Abstract
Graft-versus-tumor (GVT) reactivity mediated by donor T cells in the context of allogeneic stem cell transplantation (alloSCT) is one of the most potent forms of cellular immunotherapy. The antitumor effect against hematologic malignancies is mediated by a polyclonal T-cell response targeting polymorphic antigens expressed on hematopoietic tissues of the recipient, leaving donor hematopoiesis in the patient after transplantation unharmed. Fortunately, hematopoietic tissues (including malignant hematopoietic cell populations) are relatively susceptible to T-cell recognition. If, however, nonhematopoietic tissues of the recipient are targeted as well, graft-versus-host disease (GVHD) will occur. The balance between GVT and GVHD is influenced by the genetic disparity between donor and recipient, the number and origin of professional antigen-presenting cells provoking the immune response, the target antigen specificity, magnitude and diversity of the response, and the in vivo inflammatory environment, whereas inhibitory factors may silence the immune response. Manipulation of each of these factors will determine the balance between GVT and GVHD.
Learning Objectives
Understanding the immunobiology of alloimmune responses as a curative modality of allogeneic stem cell transplantation
Understanding factors determining success or failure of donor T cell–mediated antitumor responses after allogeneic stem cell transplantation
Introduction
The main advantage of allogeneic hematopoietic stem cell transplantation (alloSCT) over high-dose chemotherapy or autologous stem cell transplantation for the treatment of hematologic malignancies is the profound therapeutic effect of the alloimmune response mediated by donor T cells, resulting in eradication or persistent control of the malignant cell population.1 However, alloimmune responses are also responsible for the development of graft-versus-host disease (GVHD), the main complication after alloSCT. Although the immunobiologic principles of development of GVHD and graft-versus-tumor (GVT) responses are highly similar, there may be several ways to clinically separate GVT from GVHD.2 The present article discusses similarities and differences of induction and execution of these alloimmune responses, and how the balance between GVT and GVHD may be influenced.
Beneficial and detrimental alloreactivity
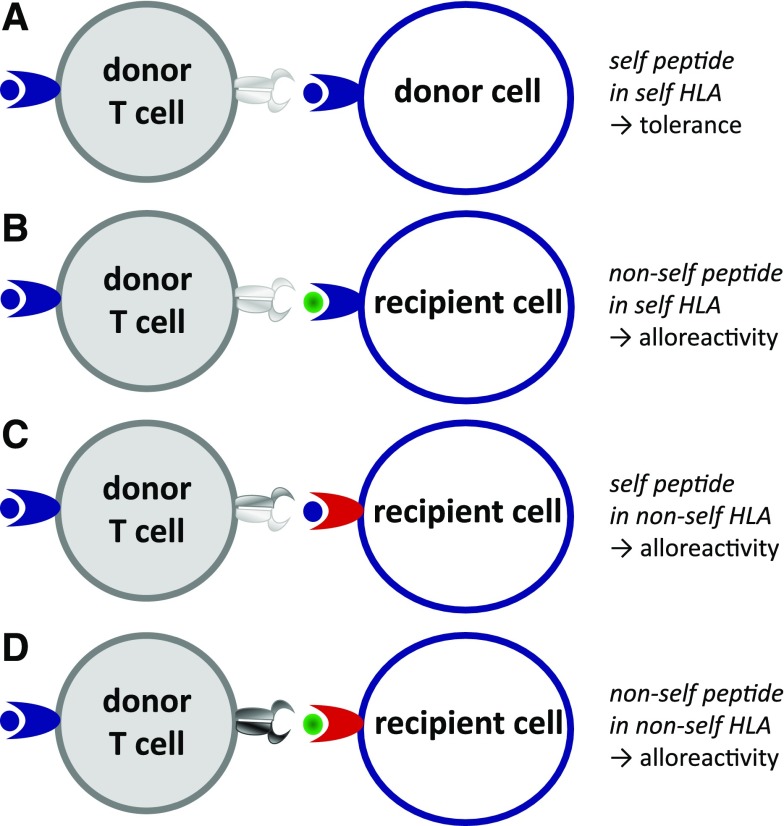
The mechanism by which donor T cells execute their alloreactivity is in essence not different from their normal function: the control of viral infections by attacking virally infected cells. Any nonself (viral or other) peptide that is presented in the context of (self) HLA molecules can be recognized by the hugely diverse repertoire of T cells. To prevent the development of autoimmune reactivities, during thymic selection, all T cells are deleted from the repertoire that are capable of recognizing “self” peptides in the context of self HLA molecules.3 The residual T-cell repertoire therefore consists of T cells that are capable of recognizing any combination of a peptide presented in an HLA molecule that is different from the self peptide/self HLA combination. Because the HLA complex is highly polymorphic and different between individuals, T cells have many possibilities to choose from to create alloreactivity. These possibilities include nonself (polymorphic) peptides presented in self HLA molecules, any peptide presented in an HLA molecule that is different from the autologous HLA, and nonself peptides presented in nonself HLA molecules. These possibilities result in a highly diverse alloreactive T-cell repertoire (Figure 1).
