Abstract
There is limited evidence linking airway inflammation and lung function impairment in older non-smoking asthmatics with fixed airflow obstruction (FAO), which can develop despite treatment with inhaled corticosteroids (ICS). We assessed lung function (spirometry, forced oscillation technique (FOT)), lung elastic recoil and airway inflammation using bronchoalveolar lavage (BAL) in non-smoking adult asthmatics with FAO, following 2 months treatment with high-dose ICS/long-acting beta-agonist. Subjects demonstrated moderate FAO, abnormal FOT indices and loss of lung elastic recoil. This cross-sectional study showed a lack of a relationship between BAL neutrophils, eosinophils, inflammatory cytokines and lung function impairment. Other inflammatory pathways or the effect of inflammation on lung function over time may explain FAO development.
Keywords: Fixed airflow obstruction, Asthma, Reduced lung elastic recoil, Airway inflammation
Irreversible or fixed airflow obstruction (FAO) can develop in long-standing asthma despite no or minimal smoking history and is associated with moderate to severe disease [1, 2]. The mechanisms of FAO in asthma are poorly understood; therefore prevention and treatment remain a challenge. Inhaled and/or oral corticosteroids improve lung function and reduce exacerbations, yet may not necessarily prevent FAO from occurring [3, 4]. Asthma severity [1, 5] and FAO development may be attributed to corticosteroid resistance or insensitivity resulting in persistent airway inflammation [6] and structural airway changes. Both eosinophilic and neutrophilic inflammation may be associated with lung function impairment and FAO in asthma, however evidence is limited and contradictory [4, 7].
In this prospective study, we investigated whether lung function impairment in older non-smokers with long-standing asthma and FAO is associated with the airway inflammation which remains after treatment with maximal dose inhaled corticosteroid (ICS). We hypothesized that the degree of lung function abnormalities would positively correlate with persistent airway inflammation in patients with asthma and FAO, thereby providing a potential mechanism for the development of FAO and its apparent steroid insensitivity.
Patients were > 40 years old, non-smokers or had a negligible smoking history with a respiratory physician diagnosis of asthma. All patients were treated with a standardized maximal dose of ICS/long-acting beta-agonist (ICS/LABA) using fluticasone/eformoterol 250 μg/10 μg metered dose inhaler via a holding chamber, two puffs twice daily, if not already taking this treatment. A baseline test skin prick test to common allergens was performed to assess atopic status. During enrolment and after 2 months of treatment, patients completed a symptom questionnaire (Asthma Control Questionnaire, ACQ-5) and performed pre-bronchodilator lung function measurements. Measurements included spirometry and the forced oscillation technique (FOT) to derive airway resistance (R5) and reactance (X5) at 5 Hz. After 2 months of treatment an oesphageal balloon was used to derive the pressure-volume (P-V) curve to assess the elastic recoil properties of the lung via the indices K, reflecting lung compliance, and B/A, reflecting lung elastic recoil [8]. FAO was assessed following 1 month of treatment and was defined as a < 200 ml and < 12% change in spirometry post-bronchodilator (400mcg inhaled salbutamol). ICS/LABA medication was withheld for at least 24 h and short acting beta-agonist medication for at least 6 h prior to testing.
Following 2 months of ICS/LABA treatment and within a week of the lung function measurements, patients then underwent bronchoscopy with bronchoalveolar lavage (BAL) from the right middle lobe [9]. Neutrophil and eosinophil counts were obtained from BAL samples as previously described [10]. Cytokines including IL-1b, IL-4, IL-6, IL-10, IL-17A, IL-17F, IL-21, IL-22, IL-23, IL-25, IL-31, IL-33, IFN-γ, scD40L and TNF-α were measured in BAL supernatant using a multiplex immunoassay (Bio-Rad® Bio-Plex Multiplex Immunoassay). Univariate correlations (Spearman rank test) between lung function indices (using z-scores) and BAL samples (using raw values) after 2 months of treatment were assessed.
