Abstract
Gastric cancer has a high morbidity and mortality. Chemotherapy regimens are routine advanced stage gastric cancer (AGC) treatment protocols, but most of these drugs have side-effects such as myelosuppression and gastrointestinal disorders. Cinobufacini, an extractive from TCM, could suppress cell proliferation and inhibit gastric cancer. In this study, we comprehensively reviewed the literature on the efficacy comparison between Cinobufacini injection combined with chemotherapy and chemotherapy solely used in AGC treatment. We extracted data for from six electronic databases to evaluate the efficacy of Cinobufacini injection on AGC patients. Twelve studies with a total of 853 patients were finally included in our study. The results indicated that Cinobufacini injection could increase response rate and disease control rate of chemotherapy on AGC, improve the life quality of AGC patients, increase leukocytes, improve anemia, improve hand-foot syndrome induced by chemotherapy, and relieve cancer pain. This study has its own limitations that prevented us from drawing a definite conclusion and more well-designed clinical trials of TCM are needed.
1. Introduction
Gastric cancer (GC) is one of the most common and lethal cancers worldwide and quite a number of GC patients are initially diagnosed with advanced stage gastric cancer (AGC) including local advanced GC (stage III and unresectable) and metastasis GC (stage IV). Chemotherapy regimens, such as FOLFOXs regimen (oxaliplatin, 5-fluorouracil, and leucovorin calcium), XELOX regimen (oxaliplatin and capecitabine), or other chemotherapeutic drugs, including paclitaxel, cisplatin, epirubicin, and etoposide [1, 2], are common AGC treatment protocols. But most of these drugs have side-effects such as myelosuppression (anemia, low count of leukocytes) and gastrointestinal tract disorders (nausea, vomiting, and diarrhea).
Traditional Chinese medicine (TCM) honors a long history in tumor treatment and it is accepted that TCM can inhibit tumor growth and metastasis, improve antitumor immunity, relieve tumor pains, and reduce side-effects of chemotherapy [3–5]. Combined treatment of TCM and modern medicine is widely used for AGC in China and studies showed TCM had an important potential value for improving the prognosis of patients with AGC [6, 7].
Cinobufacini (also called Huachansu in Chinese), extracted from the skins and parotid venom glands of the Bufo bufo gargarizans Canto, is a kind of traditional Chinese animal-derived drug used in the treatment of malignant neoplasms in ancient oriental countries. Recent studies showed that Cinobufacini could induce the apoptosis of tumor cells and downregulate protumor inflammatory signaling pathways in the tumor microenvironment [8–11]. Furthermore, researches also indicated that Cinobufacini can inhibit several kinds of human tumors in both clinical treatments and animal xenograft models [12–14].
While Cinobufacini antitumor activity has been proved, the gastrointestinal metabolic pathways of Cinobufacini remain unclear, so intravenous administration (e.g., Cinobufacini injection) is the most common route. Thus, Cinobufacini injection was increasingly used in clinical and basic studies. As there is no systemic review specifically for Cinobufacini injection on AGC treatment, this systematic review and meta-analysis comprehensively evaluated the effects of it according to the PRISMA statement for a high quality [15, 16].
2. Material and Methods
2.1. Literature Search
Studies were explored from databases including PubMed (from Jan. 1975 to Oct. 2017), Cochrane library (from Jan. 2010 to Oct. 2017), Excerpta Medica data BASE (Embase) (from Jan. 1990 to Oct. 2017), China National Knowledge Infrastructure (CNKI) (from Jan. 1979 to Oct. 2017), Weipu database (VIP) (from Jan. 1990 to Oct. 2017), and Wanfang database (WF) (from Jan. 1989 to Oct. 2017). All the studies were searched regardless of their publication types and without language restriction. The search terms were as follows: “Cinobufacini” OR “Cinobufotalin” OR “Huachansu” AND “gastric” OR “stomach”. In addition to electronic databases, printed journals and relevant textbooks were manually searched from the libraries of Beijing University of Chinese Medicine, Peking Union Medical College and Guang'anmen Hospital. Specialized experts in particular fields were consulted for necessary supplements as well.
Inclusion criteria include the following: (1) types of studies: randomized clinical trials (RCTs); (2) participants: adult human populations (⩾18 years old) who were pathologically diagnosed as gastric cancer with clinical stages III (unresectable) and IV; (3) interventions: the control group was treated with chemotherapy while the experimental group was treated with the same chemotherapeutics plus Cinobufacini injection; and (4) outcomes: short/long-term chemotherapy response rate, Karnofsky's performance score, chemotherapeutic side-effects such as myelosuppression and gastrointestinal symptoms, and pain management. Exclusion criteria include the following: (1) studies such as reviews, animal researches, observational studies without control group, or other kinds of non-RCT studies; (2) trails about other types of gastrointestinal diseases; (3) participants who had nonpathological diagnosis, previously subjected to chemotherapy, radiotherapy or surgery, concurrent infection, or other malignancies or severe illnesses; and (4) participants in the control group who were treated with other antitumor TCM drugs.
2.2. Literature Selection and Data Extraction
Two independent reviewers (Yuan Y, Qiujun G) evaluated each title, abstract, citation, and selected relevant studies according to the inclusion criteria. Disagreements were discussed with and resolved by the third reviewer (Zizhen Y). Data from included studies were extracted separately by Yupeng X by using a specific form and checked by Xing Z. The characteristics of the data included name of first author, year of publication, gender and number of cases and controls, methods of randomization, interventions, treatment period, and outcomes. The hazard ratio (HR) was calculated from the Kaplan-Meier survival curve and survival outcome events as reported by Tierney [29].
2.3. Quality Assessment of Studies
The methodological quality of each randomized controlled trials (RCTs) was independently assessed by Yuan Y and Qiujun G via the Cochrane Risk of Bi as tool [30]. Disagreements were discussed with and resolved by Baojin H.
