Abstract
The introduction of computed tomographic pulmonary angiography and its recent increasing availability has led to a significant rise in its use to help clinicians diagnose acute pulmonary embolism (PE). This has led to a significant increase in the incidence of PE diagnoses. Simultaneously, the case fatality rate of acute PE has been decreasing and no significant change in its mortality has been noted, suggesting that the additional PE diagnoses are less severe and these patients might not benefit from anticoagulation therapy. This also seems to be correlated with an increase in the diagnosis of PE localized in the subsegmental pulmonary arteries (subsegmental pulmonary embolism [SSPE]). The clinical importance of SSPE is unclear. Whereas some studies have shown that it might be reasonable to manage patients with SSPE without anticoagulation, others have not. Although the current medical literature is limited, it suggests that a subgroup of patients with SSPE might be safely managed without the use of anticoagulant therapy. Current clinical practice guidelines suggest that clinicians take an individualized approach after carefully assessing the risk/benefit ratio for patients with SSPE and negative leg limb ultrasonography results. Prospective studies are ongoing and results are eagerly awaited to help tailor the management of this patient population.
Learning Objectives
Review the incidence and clinical relevance of subsegmental pulmonary embolism (SSPE)
Propose a management strategy for patients with symptomatic SSPE
Introduction
Pulmonary embolism (PE) is a common disease that causes >100 000 deaths and complicates >300 000 hospitalizations every year in the United States.1-3 The introduction of computed tomographic pulmonary angiography (CTPA) and its recent increasing availability in hospital emergency rooms around the world has led to a significant rise in its use to help clinicians diagnose acute PE. Advances in technology (more specifically, the implementation of multirow detector CTPA in clinical practice) have led to an improvement in the sensitivity of PE diagnosis by allowing better resolution of the 2- to 3-mm-diameter subsegmental pulmonary arteries. This also seemed to led to a significant increase in the overall incidence of PE diagnosis in the United States.4 A randomized controlled trial previously showed that a management strategy using multirow detector CTPA increases the rate of PE diagnosis compared with a strategy including a ventilation-perfusion (VQ) scan.5 This increase in the incidence of PE diagnosis over time could be the result of an improvement in the effectiveness of PE diagnosis using multirow detector CTPA. On the other hand, it could also represent overdiagnosis or misdiagnosis. A final but less likely possibility is an increase in the “true” incidence of PE. Interestingly, as the incidence in PE diagnoses has increased in the past years, its case fatality (fatal PE/PE diagnosis) has decreased and no significant change in PE mortality (fatal PE/US population) has been noted. Therefore, these findings suggest that the additional subsegmental pulmonary embolism (SSPE) diagnosed using modern, multirow detector CTPA might be less severe.4 Following this reasoning and contemplating similar management of patients with other types of less severe venous thromboembolism (VTE) (eg, distal deep vein thrombosis [DVT]), some patients diagnosed with these small PEs may not benefit from anticoagulant therapy. Here, we review the incidence of SSPE diagnosis, discuss its clinical importance, and establish the risk/benefit ratio of anticoagulant therapy in this specific patient population.
Increased incidence of SSPE
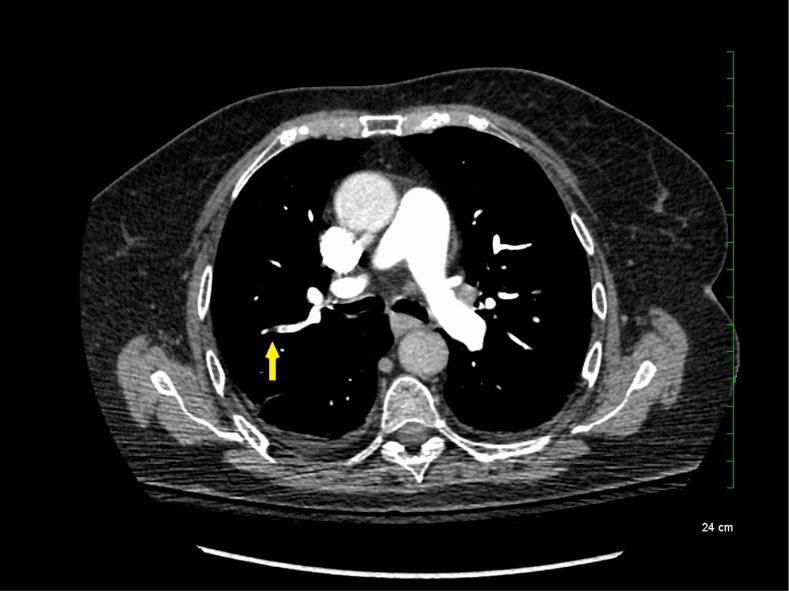
The reported increased incidence of PE diagnosis since the introduction of multirow detector CTPA seems to be correlated with an increase in the diagnosis of PE localized in the subsegmental pulmonary arteries without involvement in larger-order vessels (eg, segmental pulmonary arteries) (see Figure 1). A systematic review and meta-analysis of the literature reported that the rate of SSPE diagnosis among patients who underwent single-row detector CTPA was 4.7% compared with 9.4% for those who underwent multirow detector CTPA.6 Interestingly, the rate of SSPE diagnosis also seems to be increasing with the number of detectors used for PE diagnosis. These rates are reported to range from 7% to 15% in patients undergoing 4-row to 64-row detector CTPA, respectively.6 Similarly, a cohort study using 64-row detector CTPA reported a rate of SSPE as high as 12% among patients with confirmed PE.7 With further advancements in technology, reported rates of SSPE may increase, highlighting the importance of addressing this important knowledge gap.
