Abstract
In this paper, we examined three different sequential coprecipitation schemes based on Mg(OH)2 and CaF2 precipitation using triethylamine (TEA) and hydrofluoric acid (HF), respectively, for determination of cadmium (Cd) impurities from multivitamin/mineral (MVM) supplements by isotope dilution (ID) inductively coupled plasma mass spectrometry (ICP-MS). The schemes involved three-step coprecipitation with either TEA alone or in combination with HF and are designated as Scheme 1 (TEA-TEA-TEA), Scheme 2 (TEA-HF-TEA) and Scheme 3 (HF-TEA-TEA) according to the addition sequence of each reagent. Experiments were carried out with MVM solutions spiked with 60 μg L−1 Cd from a multielement standard solution. All schemes provided quantitative separation of Cd from MVM matrix. Scheme 1 was the least effective in removal of interfering concomitant elements, molybdenum (Mo) and tin (Sn). Scheme 2 performed better for Sn, but failed in eliminating Mo. Scheme 3 was the most effective in eliminating both Mo and Sn. Mo levels in test MVM solutions reduced from 4.3 μg mL−1 to as low as 0.014 μg mL−1 while that for Sn decreased from 0.5 μg mL−1 to 0.018 μg mL−1 allowing interference-free determination of Cd to be achieved. Salt-matrix due to Mg, Ca, P and K along with the essential elements (Mn, Fe, Cu and Zn) levels was also reduced significantly. Reagent blanks from HF and TEA were insignificant (0.008 μg L−1) allowing a limit of detection of 0.004 μg L−1 or 0.26 ng g−1 Cd to be achieved (3σ, n = 6). The performance of the coprecipitation method (Scheme 3) was validated by determination of Cd in multivitamin/multielement tablets certified reference material (SRM 3280) by ID-ICP-MS. Experimental results (ng g−1) and recoveries were 78.8 ± 4.7 (98.5%), 77.9 ± 5.2 (97.4%) and 76.5 ± 4.8 (95.6%) for 110Cd, 111Cd and 114Cd isotopes, respectively. Several commercial MVM supplements were analyzed using the method. Mean Cd concentration ranged from 21.4 ng g−1 to 93.3 ng g−1. These values are much lower than those reported to date for various MVM supplements by ICP-MS determinations without chemical separation.
Keywords: Cadmium, multivitamin/mineral supplement, coprecipitation, hydrofluoric acid, triethylamine, ICP-MS
1. Introduction
Multivitamin/mineral (MVM) dietary supplements contain various vitamins, minerals, herbs or herbal extracts, amino acids [1–9]. The consumption of these dietary supplements has significantly increased over the last two decades as low cost remedies for boosting physiological well-being [10]. Despite the increasing consumption, consumers are often not knowledgeable about the accuracy of the contents provided in the labels and the levels of heavy metal contaminants. These products are regulated under the Dietary Supplement Health and Education Act of 1994 by the US Food and Drug Administration (FDA). According to current FDA regulations, however, manufacturers are to ensure the safety and accuracy of the contents of their products which should not contain any detrimental components, such as heavy metals [7–9]. In recent years, there has been a growing community effort for characterization of elemental composition of MVM supplements to assess public safety [6,8,9,11–13]. A number of studies have shown that MVM supplements indeed contain significant levels of heavy metals, such as As and Pb [8,14–16].
Cadmium (Cd) is a toxic heavy metal found at trace levels in dietary MVM supplements that mostly originate from raw materials and chemical methods used in preparation [2]. Even at trace levels, chronic exposure to Cd is a concern due to long biological half-life (15–30 years) in the human body resulting in nephrotoxicity and kidney failure [17,18]. Thus, accurate quality control of Cd levels in MVM supplements is critical by using sensitive techniques to prevent accidental public exposure. Inductively coupled plasma mass spectrometry (ICP-MS), owing to its sensitivity and multi-element analysis capability, has been used in a number of studies to determine various essential elements and heavy metals in dietary MVM supplements [2,6,9,12,19,20]. Despite exceptional sensitivity, determination of Cd from MVM supplements by ICP-MS is, however, very difficult due to the difficulties associated with the interfering matrix. Almost all commercial MVM supplements contain substantial levels of molybdenum (Mo) and tin (Sn) as trace element nutrients. Tin (Sn) isotopes, 112Sn (0.97%), 114Sn (0.65%), 116Sn (14.53%) exhibit isobaric overlaps on 112Cd (24.13%), 114Cd (28.73%), 116Cd (7.49%). The scenario with Mo is even worse. Oxides of molybdenum, 94Mo16O, 95Mo16O, 96Mo16O, 97Mo16O, 98Mo16O, 100Mo16O overlap on 110Cd, 111Cd, 112Cd, 113Cd, 114Cd, 116Cd, respectively. Neither high resolution (HR) ICP-MS nor collision/reaction cell ICP-MS can fully eliminate the isobaric and molecular ion overlaps from Mo and Sn in complex MVM samples without removal of Mo and Sn matrices.
Besides matrix removal, the use of matrix-matched quality control or certified reference materials is important to verify the accuracy of Cd determinations in MVM samples. To date most studies concerning Cd determination from dietary MVM samples with ICP-MS are based on direct analysis utilizing plant and tissue reference materials for quality control [2,12,19]. However, plant or tissue matrices are not ideal surrogates for Cd determinations as they do not contain elevated levels of Mo and Sn, and thus would not mimic the effects of MVM matrix on Cd. A certified reference material of multivitamin/multielement tablets (SRM 3280) is available from the U.S. National Institutes of Standard and Technology (NIST). Nevertheless, direct determination of Cd accurately from SRM 3280 is virtually not feasible by ICP-MS due to high Mo (70 ± 4.5 μg g−1) and Sn (11.1 ± 0.9 μg g−1) content besides the complexity of the material. For instance, Avula et al. [19] used NIST SRM 3280 (multivitamin/multielement tablet) and SRM 1566b (oyster tissue) in their analysis of a number of dietary supplements by collision cell technology (CCT) ICP-MS, but utilized SRM 1566b for validation of Cd determinations that has relatively high Cd concentration (2.48 ± 0.08 μg g−1) without any significant Sn or Mo. This was presumably due to the difficulties in measuring low levels of Cd in SRM 3280 (0.08015 ± 0.0086 μg g−1) due to spectral interferences of Sn and molybdenum oxides, indicating the pressing need for matrix elimination approaches for Cd determinations. Recently, Thompson and Christopher developed a 4-step matrix removal method based on solid phase extraction (SPE) with thiourea and magnesium hydroxide coprecipitation followed by anion exchange separation to separate Cd from SRM 3820 matrix [21] In a follow-up report, they interfaced this method with isotope dilution (ID) for comparative validation of Cd in SRM 3280 by ID-CCT-ICP-MS and ID-HR-ICP-MS [22].