2.4. Data Synthesis and Analyses
The statistical analyses were performed using Review Manager (RevMan) 5.3.5 software (Cochrane Community, London, United Kingdom) and STATA 14 software. The total effectiveness rates of dichotomous data were pooled using risk ratios (RRs) with 95% confidence interval (CI). P < 0.05 was considered to indicate a statistically significant difference. The heterogeneity of the included studies was evaluated by the χ2 and I2 tests, and P < 0.10 or I2 > 50% was defined as indicating heterogeneity. The fixed-effect models were used in merging homogeneity data and the random-effects models were applied to merge of heterogeneous data. The publication bias was evaluated by visual assessment of the asymmetry of funnel plots (RevMan 5.3.5) and Egger's test (STATA 14) with p < 0.05 indicating potential bias. The sensitive analysis was evaluated by reanalyzing the data using different statistical approaches or eliminating a variable which takes the largest proportion.
3. Results
3.1. Included Eligible Studies
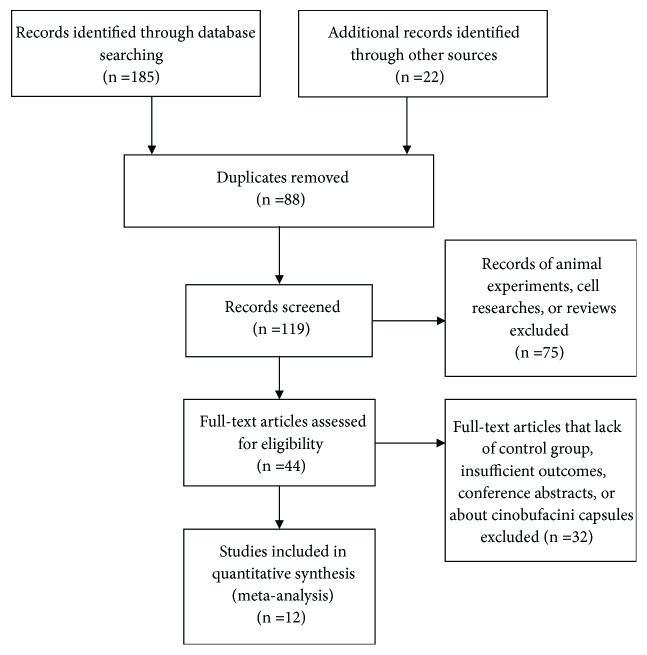
207 studies (including 22 additional records identified through other sources such as postgraduate dissertations and conference articles) were initially searched out by using the search strategy mentioned above, among which 88 duplicated studies were removed, and 75 studies were excluded because they were animal experiments, cell researches, or reviews. After reading the full text, 32 studies were excluded because they lacked control group, had insufficient outcomes conference abstracts, or were about Cinobufacini capsules. Eventually, 12 studies were included in the final research (Figure 1).
Figure 1.
Flow diagram of the literature search process.
3.1.1. Characteristics of Included Studies
Twelve studies with a total of 853 patients were finally included (423 patients in the experiment group and 430 patients in the control group). Characteristics such as sample size, gender, age, interventions, and outcomes of each study were described in Table 1.
Table 1.
Characteristics of the included studies.
| Trials | Sample size (E/C) | Gender | Age (yr) | clinical stage | Experimental group (E) | Control group (C) | Period | Outcome measure |
|---|---|---|---|---|---|---|---|---|
| Zhu W [17] | 32/32 | M: 16, F: 16/M: 15, F: 17 |
32-74 (61.7)/34-72 (62.8) | III: 15, IV: 17/III: 16, IV: 16 | Cinobufacini injection 30 ml iv. Qd + C | Xelox regimen | 4 weeks | tumor response (WHO), Kamofsky Score, Side-effects of chemotherapy (WHO) |
|
| ||||||||
| Zou H [18] | 30/30 | M: 13, F: 17/M: 21, F: 9 |
59.1/56.5 | III, IV | Cinobufacini injection 20 ml iv. Qd + C | EOF regimen | 6 weeks | tumor response (RECIST), Kamofsky Score, Side-effects of chemotherapy (WHO) |
|
| ||||||||
| Zhang C [19] | 35/32 | M: 28, F: 7/M: 23, F: 9 |
46-82 (64)/42-79 (66) | III: 15, IV: 20/III: 13, IV: 19 | Cinobufacini injection 20ml iv. Qd + C | ELF regimen | 8 weeks | tumor response (UICC), Side-effects of chemotherapy (WHO) |
|
| ||||||||
| Guo C [20] | 43/43 | M: 62, F: 24 |
43-74 (55) | IV | Cinobufacini injection 20 ml iv. Qd + C | Docetaxel | 9 weeks | tumor response (WHO), Kamofsky Score, Side-effects of chemotherapy (WHO), analgesic effect |
|
| ||||||||
| Zhang Y [21] | 28/29 | None | 42-71 (57)/35-69 (54) | IV | Cinobufacini injection 50 ml iv. Qd + C | oxaliplatin + floxuridine | 9 weeks | tumor response (WHO), Kamofsky Score, Side-effects of chemotherapy (WHO), analgesic effect, 1 year and 2 year survival time |
|
| ||||||||
| Chen G [22] | 62/86 | M: 56, F: 30/M: 39, F: 23 |
65-87 (71.8 ± 18.6)/64-89 (73.1 ± 22.3) | IV | Cinobufacini injection 10 ml iv. Tid + C | Capecitabine | 6 weeks | tumor response (WHO), Kamofsky Score, Side-effects of chemotherapy (WHO), overall survival time |
|
| ||||||||
| Xu D [23] | 30/30 | M: 20, F: 10/M: 21, F: 9 |
66.3 ± 4.6/65.0 ± 3.9 | IV | Cinobufacini injection 20 ml iv. Qd + C | Capecitabine | 6 weeks | tumor response (WHO), Kamofsky Score, Side-effects of chemotherapy (WHO), analgesic effect |
|
| ||||||||
| Zhang Z [24] | 30/30 | None | 35-79 (53)/33-75 (56) | IV | Cinobufacini injection 20ml iv. Qd + C | Hydroxycamptothecin | 6 weeks | tumor response (WHO), Kamofsky Score, Side-effects of chemotherapy (WHO), analgesic effect |
|
| ||||||||
| Lu C [25] | 31/31 | M: 34 F: 28 |
37-71 (54 ± 17) | III | Cinobufacini injection 20 ml iv. Qd + C | FOLFOX4 regimen | 9 weeks | tumor response (WHO), immune regulation |
|
| ||||||||
| Wang Y [26] | 36/32 | M: 48 F: 20 |
40-72 (54) | IV | Cinobufacini injection 20 ml iv. Qd + C | FOLFOX4 regimen | 8 weeks | tumor response (WHO), Side-effects of chemotherapy (WHO), |
|
| ||||||||
| Ren L [27] | 32/22 | Unclear | 40-68 (53) | IV | Cinobufacini injection 20 ml iv. Qd + C | FOLFOX regimen | 6 weeks | tumor response (WHO), Side-effects of chemotherapy (WHO), Kamofsky Score, |
|
| ||||||||
| Chen H [28] | 34/33 | M: 20, F: 14/M:20, F: 13 |
50.6/40.9 | III: 23, IV: 11/III: 24, IV: 9 | Cinobufacini injection 30 ml iv. Qd + C | TPF regimen | 6 weeks | tumor response (WHO), Side-effects of chemotherapy (WHO) |
ELF regimen: oxaliplatin + epirubicin + floxuridine; ELF regimen: etoposide + cisplatin+ floxuridine; Xelox regimen: oxaliplatin + capecitabine; FOLFOX4 regimen: oxaliplatin + floxuridine + leucovorin; and TPF regimen: paclitaxel + cisplatin +floxuridine.
