Abstract
Splenic marginal zone lymphoma (SMZL) and nodal marginal zone lymphoma (NMZL) are rare indolent chronic B-cell lymphomas. Prognosis is typically good with median survival around 10-15 years. Management is generally based on the presence of symptoms or high tumor burden. There are no standard treatments for these 2 entities, and therapeutic strategies are rapidly evolving. Clinical developments for these 2 entities are oriented by genomic studies, with largely overlapping mutational profiles involving the NOTCH, B-cell receptor (BcR) and nuclear factor κB (NF-κB) signaling, chromatin remodeling, and the cytoskeleton. Although new therapeutic options based on targeting signaling pathways and overcoming resistance are increasingly available, few specific prospective studies are performed for these rare subtypes, limiting the conclusions that can be drawn. Novel drugs targeting B-cell signaling have shown promise, including ibrutinib and copanlisib. The second-generation oral immunomodalator (IMiD) lenalidomide showed impressive results when combined with rituximab. Other potential solutions include targeting the NF-κB, JAK/STAT, BCL2, NOTCH, and Toll-like receptor signaling pathways; however, studies in these 2 MZL entities are yet to prove a definitive benefit. Molecular profiling is now a cornerstone of diagnostic, prognostic, and therapeutic strategies to offer patient- and disease-specific solutions. The development of a wider range of effective targeted therapies and prognostic biomarkers is keenly awaited and is expected to strongly affect the natural history of SMZL and NMZL.
Learning Objectives
To describe the novelties in the biology of the SMZL and NMZL, including the strongly biased immunoglobulin heavy-chain variable (IGHV) repertoire toward the IGHV1-2*04 allele in SMZL and the IGHV4-34 allele in NMZL, suggesting a contribution of antigen stimulation, and similar mutational profiles in the NOTCH and BcR/TLR/NF-κB pathways, chromatin remodeling, and the cytoskeleton
To explain the impact of these biological findings on the diagnosis, the prognosis, and the management of SMZL and NMZL
To describe the new therapeutic options such as those targeting BcR signaling and IMIDs on the basis of the few specific prospective studies, which are just the first of many promising drugs in SMZL and NMZL
Current management of splenic and nodal marginal zone lymphoma
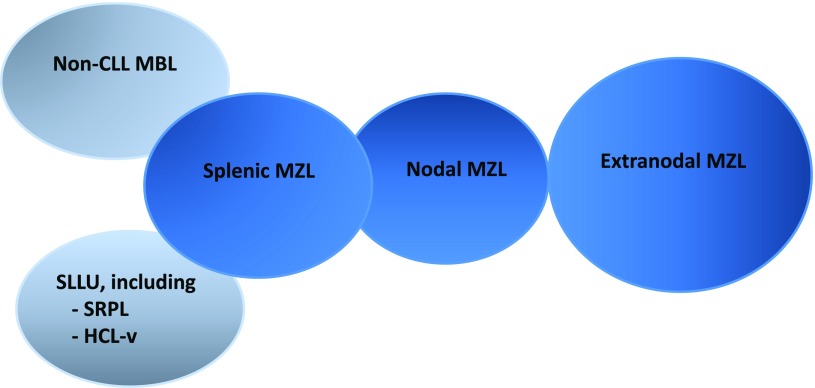
Splenic marginal zone lymphoma (SMZL) and nodal marginal zone lymphoma (NMZL) are distinct subtypes of marginal zone lymphomas (MZL) recognized in the World Health Organization classification.1 Other MZLs include extranodal MZL (EMZL) of mucosa-associated lymphoid tissue (MALT lymphoma), the most common entity of MZL, and 2 novel entities, non-chronic lymphocytic leukemia (non-CLL) monoclonal B-cell lymphocytosis, probably closely related to SMZL, and a broad category of less well-defined provisional entities primarily involving the spleen, termed splenic B-cell lymphoma/leukemia, unclassifiable (SLLU). The 2 main provisional SLLU entities are splenic diffuse red pulp small B-cell lymphoma (SDRPL) and hairy cell leukemia variant (HCL-v) (Figure 1).
Figure 1.
MZL entities described in the World Health Organization classification.1 EMZL, also known as MALT lymphomas (65% of MZL), SMZL (20%), and NMZL (5%); non-CLL, monoclonal B-cell lymphocytosis (MBL), and SLLU, including SDRPL and HCL-v.