Figure 1.
Peptide/HLA complexes as targets of T cell–mediated alloimmune reactivity. (A) Self peptides expressed in self HLA molecules induce tolerance. (B) Polymorphic nonself peptides recognized in the context of self HLA molecules can induce alloreactivity; these polymorphic nonself peptides are called minor histocompatibility antigens (MiHA). (C) Monomorphic (self) peptides presented in the context of nonself HLA molecules provoke alloreactivity. (D) Polymorphic nonself peptides in the context of nonself HLA molecules can also provoke alloreactivity.
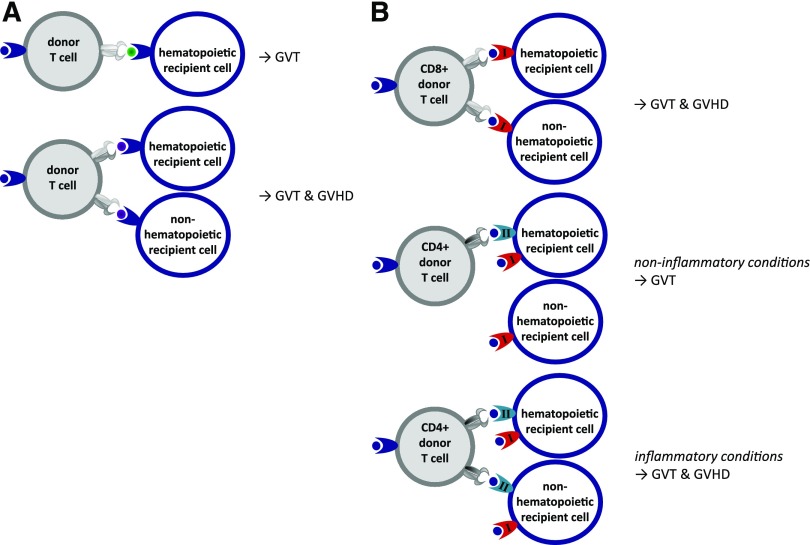
After fully HLA matched alloSCT, the only possibility for the development of alloreactivity mediated by T cells is the recognition of nonself peptides in the context of self HLA because the HLA alleles of the donor and recipient are identical. Because many genes are polymorphic and contain single nucleotide polymorphisms, proteins will often contain small amino acid differences. HLA molecules present a large representation of peptides derived from most proteins present in the cell, and therefore HLA molecules present many peptides that contain small amino acid variations which differ between individuals. Indeed, analysis of the peptidome eluted from HLA molecules has revealed that ∼10% of all peptides presented in HLA molecules differ between individuals due to genetic polymorphisms.4 Polymorphic peptides presented in the context of HLA molecules that are capable of inducing an immune response between HLA identical individuals are called minor histocompatibility antigens (MiHAs).5 Due to the high genetic diversity, the T-cell repertoire from an HLA identical donor will contain hundreds of different T cells capable of recognizing a polymorphic peptide presented in HLA molecules on tissues from the recipient. The clinical effect of the alloimmune T-cell response will be the result of the tissue expression of the peptide/HLA complexes and the magnitude of the T-cell response. If polymorphic peptides presented on hematopoietic cells of recipient origin are being attacked, the hematopoietic system from the recipient, including the malignant cells, is eliminated by donor T cells. Because, after transplantation, normal hematopoiesis is of donor origin, a T-cell response against polymorphic antigens expressed on hematopoietic cells from the recipient will lead to a profound GVT response, without damaging normal hematopoiesis in the recipient (Figure 2). This antirecipient hematopoietic tissue–directed T-cell response is the main beneficial therapeutic effect of alloSCT. The likelihood that donor T cells recognize a polymorphic antigen on recipient cells greatly exceeds the chance that donor T cells will recognize tumor-specific or tumor-associated overexpressed antigens that may play a role in autologous antitumor reactivity. If a donor T-cell response targets polymorphic peptides that are expressed on nonhematopoietic tissues from the recipient, which obviously cannot be replaced by donor tissue, GVHD will occur. Selective GVT will only occur if the alloimmune T-cell response is mainly or more specifically directed against the hematopoietic system of the recipient.
Figure 2.