In the present work, a three-step sequential coprecipitation method is described for determination of trace levels of Cd from MVM supplements by ICP-MS. The objective of the study was to (1) remove interfering Mo and Sn matrix from MVM solutions and (2) alleviate the levels of other matrix elements, including Mg, Ca, Fe, and Zn etc. Hydrofluoric acid (HF) and triethylamine (TEA) were used to selectively isolate Cd quantitatively from MVM solutions. Various coprecipitation schemes were examined for effective removal of Mo and Sn. The optimum scheme starts with HF that partially removes Mo and Sn matrices while quantitatively retaining Cd in the MVM solution. Magnesium hydroxide, Mg(OH)2, coprecipitation was performed with TEA on the resulting supernatant solution. Cd in the supernatant solution was scavenged onto Mg(OH)2. The pellet was dissolved in dilute nitric acid (HNO3) and precipitated for a third time with TEA. Under the optimized conditions, Mo and Sn were successfully eliminated from analysis solutions. Isotope dilution (ID) analysis was used for validation of the optimized method for determination of Cd in SRM 3280 by ICPMS, and then applied to determination of Cd impurities in several commercially available MVM supplements.
2. Experimental
2.1. Reagents and materials
Triethylamine (Trace metal grade, 99.8%, Lot# A0374495) was purchased from Acros Organics (Fair Lawn, NJ). Trace metal grade hydrofluoric acid (HF) and nitric acid (HNO3) were obtained from Fisher Scientific. A multielement standard solution containing 10 μg mL−1 of Al, As, Ba, Cd, Ca, Co, Cr, Cu, Fe, Ga, K, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, Tl, V and Zn in 5% HNO3 was prepared from 1000 μg mL−1 single element stock solutions (High Purity Standards, Fisher Scientific). Tin standard solution (10 μg mL−1) was made separately in 2% HCl and 2% HNO3 from 1000 μg mL−1 single element solution. Enriched cadmium metal (113Cd, 95.83%, Lot # 126-4) was purchased from Trace Sciences International, Ontario Canada. It was dissolved in 2 mL concentrated HNO3 to make a 100 μg mL−1 113Cd stock solution. A 1.0 μg mL−1 113Cd solution was prepared in 5% HNO3 for isotope dilution measurements.
Multivitamin/multielement tablets certified reference material (SRM 3280) was purchased from National Institutes of Standards and Technology (NIST, Gaithersburg, MD) and utilized for method validation. Several commercially available MVM preparations in the form of hard tablets were purchased from local pharmacy and retail stores and analyzed for Cd impurities. These included Century Adults (MVM-1), Rite Aid MVM (MVM-2), Kroger Complete MVM Supplement (MVM-3), Centrum Adults MVM (MVM-4), Equate MVM (MVM-5) and Walgreens MV (Adults 50+) (MVM-6). All standard and samples solutions are prepared with dilute HNO3 or HCl prepared on a volume by volume basis (v/v) with double deionized water (18.2 MΩ cm resistivity).
2.2. Instrumentation
Measurements were carried out by using a Varian 820MS inductively coupled plasma mass spectrometer (Varian, Australia). The instrument was equipped with a peltier-cooled double-pass glass spray chamber, an teflon Ari-mist nebulizer (SCP Science, Champlain NY), quartz torch, CRI-type Pt sampler and skimmer cones and all-digital detector (DDEM, Model AF250, ETP Australia) providing nine decades of linear dynamic range. Samples were introduced manually. The instrument was optimized daily with 5 μg L−1 138Ba, 25Mg, 115In, 140Ce, 208Pb solution for optimal sensitivity, oxides (156CeO+/140Ce+ < 3%) and doubly charged ions (138Ba2+/138Ba+ < 2%). Data collection was achieved by ICP-MS Expert software package (version 2.2 b126). The operating parameters of the instrument are summarized in Table 1. Method development studies were conducted without isotope dilution. 110Cd, 111Cd and 114Cd isotopes were monitored, and 5 μg L−1 solution of germanium (Ge), rhodium (Rh), rhenium (Re) was used as internal standard solution for compensating the effects of matrix suppression and instrumental drift. The internal standard solution was mixed on-line with the sample solution.
Table 1.
Operating parameters for Varian 820-MS ICP-MS instrument
| RF Power | 1.4 kW |
| Plasma argon flow | 17 L min−1 |
| Auxiliary argon flow | 1.7 L min−1 |
| Nebulizer argon flow | 1.1 L min−1 |
| Sheath argon flow | 0.15 L min−1 |
| Sampling depth | 6.5 mm |
| Pump rate | 6 rpm; 0.2 L min−1 |
| Stabilization time | 20 s |
| Spray chamber temperature | 4 °C |
| Scan mode | Peak hopping |
| Dwell time | 50 ms |
| Points/peak | 1 |
| Scans/peak | 5 |
| Scans/replicate | 3 |
2.3. MVM digestion procedure for preparing stock solution
For method development and optimization, an MVM stock solution was prepared by digesting a sample of RiteAid MVM tablets. This brand was chosen as it possessed the highest declared Mo concentration (160 μg per 1.5 g tablet). A sample of 3 tablets (4.5 g, n = 4) were digested in 10 mL HNO3 and 10 mL H2O2 in teflon vessels (Savillex) on a digestion block (DigiPrep, SCP Science, Champlain, NY). A total of 18 g MVM sample (12 tablets) were digested simultaneously. Initially, MVM samples were digested in 10 mL HNO3 for 3 h at 120 °C. Then, digestion vessels were opened and H2O2 was added slowly under heating. The vessels were closed and the contents were heated for another 2 at 140 °C. After digestion, the MVM solutions were heated to near dryness to evaporate HNO3. Then, the residue was dissolved with 5 mL of 0.5% HNO3, warmed gently and then transferred to a 50-mL tube. Digestion vessels were rinsed with 0.5% HNO3 and added to 50-mL tubes. Finally volume was completed to 25 mL with 0.5% HNO3. These solutions contained undissolved TiO2 and SiO2 that were removed via centrifugation. Briefly, the MVM solutions were centrifuged for 30 min at 10,000 rpm on a centrifuge (Thermo Scientific Sorvall ST 16). Then, the supernatant portions were combined in an acid-cleaned polypropylene bottle and completed to 400 mL with 0.5% HNO3.