Clinical presentation
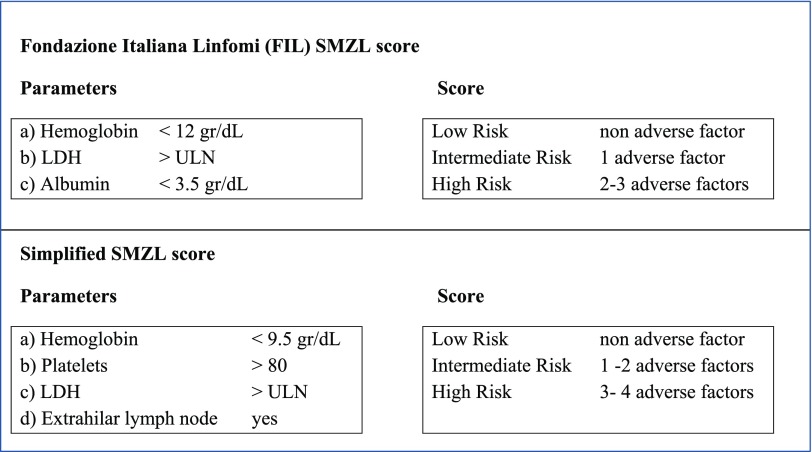
SMZL and NMZL represent rare chronic B-cell lymphomas accounting for 20% and 10% of MZL, respectively, and less than 2% of all non-Hodgkin lymphomas (NHL) in adults.2,3 The median age of diagnosis for SMZL is 65 years and is between 50 and 62 years for NMZL. Both typically present with disseminated disease, SMZL with predominant enlarged spleen without concomitant lymphadenopathy, and NMZL with disseminated, primarily nodal, involvement without extranodal or splenic disease. Nonetheless, they share several clinical features, including an indolent presentation, which can be asymptomatic for several years. Histological transformation to large-cell lymphoma remains uncommon, occurring in 10% to 20% of patients. Prognosis is usually good, with median survival around 10 to 15 years.2,3 Some degree of heterogeneity exists, and 5% to 10% of SMZL patients present with more aggressive disease and shorter survival, supporting the usefulness of prognostic indexes4,5 (Figure 2). However, for NMZL, owing to its rarity, a specific prognostic score has not yet been developed, although the follicular lymphoma (FL) international prognostic index has been reported to have some value in NMZL patients.6 In any case, all prognostic parameters published in retrospective analyses, both biological and clinical, should be interpreted with caution because of the small number of patients and heterogeneity of treatment.3
Figure 2.
Prognostic indices for SMZL.
Current therapeutic management
In the absence of standard treatment of both SMZL and NMZL, current treatment strategies recommend treating patients only in the presence of symptoms or high tumor burden,2,3 and therapeutic specificities are presented below for each.
In patients with splenic marginal zone lymphoma.
For patients with symptomatic splenomegaly, cytopenia, systemic symptoms, or progressive nodal disease, therapeutic options include splenectomy, chemotherapy, and rituximab as a single agent or combined with chemotherapy.2 Currently, rituximab monotherapy (375 mg/m2, weekly for 6 weeks) represents the most effective treatment among the conservative strategies and is associated with high response rates (∼90%), with approximately half of the responses being complete.7 Importantly, using a maintenance therapy (rituximab every 2 months for 1-2 years), many of these responses improve in quality and are long lasting. The 7-year progression-free survival (PFS) rate was reported to be 75% in a recent study by Kalpadakis et al.7 The addition of chemotherapy to rituximab does not further improve the outcome, although this issue is still under investigation. Splenectomy should be recommended only in patients fit for surgery and presenting symptomatic splenomegaly without bulky lymphadenopathy. The surgical removal of an enlarged spleen will quickly ameliorate symptoms related to splenomegaly, as well as improve cytopenia due to splenic sequestration. The median PFS after splenectomy in the largest retrospective series published to date was 8.25 years.8 In SMZL patients with active hepatitis C virus (HCV) infection, a first-line approach with antiviral treatment should be considered.
In patients with nodal marginal zone lymphoma.
For patients with NMZL, a similar strategy as that used for FL is initially proposed.3 Patients with strictly localized disease may be considered for localized radiation therapy. In cases of disseminated low tumor burden, a watchful waiting strategy is usually used, whereas in disseminated high tumor burden, immunochemotherapy (rituximab plus chemotherapy with or without an anthracycline) is considered appropriate. Several immune-chemotherapies have been evaluated, including R-CVP (cyclophosphamide, vincristine, prednisone), FCR (fludarabine, cyclophosphamide, rituximab), 2-CdA +/−R, R-CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), BR (bendamustine-rituximab), and chlorambucil, the most currently used being BR. The multicenter, randomized phase 3 study comparing BR and R-CHOP in indolent lymphomas showed a similar median PFS in the subgroup of MZL, including 67 patients (BR, 57.2 months vs R-CHOP, 47.2 months; hazard ratio 0.70, 95% confidence interval [CI] 0.34–1.43; P = .3249).9 The very small MZL patient sample size limits unequivocal extrapolation of these data to NMZL patients.
Biological insights: immunogenetics, genetic changes, and oncogenetic cooperation
SMZL and NMZL originate from B lymphocytes normally present in the marginal zone (MZ) of the secondary lymphoid follicles. The MZ is mainly present in the mucosae and the spleen but rarely in nodes. These B lymphocytes provide the first line of defense against blood-borne pathogens, with an early and rapid antibody response to both T-dependent and independent microbial antigens following dual activation of B-cell receptors (BcRs) and Toll-like-receptors (TLRs), and with the production of polyreactive low-affinity “natural” antibodies.
In splenic marginal zone lymphoma
The immunoglobulin heavy-chain variable region (IGHV) gene repertoire reveals the exact point of differentiation of the cell of origin. In SMZL, the IGHV repertoire is strongly biased and restricted to the IGHV1-2*04 allele in ∼30% of cases, with a long complementarity-determining region 3 sequence with common motifs. The skewing of the IG gene repertoire extends to IG light chains and pairing with the IG heavy chains, for example, IGHV1-2*04 with IGKV3-20, IGKV1-8, and IGL2-14.10,11 Interestingly, a study has shown that ongoing somatic hypermutations (HSM) are frequent in rearrangements, including IGHV1-2*04 and less common in rearrangements using other IGHV genes (P = .001), supporting the idea of a distinct molecular SMZL subtype.12
The largest published cytogenetic study of 330 cases showed that 72% had an abnormal karyotype, of which 53% are complex (defined as ≥3 aberrations or ≥2 clones), and a mean of 2.3/5.1 abnormalities per case (range, 0-19) was detected by SNP6.0 arrays.13 The 7q deletions are the most frequent abnormality, occurring in ∼30% to 40% of cases,14 without clear evidence for a targeted gene involved in this deletion.15,16 Other recurring abnormalities are gains of 3/3q, 9q, 12q, and 18q, and losses of 6q, 8p, 14q, and 17p.