GVT and GVHD targets in alloreactivity. (A) Donor and recipient are fully HLA identical. Polymorphic nonself peptides (MiHA) selectively expressed on hematopoietic cells provoke hematopoiesis-specific GVT responses. Polymorphic nonself peptides (MiHA) derived from broadly expressed genes can provoke combined GVT and GVHD. (B) CD8 T cells recognizing peptides presented in the context of nonself HLA class I molecules can mediate combined GVT and GVHD reactivity. CD4 T cells recognizing peptides in the context of HLA class II molecules mediate specific GVT responses under noninflammatory circumstances when HLA class II molecules are not expressed on nonhematopoietic tissues. Under inflammatory circumstances, upregulation of HLA class II on nonhematopoietic tissues causes combined GVT and GVHD by alloreactive CD4 T cells.
After (partially) HLA mismatched transplantation, the potential alloreactive donor T-cell repertoire capable of recognizing recipient-derived tissues is of even higher magnitude. Any peptide, regardless of whether it is polymorphic, that is presented in an HLA allele which is different between donor and recipient may theoretically provoke an alloimmune response.6 Because this action leads to a high diversity and magnitude of the donor antirecipient T-cell response, after HLA mismatched transplantation, the likelihood of a profound combined T cell–mediated GVT and GVHD response will be high if no measures of controlling the T-cell response are taken.
Induction of alloreactivity post-alloSCT
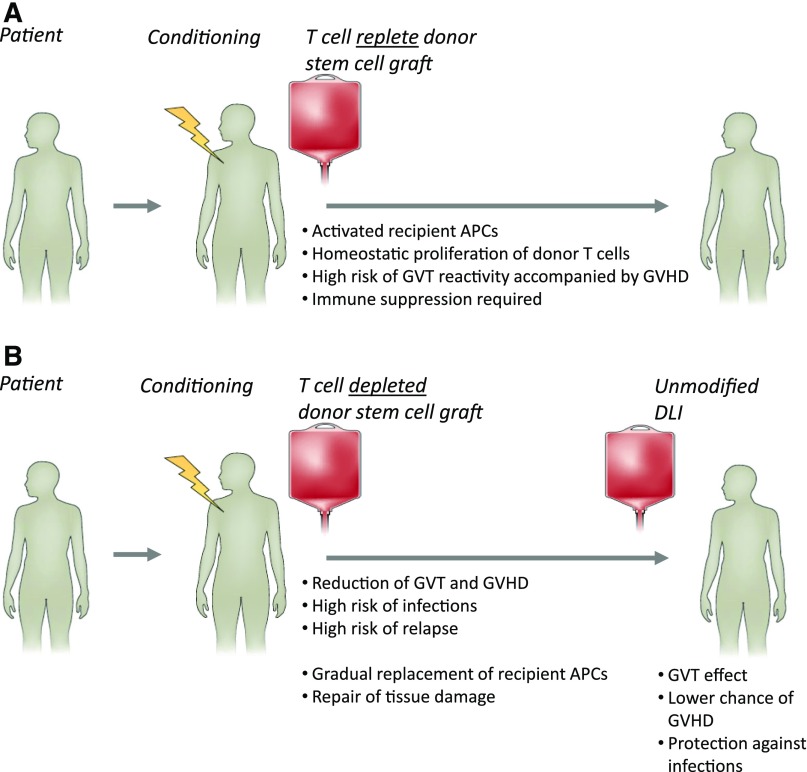
Naive T cells are likely to require stimulation by activated professional antigen-presenting cells (APC) such as maturated dendritic cells (DC) to be appropriately activated, allowing execution of their full function leading to proliferation and cytotoxic activity against the targeted cell population.7 In contrast, memory T cells that have been activated in the past will need to overcome only a low threshold of activation to perform their function. Within the T-cell repertoire of healthy individuals, the memory T-cell repertoire will mainly contain pathogen-specific T cells that have been triggered in the past by pathogen-specific peptides presented in the context of self HLA molecules expressed by infected activated APC. Only in cases of previous transfusions or pregnancies will donor T cells have the likelihood of being previously exposed to polymorphic peptides from other individuals. Therefore, in the context of HLA identical alloSCT, donor T cells capable of recognizing polymorphic peptides presented by recipient tissues will mainly be present in the naive donor T-cell repertoire.8 These donor T cells require the presence of activated patient-derived DC in an inflammatory environment to maximally execute their potential to recognize multiple MiHA presented on recipient cells.9 If donor T cells are infused together with the stem cell graft, MiHA-specific T cells will find optimal circumstances for activation. The professional APC will be of recipient origin and are likely to be activated due to the conditioning regimen, pathogens, and other danger signals. Target tissues of GVHD such as gut, skin, liver, and lung may contain large numbers of activated recipient APC and present a large variety of MiHA expressed in these tissues, resulting in the development of GVHD. Homeostatic proliferation of donor T cells caused by the lymphopenia created by the conditioning regimen will further promote the immune response. Because professional APC such as DC are derived from the hematopoietic system, their HLA peptidome will contain a large variety of MiHA that are coexpressed on many hematopoietic cells, and T-cell responses directed against these APC will include many specificities that will target recipient hematopoietic cells, resulting in GVT reactivity. Nonspecific reduction of the total number of T cells by T-cell depletion of the graft or profound immune suppression will reduce both the beneficial and the detrimental alloimmune response (Figure 3).