Nineteen patients were recruited (11 male; mean ± SD age 63 ± 9 years, asthma duration 38 ± 22 years, height 1.69 ± 0.10 m, body mass index 28.4 ± 5.8 kg/metre2); 18 completed the study. Five patients were ex-smokers with 2.2 ± 2.5 pack-years smoking history and 14/19 patients were atopic. Patients were symptomatic (ACQ-5 1.03 ± 0.92) despite taking regular asthma medications prior to enrolment (ICS 18/19; with LABA 18/19; ICS/LABA/long-acting muscarinic antagonist 5/19). One patient was on long-term low dose oral corticosteroids (Prednisone dose 5 mg) for treatment of rheumatoid arthritis.
Post-bronchodilator spirometry after 1 month of treatment showed moderate FAO (mean ± SD z-score: FEV1–2.05 ± 0.75, FVC -0.61 ± 0.95, FEV1/FVC -2.46 ± 0.90). After 2 months of treatment FOT indices were abnormal: R5 (median (IQR) z-score: 2.7(1.8–3.2)) and X5 (z-score: − 3.9(-7.3 - -2.0)). Spirometry did not change between enrolment and after 2 months of treatment, however R5 worsened. Eighteen patients performed lung elastic recoil measurements (median (IQR) z-score: K 1.57(− 1.08–3.43) and B/A -1.18(− 1.65--0.02)). Increased compliance was demonstrated in 9/18 patients (K z-score ≥ 1.64) and loss of elastic recoil in 5/18 (B/A% z-score ≤ − 1.64).
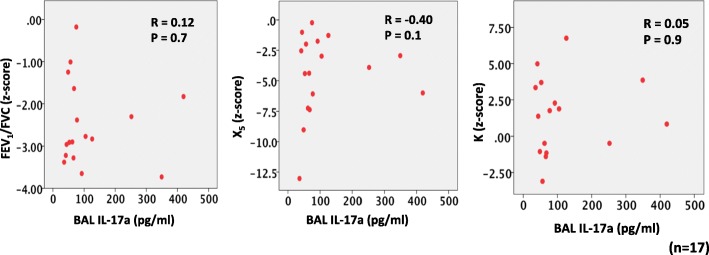
Eighteen patients performed bronchoscopy and BAL neutrophil and eosinophil cell counts were obtained in 10 patients (mean ± SD: neutrophils 9.1 ± 18.1% and eosinophils 1.9 ± 1.6%). No patients had evidence of neutrophilic airway inflammation whilst 4/10 patients had eosinophilic airway inflammation. BAL cytokines were obtained in 17 patients and results are shown in Fig. 1. BMI, spirometry, FOT and elastic recoil indices did not correlate with BAL neutrophil or eosinophil count and inflammatory cytokines (Fig. 2). Occasionally, statistically significant correlations were observed however these were the result of a single outlier.
Fig. 1.
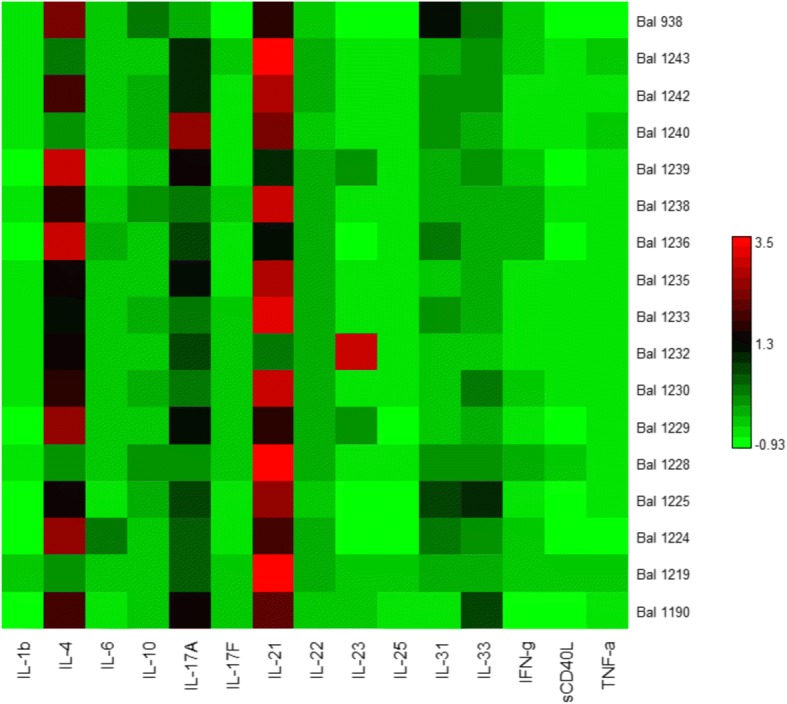
Cytokine levels measured in bronchoalveolar lavage fluid from each patient. Each row represents a patient and each column represents different cytokines. Red indicates highest levels and bright green lowest levels. IL = interleukin, IFN-g = interferon gamma, sCD40L = soluble CD40 ligand, TNF-a = tissue necrosis factor alpha
Fig. 2.