Candidate gene screening as well as whole genome and exome sequencing studies, with targeted resequencing of larger patient cohorts, allow characterization of recurring mutations in SMZL and their classification into 3 main groups: NOTCH signaling, the nuclear factor κB (NF-κB) pathway, and chromatin remodeling and the cytoskeleton.17-20 These studies reported that the zinc finger transcription factor KLF2 is mutated in 10% to 40% of SMZL cases, whereas the monoallelic mutation of exon 34 of NOTCH2 is found in 10% and 21% of cases,17-21 eliminating or truncating the PEST domain. Mutations in NOTCH1 (also in the PEST domain), SPEN, and DTX1 are found in ∼11%. All of these mutations result in upregulated activity of the NOTCH pathway. Mutations leading to NF-κB activation involving members of the BcR (CARD11), TLR (MYD88), and canonical and noncanonical NF-κB pathways (TRAF3, MAP3K14, TNFAIP3, IKBKB, BIRC3) are reported in 34% of cases. Mutations in the NOTCH and BcR/TLR/NF-κB pathways were largely mutually exclusive. Mutations were also found in chromatin remodelers such as MLL2 (6/40 cases), ARID1A (2/40), and SIN3A (3/40),17 and in CREBBP, and TP53 mutations were detected in approximately 15% of cases.
In nodal marginal zone lymphoma
The IGHV repertoire of NMZL is also strongly biased toward the IGHV4-34 allele (∼30%) with frequent HSM (∼85%), suggesting that the contribution of antigen stimulation, demonstrated by this highly restricted IG repertoire, strongly influences SMZL and NMZL pathogenesis, occurring in the context of a microenvironment (the spleen or the lymph node) in which the tumor cells have been highly selected. This will likely have consequences for the genes associated with BcR signaling, including NF-κB activation.
Deletion of 7q is not found, and trisomies 3, 18, 7, 12 and del6q are recurrent clonal abnormalities in NMZL, along with gain of chromosome 3 (affecting FOXP1, NFKBIZ, and BCL6) and 18q23 (affecting NFATC1). Inactivation of the A20 genes (on 6q23) by somatic mutation, deletion, or both has been described across all MZL subtypes and may contribute to lymphomagenesis via induction of constitutive NF-κB.
Transcriptomic analyses of 15 NMZL cases led to the proposition of an “NMZL signature” of 264 upregulated and 184 downregulated genes.22 Deregulated pathways including BcR signaling, interleukins (IL-2, IL-6, IL-10), integrin signaling (CD40), and cell survival pathways (MAPKs, tumor necrosis factor [TNF], transforming growth factor-β, and NF-κB) have been revealed as activated survival pathways in NMZL. The transmembrane activator and calcium-modulating ligand interactor (TACI), a TNF-family receptor for differentiation and NF-κB activation, and CD74 (antigen presentation and B-cell maturation) may serve as potential therapeutic targets.
Mutational analysis of another 35 cases identified 39 genes recurrently affected by mutations (30 genes). Among them, MLL2 (34% of cases), protein tyrosine phosphatase receptor δ (PTPRD) tumor suppressor (20%), and NOTCH2 (20%) were the most frequently mutated. The study identified several deregulated pathways whose combinations appear to be specific for NMZL across mature B-cell tumors, notably JAK/STAT (43% of cases), NF-κB (54%), NOTCH (40%), and TLR signaling (17%); cell cycle (43%); chromatin remodeling/transcriptional regulation in terms of epigenetic modifiers, histones, or transcriptional corepressors (71%); and immune escape via T cell–mediated tumor surveillance (14% to 17%).
In total, the genetics of SMZL and NMZL overlap remarkably (with the exception of PTPRD mutations), offering fertile ground for the development of future therapeutic new targets. Interestingly, most of these genes are those implicated in marginal zone development and function, such as NOTCH2, the master regulator of MZ B-cell differentiation, KLF2, calcium-modulating ligand interactor receptors, and downstream of NF-κB activation.
SMZL and NMZL associated with HCV: biological particularities
HCV is frequently associated with MZL and diffuse large B-cell lymphomas (DLBCL). Chronic stimulation by HCV may contribute to the development of SMZL or NMZL. The E2 glycoprotein of HCV may interact with CD81 in the B cells, causing B-cell activation via the BcR, leading to increased proliferation. The IG BcR repertoire seems restricted toward the IGHV1-69 gene. In murine models, chronic stimulation by HCV has been associated with mutations of FAS, AP12/ML, p53. A decrease in lymphoproliferation following antiviral treatments reinforces the data suggesting a contribution of chronic antigenic stimulation to the pathophysiologic process of HCV-related MZL.
Clinical significance of biology and mutations
Diagnosis
SMZL and NMZL are diagnosed using a combination of clinical, morphological, histological, and immunophenotypic data. However, diagnosis can be challenging, particularly for SMZL with other splenic lymphomas, notably SDRPL, HCL-v, and lymphoplasmacytic lymphoma. Immunogenetic and genomic data have revealed specificities that may help in diagnosis.