Figure 3.
Balance between GVT and GVHD after T cell–replete or T cell–depleted transplantation. (A) T cell–replete alloSCT: conditioning regimen induced tissue damage and pathogens mediate activation of recipient APC, lymphopenia-associated homeostatic proliferation, and an inflammatory cytokine milieu resulting in highly diverse and high-magnitude alloimmune T-cell responses causing simultaneous GVT and GVHD reactivity, requiring posttransplant immune suppression. (B) T-cell depletion abrogates GVT and GVHD early after transplantation, resulting in an increased risk of relapse and lack of control of pathogens. Postponed donor T-cell infusions (donor lymphocyte infusion [DLI]) are needed for GVT reactivity: the risk of GVHD is lower due to tissue repair, less inflammation, and replacement of recipient APC by donor APC.
After alloSCT, the in vivo circumstances will change, and this action will influence the development of the alloimmune response. Because professional APC are derived from the hematopoietic system, after alloSCT, recipient-derived DC are gradually replaced by donor DC generated from the transplanted donor stem cells.10 Tissue damage will gradually be repaired, and recurrence of lymphocytes will result in lower homeostatic proliferation. Thus, if no major complications occur resulting in profound inflammation or tissue damage after alloSCT, the circumstances for MiHA-specific T cells to be activated will gradually diminish. APC are cells of hematopoietic origin, and it is therefore likely that the specificity of donor T cells recognizing hematopoiesis-associated MiHA will be the longest preserved. This scenario is consistent with observations that profound in vivo T-cell depletion at the time of transplant or T-cell depletion of the graft followed by postponed scheduled DLI can abrogate the initial alloimmune response and reinstall GVT activity with limited GVHD if there is a sufficient time interval between transplantation and DLI.11,12 It is also consistent with the observations that higher doses of DLI can be administered with more limited risk of GVHD if the time interval between DLI and alloSCT increases. Obviously, increasing the time interval between transplantation and DLI will increase the risk of relapse within this interval. Furthermore, if the time interval becomes too long, all recipient-derived APC may be replaced by donor APC. If DLI is then administered in the absence of a danger signal, no alloreactive T-cell response may occur. Relapsed hematologic malignancies that are poor APC by themselves may not respond to DLI under these circumstances.13 Thus, the balance between GVT and GVHD can be influenced by specific timing of the infusion of stem cells of donor origin, resulting in hematopoiesis of donor origin in the patient and the administration of donor T cells necessary for execution of the antirecipient hematopoiesis-restricted immunity.
Following (partially) HLA mismatched (including haploidentical) alloSCT, the alloreactive T-cell repertoire will not only be present within the naive T-cell repertoire but also within the memory donor T-cell compartment.14 The memory T-cell repertoire contains at least in part T cells that can cross-recognize peptides/nonself–HLA complexes. Any T cell that is meant to recognize nonself peptides in the context of self HLA may by chance be cross-reactive with a peptide presented in the context of nonself HLA because during thymic selection, T cells will never have been exposed to this combination, which would have caused deletion of this repertoire.15 Therefore, pathogen-specific T cells present in the memory T-cell compartment and capable of recognizing viral peptides in the context of self HLA, may also be cross-reactive against alloHLA alleles. These memory T cells do not need professional APC to be activated and execute their function. Therefore, after HLA mismatched alloSCT, T cells from the memory repertoire have a higher likelihood of inducing both GVHD and GVT, and postponed administration of DLI after HLA mismatched alloSCT will also have a higher likelihood of resulting in GVHD due to the fact that they do not require hematopoiesis-derived APC to be activated. Profound T-cell depletion is therefore likely to be essential to prevent GVHD after haploidentical transplantation, which may be performed by in vitro selection of CD34-positive stem cells or by posttransplant depletion of alloreactive T cells by the administration of high-dose cyclophosphamide early after transplantation. Under these circumstances, the GVT effect seems to be mainly dependent on the ability of donor natural killer cells to eliminate HLA and killer immunoglobulin–like receptor ligand mismatched leukemic cells. Only in cases in which the alloHLA repertoire is (almost) exclusively present within the naive T-cell repertoire, which is the case when very young donors or umbilical cord blood cells are used, the initiation of alloimmune T-cell responses from HLA mismatched donors may follow similar rules as after HLA identical transplantation.