2.4. Optimization of coprecipitation scheme
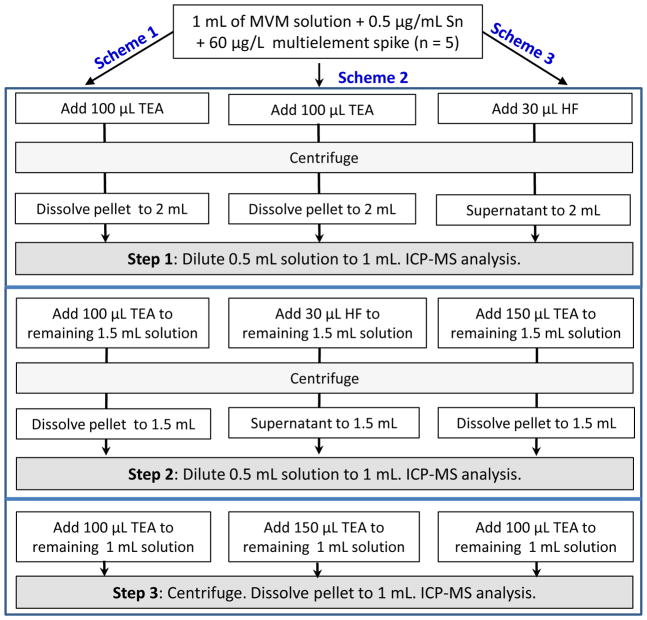
MVM supplements contain high levels of Ca and Mg matrices. Previously, we reported that both HF and TEA were effective reagents for separation of Cd from saline samples containing high Ca and Mg, such as otoliths and seawater [23, 24]. While TEA enables coprecipitation of Cd(II) onto magnesium hydroxide (Mg(OH)2), adding HF into a solution of high calcium matrix resulted in removal of Mo and Sn via calcium fluoride (CaF2) precipitation. Based on this information, sequential coprecipitations with TEA alone and in combination with HF were examined to determine the most effective route for quantitative separation of Cd and removal of interfering Mo and Sn matrices from MVM solutions. These trials are designated as Scheme 1, Scheme 2 and Scheme 3 (see Fig. 1) and are described in detail below. It should be noted here that isotope dilution analysis was not employed during these trials. All Cd spikes were added from 1.0 μg mL−1 multielement solution containing the natural isotopes of the elements listed in section 2.1.
Fig. 1.
Sequential coprecipitation schemes examined for separation of Cd from MVM matrix. Steps 1, 2, and 3 refer to sampling after treatments with TEA or HF. Five preparations were made in each scheme (n = 5).
Scheme 1 (TEA-TEA-TEA)
A 3-step sequential coprecipitation was performed with TEA. A volume of 1 mL of MVM stock solution ( n=5) was placed into a 2-mL microcentrifuge tube and spiked with 60 μL of 1.0 μg mL−1 multielement standard solution. A volume of 100 μL TEA was added to the solution to precipitate Mg available in MVM solution as Mg(OH)2. Cd(II) was scavenged from the solution onto the Mg(OH)2 colloids. The MVM solutions were allowed for 5 min for precipitation and then centrifuged at 10,000 rpm using Eppendorf 5415D centrifuge. The supernatant solution was discarded. The pellet was dissolved in 1.5 mL of 5% HNO3 and then completed to 2 mL with deionized water. Of this solution, 0.5 mL was taken and completed to 1 mL with 5% HNO3 (Step 1). The remaining 1.5 mL solution was coprecipitated with 100 μL TEA for a second time and processed similarly. The resulting pellet was dissolved in 1 mL of 5% HNO3. At this stage, 1 mL 5% HNO3 was sufficient for dissolution of the pellet due to reduced amount of Mg(OH)2 precipitation. The solution was completed to 1.5 mL with deionized water, mixed well, and then 0.5 mL was taken and completed to 1 mL with 5% HNO3 (Step 2). In the third coprecipitation, 100 μL TEA was added to the remaining 1 mL MVM solution, allowed for for 10 min and then centrifuged. After discarding the supernatant solution, the pellet was gently washed with deionized water and dissolved in 1 mL of 5% HNO3 (Step 3).
Scheme 2 (TEA-HF-TEA)
In this scheme, first coprecipitation was performed as described in Scheme 1 on a 1 mL MVM solution (n = 5) containing 60 μg L−1 multielement spike (60 μL of 1.0 μg mL−1). A volume of 0.5 mL solution was diluted to 1 mL with 5% HNO3 in a 2-mL centrifuge tube (Step 1). Onto the remaining 1.5 mL solution, 30 μL HF was added that resulted in precipitation of CaF2. The CaF2 colloids developed relatively slowly in comparison to Mg(OH)2. Solutions showed turbidity within 30 s to 1 min and thus allowed for 15 to 20 min for completion of precipitation. Then, they were centrifuged similarly. After centrifugation, the supernatant solution was transferred to another 2-mL microcentrifuge tube. A volume of 0.5 mL was transferred to a 2-mL microcentrifuge tube and completed to 1 mL with deionized water (Step 2). To the remaining 1 mL solution, 150 μL TEA was added to coprecipitate Cd(II) back onto Mg(OH)2. Solution was allowed for 15 min and then centrifuged. After centrifugation, the pellet was gently washed and dissolved in 1 mL of 5% HNO3 (Step 3).
Scheme 3 (HF-TEA-TEA)
CaF2 coprecipitation was performed first by adding 30 μL HF into a 1 mL MVM solution (n = 5) that contained 60 μg L−1 multielement spike (60 μL of 1.0 μg mL−1). Precipitation was fast in the untreated MVM solution with high Ca content. Solutions were centrifuged as described in Scheme 1. Then the supernatant portions were transferred to another 2-mL microcentrifuge tubes and completed to 2 mL with deionized water. From this solution, 0.5 mL was taken and completed to 1 mL in a 2-mL microcentrifuge tube (Step 1). To the remaining 1.5 mL solution, 150 μL TEA was added for Mg(OH)2 coprecipitation. After waiting for 10–15 min, the solutions were centrifuged. The resulting pellets were dissolved in 1 mL of 5% HNO3 and then completed to 1.5 mL with deionized water. From this solution, 0.5 mL was taken and diluted to 1 ml with 5% HNO3 (Step 2). The remaining 1 mL solution was coprecipitated for a third time with 100–120 μL TEA. After centrifugation, the pellet was dissolved in 1 mL of 5% HNO3 (Step 3).