The preferential implication of the IGHV1-2*04 gene in SMZL, in comparison with SDRPL and HCL-v (∼30% vs 5%), and the IGHV 4-34 gene family in HCL and HCL-v are valuable.10 Moreover, a study in which SMZL, HCL-v, and NMZL lymphoma patients were screened for mutations in NOTCH1, NOTCH2, BIRC3, TNFAIP3, TRAF3, IKBKB, MYD88, CD79A, CD79B, and CARD11 showed that SMZL and NMZL shared a similar genotype, whereas none of these genes were mutated in HCL-v, in which activating mutations of MAP2K1 were reported in 50% of cases.23 The 7q deletions were found in only 9 out of 170 of the other mature B-cell tumors and in 0 of 48 nonmarginal splenic lymphomas but were detected in 3 of 9 SLLU cases. Similarly, NOTCH2 mutations were not found in CLL (0/100), MCL (0/20), follicle center lymphoma (0/20), HCL (0/20), NMZL (0/18), or myeloma (0/22), and only rarely in EMZL (1/65) and DLBCL (5/134).
The MYD88 L265P mutation has recently been recognized as the hallmark of lymphoplasmacytic lymphoma/Waldenstrom macroglobulinemia, described in >90% of cases and over 50% of immunoglobin M (IgM) monoclonal gammapathy of undetermined significance,24-26 vs 5 of 84 (6%) SMZL patients. No clinical differences between cases of SMZL with or without MYD88 mutation were identified other than a higher incidence of an IgM paraprotein in the former (80% vs 19%). Mutations of BRAF1, present in virtually all cases of HCL, have not been reported in SMZL.17-20
Prognosis
The value of biomarkers in SMZL and NMZL remains unclear because of the relatively small size of the series, heterogeneity of treatment, and lack of prospective clinical trials.
The prognostic impact of NOTCH2 mutations has been investigated with conflicting results. Rossi et al reported that 5-year overall survival (OS) of patients with a NOTCH2 mutation was 93%, significantly longer than in those without (74%; P = .048). Similarly, 5-year PFS after first-line treatment was longer (83% vs 44%; P = .020).17 However, NOTCH2 mutations at diagnosis were reported in 10 of 105 SMZL patients, all of whom subsequently required treatment.21 Those with a NOTCH2 mutation had a shorter 5-year OS (32% vs 87%; P = .0001), shorter 5-year PFS (16% vs 66%; P = .0008), and an increased risk of transformation to large-cell lymphoma within 5 years (26% vs 6%; P = .0051). Multivariate analysis showed that NOTCH2 mutations were an independent predictor of OS. Similarly, Kiel et al identified NOTCH2 mutations in 25 of 99 (25%) SMZL patients. The time from tissue diagnosis to relapse, transformation, or death was shorter in NOTCH2 mutated cases (32.6 vs 107.2 months; P = .002). The poorer outcome was independent of gender, age, performance status, and stage at diagnosis.18
KLF2, TP53 abnormalities, IGHV1-2*04 gene usage, and aberrant promoter methylation represent promising prognostic biomarkers, associated with inferior outcome mostly in SMZL, and could be incorporated into current clinical prognostic models to improve risk stratification.12,27
Treatment perspectives and novelties
In the past 5 years we have seen impressive progress in the treatment of lymphoid malignancies. New knowledge in terms of biological features along with the development of novel drugs have resulted in innovative options in the therapeutic strategy for this group of diseases. This has opened the door to therapeutic options based on targeting signaling pathways and overcoming potential resistance due to mutational redundancies. However, because of the rarity of SMZL and NMZL, preclinical and clinical research specific to this entity is sporadic, with clinical trials few and far between. Those that have been performed include few patients, making it extremely challenging to obtain a comprehensive view of the efficacy of new agents in MZL. MZL entities may be incorporated into larger trials designed for a biologically similar indolent disease, and in particular follicular lymphoma. A summary of completed and ongoing studies in MZL patients, including SMZL and NMZL, is presented in Tables 1 and 2.
Table 1.
