Abstract
Follicular lymphoma is the most common indolent non-Hodgkin lymphoma in the Western hemisphere. The natural history of FL appears to have been favorably impacted by the introduction of rituximab after randomized clinical trials demonstrated that the addition of rituximab to standard chemotherapy induction has improved the overall survival. Yet, the disease is biologically and clinically heterogeneous with wide variations in outcomes for individual patients. The ability to accurately risk-stratify patients and then tailor therapy to the individual is an area of ongoing research. Historically, tumor grade, tumor burden, and the FL international prognostic index (version 1 and version 2) have been used to distinguish low-risk from high-risk patients. Biologic factors such as mutations in key genes can identify patients at high risk for poor outcomes to first-line therapy (mutational status of 7 genes [EZH2, ARID1A, MEF2B, EP300, FOX01, CREBBP, and CARD11] with Follicular Lymphoma International Prognostic Index). More recently, the quality of the response to initial therapy, as measured by either PET imaging or by remission duration, has been show to identify individuals at high risk. However, several unmet needs remain, including a better ability to identify high-risk patients at diagnosis, the development of predictive biomarkers for targeted agents, and strategies to reduce the risk of transformation.
Learning Objectives
Understand the most appropriate application of the various clinical risk stratification tools available in follicular lymphoma
Recognize the limitations regarding current biologic risk stratification tools
Introduction
Follicular lymphoma (FL) is the most common indolent non-Hodgkin lymphoma in the Western hemisphere. Although FL is considered incurable with standard chemotherapy, advances in treatment have improved disease management and clinical outcomes. However, these improvements are primarily the result of empiric application of available therapies rather than capitalization on improved understanding of FL biology. In fact, relatively few examples of targeted agents making significant impact in FL and no example of predictive biomarkers exist. FL can be compared to chronic lymphocytic leukemia (CLL), where a patient can undergo fluorescence in situ hybridization testing or IgVH mutational testing and receive information guiding the selection of frontline therapy. Available data suggest that a young patient with CLL with IgVH hypermutation and no 11q or 17p can expect outstanding results from the fludarabine, cyclophosphamide, and rituximab regimen. Alternatively, patients with CLL with 17p deletions clearly derive more benefits from BTK inhibition with ibrutinib than with traditional cytotoxic therapy. In addition, emerging literature suggest patients with CLL with 11q deletions or IgVH unmutated may also be better served by BTK inhibition. Routinely available prognostic and predictive biomarkers have yet to be implemented in frontline FL management.
FL is a biologically heterogeneous disease, and the prognosis varies widely among individuals. However, relatively recent discoveries in FL biology may lead to the development of tools for risk-adapted therapy. Gene expression profiling can identify patients with different immune signatures and different outcomes. However, attempts to define immune signatures by immunohistochemical stains have been inconsistent. Mutational profiling of key genes can identify patients at higher risk of relapse and of progression within 24 months of diagnosis (POD24). Mutational profiling also has identified key biologic pathways such as B-cell receptor (BCR) signaling and histone modification genes, which may provide clues into therapeutic targets. To date, the most powerful predictors of outcome have been the quality of response to initial therapy, which can be assessed by end-of-treatment positron emission tomography imaging or by the durability of the first remission. Assessing and assigning risk can facilitate many goals including counseling patients on expected outcomes, refining subgroup analysis from clinical trials, and adapting therapy to individual patients. This review focuses on the tools currently available, and under development, for risk stratification of patients with FL.
Using routine histologic features to determine risk
FL is derived from germinal center (GC) B cells. Its pathogenesis is closely linked to the normal GC reaction where naïve B cells from the bone marrow undergo somatic hypermutation and class-switching of the BCR in a process that generates immunoglobulin diversity and selects B cells producing high-affinity antibodies. The hallmark t(14;18)(q32;q21) translocation of FL occurs early in B-cell development, from an error in V(D)J recombination. Like normal naïve B cells, those carrying t(14;18) home to follicles where they are selected for entry and proliferation in germinal centers by follicular helper T cells. Here, t(14;18)-positive B cells likely have a survival advantage due to ectopic expression of BCL2. Whereas normal B cells exit the GC as mature memory B cells or plasma cells, t(14;18)-positive FL-like B cells that exit the GC can traffic and acquire the additional genetic changes necessary for developing their full malignant phenotype.