Control solutions (n=3) were prepared for each scheme using the particular protocol for correction of Cd blanks. All experimental solutions from steps 1, 2 and 3 in each scheme were analyzed for Cd recoveries and for residual Mo, Sn and other major matrix elements.
3. Results and discussion
3.1. Preliminary analysis of MVM stock solution
Diluted solutions (n = 4) of the MVM stock solution were analyzed by ICP-MS to determine Mo and Sn concentrations along with matrix elements. The major matrix elements were measured to be Ca (5.6 ± 0.7 mg mL−1), Mg (3.8 ± 0.4 mg mL−1), P (3.2 ± 0.4 mg mL−1), K (4.9 ± 0.6 mg mL−1), Fe (63 ± 6 μg mL−1), Cu (51 ± 8 μg mL−1), Mn (103 ± 10 μg mL−1) and Zn (482 ± 70 μg mL−1). Mo concentration was 4.3 ± 0.6 μg mL−1. This concentration was equivalent to 143 μg Mo/tablet (ca. 96 μg g−1) which was less than the declared value (162 μg/tablet). Interestingly, no significant Sn was detected in the MVM stock solution. As a result, 0.2 mL of 1000 μg mL−1 Sn solution in 10% HCl was added to MVM solution to adjust Sn concentration to 0.5 μg mL−1. This Sn concentration was determined in reference to the certified value of Sn (11.1 μg g−1) in SRM 3280 (e.g., dissolution of 18 g SRM 3280 would yield about 0.5 μg mL−1 Sn in 400 mL volume). During the method optimization studies using the described schemes, the concentrations of matrix elements were monitored as a measure of effectiveness of the particular scheme in removal of MVM matrix.
3.2. Elimination of Mo and Sn by coprecipitation schemes
Mg(OH)2 coprecipitation with NH4OH or NaOH is a popular way of scavenging trace metals from seawater, though its capacity is limited to handful elements (Fe, Cr, Mn and Pb etc.) [24]. Thompson and Christopher [21] used NaOH for Mg(OH)2 coprecipitation in their thiourea-based procedure to separate Cd(II) from thiourea matrix. However, it was critical to add the calculated amount of NaOH to avoid formation of soluble Cd-species, such as Cd(OH)42−, at higher pHs. Unlike NaOH and NH4OH, TEA is alkylamine that does not coordinate with Cd(II) nor with other metals ions and hence does not form any water-soluble metal complexes [24, 25]. From this point of view, TEA is advantageous to carry out coprecipitation of Cd(II) without adjusting the pH of the solution; excess amount results in formation of more Mg(OH)2 pellet. However, TEA-assisted Mg(OH)2 coprecipitation also scavenges Mo(VI) (e.g., MoO42−) and Sn(II) in the solution, where HF that affects differently from TEA on Cd(II), and Mo(VI) and Sn(II) plays a vital role to achieve effective removal of interfering Mo and Sn from the MVM solution.
The performances of Scheme 1, Scheme 2 and Scheme 3 are summarized in Table 2 for 110Cd, 111Cd, 114Cd and residual Mo and Sn concentrations. The expected spike concentrations in analysis solutions were 15 μg L−1 for Steps 1 and 2, and 30 μg L−1 for Step 3. These values were sufficiently high to alleviate any spectral interferences on Cd isotopes from oxides of residual 94,95,98Mo and 114Sn. In scheme 1 (TEA-TEA-TEA), Cd is sequentially (step-wise) coprecipitated with TEA three times onto Mg(OH)2. Recoveries from the dissolved pellets solutions varied from 101 ± 6 (110Cd) for first coprecipitation to 100 ± 6 (111Cd) for third precipitation indicating that Cd was quantitatively recovered from MVM solution matrix through step 1 to step 3. In contrast, Mo and Sn coprecipitated partially in each step. Mo was reduced from about 4.3 μg mL−1 to 0.38 μg mL−1 while Sn concentration decreased from 0.5 μg mL−1 to 0.181 μg mL−1. The residual Mo and Sn levels reflect a significant reduction in Mo and Sn matrices but also indicated that sequential coprecipitations with TEA were not sufficient to effectively eliminate Mo and Sn from analysis solution. The remaining Mo and Sn levels were high enough to hamper the determination of Cd impurities at low parts per billion (ppb) levels.
Table 2.
Cadmium recoveries (%) along with residual Mo and Sn concentrations obtained for MVM stock solution at different steps of the sequential coprecipitation schemes. Values are mean ± standard deviation of five separate preparations (n = 5)
| Scheme | Step | Analyzed medium | Cd recovery (%) | Residual Mo (μg mL−1) | Residual Sn (μg mL−1) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 110Cd | 111Cd | 114Cd | |||||
|
|
|||||||
| Scheme 1 | 1 | Pellet | 101 ± 6 | 97 ± 5 | 100 ± 7 | 1.20 ± 0.14 | 0.32 ± 0.04 |
| 2 | Pellet | 99 ± 10 | 99 ± 8 | 97 ± 8 | 0.78 ± 0.12 | 0.23 ± 0.06 | |
| 3 | Pellet | 93 ± 6 | 100 ± 6 | 96 ± 7 | 0.38 ± 0.08 | 0.181 ± 0.08 | |
| Scheme 2 | 1 | Pellet | 98 ± 4 | 96 ± 7 | 98 ± 5 | 0.88 ± 0.10 | 0.28 ± 0.04 |
| 2 | Supernatant | 99 ± 3 | 101 ± 7 | 99 ± 7 | 0.76 ± 0.08 | 0.20 ± 0.06 | |
| 3 | Pellet | 101 ±5 | 98 ± 6 | 101 ± 7 | 0.16 ± 0.06 | 0.052 ± 0.02 | |
| Scheme 3 | 1 | Supernatant | 98 ± 3 | 101 ± 6 | 97 ± 5 | 0.88 ± 0.11 | 0.202 ± 0.08 |
| 2 | Pellet | 96 ± 4 | 93 ± 3 | 99 ± 4 | 0.14 ± 0.08 | 0.088 ± 0.02 | |
| 3 | Pellet | 97 ± 7 | 98 ± 2 | 98 ± 6 | 0.014 ± 0.008 | 0.018 ± 0.01 | |
In Scheme 2 (TEA-HF-TEA), first precipitation is identical to that in Scheme 1. In step 2, 30 μL HF was added to the dissolved pellet solution from step 1 to coprecipitate the matrix ions via CaF2 coprecipitation. Cd(II) forms monofluoride complex in the HF medium (Cd2+ + HF ↔ CdF+ + H+; Kq = 5.8 – 6.4) [26, 27], which resulted in quantitative retention (96–101%) of Cd(II) in solution as reported elsewhere [23]. However, the cadmium fluoride complex is relatively weak (stability constant Kq = 5.8 – 6.4) [26, 27], and hence Cd(II) readily coprecipitated back onto Mg(OH)2 pellet when 150 μL TEA was added to the remaining supernatant solution of CdF+ complex (Step 3). Cd recoveries varied between 97 to 101%. The precipitation of Mo(VI) and Sn(II) was negligible with HF in step 2, which might be due to the formation of soluble fluoride complexes. Secondly, because of low residual Ca(II) concentration (e.g., 1424 μg mL−1), CaF2 precipitation was relatively slow that was also thought to impede the precipitation of Mo(VI) and Sn(II). In Step 3, Mo(VI) and Sn(II) were reduced significantly with Mg(OH)2 coprecipitation in comparison to the levels in step 2. The dissolved pellet solution contained about 0.16 μg mL−1 for Mo and 0.052 μg mL−1 for Sn. However, it was obvious that single step precipitation with TEA was not sufficient to effectively eliminate interfering Mo and Sn. Especially Mo levels were still high to cause interferences on Cd isotopes.