3.1.2. Quality Assessment of Included Studies
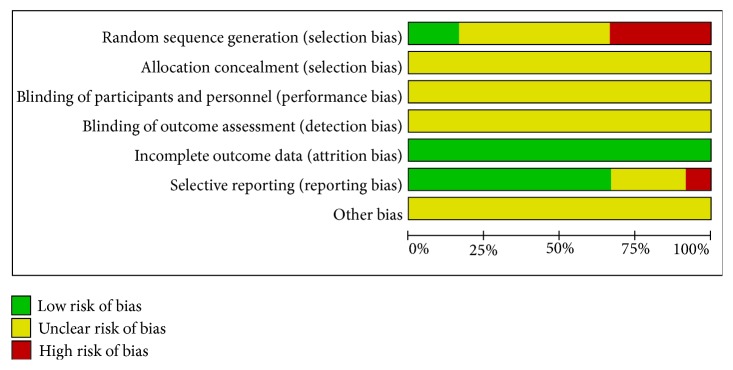
All of the included studies applied randomization methodology, but six of them did not describe the detailed random method. All of the included studies had complete data but none of them mentioned the details of allocation concealment and blinding of participants and personnel and outcome assessment. One study had high risk of reporting bias for its incompleteness of outcome, so it cannot be entered in the meta-analysis (Figures 2 and 3).
Figure 2.
Risk of bias graph.
Figure 3.
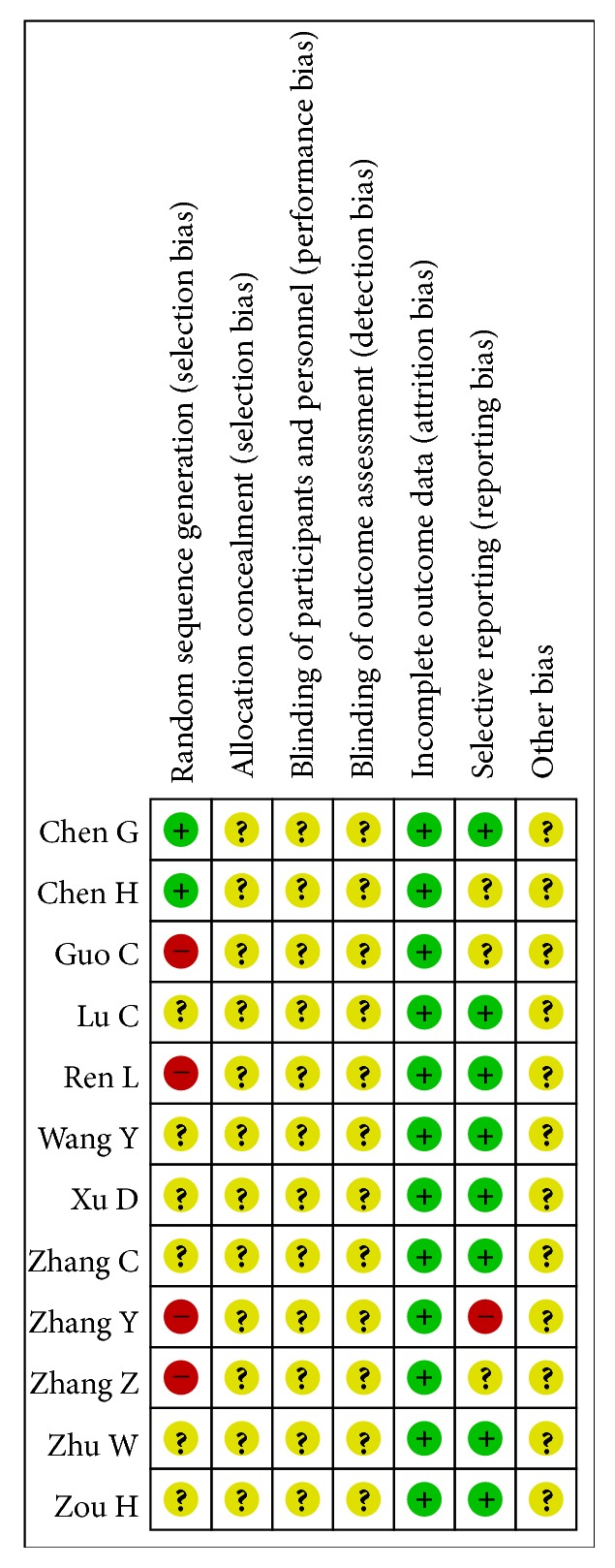
Risk of bias summary.