FL is characterized by a proliferation of neoplastic GC B cells, both centrocytes and centroblasts, with at least a partial follicular pattern.1 The current grading system for FL evaluates the proportion of centrocytes to centroblasts; cases with more centroblasts behave more aggressively and have a higher likelihood of transformation to diffuse large cell lymphoma. Grade 1 and grade 2 FL are defined as ≤15 centroblasts per high-powered field, whereas grade 3 FL has >15 centroblasts per high-powered field. Grade 3 FL is further classified as 3A or 3B, with the latter characterized by an absence of centrocytes. Accumulating evidence suggests that FL3B is a biologically distinct entity, with frequent absence of t(14;18) and CD10 expression and increased p53 and MUM1/IRF4 expression.2 Accordingly, a large retrospective analysis of more than 500 FL cases confirmed that the clinical course of FL3A is similar to FL1-2, whereas FL3B had a clinical course more similar to diffuse large B-cell lymphoma, with no relapses beyond 5 years.3 Consensus panels have recommended that grade 3B FL be managed like diffuse large B-cell lymphoma, whereas grade 3A is more appropriately managed by using the same paradigms applied to grade 1 and grade 2 FL.4
Using baseline tumor burden to determine risk
Differential outcome, based upon the tumor burden at treatment initiation, was initially identified by the Groupe d’Etude des Lymphomes Folliculaires (GELF).5 The GELF criteria were eventually used to categorize patients in need of immediate therapy vs those who would be candidates for a watch-and-wait strategy. GELF criteria for high tumor burden FL are defined as at least 1 of the following: 3 distinct nodal sites, each ≥3 cm; single nodal site ≥7 cm; symptomatic splenomegaly; organ compression or compromise; pleural effusions, ascites; B symptoms or any systemic symptoms; lactate dehydrogenase (LDH) or β2-microglobulin (B2M) above the upper limit of normal. Variations of the GELF criteria have subsequently emerged from the British National Lymphoma Investigation and the National Comprehensive Network. Presently, patients with high tumor burden are most often considered for immunochemotherapy-based treatment strategies, whereas patients with low tumor burden can be considered for single agent rituximab or a watch-and-wait strategy.6,7 Trials with uniform frontline treatments for patients with both high and low tumor burden demonstrate inferior outcomes in the high tumor burden subgroup, indicating this distinction continues to have prognostic value.8
The widespread adoption of 18F fluorodeoxyglucose-positron emission tomography–computed tomography (PET-CT) in the staging and response assessment of lymphoma has generated new methods of assessing tumor burden. The total metabolic tumor volume (TMTV) is an integration of metabolic update with tumor volumetric calculations. An international collaboration pooled data from 3 prospective multicenter trials and evaluated the prognostic utility of TMTV.9 Centrally reviewed PET-CT images from 185 patients receiving immunochemotherapy were available. The median TMTV was 298 cm3 and the optimal cutoff identified was 510 cm3, with 29% of patients falling in the high TMTV group. When high TMTV was compared to low TMTV, the 5-year PFS was 33% vs 65% (P < .001) and the 5-year OS was 85% vs 95% (P = .01), respectively. On multivariable analysis, the TMTV and the Follicular Lymphoma International Prognostic Index-2 (FLIPI-2) score were independently prognostic, and they could be combined to generate 3 distinct risk groups. TMTV calculations are not yet routinely available, but one can envision software advances making this calculation part of standard staging and even replacing Ann Arbor staging, which is not highly prognostic in FL.
Using clinical features to determine risk
The prognosis for an individual patient can be estimated based on clinical and laboratory findings. FLIPI was derived from a database of more than 4000 patients with FL treated largely in the pre-rituximab era.10 This index is often remembered by the acronym “No-LASH” because the 5 strongest prognostic factors in multivariate analysis were (1) number of nodal sites of disease (>4), (2) elevated LDH, (3) age >60 years, (4) stage III or IV disease, and (5) hemoglobin <12 g/dL. The FLIPI provides a roughly equal distribution of patients across low risk (0 to 1 factor), intermediate risk (2 factors), or high risk (≥3 factors) categories. The 10-year OS rates were 71% (low risk), 51% (intermediate risk), and 36% (high risk). Because the FLIPI was developed by using a retrospectively obtained data set, from patients treated in the pre-rituximab era, effort to develop a more contemporary index was initiated. This index, called FLIPI-2, was developed in the rituximab with chemotherapy era and identified age >60, elevated B2M, Hgb <12 g/dL, bone marrow involvement, and lymph node diameter >6 cm as independent risk factors for PFS.11 Each index has its strengths (Table 1). However, the FLIPI is more commonly used because it has been repeatedly validated as prognostic in clinical trial settings using rituximab-containing chemotherapy and was validated in the National LymphoCare Study (NLCS), a large observational cohort of 2200 patients.12,13
Table 1.