Figure 1.
Subsegmental pulmonary embolism on computed tomographic pulmonary angiography.
Although SSPE diagnoses are increasing with advancements in technology, the true incidence of SSPE remains unclear. It is unknown whether these filling defects reported by radiologists are actual thrombus or artifacts. Interobserver agreement between radiologists was shown to be low for the diagnosis of SSPE in the emergency room (κ = 0.38; 95% confidence intervals [95% CIs], 0.0 to 0.89).8,9 Previous studies reported that up to 59% of SSPE diagnoses made clinically were actually false positives upon reinterpretation by a thoracic or more experienced radiologist.10,11 Another study including reinterpretations by 5 radiologists showed that for SSPE diagnosis, at least 1 radiologist disagreed with the initial interpretation in 60% of the cases.12 Given this variability in the certainty of SSPE diagnosis, it is important for clinicians to review the CTPA results and confirm the diagnosis with a thoracic or experienced radiologist to avoid exposing patients to anticoagulant therapy for an artifactual finding.
Clinical relevance of SSPE
Indirect evidence
As discussed above, although the incidence of PE diagnosis is increasing, no changes in the overall mortality rate of PE have been noted and its case fatality rate has been decreasing.4 This suggests that multirow CTPA seems to be diagnosing PEs with a lower severity of illness or such little thrombus burden that patients might not benefit from anticoagulation therapy.4 Similarly, a randomized controlled trial comparing the utility of multirow detector CTPA to the V/Q scan for the management of patients with suspected PE showed that more PEs were diagnosed if patients had undergone a CTPA (19.2% vs 14.2%; absolute difference of 5.5%; 95% CI, 1.1% to 8.9%)5; hence, more patients diagnosed by CTPA were treated with anticoagulants. Despite this, the rate of VTE during the 3-month follow-up period was similar (0.4% vs 1.0%; absolute difference of 0.6; 95% CI, −1.6% to 0.3%) in untreated patients (ie, in whom PE was excluded), suggesting that the additional cases of PE detected by CTPA were unlikely to be clinically significant.5 Finally, a systematic review assessing the rates of SSPE according to the number of CTPA detectors did not show a lower rate of PE during the follow-up period in patients with a negative multirow detector CTPA result, despite the increased proportion of patients diagnosed with PE.6 The rate of PE diagnosed during the 3-month follow-up period in patients left untreated who had PE ruled out based on single-row detector CTPA was 0.9% (95% CI, 0.4 to 1.4) compared with 1.1% (95% CI, 0.7% to 1.4%) for patients who underwent multirow detector CTPA.6 Again, this finding suggests that the incremental SSPE diagnoses identified by multirow detector CTPA are unlikely to be clinically relevant and may not be worth diagnosing, provided that there is no evidence of DVT. It was proposed previously that one of the normal functions of the lung is to remove small emboli to prevent them from entering into the arterial circulation.13,14 Therefore, it is possible that SSPE may be part of normal physiology and may remain unrecognized until defined by multirow detector CTPA.