Univariate relationships between lung function measurements and BAL IL-17a. No significant correlations were demonstrated and similar findings were seen with other cytokines. FEV1/FVC=forced expiratory volume/forced vital capacity, K=reflects lung compliance, X5=reactance at 5Hz, BAL=bronchoalveolar lavage, IL=interleukin
Variable levels of neutrophils, eosinophils and cytokines were detected in this small cohort however there was a disconnect between measures of airway inflammation and lung function. The lack of a relationship in this cohort suggests persisting airway inflammation does not affect lung function following ICS treatment. However, the effect of previous inflammation and inflammation over time on lung function and FAO development remains unknown. A standardized period and dose of ICS treatment was used to minimize potential confounders however most patients were not steroid naïve thus the study treatment may have had minimal additional effect on the inflammatory profile. This is supported by the absence of any significant change in spirometry from enrolment and after the two-month study period. Adherence to study treatment was assessed after 1 month and at the two-month mark to ensure non-adherence did not play a role.
Somewhat surprisingly and in contrast to previous studies [11], this older cohort did not demonstrate neutrophilic airway inflammation although small subject numbers are a limiting factor. Furthermore, neutrophil activation was not measured and may have been increased due to inhibition of neutrophil apoptosis by inhaled corticosteroids. Despite treatment with high-dose ICS/LABA, eosinophilic inflammation persisted in a few patients and cytokines were still detectable, suggesting a steroid unresponsive inflammatory pathway. The fact that FAO develops despite treatment suggests inhaled corticosteroids may have minimal effect on airway remodeling in older people with asthma. Instead FAO in this cohort may predominantly be due to other mechanisms such as the loss of elastic recoil observed in this study, which in turn may occur as a result of lung tissue changes (i.e. lung remodeling) [2]. Lung tissue changes could be due to proteolytic enzymes disrupting lung parenchyma-terminal bronchiole attachments [12]. Inflammation in the lung tissue cannot be ignored, however our study lacks the ability to assess this. Less invasive tests such as a computer tomography (CT) scan to assess for possible lung tissue changes like emphysema [13] was also not done. A recent study demonstrated micro-emphysema, only on microscopic examination of post-mortem asthmatic lungs, which was not evident on CT imaging [2], therefore inclusion of CT imaging may not have be adequate to demonstrate lung tissue changes in our study.
Conclusion
In summary, this exploratory cross-sectional study has shown a lack of relationship between persistent airway inflammation and lung function impairment following a short period of maximal ICS treatment. However, other cellular mechanisms, lung tissue inflammation and the potential longitudinal effect of inflammation over time in the development of FAO in asthma warrant further investigation.
Acknowledgments
Funding
The project was partially funded by The University of Sydney Bridging Grant.