3.2. Meta-Analysis of Cinobufacini Injection on AGC Treatment
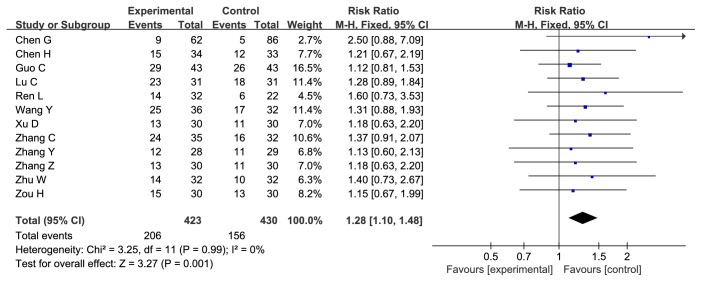
Cinobufacini Injection Could Enhance Response Rate (RR) of Chemotherapy on AGC. All of the twelve studies evaluated the RR. The RR in the experiment group (Cinobufacini injection combined with chemotherapy) was significantly higher than that in the control group (chemotherapy only), with the risk ratio = 1.28, 95% CI: 1.10-1.48, P = 0.001 in the Z test. The result did not indicate the heterogeneity with the Chi2 = 3.25, df = 11, P = 0.99, I2 = 0% (Figure 4).
Figure 4.
Forest plot of RR (risk ratio) for evaluation of response rate in fixed-effect model. The RR of chemotherapy response rate in Cinobufacini injection and chemotherapy group was compared with the chemotherapy group. Individual study is shown in the square with blue color, and the pooled datasets were shown in the diamond, representing the 95% confidence interval (CI) of each study. RR > 1 implied a better chemotherapy response rate of the experimental group. The size of each investigation represented the weighting factor (1/SE) assigned to the study.
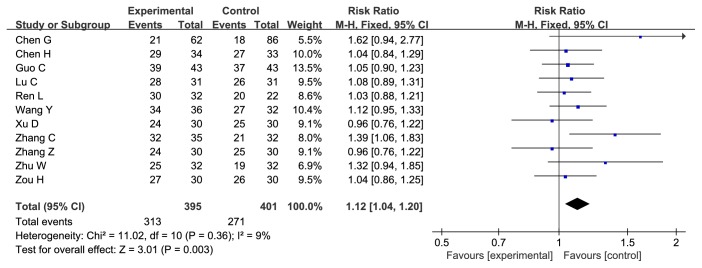
Cinobufacini Injection Could Enhance Disease Control Rate (DCR) of Chemotherapy on AGC. Eleven studies evaluated the DCR which in the experiment group was significantly higher than that in the control group, with the risk ratio = 1.12, 95% CI: 1.04-1.20, P = 0.003 in the Z test. The result did not indicate the heterogeneity with the Chi2 = 11.02, df = 10, P = 0.36, I2 = 9% (Figure 5).
Figure 5.
Forest plot of RR for evaluation of disease control rate in fixed-effect model. The RR of disease control rate in Cinobufacini injection and chemotherapy group was compared with the chemotherapy group. Individual study is shown in the square with blue color, and the pooled datasets were shown in the diamond, representing the 95% confidence interval (CI) of each study. RR > 1 implied a better disease control rate of the experimental group. The size of each investigation represented the weighting factor (1/SE) assigned to the study.
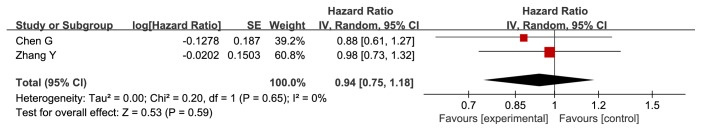
Cinobufacini Injection Could Not Prolong the Overall Survival Time (OS) of AGC Patients. Two studies evaluated the OS of AGC patients. We pooled the hazard ratios (HRs) of OS and the result showed that pooled HR = 0.94, with 95% CI: 0.75-1.18, P = 0.59 in the Z test. The result did not indicate the heterogeneity with the Chi2 = 0.20, df = 1, P = 0.65, I2 = 0% (Figure 6).
Figure 6.
Forest plot of HR (hazard ratio) for evaluation of overall survival in fixed-effect model. The HR of overall survival in Cinobufacini injection and chemotherapy group was compared with the chemotherapy group. Individual studies are shown in the red-colored squares, and the pooled datasets are shown by the diamond, representing the 95% confidence interval (CI) of each study. HR < 1 implied improved overall survival in the experimental group. The size of each investigation represented the weighting factor (1/SE) assigned to the study.
Cinobufacini Injection Could Improve the Life Quality of AGC Patients. KPS is a recognized method for evaluating the quality of life, scoring integer 100 to 0 degressively with the decreased quality of life. Six studies included the KPS evaluation. Cinobufacini injection could improve KPS (KPS enhancement ≥ 10) when combined with chemotherapy, with the risk ratio = 1.83, 95% CI: 1.40-2.39, P < 0.00001 in the Z test. The result did not indicate the heterogeneity with the Chi2 = 4.61, df = 5, P = 0.46, I2 = 0% (Table 2).
Table 2.
Meta-analysis of KPS, side-effects and tumor-related pain.