More direct evidence
A number of retrospective cohort studies and post hoc analyses assessing the efficacy and safety of anticoagulation therapy for patients with isolated SSPE have been published.1,14-16 Whereas some studies have shown that it might be reasonable to manage patients with isolated SSPE14-16 without anticoagulation, others have not.1 A post hoc analysis combining 2 cohort studies showed that the risks of recurrent VTE were similar for patients with isolated SSPE compared with those with more proximal thrombus, suggesting that patients with SSPE should potentially receive anticoagulant therapy.1 Interestingly, the rate of recurrent VTE reported for these anticoagulated patients with SSPE (3.6%) was higher than previously published. The reasons for this higher risk of recurrent events in this particular study are unclear, but they might be attributable to the absence of lower limb ultrasonography in patients with leg symptoms resulting in a potentially higher risk of undetected DVT. In addition, 18% of patients with SSPE had active cancer, a condition known to be associated with a high risk of recurrent VTE.1 A more recently published cohort study suggested that some patients with isolated SSPE might have a low risk of recurrent events and may not require anticoagulation therapy.15 Of the 550 patients with confirmed PE included in this study, 82 (15%) had SSPE. A total of 52% of the patients with SSPE did not receive anticoagulation therapy. None of the patients had recurrent VTE during the follow-up period and 2 of the anticoagulated patients had major bleeding episodes.15 Although the study findings are likely limited by treatment bias, they suggest that a subgroup of patients with isolated SSPE can be safely managed without the use of anticoagulant therapy. Other studies have also suggested that the risks of anticoagulation therapy may exceed the benefits in patients with SSPE.14,16 Therefore, although the clinical impact of SSPE remains unknown, current data suggest that some patients with SSPE might be managed without the use of anticoagulation therapy. However, concurrent DVT is a strong indication for anticoagulation because it is an important risk factor for recurrent PE. Lower-extremity ultrasonography to rule out DVT is therefore an important component in the decision on the type of management for the individual patient with isolated SSPE.3
In addition to the risk of recurrent VTE, the risks of anticoagulant therapy are also important to consider when deciding on the optimal management of patients with SSPE. Major bleeding episodes are shown to be associated with high case fatality rates (11.3%; 95% CI, 7.5% to 15.9%),17 although the risk of fatal recurrent PE in patients with SSPE who remain untreated is unknown. A retrospective cohort study reported that 5.3% of patients with isolated SSPE receiving anticoagulation experienced a bleeding episode during follow-up.16 A systematic review of the literature assessing the risks and benefits of anticoagulation in patients with SSPE reported a low rate of recurrent VTE (0%) in patients left untreated, but there was a relatively high rate of major bleeding episodes that affected 7% of patients managed with anticoagulant therapy.14 Admittedly, these rates of major bleeding episodes are reflective of the underlying risk associated with vitamin K antagonists. It is unclear whether these same risks are applicable for patients managed using direct oral anticoagulants, which have a significantly better safety profile.18 These findings suggest that the risk/benefit ratio of anticoagulant therapy for patients with SSPE should be made on a case-by-case basis.