Novel treatments in clinical trials, including MZL patients with relapsed/refractory non-Hodgkin’s lymphoma or relapsed B-cell lymphoma2,3,30,32,33
| Pathway | Drug | Target | N evaluable MZL patients* | Overall response rate | Complete response rate | Duration of response months (months) | Limiting toxicity |
|---|---|---|---|---|---|---|---|
| PI3K/AKT/mTOR | Everolimus | mTOR | 24 MZL (16 MALT; 4 NMZL) | 28% | 4% | — | Hematologic (46%, G3/4), interstitial pneumonia (4%) |
| Idelalisib | PI3K-d | 15 MZL | 57% | 6% | 18.4 | Neutropenia (27%, G3/4), aminotransferase elevations (13%, G3/4), diarrhea (13%, G3/4), pneumonia (7%, G3/4) | |
| Copanlisib | PI3K-d and PI3K-α | 23 MZL/141 | 69.6% | 8.7% | Not reached | hypertension (49%, G3/4), neutropenia (30%, G3/4), hyperglycemia (30%, G3/4), anemia (15%, G3/4) | |
| BcR | Ibrutinib | BTK | 63 MZL | 48% | 3% | Not reached at a median follow-up at 19.4 (95% CI, 17.6-22.3) | Pneumonia (8%, G3/4) |
| Anemia 1 (4%, G3/4) | |||||||
| Diarrhea (5%, G3/4) | |||||||
| Apoptosis | Venetoclax | BCL2 | 3 MZL/106 | 67% | 0% | 2.3 and 23.6 | Neutropenia (8% to 14%,† G3/4), anemia (19%), thrombocytopenia (8% to 32%†), febrile neutropenia (2%†) |
| Microenvironment | Lenalidomide | Immune modulator | 30 MZL | 89% | 67% | Median PFS 53.8 (95% CI, 50.6 to NA) | Neutropenia (35%); muscle pain (9%); rash (7%); cough, dyspnea, or other pulmonary symptoms (5%); thrombosis (5%); thrombocytopenia (4%) |
| Proteasome | Bortezomib | Proteasome | |||||
| Rituximab + bortezomib 1.3 mg/m2 twice weekly or 1.6 mg/m2 weekly | 9 r/r MZL and 70 FL | 49% | 14% | Median TTP 7.0 | Thrombocytopenia (10%, G3/4) | ||
| 43% | 10% | Median TTP 10.0 | Neuropathy (10%, G3/4) | ||||
| Diarrhea (15%, G3/4) |
BTK = Bruton’s tyrosine kinase; CR = complete remission; G = grade; NA, not applicable; ORR = overall response rate; PI3K = phosphoinositol 3 kinase; r/r, relapse/refractory; TTP, time to progression.
The number of patients with SMZL and NMZL not systematically available.
From all doses (400, 600, 900, or 1200 mg).
Table 2.
Ongoing trials for MZL, including SMZL and NMZL patients
| Compound | Clinical setting | Class | Target | Ongoing trial | ||||
|---|---|---|---|---|---|---|---|---|
| Phase | N patients | Comparator | Primary endpoint | Trial status | ||||
| Obinutuzumab | Rituximab-refractory MZL (among iNHL) | mAb | CD20 | III (combo with bendamustine) | 414 | Bendamustine | PFS | NCT01059630; complete |
| r/r MZL (among B-cell NHL) | I/II (combo with lenalidomide) | 72 | MTD, DLT | NCT01995669; recruiting (est. completion May 2018) | ||||
| r/r MZL (among B-cell NHL) | I (combo with venetoclax and lenalidomide) | 60 | ORR | NCT02992522; recruiting (est. completion January 2020) | ||||
| Ibrutinib | r/r MZL | iTK | BTK, ITK | II | 60 | — | ORR | NCT01980628; complete |
| Relapsed MZL (among B-cell NHL) | I (combo with lenalidomide) | 34 | MTD | NCT01955499; active, not recruiting (est. analysis December 2019) | ||||
| First line; untreated MZL (together with follicular lymphoma) | I (combo with rituximab and lenalidomide) | 60 | PFS | NCT02532257; active, recruiting (est. completion April 2019 | ||||
| Lenalidomide | r/r MZL (together with follicular lymphoma) | II (combo with rituximab) | 357 | NCT01938001; active, not recruiting (est. completion December 2017 | ||||
| r/r MZL (together with follicular lymphoma) | III (combo with rituximab) | 500 | PFS | NCT01996865; active (est. completion March 2023) | ||||
| r/r MZL (among B-cell NHL) | I/II (combo with nivolumab) | 102 | MTD, ORR | NCT03015896; not yet recruiting (est. completion April 2020) | ||||
| r/r MZL (among NHL) | I (combo with blinatumomab) | 36 | MTD | NCT02568553; active (est. completion June 2018) | ||||
| Copanlisib | r/r MZL (among indolent NHL) | Small molecule | PI3K-δ and PI3K-α | III (combo with rituximab) | 514 | Rituximab + placebo | PFS [time frame: 59 mo] | NCT02367040; currently recruiting (est. completion March 2020) |
| First-line indolent NHL | III (combo with standard chemo) | 621 | Copanlisib/placebo + R-B or R-CHOP | PFS [time frame: 53 mo] | NCT02626455; currently recruiting (est. completion September 2021) | |||
| TGR-1202 | r/r MZL (among B-cell NHL) | Small molecule | PI3K-δ | II/III (combo with ublituximab +/−βendmaustine) | 500 | ORR | NCT02793583; active (est. completion July 2019) | |
| Venetoclax | r/r MZL (among B-cell NHL) | Small molecule | Bcl2 | I (combo with obinutuzumab, and lenalidomide | 60 | — | ORR | NCT02992522; currently recruiting (est. completion December 2019) |
| Pembrolizumab | r/r MZL (among B-cell NHL | I/Ib (combo with ibrutinib) | 58 | MTD | NCT02950220; currently recruiting (est. completion December 2019) | |||
| r/r MZL (among r/r iNHL or CLL) | mAB | Anti-PD-1 | II (combo with idelalisib or Ibrutinib) | 68 | — | ORR | NCT02332980; currently recruiting (est. completion January 2020) | |
DLT, dose-limiting toxicity; est., estimated; iNHL, indolent non-Hodgkin lymphoma; MTD, maximal tolerated dose.