Balance between GVT and GVHD
The balance between GVT and GVHD depends on multiple factors. Obviously, if an immune response occurs, with donor T cells only recognizing polymorphic antigens selectively expressed on hematopoietic cells of the recipient, GVT will occur in the absence of severe GVHD. However, induction of such a targeted in vivo immune response may be difficult to achieve. Several approaches have been explored to obtain a hematopoiesis-specific T-cell response, including in vitro selection of T-cell clones and lines that only recognize recipient cells of hematopoietic origin.16,17 Although in individual cases, this goal may have been achieved, thus far most in vitro procedures have been very labor intensive and not always highly reproducible, and only anecdotal positive clinical results have been reported. In addition, attempts to boost the hematopoiesis-restricted immune response in vivo by activating the immune system using in vitro cultured DC-expressing hematopoiesis-associated MiHA have not resulted in significant clinical responses.18
Factors other than the antigen specificity of the T-cell response contribute to the tissue specificity. We recently evaluated the development of alloimmune responses in a clinically defined model. After T cell–depleted alloSCT without posttransplant immune suppression, the effects of preemptive DLI were investigated according to an in-depth comparative analysis of in vivo immune responses in patients who developed GVT reactivity in the presence or absence of GVHD.19 We illustrated that antigen specificity was only in part responsible for this difference. More significantly, the magnitude and diversity of the T-cell response recognizing a variety of MiHA determined whether GVHD developed. In the absence of an inflammatory environment, T cells potentially capable of recognizing nonhematopoietic tissues frequently did not exert their action. This situation is at least in part due to the lack of expression of appropriate adhesion molecules and relatively low expression of HLA molecules in these GVHD target tissues under steady-state conditions.20 Only if the combination of a high magnitude and diversity of the immune response with appropriate target antigen specificity occurs will the threshold of activation leading to severe GVHD be overcome. In contrast, many cells from the hematopoietic system, including hematologic malignancies such as myeloid leukemia cells and some of the B-cell malignancies, may express sufficient costimulatory and adhesion molecules, as well as HLA class I and HLA class II expression, to allow targeting under more restricted circumstances resulting in GVT reactivity.21
The relatively selected expression of HLA class II molecules on hematopoietic cells may be an alternative way to direct the alloimmune response toward specific GVT reactivity. Under noninflammatory circumstances, HLA class II molecules, which are targeted by CD4 T cells, are relatively highly expressed on cells of hematopoietic origin, including the malignant counterpart. Based on these findings, administration of purified CD4 T cells instead of unmanipulated DLI is being explored as a strategy to target recipient hematopoiesis in the absence of GVHD. This approach may be of special interest when HLA “matched” unrelated donors are used for transplantation. During donor selection, usually only HLA-ABC, HLA-DR, and HLA-DQ are taken into account in HLA matching but not HLA-DP. This method results in frequencies of 80% of donor-recipient pairs that have a disparity for 1 or 2 HLA-DP alleles. This disparity allows evaluation of specific targeting of allo HLA-DP after alloSCT and (CD4 purified) DLI. Several clinical studies have shown that clinical outcome of alloSCT and DLI is influenced by the disparity of HLA-DP between donor and recipient, and it depends in part on the immunogenicity of the mismatched HLA-DP allele for the donor T-cell repertoire.22,23 Administration of CD4 T cells specific for allo HLA-DP alleles may result in a specific GVT response, as illustrated both in preclinical models and in patients treated with CD4 T cells from HLA-DP–disparate donors.24,25 However, we also illustrated that administration of CD4 T cells reactive against allo HLA-DP may result in significant GVHD under inflammatory circumstances (eg, during viral infections) caused by upregulation of HLA class II on nonhematopoietic tissues.26 These results again illustrate that not only the specificity of the T-cell response but also the clinical circumstances (in particular inflammation) determine the balance between GVT and GVHD.9
Reduction of GVHD while preserving GVT
Several strategies have been explored to reduce GVHD while preserving GVT reactivity. As expected, complete removal of donor T cells by in vivo or in vitro T-cell depletion abrogates GVHD but also GVT reactivity. As illustrated in the previous section, scheduled postponed unmanipulated DLI may restore GVT reactivity with more limited risk of GVHD.27 Complete removal of donor T cells also abrogates the necessary T-cell response against a variety of viruses in the early posttransplant period, leading to a high risk of viral complications, including cytomegalovirus, Epstein-Barr virus, varicella zoster, or adenovirus reactivations or infections. This lack of immunity against pathogens may be restored by selective depletion of only naive T cells from the graft, or posttransplant administration of in vitro selected pathogen-specific T cells.28 Although this method may be effective in reducing the risk of viral infections posttransplant, GVT reactivity still needs to be installed.