FLIPI and FLIPI-2
| Risk group | No. of risk factors | Outcome, % | |
|---|---|---|---|
| 5-y OS | 10-y OS | ||
| FLIPI | |||
| Low | 0-1 | 91 | 71 |
| Intermediate | 2 | 78 | 51 |
| High | 3-5 | 53 | 36 |
| 3-y PFS | 5-y PFS | ||
| FLIPI-2 | |||
| Low | 0 | 91 | 80 |
| Intermediate | 1-2 | 69 | 51 |
| High | 3-5 | 51 | 19 |
Risk factors for FLIPI are age >60 y, stage III/IV, hemoglobin <12 g/dL, LDH elevated, and >4 nodal sites; risk factors for FLIPI-2 are age >60 y, B2M elevated, hemoglobin <12 g/dL, bone marrow involvement, and lymph node diameter > 6 cm. OS, overall survival; PFS, progression-free survival.
The clinical heterogeneity of FL is substantial, and presumably is a reflection of underlying biological differences. Effort to precisely identify these biologic factors has been underway for many years and remains a challenge for the field.
Using the microenvironment to assign risk
Work performed out of the Leukemia/Lymphoma Molecular Profiling Project demonstrated the significance of the tumor microenvironment when gene expression signatures of the nonmalignant stromal cells were found to be prognostically more important than the neoplastic B cells.14 The gene expression signature associated with favorable outcomes was enriched for genes expressed by T cells, whereas the expression signature associated with less favorable outcomes was enriched for genes expressed by macrophages and follicular dendritic cells, suggesting the balance between immune surveillance and a permissive tumor microenvironment plays a role in dictating the disease course. Many subsequent immunohistochemical studies have attempted to translate these findings to the clinical laboratory by enumerating T-cell subsets and macrophages on biopsy specimens, but results have been inconsistent and have yet to engender any practice changes.15-20 Understanding and translating the complex relationship between the neoplastic cells and their tumor microenvironment to actionable prognostic assays or companion diagnostics has been unfruitful to date and remains an area of investigation.
Some evidence suggests that tonic signaling through the BCR and its downstream pathways may provide a key survival signal to FL cells. Somatic hypermutation of the BCR is capable of introducing N-glycosylation sites to the FL BCR variable regions that ultimately bear mannose-terminated glycans.21 Introduction of these mannose-terminated glycans facilitates BCR interaction with mannose-binding lectins found on dendritic cells, macrophages, and commonly occurring bacteria, thereby allowing the GC microenvironment to support malignant B-cell survival in the absence of cognate antigen.22,23 Specifically, dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) is a mannose-binding lectin, overexpressed in FL dendritic cells and TAMs, that binds FL BCR and triggers BCR signaling. DC-SIGN–mediated FL BCR signaling can be attenuated by BCR pathway inhibitors and reduces the viability of FL cells in vitro, illuminating the therapeutic potential of targeting the FL microenvironment.24,25
Using mutational analysis to determine risk
The mutational landscape of FL is dominated by 2 recurrent alterations: (1) the t(14;18) translocation and (2) inactivating mutations of the KMT2D gene. The t(14;18) translocation is found in 85% of FL and places the BCL2 gene under the IGH regulatory elements. Dysregulation of BCL2 expression alone is not sufficient to induce lymphomagenesis, but it provides a survival advantage through activation of anti-apoptotic programs that are typically repressed by BCL6 in GC B cells. Inactivating mutations of KMT2D (MLL2) are found in >80% of FL and interfere with the ability of KMT2D to activate gene transcription through histone methylation.26 Like t(14;18), KMT2D inactivation appears to be an early event in FL, suggesting that epigenetic dysregulation combined with dysregulated BCL2 may drive malignant transformation of GC B cells.27 Mutations of histone modifiers CREBBP, EZH2, MEF2B, and EP300 are found in ∼33%, 27%, 15%, and 9% of FL, respectively.26-29