BcR pathways
Critical evidence has been accumulated, confirming the central role of BcR signaling in the pathogenesis of B-cell malignancies. Small-molecule inhibitors of kinases involved in B-cell signaling represent a booming area of research, including phosphatidylinositol-3-kinase (PI3K) and Bruton’s tyrosine kinase. Several inhibitors of the PI3K/AKT/mTOR pathway have been tested in MZL patients, including everolimus,28 and idelalisib,29 with overall response rates (ORR) in the relapse setting of 28% (complete remission [CR] 4%) and 57% (CR 6%), respectively. Ibrutinib, targeting BcR signaling via Bruton’s tyrosine kinase, has demonstrated interesting data in 63 patients in the relapsed MZL setting. The ORR was 46% (95% CI: 33.4-59.1), with 3.2% of patients achieving a CR and 42.9% achieving a partial response (PR). Efficacy was observed across all 3 MZL subtypes (ORR of 46.9%, 41.2%, and 50.0% for MALT, nodal, and splenic subtypes, respectively). The median duration of response was not reached (range, 16.7 to not reached), with median follow-up of 19.4 months. The median time to initial response was 4.5 months (range, 2.3-16.4).30 These results led to an accelerated approval of ibrutinib in relapsed MZL by the U.S. Food and Drug Administration in January 2017. Discussions over combining rituximab with ibrutinib have already been initiated. Copanlisib is an intravenous pan-class I PI3K inhibitor with predominant inhibitory activity against both PI3K-δ and PI3K-α isoforms. In vitro, its activity has been demonstrated in preclinical models of B-cell lymphomas, including MZL cell lines (Karpas1718, VM51, SSK41, ESKOL, HAIR-M, HC-1), as a single agent and in combination with conventional and targeted agents including venetoclax (BCL2 inhibitor) and palbociclib (CDK4/6 inhibitor).31 Interestingly, low expression of cell cycle genes and high expression of genes involved in interferon signaling, oxidative phosphorylation, fatty acid metabolism, apoptosis, PI3K/AKT/mTOR, and IL6/JAK/STAT signaling were associated with synergism with copanlisib/venetoclax. The palbociclib combination was more active with high expression of E2F/MYC targets and cell cycle genes and low expression of genes involved in interferon PI3K/AKT/mTOR and IL6/JAK/STAT signaling.31 In a clinical trial, copanlisib efficacy was recently reported in 141 indolent lymphoma patients, including 23 MZL. The ORR in the MZL patients was 70%, including 9% CR and 61% PR.32 On the basis of these results, a randomized, double-blind, placebo-controlled study was initiated to investigate the efficacy and safety of copanlisib in rituximab refractory indolent NHL patients previously treated with rituximab and alkylating agents (NCT02369016).
The NF-κB pathway
This signaling pathway is unequivocally deregulated in MZL. The proteasome inhibitor, bortezomib, targeting the NF-κB pathway, has demonstrated promising activity in both NMZL and MALT lymphoma, achieving complete or partial remissions in patients failing previous therapies. In patients with relapsed/refractory MALT lymphoma receiving bortezomib, among the 29 patients accessible for response, the ORR was 48%, including 9 patients who achieved a CR. Similar activity was seen when bortezomib was combined with rituximab, with 2 combination regimens proving effective in relapsed/refractory MZL in a randomized trial in which 81 patients were treated with 2 alternative regimens of rituximab and bortezomib. The ORR was 49% with twice weekly bortezomib and 43% with the weekly regimen (including 10% CR).3 Disappointingly, the high rate of neuropathy (up to 65% of patients) dramatically limits the clinical usefulness of this agent to patients.
BCL2 is overexpressed in SMZL and NMZL, making targeting apoptosis via BCL2 inhibitors such as ABT-199 another potential option. Again, few data are available in MZL, with only 3 patients treated with the highly selective BCL-2 inhibitor venetoclax (M12-175) in a phase 1 study dedicated to relapsed or refractory NHL. Two patients responded, one lasting 2.3 months and the other 23.6 months.33
IMiDs are oral immunomodulators with direct antineoplastic activity and immunologic effects, including blocking tumor cell proliferation and angiogenesis, and stimulating T-cell and natural killer (NK) cell-mediated cytotoxicity in experimental models.3 Antineoplastic and antiproliferative effects as well as increased NK cell numbers and activity have been observed in vitro and in vivo against malignant lymphoma B cells in general and specifically against DLBCL, follicular, and mantle cell lymphoma cells. The first-generation ImiD, thalidomide, showed a lack of efficacy when administered as monotherapy in a small pilot study; however, the second-generation molecule lenalidomide, administered alone or in combination with rituximab in MALT lymphoma, showed a 70% ORR in a phase 2 trial as monotherapy and an impressive 86% when combined with rituximab.34,35
The JAK/STAT signaling pathway contributes to cell survival in NMZL in a specific manner in comparison with other MZL entities, as is demonstrated by the exome-sequencing analysis36 with mutations in numerous kinases in this pathway. Specific inhibitors such as ruxolitinib (INCB018424), a small-molecule ATP mimetic, inhibits both JAK1 and JAK2. It is already used to treat myelofibrosis, a bone marrow disorder, and is being investigated for the treatment of certain cancers and autoimmune diseases.37
As was previously discussed, NOTCH signaling appears to be of major importance in MZL, including NMZL and SMZL, with NOTCH1 and NOTCH2 among the most commonly mutated genes in these diseases. The development of a novel agonist specifically targeting the NOTCH2 receptor may represent a promising strategy for NMZL.2,3
Toll-like receptor signaling is a key deregulated signaling pathway in SMZL and NMZL. MYD88 is an important linker protein in signaling via TLRs 7, 8, 9, and IL-1-R. After ligand stimulation, TLR/IL-1-R interacts with MYD88 via the Toll/interleukin-1 receptor domain. MYD88 binds subsequently via its death domain IRAK1, which in turn phosphorylates and activates IRAK4. In association with TRAF6 and TAK1, the IRAK1/IRAK4/MYD88 complex activates the NF-κB pathway. Strategies targeting TLR, IRAK4, and TAK1 mediator proteins of MYD88 signaling or MYD88 homodimerization are in clinical and preclinical development, but research to date is essentially in DLBCL. In addition there is no doubt that, as in all cancers, the microenvironment is of major importance and that targeting with anti-PD-1 or anti-PD-L1/2 is a potentially important next step.