Management strategy for SSPE
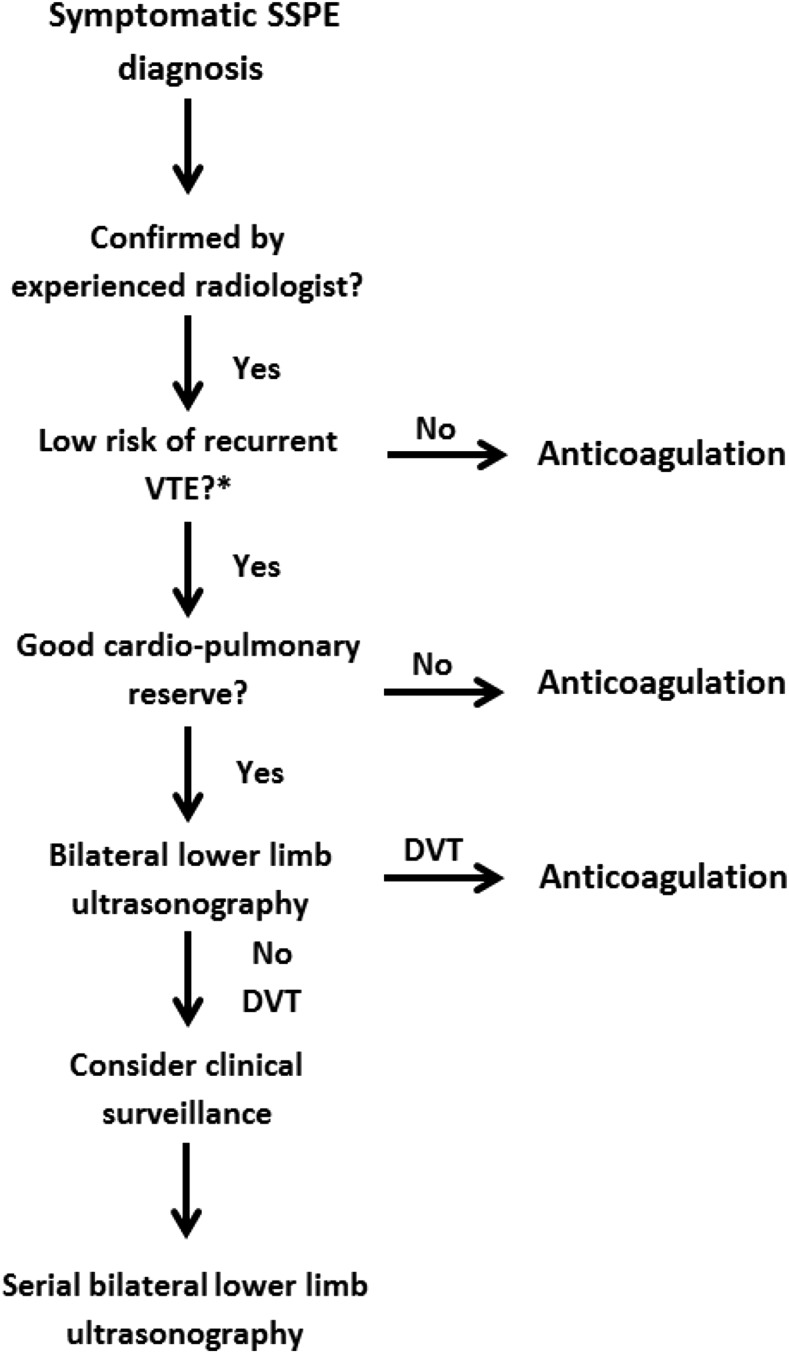
Although the clinical importance of SSPE remains debatable, it is important to establish a safe management strategy on a case-by-case basis that accounts for the risk of recurrent VTE and bleeding episodes and patient preference (see Figure 2). If a decision is made to not initiate anticoagulant therapy in patients with isolated SSPE, clinicians must have a standardized approach to manage these patients safely (Figure 2).
Figure 2.
Management strategy for symptomatic subsegmental pulmonary embolism. DVT, deep vein thrombosis; SSPE, subsegmental pulmonary embolism; VTE, venous thromboembolism.
A recently published Cochrane review failed to identify any randomized controlled trials to guide clinicians on the management of patients with SSPE.19 Therefore, physicians have to rely on clinical data from other patient populations, including those with suspected PE or a larger PE burden. SSPEs were previously shown to be frequent among patients with suspected PE and nondiagnostic V/Q scans. In the Prospective Evaluation of Pulmonary Embolism Diagnosis (PIOPED) study, 17% of patients with a low probability V/Q scan had SSPE on pulmonary angiography.20 Therefore, it is probably reasonable to presume that many patients with SSPE on multirow CTPA would have had a nondiagnostic VQ scan. Over the last decades, many prospective management studies have shown that patients with suspected PE and nondiagnostic V/Q scans can be safely managed without the use of anticoagulant therapy, provided that there is no DVT in the lower extremities.21-23 Similarly to patients with nondiagnostic VQ scans, patients with SSPE without DVT on lower limb ultrasonography might be managed conservatively without anticoagulant therapy. It is particularly important to rule out DVT in patients with SSPE, given that rates of concomitant proximal lower limb DVT have been reported at 7.1% (95% CI, 1.2% to 31.5%) in this patient population.24
This is consistent with the 2016 American College of Chest Physicians (ACCP) clinical practice guideline, which suggests using clinical surveillance instead of anticoagulation for patients with SSPE and no proximal DVT in the legs who have a low risk of recurrent VTE (grade 2C).25 Clinical surveillance should be supplemented by serial ultrasonography of the lower extremities within 5 to 7 days. Clinicians might want to favor this treatment option if the patient has good cardiopulmonary reserve or has a high risk of bleeding. However, if patients have a high risk of recurrent VTE, the latest version of the ACCP guidelines suggests anticoagulation therapy over clinical surveillance (grade 2C). Numerous risk factors for recurrent VTE have been proposed to help guide clinicians on stratifying the underlying risk of recurrent VTE. These risk factors include hospitalization, immobility, active cancer (especially metastatic disease or ongoing chemotherapy), or an unprovoked event.25 Similarly, the European Society of Cardiology clinical practice guideline suggests that an individualized approach be taken after careful assessment of the risk/benefit ratio for patients with isolated SSPE and negative leg ultrasonography results.26 Therefore, serial ultrasonography of the lower extremities might be a reasonable alternative to anticoagulation in low-risk patients.