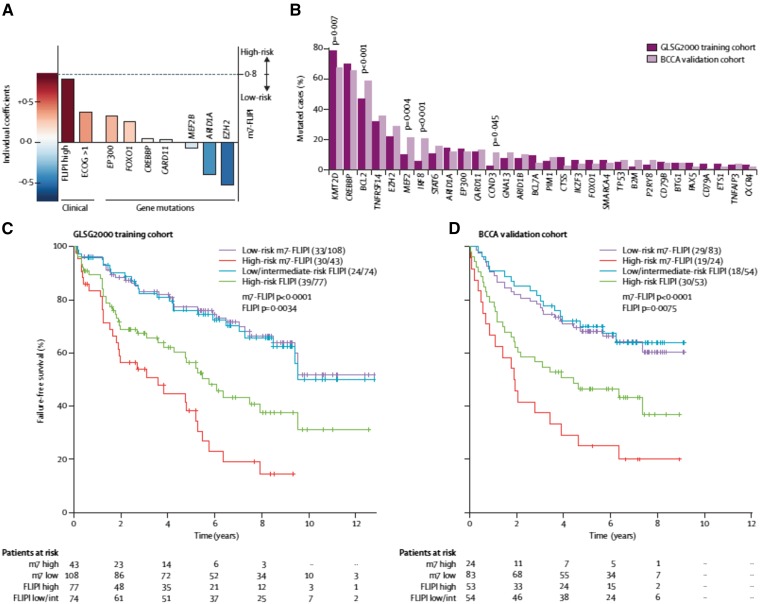
An international, multigroup effort analyzed the mutation status of 74 genes in 151 FL biopsy specimens obtained from patients who received rituximab-cyclophosphamide-adriamycin-vincristine-prednisone (R-CHOP) therapy on the GLSG2000 protocol.30 The analysis produced a clinicogenetic risk model that integrates the mutational status of 7 genes (EZH2, ARID1A, MEF2B, EP300, FOX01, CREBBP, and CARD11) with the FLIPI (termed m7-FLIPI). A validation set, using a population-based cohort of 107 patients, confirmed the prognostic value of the index. The m7-FLIPI identified a high-risk group of patients (28% of the training cohort and 22% of the validation cohort) with a 5-year failure-free survival of 29% and 25%, respectively. In contrast, the 5-year failure-free survival of the high-risk cohort identified by FLIPI alone was 46%, indicating the superiority of the m7-FLIPI for identifying a high-risk population (Figure 1). No individual gene mutation was as predictive as having a high-risk FLIPI score or a poor ECOG performance status, suggesting a constellation of genetic events is required to have a profound clinical impact in FL.
Figure 1.
(A) Individual coefficient of risk for high-risk FLIPI; ECOG performance status; and mutations in EP300, FOX01, CREBBP, CARD11, MEF2B, ARID1A, and EZH2. Mutations in MEF2B, ARID1A, and EZH2 are all “favorable” findings. (B) Mutational frequency of the various genes in the training set and in the validation cohort. (C) Failure-free survival by FLIPI risk and by m7-FLIPI risk in the training cohort. (D) Failure-free survival by FLIPI risk and by m7-FLIPI risk in the validation cohort.
A separate analysis assessed the mutation status of 1716 genes from 105 patients with FL.31 These investigators demonstrated that histone gene mutations often cooccur in patients with FL and observed a higher frequency of histone mutations than was previously reported. They also observed mutations affecting BCR and CXCR signaling pathways at a frequency higher than previously reported. These observations may have therapeutic implications for selection of targeted agents in FL. For example, the observation that EZH2 is recurrently mutated in FL has led to the evaluation of the EZH2 inhibitor tazemetostat in patients with recurrent FL. In a preliminary analysis, 12 of 13 (92%) patients with EZH2 mutations responded to therapy, whereas only 14 of 54 (26%) of patients without mutations responded.32
Using response to therapy to determine risk
It is intuitive that a patient’s response to therapy would be a strong predictor of PFS and likely OS. The discriminatory ability of an end-of-treatment PET-CT scan was assessed in a pooled analysis of 3 large multicenter clinical trials.33 In this analysis, 246 patients treated with rituximab with chemotherapy had centrally reviewed post-induction imaging, scored by 3 investigators using the 5-point scale. Scores of 4 or 5 were considered “positive,” and 17% of patients were considered positive by these criteria. The 4-year PFS was 23% for patients with a positive scan vs 63% for those with a negative scan (P < .0001), whereas the 4-year OS was 87% vs 97% (P < .0001). These results indicate that inability to achieve a complete response to frontline immunochemotherapy is a strong adverse prognostic marker.