In Scheme 3, CaF2 coprecipitation was performed first since subsequent treatment with TEA was more effective for removal of Mo and Sn from MVM solutions. Initially, Ca levels were very high (5.6 ± 0.7 mg mL−1) in the MVM solution, therefore, intense precipitation occurred when HF was added. While Cd(II) was retained in solution (95–101%) successfully, both Mo and Sn coprecipitated significantly with CaF2 (see Step 1). Mo concentration in the solution decreased from 4.3 μg mL−1 to 0.88 μg mL−1 and that for Sn decreased from 0.5 μg mL−1 to 0.202 μg mL−1. Addition of TEA to the supernatant solution showed similar effect as in Scheme 2 reducing Mo and Sn levels to 0.14 μg mL−1 and 0.088 μg mL−1, respectively (Step 2). Mo and Sn levels were further reduced to 0.014 μg mL−1 and 0.018 μg mL−1, respectively, when the remaining dissolved pellet solution from Step 2 was coprecipitated with 100 μL TEA for a third time (Step 3). These residual concentrations for Mo and Sn reflect that 99.7% of Mo and 96.5% of Sn were removed from the MVM solution without any significant loss of Cd. The recoveries for the Cd isotopes in Step 3 were between 96% and 99%.
3.3. Performances of coprecipitation schemes on elimination of MVM salt matrix
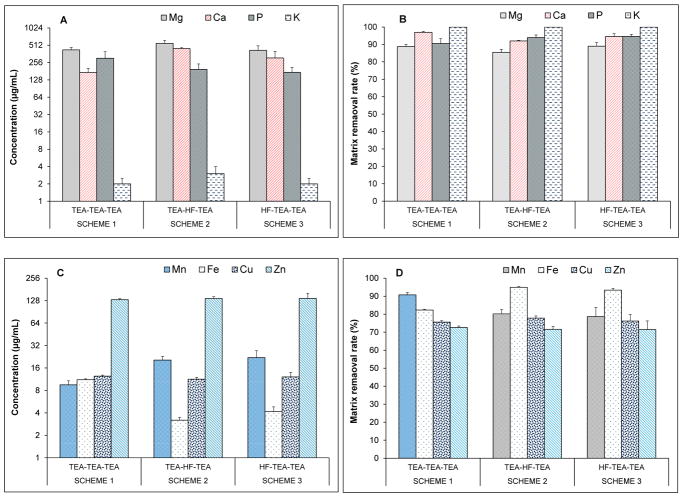
Among the coprecipitation schemes, Scheme 3 was the most effective for removal of Mo and Sn matrices followed by Scheme 2 in a three-step coprecipitation. Nonetheless, MVM solutions contain Ca, Mg, P, K at per cent levels (see section 3.1) besides Cu, Fe, Mn and Zn that are present at high part per million (ppm) levels. Especially, the removal the major matrix elements (e.g., Ca, Mg, P and K) that make up the salt matrix is important to avoid matrix suppression and salt build-up on ICP-MS components. The concentrations for Ca, Mg, P and K remained in solutions after the third coprecipitation step are illustrated in Fig. 2A for each coprecipitation scheme along with per cent removal rates in Fig. 2B. Analysis solutions contained about 418 to 552 μg mL−1 Mg, 172 to 448 μg mL−1 Ca and 172 to 304 μg mL−1 P, while K (2–3 μg mL−1) was mostly eliminated. In comparison to initial concentrations in the MVM stock solution, up to 89% of Mg, 97% of Ca, 95% of P and 99.9 of K was removed prior to ICP-MS analysis. In general, the coprecipitation schemes performed comparably in eliminating the matrix elements affording a significant reduction in the total dissolved salt matrix. Mg was inherently carried into the analysis solution through the Mg(OH)2 precipitation, yet its concentration was reduced by about 8-fold from 3.8 ± 0.4 mg mL−1. TEA-based coprecipitation (Scheme 1) was more effective in reducing Ca levels. Relatively higher of Ca (ca. 306–448 μg mL−1) remained in the analysis solutions in Scheme 2 and Scheme 3 since not all Ca precipitated as CaF2. A similar pattern was observed in removal of calcium matrix in fish otoliths [23]. Phosphorous matrix was removed more effectively in Scheme 2 and 3 for which P concentrations in analysis solutions were about 172 – 194 μg mL−1.
Fig. 2.
Performances of coprecipitation schemes on removal of major element matrix (A), and essential element matrix (C). Bars (A and C) show the concentration of matrix elements remained in MVM solution after third coprecipitation with each scheme. Bars (B and D) show percent removal of the matrix elements. Results are mean ± standard deviation for five replicates (n = 5).