The ACCP and the European Society of Cardiology recommendations seem to be reflective of clinical practice in certain settings and countries. In 2011, a Thrombosis Canada cross-sectional survey of Canadian thrombosis experts assessed the current diagnostic and therapeutic management of patients with isolated SSPE, and the survey results demonstrated considerable variability in clinical practices.27 More than 76% of clinicians responded that they manage low-risk patients with SSPE without anticoagulant therapy.27
Incidental SSPE
The term “incidental PE” is used for unsuspected filling defects of the pulmonary artery, corresponding to PE detected on routine computed tomography (CT) scans of the chest, with an incidence frequency of 1.1% in coronary CT and 3.6% in oncological CT. This prevalence and, in particular, the incidence of SSPE has also increased over the past years with advancing CT technology.28 In contrast with the patients with low-risk SSPE discussed above, the majority of patients with cancer-associated incidental SSPE should be considered for anticoagulant therapy. The higher risk of recurrent VTE in patients with cancer supports a decision to initiate anticoagulation, especially for patients with multiple SSPE, although large outcome studies in these latter patients are lacking. Nonetheless, one study that compared patients with cancer with incidental SSPE to those with more proximally located PE showed that the VTE recurrence risk was not different between the 2 groups (hazard ratio, 1.1; 95% CI, 0.50 to 2.4).29 A recently published guidance document from the International Society on Thrombosis and Haemostasis put forward some suggestions to help clinicians with these challenging clinical situations.30
Next steps
With conflicting studies, prospective studies providing safety data would be potentially practice changing if it could be shown that a group of patients with SSPE could be managed more conservatively without anticoagulation. This would allow these patients to avoid the inconvenience and known risks and complications of systematic anticoagulation. One ongoing international (Canada, Netherlands, France, and Switzerland) prospective management cohort study (ClinicalTrials.gov NCT01455818) is enrolling patients with SSPE and withholding anticoagulation in patients without DVT. The study has recruited >50% of the targeted sample size (n = 300) and its final results will shed more light on the optimal care of patients with symptomatic SSPE.
In conclusion, there is still considerable clinical controversy with regard to the management of patients with SSPE (symptomatic or incidental cancer associated) associated with significant clinical practice variation. The practice of withholding anticoagulation in patients with SSPE with serial negative leg Doppler imaging results is currently being assessed with a prospective management cohort study and can be considered in low-risk patients without cancer and DVT on serial ultrasonography of the lower limbs, especially if the patient is at high risk of major bleeding.
References
- 1.den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013;122(7):1144-1149, quiz 1329. [DOI] [PubMed] [Google Scholar]
- 2.US Office of the Surgeon General. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. Rockville, MD: Office of the Surgeon General; 2008. [PubMed]
- 3.Raskob GE. Importance of subsegmental pulmonary embolism. Blood. 2013;122(7):1094-1095. [DOI] [PubMed] [Google Scholar]
- 4.Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson DR, Kahn SR, Rodger MA, et al. Computed tomographic pulmonary angiography vs ventilation-perfusion lung scanning in patients with suspected pulmonary embolism: a randomized controlled trial. JAMA. 2007;298(23):2743-2753. [DOI] [PubMed] [Google Scholar]
- 6.Carrier M, Righini M, Wells PS, et al. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8(8):1716-1722. [DOI] [PubMed] [Google Scholar]
- 7.Pesavento R, de Conti G, Minotto I, et al. ; TACEP study. The value of 64-detector row computed tomography for the exclusion of pulmonary embolism. Thromb Haemost. 2011;105(5):901-907. [DOI] [PubMed] [Google Scholar]
- 8.Stein PD, Fowler SE, Goodman LR, et al. ; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354(22):2317-2327. [DOI] [PubMed] [Google Scholar]