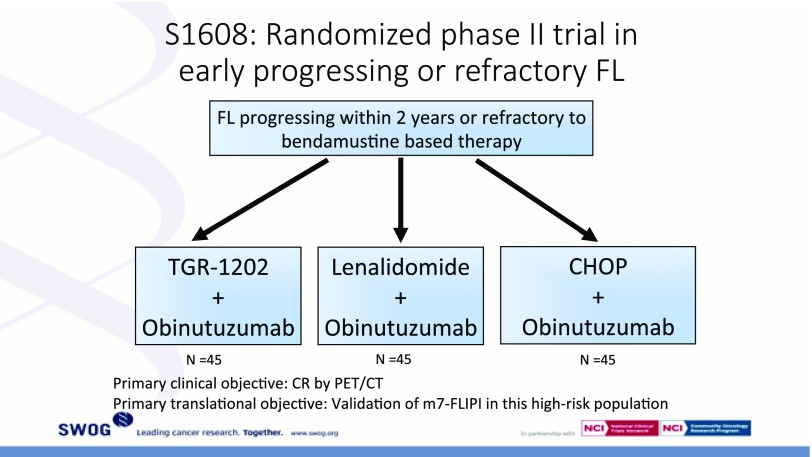
Perhaps the strongest predictor of long-term outcomes is the length of first remission after a standard immunochemotherapy induction. An analysis from the NLCS examined outcomes in 588 patients receiving R-CHOP as initial FL therapy.34 Approximately 20% of patients experienced progressive disease (PD) within 2 years of diagnosis. The 5-year OS was 50% in the early PD group compared with 90% in patients without early PD. This observation was confirmed in an analysis from the Iowa/Mayo Molecular Epidemiology Resource (MER) and validated using databases from Lyon, France.35 Early relapse was associated with a markedly increased risk of death, similar to that observed in the LymphoCare analysis, whereas patients with FL who did not experience early relapse had OS that was similar to age-matched controls without a lymphoma diagnosis. Neither the FLIPI, nor the FLIPI-2, nor the m7-FLIPI is able to identify a group of patients at such a high risk for early death. The optimal management strategy for these patients is unclear, and clinical trials are needed specifically for this patient population. The US intergroup has designed such a trial, S1608 (Figure 2). In addition to the therapeutic interventions being tested, tissue from the original diagnostic biopsy will be analyzed as part of the ongoing effort to identify biomarkers capable of identifying patients with POD24 earlier in their disease course.
Figure 2.
The schema of S1608 is depicted. Patients with POD24 after bendamustine-rituximab induction therapy will be randomized to either the phosphatidylinositol 3-kinase inhibitor TG1202 plus obinutuzumab (Arm A), lenalidomide-obinutuzumab (Arm B), or R-CHOP (Arm C).