The use of posttransplant cyclophosphamide is also a method to deplete alloreactive T cells by interfering with those T cells that rapidly respond to alloantigens early after transplantation. If only part of the alloimmune T-cell response is removed by using this method, leaving an immune response with more limited diversity in magnitude in the patient, reduction of GVHD while preserving GVT may be possible.29 A similar approach is being explored using in vitro depletion of alloreactive T cells.30 Fine-tuning is still a major challenge with this approach, as illustrated by the high risk of relapse in patients after in vitro T-cell depletion using posttransplant cyclophosphamide treatment.
Introduction of a suicide gene in T cells before administration is another method to control GVHD after administration of donor T cells.31 This approach may allow administration of larger doses of T cells to patients, permitting control of the immune response if the magnitude and diversity will be too high, or if clinical circumstances with a high risk of developing GVHD will occur. Alternatively, suppressive T cells such as regulatory T cells may be helpful in damping GVHD.32
In vitro selection of T cells capable of specifically targeting only hematopoietic cells of recipient origin seems to be the most logical method but has been found to be challenging. Several approaches that are being explored include specific vaccination after transplantation with DC of donor origin forced to express patient-specific hematopoiesis-restricted MiHA, in vitro selection and expansion of T-cell lines and clones identified to selectively target hematopoiesis of recipient origin, and T-cell receptor gene transfer with T-cell receptors specific for hematopoiesis-restricted MiHA. Administration of purified CD4 T cells targeting only HLA class II–expressing cells is relatively simple but may not be sufficient to appropriately reduce GVHD while preserving GVT reactivity.26 Other approaches include the exploration of the use of donor T cells or donor T-cell receptors specific for tumor-associated antigens that are overexpressed in hematologic malignancies such as targeting WT-1 specific peptides.33 These approaches thus far resulted in only anecdotal clinical responses.
Failure of GVT responses
Alloimmune T-cell responses after alloSCT are clearly the most widely used and efficient forms of cellular immunotherapy. The efficacy of this treatment is determined by a polyclonal immune response against multiple antigens with limited diversity and magnitude targeting polymorphic antigens expressed on hematopoietic tissues of recipient origin. Despite the efficacy, several factors may result in failure of this immune response, leading to persisting or relapsed disease. Obviously, profound T-cell depletion or immune suppression posttransplant resulting in abrogation of the alloimmune response will also abolish the antitumor reactivity. Complete replacement or depletion of recipient-derived professional APC posttransplant may also result in an inability to provoke an alloimmune response, resulting in GVT reactivity. Late after transplantation, large doses of DLI can be administered to patients with relapsed disease without the occurrence of any alloimmune response. Only if the hematologic malignancy of recipient origin can act or be induced to perform as professional APC, recipient-derived normal DC may not be necessary to provoke an appropriate immune response. Naturally occurring DC derived from chronic myeloid leukemia precursor cells, or APC-like cells from patients with acute myeloid leukemia or chronic B-cell malignancies, may fulfill these criteria in some cases. In vivo induction of costimulatory and adhesion molecules on the malignant cell populations by immune stimulatory drugs such as interferons may increase their ability to provoke a relevant alloimmune response.
Exhaustion of the immune response or in vivo inhibition of T-cell responses at the target tissue site may be other causes of failure of the immune response. High expression of checkpoint molecules in nonresponding patients who relapsed, and induction of in vivo immune responses by checkpoint inhibitors resulting in both GVHD and GVT responses, have been reported.34,35 Due to the potential diversity of the immune responses after alloSCT, it may be difficult to predict whether a relatively selective GVT response can be provoked by checkpoint inhibition. Appropriate homing to all tumor sites is also an essential feature in controlling the malignant cell population. This feature may not be a frequent cause of failure of GVT responses in patients with leukemia because T cells preferentially appear to traffic to the bone marrow compartment. In contrast, in a cohort of patients with multiple myeloma treated with DLI for persistence of disease, we recently observed that appropriate control could be obtained in bone marrow resulting in full donor chimerism, while extramedullary focal lesions were frequently resistant to T-cell therapy in the same patients. Only when local tissue damage and inflammation could be induced (eg, by irradiation) were these focal lesions targeted.