- 9.Ghanima W, Nielssen BE, Holmen LO, Witwit A, Al-Ashtari A, Sandset PM. Multidetector computed tomography (MDCT) in the diagnosis of pulmonary embolism: interobserver agreement among radiologists with varied levels of experience. Acta Radiol. 2007;48(2):165-170. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson BD, Navin P, Marom EM, Truong MT, Bruzzi JF. Overdiagnosis of pulmonary embolism by pulmonary CT angiography. AJR Am J Roentgenol. 2015;205(2):271-277. [DOI] [PubMed] [Google Scholar]
- 11.Pena E, Kimpton M, Dennie C, Peterson R, LE Gal G, Carrier M. Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. J Thromb Haemost. 2012;10(3):496-498. [DOI] [PubMed] [Google Scholar]
- 12.Miller WT Jr, Marinari LA, Barbosa E Jr, et al. Small pulmonary artery defects are not reliable indicators of pulmonary embolism. Ann Am Thorac Soc. 2015;12(7):1022-1029. [DOI] [PubMed] [Google Scholar]
- 13.Gurney JW. No fooling around: direct visualization of pulmonary embolism. Radiology. 1993;188(3):618-619. [DOI] [PubMed] [Google Scholar]
- 14.Stein PD, Goodman LR, Hull RD, Dalen JE, Matta F. Diagnosis and management of isolated subsegmental pulmonary embolism: review and assessment of the options. Clin Appl Thromb Hemost. 2012;18(1):20-26. [DOI] [PubMed] [Google Scholar]
- 15.Goy J, Lee J, Levine O, Chaudhry S, Crowther M. Sub-segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost. 2015;13(2):214-218. [DOI] [PubMed] [Google Scholar]
- 16.Donato AA, Khoche S, Santora J, Wagner B. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010;126(4):e266-e270. [DOI] [PubMed] [Google Scholar]
- 17.Carrier M, Le Gal G, Wells PS, Rodger MA. Systematic review: case-fatality rates of recurrent venous thromboembolism and major bleeding events among patients treated for venous thromboembolism. Ann Intern Med. 2010;152(9):578-589. [DOI] [PubMed] [Google Scholar]
- 18.van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(3):320-328. [DOI] [PubMed] [Google Scholar]
- 19.Yoo HH, Queluz TH, El Dib R. Anticoagulant treatment for subsegmental pulmonary embolism. Cochrane Database Syst Rev. 2014;(4):CD010222. [DOI] [PubMed] [Google Scholar]
- 20.Stein PD, Henry JW. Prevalence of acute pulmonary embolism in central and subsegmental pulmonary arteries and relation to probability interpretation of ventilation/perfusion lung scans. Chest. 1997;111(5):1246-1248. [DOI] [PubMed] [Google Scholar]
- 21.Perrier A, Desmarais S, Miron MJ, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353(9148):190-195. [DOI] [PubMed] [Google Scholar]
- 22.Perrier A, Bounameaux H, Morabia A, et al. Diagnosis of pulmonary embolism by a decision analysis-based strategy including clinical probability, D-dimer levels, and ultrasonography: a management study. Arch Intern Med. 1996;156(5):531-536. [PubMed] [Google Scholar]
- 23.Salaun PY, Couturaud F, Le Duc-Pennec A, et al. Noninvasive diagnosis of pulmonary embolism. Chest. 2011;139(6):1294-1298. [DOI] [PubMed] [Google Scholar]
- 24.Le Gal G, Righini M, Sanchez O, et al. A positive compression ultrasonography of the lower limb veins is highly predictive of pulmonary embolism on computed tomography in suspected patients. Thromb Haemost. 2006;95(6):963-966. [DOI] [PubMed] [Google Scholar]
- 25.Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report [published correction appears in Chest. 2016;150(4):988]. Chest. 2016;149(2):315-352. [DOI] [PubMed] [Google Scholar]
- 26.Konstantinides SV, Torbicki A, Agnelli G, et al. ; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-3069, 3069a-3069k. [DOI] [PubMed] [Google Scholar]
- 27.Carrier M, Kimpton M, LE Gal G, et al. The management of a sub-segmental pulmonary embolism: a cross-sectional survey of Canadian thrombosis physicians. J Thromb Haemost. 2011;9(7):1412-1415. [DOI] [PubMed] [Google Scholar]
- 28.Klok FA, Huisman MV. Management of incidental pulmonary embolism. Eur Respir J. 2017;49(6):1700275. [DOI] [PubMed]
- 29.van der Hulle T, den Exter PL, Planquette B, et al. Risk of recurrent venous thromboembolism and major hemorrhage in cancer-associated incidental pulmonary embolism among treated and untreated patients: a pooled analysis of 926 patients. J Thromb Haemost. 2016;14(1):105-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Nisio M, Lee AY, Carrier M, Liebman HA, Khorana AA; Subcommittee on Haemostasis and Malignancy. Diagnosis and treatment of incidental venous thromboembolism in cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2015;13(5):880-883. [DOI] [PubMed] [Google Scholar]