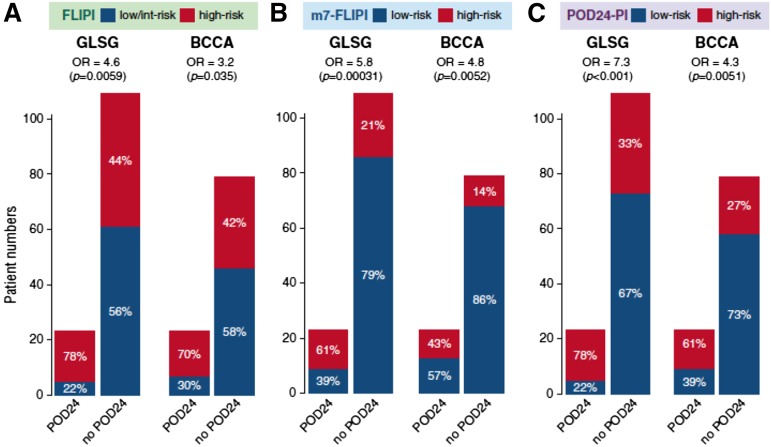
The current challenge is to identify this very-high-risk group of patients at diagnosis rather than waiting for early relapse to define them. Using the data set used to develop the m7-FLIPI, a multinational group of investigators developed a clinicogenetic risk model to predict early progression of FL after first-line immunochemotherapy.36 These investigators confirmed that POD24, which occurred in ∼20% of patients, is an accurate predictor of poor OS. The 5-year OS was 41% in patients with POD24, compared with 91% in patients without POD24. Results were similar in the validation cohort. High-risk FLIPI status correctly identified 78% of patients destined to have POD24 but also misclassified 44% of the patients without POD24. High risk by m7-FLIPI correctly identified only 61% of the patients with POD24, but was better at avoiding “false positives,” misclassifying only 21% of the patients without POD24. The investigators built a new clinicogenomic risk model called the POD24 prognostic index (POD24-PI). The POD24-PI was better than the m7-FLIPI at identifying patients with POD24, but worse than the m7-FLIPI at identifying patients without POD24. In summary, both the m7-FLIPI and the POD24-PI could identify patients likely to be in the POD24 category and likely to be in the not-POD24 category (Figure 3). However, the positive predictive value and negative predictive value will need to improve before clinicians would be comfortable making treatment allocations based upon these risk scores.
Figure 3.
The accuracy of 3 pretreatment risk models to predict POD24 status is represented in bar graphs. (A) FLIPI. (B) m7-FLIPI. (C) POD24 prognostic index (POD24-PI).
Using risk of histologic transformation to determine risk
The FL disease course, which can spontaneously remit, even in the absence of treatment, may be best modeled by the idea of a dominant clone that fluctuates under the selective pressure of inherent mutations and the associated microenvironment.37 The tracking of multiple clones in patients shows that disease progression occurs either by direct clonal evolution or by divergent evolution from a common progenitor cell. Similarly, transformation to an aggressive B-cell lymphoma can also occur by either of these routes.38,39 Although no cytogenetic or molecular biomarkers are in routine use to assess a patient’s risk for transformation, mutations in the neoplastic cells including upregulation of MYC expression and TP53 mutations and expression of IRF4, as well as changes in the tumor microenvironment, have been associated with transformation.39-41
A recent report from the NLCS evaluated outcomes in 2652 patients and found the risk of transformation remains 2% to 3% per year in the R-chemo era.42 The risk was similar in R-CHOP– and R-CVP–treated patients, suggesting no risk reduction with the up-front inclusion of anthracyclines. However, the risk was reduced in patients receiving maintenance rituximab (HR, 0.67; 95% CI, 0.46-0.97). Of particular note, the median OS after transformation was 5 years, which was markedly better than historical reports. Work from the Iowa/Mayo Clinic Specialized Program of Research Excellence (SPORE) also found outcomes after transformation were substantially better than historical data indicated.43 Additionally, their data suggested initial treatment with rituximab or rituximab containing chemotherapy reduced the risk of transformation. These provocative observations will require confirmation from other databases.
Summary
Many measures, perhaps too many, can be applied to a patient with FL to estimate risk. Finding a measure that is clinically useful is the challenge. The outstanding outcomes in FL make this exercise more difficult than it is in other cancers. Despite the excellent outcomes for the group as a whole, some individual patients do not enjoy such a good prognosis. The observation from the NLCS that POD24 identifies 20% of patients with 5-year OS of <50% is powerful. Furthermore, the observation from the Iowa/Mayo MER database that the 80% of patients who do not have POD24 have OS similar to age-matched controls without lymphoma is equally powerful. If accurate, one could argue that 80% of the population with FL does not have an unmet need, and our efforts should focus on the 20% who do. The problem is that response to therapy is the only tool currently available for reliable identification of this very-high-risk group. Future research should (1) expand efforts to identify prognostic biomarkers capable of identifying high-risk patients at diagnosis, (2) continue to develop targeted agents used in association with predictive biomarkers, and (3) test interventions designed to reduce the risk for histologic transformation. Achievement of these goals would facilitate a more personalized approach to the management of FL.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. . The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horn H, Schmelter C, Leich E, et al. . Follicular lymphoma grade 3B is a distinct neoplasm according to cytogenetic and immunohistochemical profiles. Haematologica. 2011;96(9):1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahlin BE, Yri OE, Kimby E, et al. . Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156(2):225-233. [DOI] [PubMed] [Google Scholar]