Tumor tissue–associated antigen escape variants may be another cause of inability of the immune response to target the malignancy. This factor has been a frequent cause of failure of CD19-targeted antibody or chimeric antigen receptor T-cell therapy. Due to the polyclonality and diversity of the immune response after alloSCT/DLI, loss of a single antigen is unlikely to result in resistance. However, complete loss of HLA alleles necessary to present the relevant target antigens has been described as a frequent cause of resistance of the malignancy after haploidentical transplantation.36 This outcome may suggest that even after haploidentical transplantation with profound T-cell depletion, not only alloreactive natural killer cells but also residual alloHLA-reactive T cells contribute to the antileukemic effect. After HLA-matched transplantation, complete loss of HLA alleles has not been described as a frequent event.
Conclusions
The induction of alloimmune responses in the context of alloSCT is the most widely used and efficient form of cellular immunotherapy. Targeting polymorphic antigens expressed on hematopoietic tissues of the recipient by alloreactive donor T cells will result in a highly specific antitumor response while preserving normal hematopoiesis of donor origin in the patient. Recipient-derived professional APC of hematopoietic origin in the patient after transplantation are likely to play a key role in the induction of the immune response. Cells of hematopoietic origin, including the malignant cell population, are most susceptible to the alloimmune response by relatively high and constitutive expression of HLA class I and HLA class II molecules and expression of costimulatory and/or adhesion molecules, resulting in the preferential targeting of hematopoietic tissues compared with target tissues of GVHD. By influencing the antigen specificity, the magnitude and diversity of the immune response and the inflammatory environment in the patient, the balance between GVHD and GVT can be favorably influenced. Selection and timing of T-cell response are key factors in promoting the clinical efficacy.
References
- 1.Falkenburg JH, Jedema I. Allo-reactive T cells for the treatment of hematological malignancies. Mol Oncol. 2015;9(10):1894-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JS, Warren EH, van den Brink MR, et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on the biology underlying recurrence of malignant disease following allogeneic HSCT: graft-versus-tumor/leukemia reaction. Biol Blood Marrow Transplant. 2010;16(5):565-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139-176. [DOI] [PubMed] [Google Scholar]
- 4.Hassan C, Kester MG, de Ru AH, et al. The human leukocyte antigen-presented ligandome of B lymphocytes. Mol Cell Proteomics. 2013;12(7):1829-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffioen M, van Bergen CA, Falkenburg JH. Autosomal minor histocompatibility antigens: how genetic variants create diversity in immune targets. Front Immunol. 2016;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amir AL, van der Steen DM, Hagedoorn RS, et al. Allo-HLA-reactive T cells inducing graft-versus-host disease are single peptide specific. Blood. 2011;118(26):6733-6742. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11(11):1244-1249. [DOI] [PubMed] [Google Scholar]
- 8.Bleakley M, Otterud BE, Richardt JL, et al. Leukemia-associated minor histocompatibility antigen discovery using T-cell clones isolated by in vitro stimulation of naive CD8+ T cells. Blood. 2010;115(23):4923-4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mielcarek M, Kirkorian AY, Hackman RC, et al. Langerhans cell homeostasis and turnover after nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation. Transplantation. 2014;98(5):563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yun HD, Waller EK. Finding the sweet spot for donor lymphocyte infusions. Biol Blood Marrow Transplant. 2013;19(4):507-508. [DOI] [PubMed] [Google Scholar]
- 12.Eefting M, de Wreede LC, Halkes CJ, et al. Multi-state analysis illustrates treatment success after stem cell transplantation for acute myeloid leukemia followed by donor lymphocyte infusion. Haematologica. 2016;101(4):506-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoogendoorn M, Jedema I, Barge RM, et al. Characterization of graft-versus-leukemia responses in patients treated for advanced chronic lymphocytic leukemia with donor lymphocyte infusions after in vitro T-cell depleted allogeneic stem cell transplantation following reduced-intensity conditioning. Leukemia. 2007;21(12):2569-2574. [DOI] [PubMed] [Google Scholar]
- 14.Melenhorst JJ, Scheinberg P, Williams A, et al. Alloreactivity across HLA barriers is mediated by both naïve and antigen-experienced T cells. Biol Blood Marrow Transplant. 2011;17(6):800-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amir AL, D’Orsogna LJ, Roelen DL, et al. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115(15):3146-3157. [DOI] [PubMed] [Google Scholar]