| Meta-analysis | No. of study | Risk Ratio [%95 CI] | heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| Fix-model | P value | Random-model | P value | I2(%) | P value | ||
| KPS | 6 | 1.83 [1.40, 2.39] | P < 0.00001 | 1.76 [1.35, 2.29] | P < 0.0001 | 0 | 0.46 |
| leukocytopenia | 6 | 0.78 [0.65, 0.93] | P = 0.007 | 0.76 [0.58, 0.99] | P = 0.04 | 50 | 0.07 |
| Grades III-IV leukocytopenia | 4 | 0.61 [0.33, 1.14] | P = 0.12 | 0.58 [0.23, 1.46] | P = 0.25 | 20 | 0.29 |
| nausea and vomiting | 5 | 0.68 [0.53, 0.86] | P = 0.001 | 0.68 [0.48, 0.96] | P = 0.03 | 47 | 0.11 |
| Grades III-IV nausea and vomiting | 4 | 0.34 [0.14, 0.82] | P = 0.02 | 0.44 [0.17, 1.13] | P = 0.09 | 4 | 0.37 |
| hand-foot syndrome | 3 | 0.55 [0.33, 0.91] | P = 0.02 | 0.54 [0.32, 0.91] | P = 0.02 | 0 | 0.48 |
| tumor-related pain | 2 | 1.81 [1.30, 2.54] | P = 0.0005 | 1.83 [1.31, 2.55] | P = 0.0004 | 0 | 0.73 |
| anemia | 3 | 0.79 [0.58, 1.08] | P = 0.14 | 0.80 [0.59, 1.09] | P = 0.15 | 0 | 0.83 |
| diarrhea | 5 | 0.77 [0.52, 1.15] | P = 0.21 | 0.76 [0.51, 1.14] | P = 0.19 | 0 | 0.8 |
| peripheral neurotoxicity | 3 | 0.64 [0.52, 0.80] | P < 0.0001 | 0.57 [0.23, 1.43] | P = 0.23 | 91 | <0.00001 |
| oral mucositis | 2 | 0.46 [0.25, 0.83] | P = 0.01 | 0.37 [0.04, 3.47] | P = 0.39 | 88 | 0.004 |
Cinobufacini Injection Could Reduce the Declination of Leucocyte Count but Could Not Inhibit the Severe Declination (III-IV Degrees). Six studies evaluated the low count of leukocytes of AGC patients. As the result showed Chi2 = 10.08, df = 5, P = 0.07, I2 = 50% which indicated possible heterogeneity. The P values of Z test between experimental group and control group were 0.04 (random-effect model). These results indicated Cinobufacini injection could improve the situation of the low count of leukocytes due to the chemotherapy (Table 2). Four studies evaluated the severe situation of low count of leukocytes and the results showed that Cinobufacini injection could not inhibit the III-IV-degree declination of leukocytes count, with the risk ratio = 0.61, 95% CI: 0.33-1.14, P = 0.12 in the Z test. The result did not indicate the heterogeneity with the Chi2 = 3.77, df = 3, P = 0.29, I2 = 20% (Sup 2, Fig 3).
Cinobufacini Injection Could Reduce the Morbidity of (Severe) Nausea and Vomiting Caused by Chemotherapy. Five studies evaluated the incidence of nausea and vomiting between the two groups and the results showed a significant difference with the risk ratio = 0.68, 95% CI: 0.53-0.86, P = 0.001 in the Z test. The results did not indicate the heterogeneity with the Chi2 = 7.52, df = 4, P = 0.11, I2 = 47% (Table 2). The similar results were seen in four studies that involved Grades III-IV of nausea and vomiting, with the risk ratio = 0.34, 95% CI: 0.14-0.82, P = 0.02 in the Z test. The results did not indicate the heterogeneity with the Chi2 = 3.11, df = 3, P = 0.37, I2 = 4% (Sup 2, Fig 5).
Cinobufacini Injection Could Alleviate Hand-Foot Syndrome (HFS) Induced by Chemotherapy. Some chemotherapeutic drugs such as novel-fluorouracil derivatives could induce HFS sluggish feelings and red or black spots on hands and feet. Three studies evaluated number of HFS cases and the results showed Cinobufacini injection could reduce the morbidity of HFS. The result showed a significant difference with the risk ratio = 0.55, 95% CI: 0.33-0.91, P = 0.02 in the Z test. The results did not indicate heterogeneity with the Chi2 = 1.48, df = 2, P = 0.48, I2 = 0% (Table 2).
Cinobufacini Injection Could Relieve Tumor Pain. Two studies were conducted to evaluate the effectiveness of Cinobufacini injection in managing cancer pain. The result indicated that Cinobufacini injection significantly relieves pain with the risk ratio = 0.1.81, 95% CI: 1.30-2.54, P = 0.0.0005 in the Z test. The result did not indicate heterogeneity with the Chi2 = 0.12, df = 1, P = 0.73, I2 = 0% (Table 2).
Cinobufacini injection could not reduce the incidence of anemia, diarrhea, peripheral neurotoxicity, and oral mucositis caused by chemotherapy (Sup 2, Fig 8-11).
Three studies were conducted to compare the incidence of anemia between experimental and control groups. There were no significant differences in the incidence of anemia between two groups, with the risk ratio = 0.79, 95% CI: 0.58-1.08, P = 0.14 in the Z test. The results did not indicate heterogeneity with the Chi2 = 0.37, df = 2, P = 0.83, I2 = 0%.
Cinobufacini injection could not reduce the morbidity of diarrhea induced by chemotherapy. There was no significant difference between the two groups, with the risk ratio = 0.77, 95% CI: 0.52-1.15, P = 0.21 in the Z test. The results did not indicate the heterogeneity with the Chi2 = 1.65, df = 4, P = 0.80, I2 = 0%. The similar results were shown in four studies that involved the incidence of III-IV degree diarrhea with the risk ratio = 0.33, 95% CI: 0.08-1.38, P = 0.13 in the Z test. The results did not indicate heterogeneity with the Chi2 = 0.18, df = 2, P = 0.91, I2 = 0%.
Cinobufacini injection could not reduce the incidence of peripheral neurotoxicity and oral mucositis. Three studies and two studies evaluated the recurrence of peripheral neurotoxicity and oral mucositis accordingly. There were no significant differences between experimental group and control group in the incidence of peripheral neurotoxicity and oral mucositis with the P = 0.23 and 0.39 accordingly. Significant heterogeneities were detected with P < 0.0.01 and I2 = 91% and 88% accordingly.
3.3. Sensitivity Analysis
We conducted the sensitivity analysis to strengthen the reliability of the results of response rate and disease control rate. The sensitivity analysis showed the same effect sizes among a fixed-effect model and a random-effect model of the response rate analysis, disease control rate analysis, and hazard ratio analysis. The same effects were shown in other outcome measures in the sensitive analysis except in the analysis of Grades III-IV nausea and vomiting, peripheral neuropathy, and oral mucositis (Table 2). By taking into consideration the heterogeneity, we adopted the corresponding result when there were inconsistent results in sensitivity analysis.
