidelines for Diagnosis and Management, Fourth Edition. Eugene, Oregon: Fanconi Anemia Research Fund, 2014. [Google Scholar]
- 14.Fargo JH, Rochowski A, Giri N, Savage SA, Olson SB, Alter BP. Comparison of chromosome breakage in non-mosaic and mosaic patients with Fanconi anemia, relatives, and patients with other inherited bone marrow failure syndromes. Cytogenet Genome Res. 2014;144(1):15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross M, Hanenberg H, Lobitz S, et al. . Reverse mosaicism in Fanconi anemia: natural gene therapy via molecular self-correction. Cytogenet Genome Res. 2002;98(2-3):126-135. [DOI] [PubMed] [Google Scholar]
- 16.Gregory JJ Jr, Wagner JE, Verlander PC, et al. . Somatic mosaicism in Fanconi anemia: evidence of genotypic reversion in lymphohematopoietic stem cells. Proc Natl Acad Sci USA. 2001;98(5):2532-2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soulier J, Leblanc T, Larghero J, et al. . Detection of somatic mosaicism and classification of Fanconi anemia patients by analysis of the FA/BRCA pathway. Blood. 2005;105(3):1329-1336. [DOI] [PubMed] [Google Scholar]
- 18.Savage SA. Dyskeratosis congenita. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2017. [updated 23 February 2017]. [Google Scholar]
- 19.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011;117(21):5607-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362(9396):1628-1630. [DOI] [PubMed] [Google Scholar]
- 22.Baerlocher GM, Vulto I, de Jong G, Lansdorp PM. Flow cytometry and FISH to measure the average length of telomeres (flow FISH). Nat Protoc. 2006;1(5):2365-2376. [DOI] [PubMed] [Google Scholar]
- 23.Alter BP, Rosenberg PS, Giri N, Baerlocher GM, Lansdorp PM, Savage SA. Telomere length is associated with disease severity and declines with age in dyskeratosis congenita. Haematologica. 2012;97(3):353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter BP, Giri N, Savage SA, Rosenberg PS. Telomere length in inherited bone marrow failure syndromes. Haematologica. 2015;100(1):49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armanios MY, Chen JJ, Cogan JD, et al. . Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356(13):1317-1326. [DOI] [PubMed] [Google Scholar]
- 26.Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010;116(19):3715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlachos A, Ball S, Dahl N, et al. ; Participants of Sixth Annual Daniella Maria Arturi International Consensus Conference. Diagnosing and treating Diamond Blackfan anaemia: results of an international clinical consensus conference. Br J Haematol. 2008;142(6):859-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinton C, Gazda HT. Diamond-Blackfan anemia. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2017. [updated 23 February 2017]. [Google Scholar]
- 29.Vlachos A, Rosenberg PS, Kang J, Atsidaftos E, Alter BP, Lipton JM Myelodysplastic syndrome and gastrointestinal carcinomas characterize the cancer risk in Diamond Blackfan anemia: a report from the Diamond Blackfan anemia registry [abstract]. Blood. 2016;128(22). Abstract 333. [Google Scholar]
- 30.Fargo JH, Kratz CP, Giri N, et al. . Erythrocyte adenosine deaminase: diagnostic value for Diamond-Blackfan anaemia. Br J Haematol. 2013;160(4):547-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinner MA, Sanchez LA, Hsu AP, et al. . GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. Br J Haematol. 2015;169(2):173-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquet M, Bellanné-Chantelot C, Tavitian S, et al. . High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121(5):822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hahn CN, Chong CE, Carmichael CL, et al. . Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat Genet. 2011;43(10):1012-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donadieu J, Fenneteau O, Beaupain B, et al. ; Associated investigators of the French Severe Chronic Neutropenia Registry*. Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica. 2012;97(9):1312-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myers KC, Davies SM, Shimamura A. Clinical and molecular pathophysiology of Shwachman-Diamond syndrome: an update. Hematol Oncol Clin North Am. 2013;27(1):117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myers KC, Bolyard AA, Otto B, et al. . Variable clinical presentation of Shwachman-Diamond syndrome: update from the North American Shwachman-Diamond Syndrome Registry. J Pediatr. 2014;164(4):866-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stepensky P, Chacón-Flores M, Kim KH, et al. . Mutations in EFL1, an SBDS partner, are associated with infantile pancytopenia, exocrine pancreatic insufficiency and skeletal anomalies in aShwachman-Diamond like syndrome. J Med Genet. 2017;54(8):558-566. [DOI] [PubMed] [Google Scholar]
- 39.Tummala H, Walne AJ, Williams M, et al. . DNAJC21 mutations link a cancer-prone bone marrow failure syndrome to corruption in 60S ribosome subunit maturation. Am J Hum Genet. 2016;99(1):115-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhanraj S, Matveev A, Li H, et al. Biallelic mutations in DNAJC21 cause Shwachman-Diamond syndrome. Blood. 2017;129(11):1557-1562. [DOI] [PubMed] [Google Scholar]
- 41.Klein C. Genetic defects in severe congenital neutropenia: emerging insights into life and death of human neutrophil granulocytes. Annu Rev Immunol. 2011;29:399-413. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg PS, Zeidler C, Bolyard AA, et al. . Stable long-term risk of leukaemia in patients with severe congenital neutropenia maintained on G-CSF therapy. Br J Haematol. 2010;150(2):196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boztug K, Klein C. Genetics and pathophysiology of severe congenital neutropenia syndromes unrelated to neutrophil elastase. Hematol Oncol Clin North Am. 2013;27(1):43-60. [DOI] [PubMed] [Google Scholar]
- 44.Online Mendelian Inheritance in Man (OMIM). Johns Hopkins University, Baltimore, MD. [updated 8 August 2017]. https://www.omim.org/. [Google Scholar]
- 45.Makaryan V, Rosenthal EA, Bolyard AA, et al. ; UW Center for Mendelian Genomics. TCIRG1-associated congenital neutropenia. Hum Mutat. 2014;35(7):824-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kirwan M, Walne AJ, Plagnol V, et al. . Exome sequencing identifies autosomal-dominant SRP72 mutations associated with familial aplasia and myelodysplasia. Am J Hum Genet. 2012;90(5):888-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Narumi S, Amano N, Ishii T, et al. . SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48(7):792-797. [DOI] [PubMed] [Google Scholar]
- 48.Chen DH, Below JE, Shimamura A, et al. . Ataxia-pancytopenia syndrome is caused by missense mutations in SAMD9L. Am J Hum Genet. 2016;98(6):1146-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toriello HV. Thrombocytopenia absent radius syndrome. In: Pagon RA, Adam MP, Ardinger HH, et al., eds. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993-2017. [updated 23 February 2017]. [Google Scholar]
- 50.Touw IP. Game of clones: the genomic evolution of severe congenital neutropenia. Hematology Am Soc Hematol Educ Program 2015;2015:1-7. [DOI] [PubMed]
- 51.Skokowa J, Steinemann D, Katsman-Kuipers JE, et al. . Cooperativity of RUNX1 and CSF3R mutations in severe congenital neutropenia: a unique pathway in myeloid leukemogenesis. Blood. 2014;123(14):2229-2237. [DOI] [PubMed] [Google Scholar]
- 52.Stanley SE, Gable DL, Wagner CL, et al. . Loss-of-function mutations in the RNA biogenesis factor NAF1 predispose to pulmonary fibrosis-emphysema. Sci Transl Med. 2016;8(351):351ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirabello L, Khincha PP, Ellis SR, et al. . Novel and known ribosomal causes of Diamond-Blackfan anaemia identified through comprehensive genomic characterisation. J Med Genet. 2017;54(6):417-425. [DOI] [PubMed] [Google Scholar]
- 54.Albers CA, Paul DS, Schulze H, et al. . Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome. Nat Genet 2012;44(4):435-439. [DOI] [PMC free article] [PubMed]