The salts of essential elements, including Mn, Fe, Cu and Zn make up about 5–8% of an MVM sample. The removal of these matrix elements is advantageous to alleviate adverse effects of salt matrix in determinations of low levels of Cd impurities. As shown in Fig. 2C, Mn, Fe, Cu and Zn levels were significantly reduced in analysis solutions. Percent removal rates varied between 89% to 91% for Mn, 82% to 95% for Fe, 76% to 78% for Cu and 71% to 73% for Zn (Fig. 2D). All three schemes performed similarly on reducing Cu and Zn levels; Cu concentrations decreased from 51 μg mL−1 to about 12 μg mL−1 and that for Zn decreased from 482 μg mL−1 to 136 μg mL−1. Mn levels were relatively higher in Scheme 2 (20 μg mL−1) and Scheme 3 (22 μg mL−1) in comparison to that in Scheme 1 (9 μg mL−1). This was due the fact that Mn largely remained in solution as fluoride complex when HF was added. These Mn complexes then coprecipitated with Mg(OH)2 and were carried into the pellet through Mg(OH)2 coprecipitation. In contrast, Fe yields insoluble fluorides with CaF2. As a result, Scheme 2 and 3 was the most effective for reducing Fe matrix; Fe concentrations in analysis solutions decreased from 63 μg mL−1 to around 3.1 and 4.2 μg mL−1, respectively.
3.4. Optimization of Scheme 3 for a two-step matrix removal
Among the coprecipitation schemes, Scheme 3 (HF-TEA-TEA) was most suitable as it provided the most effective removal of Mo and Sn matrices via three-step coprecipitations along with comparable performance for the removal of other matrix salts. In a separate experiment, the effect of HF was examined to achieve removal of Mo and Sn in a two-step coprecipitation (e.g., HF-TEA). The protocols outlined in Fig. 1 for Scheme 3 are repeated until Step 2. In first coprecipitation step, the volume of HF was increased from 20 μL to 80 μL for a 1 mL MVM solution (n = 5) containing 60 μg L−1 multielement and 0.5 μg mL−1 Sn spike. The results are summarized in Table 3. Up to 40 μL HF, no significant improvement occurred in removal of Mo and Sn. The analysis solutions contained about 0.12 – 0.14 μL mL−1 Mo and 0.056 – 0.062 μL mL−1 Sn. A matrix of 0.12 – 0.14 μg mL−1 Mo was still high considering sub-ppb (μg L−1) Cd impurities in MVM solutions. At and above 60 μL HF, both Mo and Sn concentration decreased significantly to as low as 0.014 μg mL−1 and 0.016 μg mL−1, respectively. Nonetheless, Cd recoveries did decrease with 60 μL HF to about 72–78%. When 80 μL HF was added in the first coprecipitation, Cd could not be coprecipitated back with Mg(OH)2. This was attributed to the fact that the resulting supernatant solution of cadmium fluoride complex was still acidic even after adding 150 μL TEA. When TEA volume was increased up to 250–300 μL, Cd recoveries improved, but Mo and Sn concentrations also increased to about 0.15 μL mL−1 and 0.065 μL mL−1, respectively. These results indicated that it would not be feasible to effectively remove Mo and Sn via two-step coprecipitation using HF and TEA. Scheme 3 comprised of three-step coprecipitation was optimum for analysis of MVM solutions.
Table 3.
Effect of HF volume on Cd recoveries (%) and removal of Mo and Sn in MVM solution in a two-step coprecipitation using HF and TEA. Values are mean ± standard deviation of five separate preparations (n = 5)
| HF volume (μL) | Step | Analyzed medium | Cd recovery (%) | Residual Mo (μg mL−1) | Residual Sn (μg mL−1) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 110Cd | 111Cd | 114Cd | |||||
|
|
|||||||
| 20 | 1 | Supernatant | 94 ± 5 | 96 ± 5 | 92 ± 6 | 0.87 ± 0.04 | 0.18 ± 0.02 |
| 2 | Pellet | 98 ± 6 | 101 ± 3 | 96 ± 6 | 0.14 ± 0.02 | 0.062 ± 0.02 | |
| 40 | 1 | Supernatant | 98 ± 3 | 101 ± 4 | 98 ± 4 | 0.90 ± 0.08 | 0.20 ± 0.04 |
| 2 | Pellet | 99 ± 2 | 97 ± 5 | 99 ± 3 | 0.12 ± 0.06 | 0.056 ± 0.05 | |
| 60 | 1 | Supernatant | 100 ± 2 | 100 ± 5 | 94 ± 6 | 0.092 ± 0.04 | 0.26 ± 0.06 |
| 2 | Pellet | 75 ± 6 | 78 ± 4 | 72 ± 5 | 0.020 ± 0.02 | 0.024 ± 0.02 | |
| 80 | 1 | Supernatant | 97 ± 4 | 94 ± 4 | 95 ± 3 | 0.94 ± 0.07 | 0.35 ± 0.08 |
| 2 | Pellet | 6.8 ± 0.6 | 7.3 ± 0.2 | 5.8 ± 0.5 | 0.014 ± 0.01 | 0.016 ± 0.04 | |
3.5. Method validation and analysis of commercial MVM tablets
Samples of multivitamin/multielement tablets certified reference material (SRM 3280) was analyzed using Scheme 3. A sample of 6 g (4 tablets) of SRM 3280 was ground with agate mortar and mill. About 0.15 g samples (n = 5) were weighed in teflon vessels and digested in 3 mL HNO3 and 2 mL H2O2 using the procedure described in section 2.3. After digestion, the residue was dissolved in 1 mL 0.5% HNO3 and transferred to a 2-mL microcentrifuge tube. The digestion vessel was rinsed with 1 mL water and added into the microcentrifuge tube. To get rid of undissolved TiO2 and SiO2 matrix, the samples were first centrifuged at 10000 rpm for 20 min. After centrifugation, the supernatant solutions were transferred to new 2-mL microcentrifuge tubes and treated according to the coprecipitation protocol described in Scheme 3. A volume of 40 μL of 1.0 μg mL−1 enriched 113Cd (95.83%) isotope solution (40 μg L−1 113Cd) was spiked into final solution after each sampling at Step 1, 2 and 3. Blank solutions (n = 6) were prepared with 30 μL HF and 250 μL TEA in 2-mL centrifuge tubes. These solutions were first evaporated on a hot-block digester at 100 °C, then spiked with 113Cd isotope solution similarly and completed to 2 mL with 0.5% HNO3. Multielement external calibration solutions from 0 to 100 μg L−1 of each element listed in section 2.1 were prepared in 2% HNO3. Appropriate volume of enriched 113Cd isotope solution was added to all calibration solutions to yield 40 μg L−1 113Cd. The calculations for Cd were performed with the isotope dilution method using standards from 0 to 20 μg L−1 [28]. Calculations for all other elements (e.g., Mo and Sn) were made using external calibration method.