3.4. Publication Bias
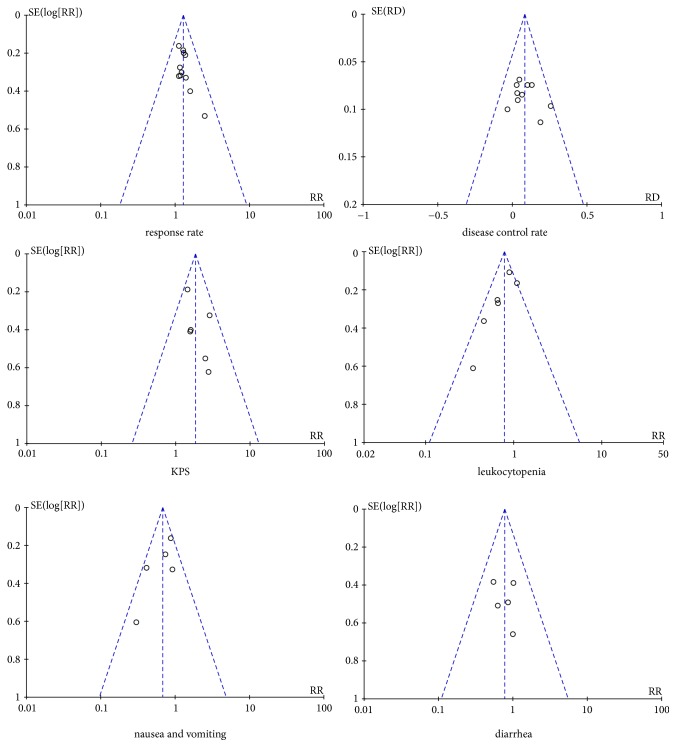
The funnel plots (Figure 7) were not strictly symmetrical in the meta-analysis of response rate, disease control rate, KPS, and diarrhea. But Egger's test (Table 3) showed that there was no significant publication bias among the studies except the meta-analysis of disease control rate (P = 0.004) and diarrhea (P = 0.026).
Figure 7.
Funnel plots of response rate, disease control rate, KPS, leukocytopenia, and nausea, vomiting, and diarrhea.
Table 3.
Egger's test.
| Meta-analysis of publication bias | P value |
|---|---|
| response rate | 0.114 |
| disease control rate | 0.004 |
| KPS | 0.250 |
| leukocytopenia | 0.224 |
| nausea and vomiting | 0.177 |
| diarrhea | 0.026 |
4. Discussion
Gastric cancer has a high morbidity around the world. The comprehensive treatment including surgery, chemotherapy, radiotherapy, targeted therapy, support treatment, and treatment of TCM is the optimal treatment for gastric tumor. Chemotherapy is one of the most important treatments for advanced gastric cancer (AGC), but the response rate is far from satisfactory so far. The combination of TCM and modern medical treatments has been proved effective on AGC. For instance, a research showed that TCM herbal formula of invigorating spleen could prolong the median overall survival time and improve the prognosis of patients with AGC [7]. On fundamental research, A. cucullata, an extractive from TCM herb Alocasia cucullata (Lour.) G. Don, was reported to have a potent antigastric cancer activity both in vitro and in vivo via antiproliferation of G0/G1 arrest and cell proapoptosis, including PI3K/Akt pathway, ERK activity, stimulated cytochrome C release, and caspase 3/7 activity accompanied with an increase of Bax/Bcl-2 ratio [31]. As a kind of TCM extractive, Cinobufacini could suppress the cell proliferation of BGC-823 human gastric cancer cells via targeting BAG-1 (an antiapoptosis gene) and inhibit tumor growth and metastasis in xenograft models [8, 14, 32]. These may partially explain the mechanisms of how TCM and Cinobufacini injection inhibit gastric cancer. Some researchers started to work on the antitumor components of Cinobufacin injection, and Bufadienolides might be one of the antitumor agents in treating gastric cancer [33]. Further studies are needed to clarify how Cinobufacini injection could benefit cancer patients.
In this review, we comprehensively reviewed the literature on the efficacy comparison between Cinobufacini injection combined with chemotherapy and chemotherapy solely used in AGC treatment. Our results indicated that Cinobufacini injection could enhance the response rate and disease control rate of chemotherapy, which meant the experiment group had a better short-term efficacy than that in the control group. However, due to insufficient data, only two of our included studies included overall survival time, and our results showed that Cinobufacini injection could not prolong the overall survival time. High life quality is also important for tumor patients' living and recovery. Our study showed that Cinobufacini injection improved the life quality of AGC patients receiving chemotherapy by enhancing their KPS.
Side-effects such as myelosuppression and gastrointestinal toxicity constantly occur in tumor patients undergoing chemotherapy, which cause them great trouble. TCM plays an important role in alleviating side-effects when used in combination with chemotherapy. For instance, a double-blind clinical trial showed that the standardized ginger extract (the extract from a kind of traditional medicine in Asian countries to treat nausea and vomiting) acted as an antiemetic against chemotherapy-induced nausea and vomiting [34]. A meta-analysis based on eight trails indicated that Chinese herb medicine significantly protected peripheral blood WBCs from decreasing during the course of chemotherapy or radiotherapy [35]. Astragalus membranaceus was also proved to have a myelo-protective and myelo-therapeutic capacity against the chemotherapy-induced myelosuppression, evidenced at both laboratory and morphological levels in basic study [36]. Our results indicated that Cinobufacini injection could inhibit the declination of leukocytes in peripheral blood and allay nausea and vomiting caused by chemotherapy, but it could not prevent myelosuppression or gastrointestinal toxicity which commonly present as anemia or diarrhea. Most tumor patients suffer from cancer pain, which even painkillers cannot cure. Some Chinese herbal injectionsare proved to improve clinical efficacy and relieve adverse reactions when combined with the FOLFOX regimen in treating gastric cancer [37]. Chinese medicine such as Fufang Kushen injection could reduce cancer pain directly by blocking TRPV1 signaling pathway [38]. Cinobufacini injection could help to relieve cancer pain as well based on our evaluation, but the exact mechanisms of these effects remain unclear.