Availability of data and materials
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACQ
Asthma control questionnaire
- B/A%
Reflects lung elastic recoil
- BAL
Bronchoalveolar lavage
- FAO
Fixed airflow obstruction
- FEV1
Forced expiratory volume in 1 s
- FOT
Forced oscillation technique
- FVC
Forced vial capacity
- ICS
Inhaled corticosteroid
- IFN-γ
Interferon- γ
- IL
Interleukin
- K
Reflects lung compliance
- LABA
Long acting-beta-agonist
- P-V
Pressure-volume
- R5
Resistance at 5 Hz
- scD40L
Soluble cD40 ligand
- TNF-α
Tissue necrosis factor-α
- X5
Reactance at 5 Hz
Authors’ contributions
KOT, GGK and BGO contributed to conception and design of the study, analysis and interpretation of data and preparation of the manuscript. KOT, FST, JS and PS contributed to data collection and analysis. CSF, CT, PS and DGC contributed to interpretation of data and preparation of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Sydney Local Health District Human Research Ethics Committee - CRGH (protocol no. HREC/14/CRGH/75). Written informed consent was obtained from all recruited patients.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ulrik C, Backer V. Nonreversible airflow obstruction in life-long nonsmokers with moderate to severe asthma. Eur Respir J. 1999;14:892–896. doi: 10.1034/j.1399-3003.1999.14d27.x. [DOI] [PubMed] [Google Scholar]
- 2.Gelb AF, Yamamoto A, Verbeken EK, Schein MJ, Moridzadeh R, Tran D, et al. Further studies of unsuspected emphysema in nonsmoking patients with asthma with persistent expiratory airflow obstruction. Chest. 2018;153(3):618–629. doi: 10.1016/j.chest.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Backman KS, Greenberger PA, Patterson R. Airways obstruction in patients with long-term asthma consistent with 'irreversible asthma'. Chest. 1997;112:1234–1240. doi: 10.1378/chest.112.5.1234. [DOI] [PubMed] [Google Scholar]
- 4.ten Brinke A, Zwinderman AH, Sterk PJ, Rabe KF, Bel EH. Factors associated with persistent airflow limitation in severe asthma. Am J Respir Crit Care Med. 2001;164(5):744–748. doi: 10.1164/ajrccm.164.5.2011026. [DOI] [PubMed] [Google Scholar]
- 5.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanovic R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161(1):9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160(3):1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 7.Shaw DE, Berry MA, Hargadon B, McKenna S, Shelley MJ, Green RH, et al. Association between neutrophilic airway inflammation and airflow limitation in adults with asthma. Chest. 2007;132(6):1871–1875. doi: 10.1378/chest.07-1047. [DOI] [PubMed] [Google Scholar]
- 8.Colebatch H, Ng C, Nikov N. Use of an exponential function for elastic recoil. J Appl Physiol. 1979;46(2):387–393. doi: 10.1152/jappl.1979.46.2.387. [DOI] [PubMed] [Google Scholar]
- 9.Rankin JA, Snyder PE, Schachter EN, Matthay RA. Bronchoalveolar lavage. Its safety in subjects with mild asthma. CHEST J. 1984;85(6):723–728. doi: 10.1378/chest.85.6.723. [DOI] [PubMed] [Google Scholar]
- 10.Wenzel SE, Szefler SJ, Leung DYM, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Am J Respir Crit Care Med. 1997;156(3):737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 11.Nyenhuis SM, Schwantes EA, Evans MD, Mathur SK. Airway neutrophil inflammatory phenotype in older asthma subjects. J Allergy Clin Immunol. 2010;125(5):1163–1165. doi: 10.1016/j.jaci.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauad T, Silva LF, Santos MA, Grinberg L, Bernardi FD, Martins MA, et al. Abnormal alveolar attachments with decreased elastic fiber content in distal lung in fatal asthma. Am J Respir Crit Care Med. 2004;170(8):857–862. doi: 10.1164/rccm.200403-305OC. [DOI] [PubMed] [Google Scholar]
- 13.Jang A-S, Lee J-H, Park SW, Park J-S, Kim D-J, Park C-S. Risk factors related to fixed airway obstruction in patients with asthma after antiasthma treatment. Ann Allergy Asthma Immunol. 2007;99(5):408–412. doi: 10.1016/S1081-1206(10)60564-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.