No significant Cd impurities were detected in the reagent blanks. Average Cd concentration in the blank solutions was about 0.008 μg L−1. A limit of detection (LOD) of 0.004 μg L−1 was calculated based on three times the standard deviation of blank signals (3σ) (n = 6). This LOD was equivalent to 0.26 ng g−1 Cd for a sample size of 0.15 g, and was sufficiently low for determination of Cd levels in SRM 3280 in that certified Cd level is 80.15 ± 0.86 ng g−1. The certified and reference values for Mo and Sn are 70.7 ± 4.5 μg g−1 and 11.1 ± 0.9 μg g−1, respectively. For 0.15 g sample, these concentrations translate to 6.01 μg L−1 Cd in SRM 3280 solution along with 5.3 μg mL−1 Mo and 0.832 μg mL−1 Sn in 2 mL volume. The results for the isotope dilution (ID) ICP-MS analysis of SRM 3280 samples are summarized in Table 4. To demonstrate the impact of Mo and Sn matrix on accuracy, dry-basis Cd concentrations determined in each step of Scheme 3 are provided along with the solution concentrations of Mo and Sn. As can be seen, Cd concentrations measured using 110Cd, 111Cd and 114Cd were inaccurately high (49% to 70%) after coprecipitation with HF (Step 1) which were due mainly to the interferences from 94Mo16O, 95Mo16O and 98Mo16O, respectively, originating from high Mo levels (ca. 1.27 μg mL−1). The remaining Mo concentration in Step 1 was about 423-fold higher than that of Cd (ca. 3 μg L−1). After second coprecipitation with TEA (Step 2), Mo levels decreased to 0.32 μg mL−1, but Mo/Cd ratio was still substantial around 106-fold. As a result, Cd levels were 20% to 40% higher than the certified value. It should also be noted that the results of 111Cd were consistently higher while that for 114Cd were lower for the determinations in Step 1 and Step 2. The latter appears to an overcorrection of 114Sn overlap on 114Cd though Sn was removed to a greater extent after the first coprecipitation. The former was attributed to additional contribution from 80Se31P, because SRM 3280 solutions contained about 0.4 and 0.2 μg mL−1 Se along with 440 to 350 μg mL−1 P in Step 1 and Step 2, respectively. After the third coprecipitation, Mo levels in solution depleted to about 0.018 μg mL−1, and in turn, a good agreement was achieved for the results of the Cd isotopes with the certified value (80.15 ± 0.86 ng g−1) within 95% confidence interval (n = 5). These results corroborated the fact that a three-step coprecipitation procedure was critical to eliminate the interferences of MoO on Cd isotopes. In addition, the results for 111Cd agreed with those of 110Cd and 114Cd as Se levels also depleted to about 0.035 μg mL−1 in Step 3 alleviating the effects from 80Se31P on 111Cd.
Table 4.
Dry mass concentrations of Cd determined in SRM 3280 by ID-ICPMS along with residual solution concentrations of Mo and Sn measured in different of Scheme 3. Values are mean ± standard deviation of five separate preparations (n = 5). Values in parenthesis indicate per cent deviation (inaccuracy) from the certified value of Cd and those for Mo and Sn reflect per cent removal of Mo and Sn from SRM 3280 solution.
| Step | Analyzed medium | Cd (ng g−1) | Residual Mo (μg mL−1) | Residual Sn (μg mL−1) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| 110Cd/113Cd | 111Cd/113Cd | 114Cd/113Cd | ||||
|
|
||||||
| 1 | Supernatant | 129 ± 10 | 136 ± 9 | 119 ± 12 | 1.27 ± 0.30 | 0.110 ± 0.012 |
| (+61%) | (+70%) | (+49%) | (76.0%) | (86.7%) | ||
| 2 | Pellet | 102 ± 8 | 112 ± 11 | 95.6 ± 7.1 | 0.32 ± 0.08 | 0.053 ± 0.006 |
| (+28%) | (+40%) | (+20%) | (93.9%) | (93.6) | ||
| 3 | Pellet | 78.8 ± 4.7 | 77.9 ± 5.2 | 76.5 ± 4.8 | 0.018 ± 0.008 | 0.019 ± 0.004 |
| (−1.5%) | (−2.6%) | (−4.4%) | (99.6%) | (97.7%) | ||
For analysis of the commercial MVM tablets, 0.1 g samples (n = 5) from ground samples (5 to 6 tablets) were digested similarly and coprecipitated as described for the SRM 3280 samples. The results obtained after third coprecipitation are provided in Table 5. Mean Cd levels varied from 21.4 to 93.3 ng g−1 in the tablets. The precision, relative standard deviation (%RSD), for five replicate analyses varied between 3.4% and 12.7%. Lower precision (e.g., high %RSD) was likely due to the inhomogeneity of ground sample as well as digestion of small masses (0.1 g). The Cd values measured in the MVM tablets translate to about 0.027 to 0.14 μg Cd day−1 (e.g. serving size or per tablet) and are well below the minimum risk level (MRL) of 0.5 μg kg−1 day−1 [29]. Avula et al. [19] reported 0.3 to 3.8 μg Cd day−1 in a survey of 35 MVM supplements for essential and toxic elements by direct analysis (e.g., without any matrix removal) using CCT-ICPMS. The values measured in this study using the sequential coprecipitation ICP-MS procedure are at least 10-fold less than those Cd levels reported [19], which could indicate that Cd contamination in dietary MVM supplements could be much less than that measured directly by ICP-MS or HR-ICP-MS; and hence suitable analytical separation approaches should be utilized for interference-free determination of Cd from these complex MVM supplements.
Table 5.