However, this study has its own limitations. First, allocation concealment and blinding of all the included studies were unclear and there was publication bias in some evaluations since the included studies were all published in Chinese. Second, we failed to evaluate the long-term effects, because the treatment periods of included studies were generally short and they did not include long-term follow-ups. Thus, the long-term effects of Cinobufacini injection on AGC patients remain unclear. Third, the criteria for the evaluation of tumor response varied from one study to another, which might bring different results in subgroup analysis in RR and DCR evaluation. Taking into consideration all the above reasons, the evidence for this study might be insufficient. Although the above questions might exist that prevent us from drawing a definite conclusion about Cinobufacini injection, our study still provided helpful information for clinical practice that Cinobufacini injection could enhance the efficacy of other treatments in AGC patients, reduce the side-effects induced by chemotherapy, and help to relieve cancer pain, which might be helpful for clinical medication. However, in order to draw precise conclusion, more well-designed clinical trials with long-term follow-ups of Cinobufacini injection are needed for future study.
Acknowledgments
This work is supported by the National Natural Science Foundation (81774294 and 81673961).
Contributor Information
Qiujun Guo, Email: drguoqiujun@126.com.
Honggang Zheng, Email: honggangzheng@126.com.
Baojin Hua, Email: dr.huabaojin@hotmail.com.
Data Availability
All the data are included in this article and its supplementary information files.
Conflicts of Interest
The authors disclose no conflicts of interest.
Supplementary Materials
Supplementary File: forest plots of KPS, side-effects, and tumor-related pain (DOC). Supplement 1: forest plots of response rate, overall survival, and disease control rate (DOC). Supplement 2: sensitivity analysis (DOC). Supplement 3: publication bias of the meta-analysis (DOC).
References
- 1.Xu H.-B., Huang F., Su R., Shen F.-M., Lv Q.-Z. Capecitabine plus oxaliplatin (XELOX) compared with 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOXs) in advanced gastric cancer: Meta-analysis of randomized controlled trials. European Journal of Clinical Pharmacology. 2015;71(5):589–601. doi: 10.1007/s00228-015-1828-9. [DOI] [PubMed] [Google Scholar]
- 2.Sudo K., Yamada Y. Advancing pharmacological treatment options for advanced gastric cancer. Expert Opinion on Pharmacotherapy. 2015;16(15):2293–2305. doi: 10.1517/14656566.2015.1080238. [DOI] [PubMed] [Google Scholar]
- 3.Guo Q., Lin J., Liu R., et al. Review on the Applications and Molecular Mechanisms of Xihuang Pill in Tumor Treatment. Evidence-Based Complementary and Alternative Medicine. 2015;2015:10. doi: 10.1155/2015/854307.854307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao Y., Wang G., Gao Y., et al. Topical treatment with Xiaozheng Zhitong Paste alleviates bone cancer pain by inhibiting proteinase-activated receptor 2 signaling pathway. Oncology Reports. 2015;34(3):1449–1459. doi: 10.3892/or.2015.4073. [DOI] [PubMed] [Google Scholar]
- 5.Guo Q., Li J., Lin H. Effect and Molecular Mechanisms of Traditional Chinese Medicine on Regulating Tumor Immunosuppressive Microenvironment. BioMed Research International. 2015;2015:12. doi: 10.1155/2015/261620.261620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu H., Liu T.-G., Zhang Z., Yi C. Malignant gastric cancer cured by short-term chemotherapy and long-term use of combined Chinese medicine: A case report. Chinese Journal of Integrative Medicine. 2012;18(10):788–789. doi: 10.1007/s11655-012-1232-6. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y., Zhao A. G., Li Z. Y., et al. Survival benefit of traditional chinese herbal medicine (a herbal formula for invigorating spleen) for patients with advanced gastric cancer. Integrative Cancer Therapies. 2013;12(5):414–422. doi: 10.1177/1534735412450512. [DOI] [PubMed] [Google Scholar]
- 8.Shen Z., Li Y., Zhao C., Wang F., Zhou R., Chen G. MiR-494-BAG-1 axis is involved in cinobufacini-induced cell proliferation and apoptosis in gastric cancer. Molecular Medicine Reports. 2018;17(5):7435–7441. doi: 10.3892/mmr.2018.8788. [DOI] [PubMed] [Google Scholar]
- 9.Chen D., Chen J., Guo Y., Li Y. Cinobufacini promotes apoptosis of bladder cancer cells by influencing the expression of autophagy-related genes. Oncology Letters. 2018;15(5):7104–7110. doi: 10.3892/ol.2018.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi F., Li A., Inagaki Y., et al. Induction of apoptosis by cinobufacini preparation through mitochondria- and Fas-mediated caspase-dependent pathways in human hepatocellular carcinoma cells. Food and Chemical Toxicology. 2012;50(2):295–302. doi: 10.1016/j.fct.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 11.Wang J.-Y., Chen L., Zheng Z., Wang Q., Guo J., Xu L. Cinobufocini inhibits NF-κB and COX-2 activation induced by TNF-α in lung adenocarcinoma cells. Oncology Reports. 2012;27(5):1619–1624. doi: 10.3892/or.2012.1647. [DOI] [PubMed] [Google Scholar]
- 12.Jiarui W., Jiaping X., Kaihuan W., Mengwei N., Dan Z., Xiaojiao D. Meta-analysis on the Randomized Controlled Trials of Huachansu Injection in the Treatment of Liver Cancer. Chinese Journal of Pharmacoepidemiology. 2018;27(2):92–97. [Google Scholar]