- 16.Warren EH, Fujii N, Akatsuka Y, et al. Therapy of relapsed leukemia after allogeneic hematopoietic cell transplantation with T cells specific for minor histocompatibility antigens. Blood. 2010;115(19):3869-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meij P, Jedema I, van der Hoorn MA, et al. Generation and administration of HA-1-specific T-cell lines for the treatment of patients with relapsed leukemia after allogeneic stem cell transplantation: a pilot study. Haematologica. 2012;97(8):1205-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oostvogels R, Kneppers E, Minnema MC, et al. Efficacy of host-dendritic cell vaccinations with or without minor histocompatibility antigen loading, combined with donor lymphocyte infusion in multiple myeloma patients. Bone Marrow Transplant. 2017;52(2):228-237. [DOI] [PubMed] [Google Scholar]
- 19.van Bergen CA, van Luxemburg-Heijs SA, de Wreede LC, et al. Selective graft-versus-leukemia depends on magnitude and diversity of the alloreactive T cell response. J Clin Invest. 2017;127(2):517-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Zouwen B, Kruisselbrink AB, Jordanova ES, et al. Alloreactive effector T cells require the local formation of a proinflammatory environment to allow crosstalk and high avidity interaction with nonhematopoietic tissues to induce GVHD reactivity. Biol Blood Marrow Transplant. 2012;18(9):1353-1367. [DOI] [PubMed] [Google Scholar]
- 21.Brouwer RE, Zwinderman KH, Kluin-Nelemans HC, van Luxemburg-Heijs SA, Willemze R, Falkenburg JH. Expression and induction of costimulatory and adhesion molecules on acute myeloid leukemic cells: implications for adoptive immunotherapy. Exp Hematol. 2000;28(2):161-168. [DOI] [PubMed] [Google Scholar]
- 22.Petersdorf EW, Malkki M, O’hUigin C, et al. High HLA-DP expression and graft-versus-host disease. N Engl J Med. 2015;373(7):599-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fleischhauer K, Beelen DW. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin Hematol. 2016;53(2):57-64. [DOI] [PubMed] [Google Scholar]
- 24.Stevanović S, Griffioen M, Nijmeijer BA, et al. Human allo-reactive CD4+ T cells as strong mediators of anti-tumor immunity in NOD/scid mice engrafted with human acute lymphoblastic leukemia. Leukemia. 2012;26(2):312-322. [DOI] [PubMed] [Google Scholar]
- 25.Rutten CE, van Luxemburg-Heijs SA, Halkes CJ, et al. Patient HLA-DP-specific CD4+ T cells from HLA-DPB1-mismatched donor lymphocyte infusion can induce graft-versus-leukemia reactivity in the presence or absence of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19(1):40-48. [DOI] [PubMed] [Google Scholar]
- 26.Stevanovic S, van Bergen CA, van Luxemburg-Heijs SA, et al. HLA class II upregulation during viral infection leads to HLA-DP-directed graft-versus-host disease after CD4+ donor lymphocyte infusion. Blood. 2013;122(11):1963-1973. [DOI] [PubMed] [Google Scholar]
- 27.Eefting M, Halkes CJ, de Wreede LC, et al. Myeloablative T cell-depleted alloSCT with early sequential prophylactic donor lymphocyte infusion is an efficient and safe post-remission treatment for adult ALL. Bone Marrow Transplant. 2014;49(2):287-291. [DOI] [PubMed] [Google Scholar]
- 28.Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015;125(7):2677-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson TM, O’Donnell PV, Fuchs EJ, Luznik L. Haploidentical bone marrow and stem cell transplantation: experience with post-transplantation cyclophosphamide. Semin Hematol. 2016;53(2):90-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bastien JP, Roy J, Roy DC. Selective T-cell depletion for haplotype-mismatched allogeneic stem cell transplantation. Semin Oncol. 2012;39(6):674-682. [DOI] [PubMed] [Google Scholar]
- 31.Cieri N, Mastaglio S, Oliveira G, Casucci M, Bondanza A, Bonini C. Adoptive immunotherapy with genetically modified lymphocytes in allogeneic stem cell transplantation. Immunol Rev. 2014;257(1):165-180. [DOI] [PubMed] [Google Scholar]
- 32.Di Ianni M, Falzetti F, Carotti A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921-3928. [DOI] [PubMed] [Google Scholar]
- 33.Chapuis AG, Ragnarsson GB, Nguyen HN, et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci Transl Med. 2013;5(174):174ra27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norde WJ, Maas F, Hobo W, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011;71(15):5111-5122. [DOI] [PubMed] [Google Scholar]
- 35.Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin’s lymphoma. Blood. 2017;129(18):2471-2478. [DOI] [PubMed] [Google Scholar]
- 36.Vago L, Perna SK, Zanussi M, et al. Loss of mismatched HLA in leukemia after stem-cell transplantation. N Engl J Med. 2009;361(5):478-488. [DOI] [PubMed] [Google Scholar]