Conclusions
SMZL and NMZL are distinct entities among NHL and more particularly among the indolent small B-cell lymphomas. Although characterized by a different clinical presentation, striking similarities in their epidemiology and tumor cell biology support a common origin from the B cells of the marginal zone, and outcomes of clinical trials in these 2 marginal zone lymphoma entities are an area of strong interest not only to clinicians but also to patients.
Until now, splenectomy in SMZL and immunochemotherapy in both SMZL and NMZL have formed the backbone of treatment, but this situation is rapidly evolving. Personalized medicine for SMZL and NMZL patients will integrate clinicopathologic features of the disease combined with state-of-the-art molecular profiling to create diagnostic, prognostic, and therapeutic strategies tailored to specific patient and disease requirements. This will improve therapeutic efficacy with targeted treatments, although for now, few data have been generated to allow novel strategies to be defined. Biological insights and knowledge of the toxicity profiles of the novel available drugs provide guidance to physicians when deciding whether or not to combine novel agents with classical chemotherapy. During the past 5 years, an extensive collaborative effort among biologists, pathologists, and clinicians has resulted in agreement on more stringent criteria for disease diagnosis and evaluation of clinical response. These efforts support the design of further prospective clinical trials to define the optimal targeted therapeutic approach for SMZL and NMZL.
Acknowledgments
This work was supported by the Nella and Amadeus Barletta Foundation.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arcaini L, Rossi D, Paulli M. Splenic marginal zone lymphoma: from genetics to management. Blood. 2016;127(17):2072-2081. [DOI] [PubMed] [Google Scholar]
- 3.Thieblemont C, Molina T, Davi F. Optimizing therapy for nodal marginal zone lymphoma. Blood. 2016;127(17):2064-2071. [DOI] [PubMed] [Google Scholar]
- 4.Montalbán C, Abraira V, Arcaini L, et al. ; Splenic Marginal Zone Lymphoma Study Group. Risk stratification for Splenic Marginal Zone Lymphoma based on haemoglobin concentration, platelet count, high lactate dehydrogenase level and extrahilar lymphadenopathy: development and validation on 593 cases. Br J Haematol. 2012;159(2):164-171. [DOI] [PubMed] [Google Scholar]
- 5.Montalban C, Abraira V, Arcaini L, et al. ; Splenic Marginal Zone Lymphoma Study Group (SMZLSG). Simplification of risk stratification for splenic marginal zone lymphoma: a point-based score for practical use. Leuk Lymphoma. 2014;55(4):929-931. [DOI] [PubMed] [Google Scholar]
- 6.Heilgeist A, McClanahan F, Ho AD, Witzens-Harig M. Prognostic value of the Follicular Lymphoma International Prognostic Index score in marginal zone lymphoma: an analysis of clinical presentation and outcome in 144 patients. Cancer. 2013;119(1):99-106. [DOI] [PubMed] [Google Scholar]
- 7.Kalpadakis C, Pangalis G, Vassilakopoulos T, et al. Splenic marginal zone lymphoma (SMZL) treated with rituximab (R) monotherapy: a long term follow-up study on 104 patients [abstract]. Haematologica. 2017;102. Abstract S416. [Google Scholar]
- 8.Lenglet J, Traullé C, Mounier N, et al. Long-term follow-up analysis of 100 patients with splenic marginal zone lymphoma treated with splenectomy as first-line treatment. Leuk Lymphoma. 2014;55(8):1854-1860. [DOI] [PubMed] [Google Scholar]
- 9.Rummel MJ, Niederle N, Maschmeyer G, et al. ; Study Group Indolent Lymphomas (StiL). Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381(9873):1203-1210. [DOI] [PubMed] [Google Scholar]
- 10.Baliakas P, Strefford JC, Bikos V, Parry M, Stamatopoulos K, Oscier D. Splenic marginal-zone lymphoma: ontogeny and genetics. Leuk Lymphoma. 2015;56(2):301-310. [DOI] [PubMed] [Google Scholar]
- 11.Ghia P, Nadel B, Sander B, Stamatopoulos K, Stevenson FK. Early stages in the ontogeny of small B-cell lymphomas: genetics and microenvironment [published online ahead of print 10 April 2017]. J Intern Med. [DOI] [PubMed] [Google Scholar]
- 12.Bikos V, Karypidou M, Stalika E, et al. An immunogenetic signature of ongoing antigen interactions in splenic marginal zone lymphoma expressing IGHV1-2*04 receptors. Clin Cancer Res. 2016;22(8):2032-2040. [DOI] [PubMed] [Google Scholar]
- 13.Salido M, Baró C, Oscier D, et al. Cytogenetic aberrations and their prognostic value in a series of 330 splenic marginal zone B-cell lymphomas: a multicenter study of the Splenic B-Cell Lymphoma Group. Blood. 2010;116(9):1479-1488. [DOI] [PubMed] [Google Scholar]
- 14.Bikos V, Darzentas N, Hadzidimitriou A, et al. Over 30% of patients with splenic marginal zone lymphoma express the same immunoglobulin heavy variable gene: ontogenetic implications. Leukemia. 2012;26(7):1638-1646. [DOI] [PubMed] [Google Scholar]