- 13.Zhou B., Wu F., Yuan L., Miao Z., Zhu S. Is Huachansu Beneficial in Treating Advanced Non-Small-Cell Lung Cancer? Evidence from a Meta-Analysis of Its Efficacy Combined with Chemotherapy. Evidence-Based Complementary and Alternative Medicine. 2015;2015:11. doi: 10.1155/2015/408145.408145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin J., Zhu X., Shi W., Liu L. Huachansu injection inhibits metastasis of pancreatic cancer in mice model of human tumor xenograft. BMC Complementary and Alternative Medicine. 2014;14(1) doi: 10.1186/1472-6882-14-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100.e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu W., Li Y., Hou F., Chen M., Zhou Y. Efficacy of Cinobufacini combined with CapeOX regimen in treatment of advanced gastric cancer. China Medical Herald. 2012;09(5):35–36. [Google Scholar]
- 18.Zou H., Guo X., Zhu Y. Clinical Research on Huachansu with EOF Regimen in Patients with Advanced Gastric Cancer. Chinese Journal of Clinical Medicine. 2012;19(2):140–141. [Google Scholar]
- 19.Zhang C., Wang Q. Efficacy of Cinobufacini combined with ELF regimen in treatment of advanced gastric cancer: a report of 35 cases. Journal of Anhui Traditional Chinese Medical College. 2001;20(4):18–19. [Google Scholar]
- 20.Guo C., Yu T., Zhang H., Xing J. The observation of clinical therapeutic effect of Cinobufacini combined with Docetaxel on advanced stomach cancer. China Medical Herald. 2011;8(28):54–55. [Google Scholar]
- 21.Zhang Y., Zhu M., Cao Y., Zhang P., Yao L., Hong H. Effect of Cinobufacini combined with LF+ L-OHP regimen on middle and advanced stomach cancer. Henan Joural of Oncology. 2005;18(5):359–360. [Google Scholar]
- 22.Chen G., Jin D., Li M. Efficacy of Cinobufacini Combined with Xeloda in Treatment of Older Patients with Advanced Gastric Cancer: A Report of 62 Cases. zhejiang Journal of Traditional Chinese Medicine. 2012;47(6):462–463. [Google Scholar]
- 23.Xu D., Liu L. Clinical Observation of Cinobufotalin Combined with Capecitabine for Gastric Cancer in Elderly Patients. The Practical Journal of Cancer. 2015;30(3):405–407. [Google Scholar]
- 24.Zhang Z., Wang Y., Wang D. The short-term therapeutic effect of cinobufacini combined with hydroxycamptothecin for advanced stomach cancer. Practical Journal of Medicine Pharmacy. 2006;23(7):794–795. [Google Scholar]
- 25.Lu C., Hong M., Liu K., You J. Clinical observations of Cinobufacini combined with neoadjuvant chemotherapy in the treatment of advanced stomach cancer. Traditional Chinese Medicine Journal. 2002;13(3):41–43. [Google Scholar]
- 26.Wang Y. The observation of clinical therapeutic effect of Cinobufacini injection combined with FOLFOX4 regimen on advanced stomach cancer. Jiangxi Journal of Traditional Chinese Medicine. 2009;40(4):31–32. [Google Scholar]
- 27.Ren L., Wang Y., Ha M. Efficacy of Cinobufacini in treatment of advanced gastric cancer. China Journal of Chinese Materia Medica. 2008;33(12):1474–1475. [Google Scholar]
- 28.Chen H. Efficacy of Cinobufacini combined with TPF regimen in treatment of advanced gastric cancer. Journal of Emergency in Traditional Chinese Medicine. 2009;18(1):437–448. [Google Scholar]
- 29.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8, article 16 doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins J. P. T., Altman D. G., Gøtzsche P. C., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. British Medical Journal. 2011;343(7829) doi: 10.1136/bmj.d5928.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei P., Zhiyu C., Xu T., Xiangwei Z. Antitumor effect and apoptosis induction of Alocasia cucullata (Lour.) G. Don in human gastric cancer cells in vitro and in vivo. BMC Complementary and Alternative Medicine. 2015;15(1) doi: 10.1186/s12906-015-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou R.-P., Chen G., Shen Z.-L., Pan L.-Q. Cinobufacin suppresses cell proliferation via miR-494 in BGC-823 gastric cancer cells. Asian Pacific Journal of Cancer Prevention. 2014;15(3):1241–1245. doi: 10.7314/APJCP.2014.15.3.1241. [DOI] [PubMed] [Google Scholar]
- 33.Wei X., Si N., Zhang Y., et al. Evaluation of Bufadienolides as the Main Antitumor Components in Cinobufacin Injection for Liver and Gastric Cancer Therapy. PLoS ONE. 2017;12(1) doi: 10.1371/journal.pone.0169141.e0169141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marx W., McCarthy A. L., Ried K., et al. The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: A double blind, randomized, placebo controlled trial. Nutrients. 2017;9(8) doi: 10.3390/nu9080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jia Y., Du H., Yao M., et al. Chinese Herbal Medicine for Myelosuppression Induced by Chemotherapy or Radiotherapy: A Systematic Review of Randomized Controlled Trials. Evidence-Based Complementary and Alternative Medicine. 2015;2015:12. doi: 10.1155/2015/690976.690976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismail Z. M. K., Amin N. M. A., Yacoub M. F. Y., Mohamed A. M. O. Myelo-enhancement by astragalus membranaceus in male albino rats with chemotherapy myelo-suppression. Histological and immunohistochemical study. International Journal of Stem Cells. 2014;7(1):12–22. doi: 10.15283/ijsc.2014.7.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang D., Zheng J., Ni M., et al. Comparative efficacy and safety of Chinese herbal injections combined with the FOLFOX regimen for treating gastric cancer in China: a network meta-analysis. Oncotarget . 2017;8(40):68873–68889. doi: 10.18632/oncotarget.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Z., Fan H., Higgins T., et al. Fufang Kushen injection inhibits sarcoma growth and tumor-induced hyperalgesia via TRPV1 signaling pathways. Cancer Letters. 2014;355(2):232–241. doi: 10.1016/j.canlet.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File: forest plots of KPS, side-effects, and tumor-related pain (DOC). Supplement 1: forest plots of response rate, overall survival, and disease control rate (DOC). Supplement 2: sensitivity analysis (DOC). Supplement 3: publication bias of the meta-analysis (DOC).
Data Availability Statement
All the data are included in this article and its supplementary information files.