- 4.Zelenetz AD, Abramson JS, Advani RH, et al. . NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin’s lymphomas. J Natl Compr Canc Netw. 2010;8(3):288-334. [DOI] [PubMed] [Google Scholar]
- 5.Brice P, Bastion Y, Lepage E, et al. . Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: a randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 1997;15(3):1110-1117. [DOI] [PubMed] [Google Scholar]
- 6.Ardeshna KM, Qian W, Smith P, et al. . Rituximab versus a watch-and-wait approach in patients with advanced-stage, asymptomatic, non-bulky follicular lymphoma: an open-label randomised phase 3 trial. Lancet Oncol. 2014;15(4):424-435. [DOI] [PubMed] [Google Scholar]
- 7.Kahl BS, Hong F, Williams ME, et al. . Rituximab extended schedule or re-treatment trial for low-tumor burden follicular lymphoma: eastern cooperative oncology group protocol e4402. J Clin Oncol. 2014;32(28):3096-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barta SK, Li H, Hochster HS, et al. . Randomized phase 3 study in low-grade lymphoma comparing maintenance anti-CD20 antibody with observation after induction therapy: A trial of the ECOG-ACRIN Cancer Research Group (E1496). Cancer. 2016;122(19):2996-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meignan M, Cottereau AS, Versari A, et al. . Baseline metabolic tumor volume predicts outcome in high-tumor-burden follicular lymphoma: a pooled analysis of three multicenter studies. J Clin Oncol. 2016;JCO669440. [DOI] [PubMed] [Google Scholar]
- 10.Solal-Céligny P, Roy P, Colombat P, et al. . Follicular lymphoma international prognostic index. Blood. 2004;104(5):1258-1265. [DOI] [PubMed] [Google Scholar]
- 11.Federico M, Bellei M, Marcheselli L, et al. . Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555-4562. [DOI] [PubMed] [Google Scholar]
- 12.Buske C, Hoster E, Dreyling M, Hasford J, Unterhalt M, Hiddemann W. The Follicular Lymphoma International Prognostic Index (FLIPI) separates high-risk from intermediate- or low-risk patients with advanced-stage follicular lymphoma treated front-line with rituximab and the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) with respect to treatment outcome. Blood. 2006;108(5):1504-1508. [DOI] [PubMed] [Google Scholar]
- 13.Nooka AK, Nabhan C, Zhou X, et al. Examination of the follicular lymphoma international prognostic index (FLIPI) in the National LymphoCare study (NLCS): a prospective US patient cohort treated predominantly in community practices. Ann Oncol 2013;24:441-448. [DOI] [PubMed]
- 14.Dave SS, Wright G, Tan B, et al. . Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21):2159-2169. [DOI] [PubMed] [Google Scholar]
- 15.Canioni D, Salles G, Mounier N, et al. . High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26(3):440-446. [DOI] [PubMed] [Google Scholar]
- 16.Carreras J, Lopez-Guillermo A, Fox BC, et al. . High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108(9):2957-2964. [DOI] [PubMed] [Google Scholar]
- 17.Farinha P, Masoudi H, Skinnider BF, et al. . Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL). Blood. 2005;106(6):2169-2174. [DOI] [PubMed] [Google Scholar]
- 18.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115(2):289-295. [DOI] [PubMed] [Google Scholar]
- 19.Glas AM, Knoops L, Delahaye L, et al. . Gene-expression and immunohistochemical study of specific T-cell subsets and accessory cell types in the transformation and prognosis of follicular lymphoma. J Clin Oncol. 2007;25(4):390-398. [DOI] [PubMed] [Google Scholar]
- 20.Taskinen M, Karjalainen-Lindsberg ML, Nyman H, Eerola LM, Leppä S. A high tumor-associated macrophage content predicts favorable outcome in follicular lymphoma patients treated with rituximab and cyclophosphamide-doxorubicin-vincristine-prednisone. Clin Cancer Res. 2007;13(19):5784-5789. [DOI] [PubMed] [Google Scholar]
- 21.Zhu D, McCarthy H, Ottensmeier CH, Johnson P, Hamblin TJ, Stevenson FK. Acquisition of potential N-glycosylation sites in the immunoglobulin variable region by somatic mutation is a distinctive feature of follicular lymphoma. Blood. 2002;99(7):2562-2568. [DOI] [PubMed] [Google Scholar]