- 15.Fresquet V, Robles EF, Parker A, et al. High-throughput sequencing analysis of the chromosome 7q32 deletion reveals IRF5 as a potential tumour suppressor in splenic marginal-zone lymphoma. Br J Haematol. 2012;158(6):712-726. [DOI] [PubMed] [Google Scholar]
- 16.Watkins AJ, Hamoudi RA, Zeng N, et al. An integrated genomic and expression analysis of 7q deletion in splenic marginal zone lymphoma. PLoS One. 2012;7(9):e44997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi D, Trifonov V, Fangazio M, et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J Exp Med. 2012;209(9):1537-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiel MJ, Velusamy T, Betz BL, et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J Exp Med. 2012;209(9):1553-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez N, Almaraz C, Vaqué JP, et al. Whole-exome sequencing in splenic marginal zone lymphoma reveals mutations in genes involved in marginal zone differentiation. Leukemia. 2014;28(6):1334-1340. [DOI] [PubMed] [Google Scholar]
- 20.Parry M, Rose-Zerilli MJJ, Gibson J, et al. Whole exome sequencing identifies novel recurrently mutated genes in patients with splenic marginal zone lymphoma. PLoS One. 2013;8(12):e83244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Algrin C, Brisou G, Beldjord K, Mounier N. Prognostic impact of somatic NOTCH2 mutation in splenic marginal zone lymphoma [abstract]. Blood. 2013;122(21). Abstract 4247. [Google Scholar]
- 22.Arribas AJ, Campos-Martín Y, Gómez-Abad C, et al. Nodal marginal zone lymphoma: gene expression and miRNA profiling identify diagnostic markers and potential therapeutic targets. Blood. 2012;119(3):e9-e21. [DOI] [PubMed] [Google Scholar]
- 23.Waterfall JJ, Arons E, Walker RL, et al. High prevalence of MAP2K1 mutations in variant and IGHV4-34-expressing hairy-cell leukemias. Nat Genet. 2014;46(1):8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treon SP, Xu L, Yang G, et al. MYD88 L265P somatic mutation in Waldenström’s macroglobulinemia. N Engl J Med. 2012;367(9):826-833. [DOI] [PubMed] [Google Scholar]
- 25.Treon SP, Cao Y, Xu L, Yang G, Liu X, Hunter ZR. Somatic mutations in MYD88 and CXCR4 are determinants of clinical presentation and overall survival in Waldenstrom macroglobulinemia. Blood. 2014;123(18):2791-2796. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Hunter ZR, Yang G, et al. Detection of MYD88 L265P in peripheral blood of patients with Waldenström’s macroglobulinemia and IgM monoclonal gammopathy of undetermined significance. Leukemia. 2014;28(8):1698-1704. [DOI] [PubMed] [Google Scholar]
- 27.Parry M, Rose-Zerilli MJJ, Ljungström V, et al. Genetics and prognostication in splenic marginal zone lymphoma: revelations from deep sequencing. Clin Cancer Res. 2015;21(18):4174-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conconi A, Raderer M, Franceschetti S, et al. Clinical activity of everolimus in relapsed/refractory marginal zone B-cell lymphomas: results of a phase II study of the International Extranodal Lymphoma Study Group. Br J Haematol. 2014;166(1):69-76. [DOI] [PubMed] [Google Scholar]
- 29.Gopal AK, Kahl BS, de Vos S, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noy A, de Vos S, Thieblemont C, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129(16):2224-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudio E, Kwee I, Spriano F, et al. The phosphatidylinositol-3-kinase (PI3K) inhibitor (i) copanlisib is active in preclinical models of B-cell lymphomas as single agent and in combination with conventional and targeted agents including venetoclax and palbociclib [Poster 154/21]. Paper presented at the 107th AACR Annual Meeting. April 2017. Washington, DC. [Google Scholar]
- 32.Dreyling M, Santoro A, Leppa S, et al. Copanlisib in patients with relapsed or refractory indolent B-cell lymphoma: primary results of the pivotal Chronos-1 study [Abstract CT149]. Paper presented at the 107th AACR Annual Meeting. April 2017. Washington, DC. [Google Scholar]
- 33.Davids MS, Roberts AW, Seymour JF, et al. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiesewetter B, Troch M, Dolak W, et al. A phase II study of lenalidomide in patients with extranodal marginal zone B-cell lymphoma of the mucosa associated lymphoid tissue (MALT lymphoma). Haematologica. 2013;98(3):353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fowler NH, Davis RE, Rawal S, et al. Safety and activity of lenalidomide and rituximab in untreated indolent lymphoma: an open-label, phase 2 trial. Lancet Oncol. 2014;15(12):1311-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spina V, Khiabanian H, Bruscaggin A, et al. The coding genome of nodal marginal zone lymphoma reveals recurrent molecular alterations of PTPRD and other Jak/Stat signaling genes [abstract]. Blood. 2014;124(21). Abstract 705. [Google Scholar]
- 37.Tefferi A. JAK inhibitors for myeloproliferative neoplasms: clarifying facts from myths. Blood. 2012;119(12):2721-2730. [DOI] [PubMed] [Google Scholar]