- 22.Coelho V, Krysov S, Ghaemmaghami AM, et al. . Glycosylation of surface Ig creates a functional bridge between human follicular lymphoma and microenvironmental lectins. Proc Natl Acad Sci USA. 2010;107(43):18587-18592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider D, Dühren-von Minden M, Alkhatib A, et al. . Lectins from opportunistic bacteria interact with acquired variable-region glycans of surface immunoglobulin in follicular lymphoma. Blood. 2015;125(21):3287-3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amin R, Mourcin F, Uhel F, et al. . DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood. 2015;126(16):1911-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linley A, Krysov S, Ponzoni M, Johnson PW, Packham G, Stevenson FK. Lectin binding to surface Ig variable regions provides a universal persistent activating signal for follicular lymphoma cells. Blood. 2015;126(16):1902-1910. [DOI] [PubMed] [Google Scholar]
- 26.Morin RD, Mendez-Lago M, Mungall AJ, et al. . Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476(7360):298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasqualucci L, Dominguez-Sola D, Chiarenza A, et al. . Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471(7337):189-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bödör C, Grossmann V, Popov N, et al. . EZH2 mutations are frequent and represent an early event in follicular lymphoma. Blood. 2013;122(18):3165-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okosun J, Bödör C, Wang J, et al. . Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet. 2014;46(2):176-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pastore A, Jurinovic V, Kridel R, et al. . Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16(9):1111-1122. [DOI] [PubMed] [Google Scholar]
- 31.Krysiak K, Gomez F, White BS, et al. . Recurrent somatic mutations affecting B-cell receptor signaling pathway genes in follicular lymphoma. Blood. 2017;129(4):473-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morschhauser F, Salles G, Mckay H, et al. . Interim Report from a phase 2 multicenter study off tazemetostat, an ezh2 inhibitor, in patients wiht relapsed or refractory follicular lymphoma. Hematol Oncol. 2017;35(S2):24-25. [Google Scholar]
- 33.Trotman J, Luminari S, Boussetta S, et al. . Prognostic value of PET-CT after first-line therapy in patients with follicular lymphoma: a pooled analysis of central scan review in three multicentre studies. Lancet Haematol. 2014;1(1):e17-e27. [DOI] [PubMed] [Google Scholar]
- 34.Casulo C, Byrtek M, Dawson KL, et al. . Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the national lymphocare study. J Clin Oncol. 2015;33(23):2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maurer MJ, Bachy E, Ghesquières H, et al. . Early event status informs subsequent outcome in newly diagnosed follicular lymphoma. Am J Hematol. 2016;91(11):1096-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurinovic V, Kridel R, Staiger AM, et al. . Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood. 2016;128(8):1112-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J Clin Invest. 2012;122(10):3424-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlotti E, Wrench D, Matthews J, et al. . Transformation of follicular lymphoma to diffuse large B-cell lymphoma may occur by divergent evolution from a common progenitor cell or by direct evolution from the follicular lymphoma clone. Blood. 2009;113(15):3553-3557. [DOI] [PubMed] [Google Scholar]
- 39.Kridel R, Mottok A, Farinha P, et al. . Cell of origin of transformed follicular lymphoma. Blood. 2015;126(18):2118-2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kridel R, Sehn LH, Gascoyne RD. Can histologic transformation of follicular lymphoma be predicted and prevented? Blood. 2017;130(3):258-266. [DOI] [PubMed] [Google Scholar]
- 41.Lossos IS, Alizadeh AA, Diehn M, et al. . Transformation of follicular lymphoma to diffuse large-cell lymphoma: alternative patterns with increased or decreased expression of c-myc and its regulated genes. Proc Natl Acad Sci USA. 2002;99(13):8886-8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner-Johnston ND, Link BK, Byrtek M, et al. . Outcomes of transformed follicular lymphoma in the modern era: a report from the National LymphoCare Study (NLCS). Blood. 2015;126(7):851-857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Link BK, Maurer MJ, Nowakowski GS, et al. . Rates and outcomes of follicular lymphoma transformation in the immunochemotherapy era: a report from the University of Iowa/MayoClinic Specialized Program of Research Excellence Molecular Epidemiology Resource. J Clin Oncol. 2013;31(26):3272-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]