Cadmium concentration (ng g−1) determined in various multivitamin/mineral dietary supplements by sequential coprecipitation ID-ICPMS. Values are mean ± standard deviation of five separate preparations (n = 5). Serving size (daily) concentration (μg) is calculated as the average of 110Cd, 111Cd and 114Cd values per tablet
| Sample | Cd (ng g−1) | Average Cd (μg/serving size) | ||
|---|---|---|---|---|
|
| ||||
| 110Cd/113Cd | 111Cd/113Cd | 114Cd/113Cd | ||
|
|
||||
| MVM-1 | 21.9 ± 2.5 | 22.1 ± 1.4 | 20.5 ± 1.6 | 0.027 |
| MVM-2 | 92.5 ± 3.0 | 93.2 ± 4.8 | 93.0 ± 7.2 | 0.140 |
| MVM-3 | 81.8 ± 5.6 | 83 ± 4.9 | 83.8 ± 6.2 | 0.108 |
| MVM-4 | 45.5 ± 5.8 | 48.4 ± 3.8 | 46.2 ± 4.2 | 0.058 |
| MVM-5 | 70.3 ± 6.2 | 68.4 ± 5.7 | 66.7 ± 5.5 | 0.099 |
| MVM-6 | 62.1 ± 4.6 | 59.5 ± 4.3 | 58.0 ± 5.2 | 0.087 |
4. Conclusion
In this study, we developed a sequential coprecipitation procedure for separation of Cd impurities from MVM matrix. HF and TEA were used judiciously for effective removal of spectrally interfering Mo and Sn concomitants. In a three-step coprecipitation scheme of HF-TEA-TEA, Mo and Sn levels were virtually eliminated in analysis solutions to achieve interference-free Cd determination. The procedure was also effective in reducing the MVM salt matrix and essential element matrices to avoid exposure of ICP-MS to elevated levels of transition elements. Isotope dilution (ID) provided accurate compensation for the effects of other residual matrix components, including Mg, Ca and P. Application of the coprecipitation procedure to analysis of commercial MVM samples showed very low Cd impurities in comparison to those reported in literature previously, pointing to the fact that determinations of toxic heavy metals impurities in MVM samples should be carried with suitable analytical methods. Further, the coprecipitation method is simple and relatively fast in contrast to solid phase extraction approaches; 20 samples can be prepared within an hour affording about 3 min per sample throughput. Future studies will involve further improving the coprecipitation scheme to achieve determinations of other heavy metals impurities, such as As, Pb, Tl, and U in MVM samples.
Highlights.
Sequential coprecipitation was performed for determining Cd in multivitamin/mineral (MVM) supplements.
Triethylamine and hydrofluoric acid were utilized for scavenging Cd from MVM matrix.
Interfering molybdenum and tin matrix were eliminated effectively from solutions.
Cadmium was accurately determined in multivitamin/multielement tablets (SRM 3280) by ID-ICPMS.
Commercial MVM supplements contain lower Cd impurities than minimum risk levels (MRLs).
Acknowledgments
This project is funded in part by grants from the National Institutes of Health (NIH) through Research Centers in Minority Institutions (RCMI) Program (Grant No: G12RR013459) and from the National Science Foundation Research Experience for Undergraduates (NSF-REU) Program at Jackson State University (Grant No: 1461143). The views expressed herein are those of authors and do not necessarily represent the official views of the funding agencies, and any of their sub-agencies. The authors are grateful the Scientific and Technological Council of Turkey (TUBITAK) for post-doctoral fellowship to Dr. Vedat Yılmaz during the course of this project at Jackson State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Department of Health and Human Services. What’s in the bottle? An introduction to dietary supplements. 2007. [Google Scholar]
- 2.Dolan SP, Nortrup DA, Bolger PM, Capar SG. Analysis of dietary supplements for arsenic, cadmium, mercury, and lead using inductively coupled plasma mass spectrometry. J Agric Food Chem. 2003;51:1307–1312. doi: 10.1021/jf026055x. [DOI] [PubMed] [Google Scholar]
- 3.Costello RB, Saldanha LG. Annual bibliography of significant advances in dietary supplement research 2004. n.d. [Google Scholar]
- 4.Sander LC, Sharpless KE, Wise SA. Dietary supplement standard reference materials. Life Sci. 2006;78:2044–2048. doi: 10.1016/j.lfs.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Rapaka RS, Coates PM. Dietary supplements and related products: A brief summary. Life Sci. 2006;78:2026–2032. doi: 10.1016/j.lfs.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Bu K, Cizdziel JV, Reidy L. Analysis of herbal supplements for selected dietary minerals and trace elements by laser ablation- and solution-based ICPMS. Microchem J. 2013;106:244–249. doi: 10.1016/j.microc.2012.07.011. [DOI] [Google Scholar]
- 7.van der Voet GB, Sarafanov A, Todorov TI, Centeno JA, Jonas WB, Ives JA, Mullick FG. Clinical and analytical toxicology of dietary supplements: a case study and a review of the literature. Biol Trace Elem Res. 2008;125:1–12. doi: 10.1007/s12011-008-8157-0. [DOI] [PubMed] [Google Scholar]
- 8.Mindak WR, Cheng J, Canas BJ, Bolger PM. Lead in women’s and children’s vitamins. J Agric Food Chem. 2008;56:6892–6896. doi: 10.1021/jf801236w. [DOI] [PubMed] [Google Scholar]
- 9.Krejčova A, Ludvíková I, Černohorsky T, Pouzar M. Elemental analysis of nutritional preparations by inductively coupled plasma mass and optical emission spectrometry. 2012;132:588–596. doi: 10.1016/j.foodchem.2011.10.076. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay M, Kessler RC. Trends in alternative medicine use in the United States, 1990–1997. JAMA. 1998;280:1569. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- 11.Raman P, Patino LC, Nair MG. Evaluation of metal and microbial contamination in botanical supplements. J Agric Food Chem. 2004;52:7822–7827. doi: 10.1021/jf049150+. [DOI] [PubMed] [Google Scholar]
- 12.Korfali SI, Hawi T, Mroueh M. Evaluation of heavy metals content in dietary supplements in Lebanon. Chem Cent J. 2013;7:10. doi: 10.1186/1752-153X-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marrero J, Jiménez R, Leiva E, Londonio A, Smichowski P. Inductively coupled plasma optical emission spectrometric determination of fifteen elements in dietary supplements: Are the concentrations declared in the labels accurate? Microchem J. 2013;108:81–86. doi: 10.1016/j.microc.2012.12.013. [DOI] [Google Scholar]
- 14.Amster E, Tiwary A, Schenker MB. Case report: Potential arsenic toxicosis secondary to herbal kelp supplement. Environ Health Perspect. 2007;115:606–608. doi: 10.1289/ehp.9495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN. Lead, mercury, and arsenic in US- and Indian-manufactured Ayurvedic medicines sold via the Internet. JAMA. 2008;300:915–923. doi: 10.1001/jama.300.8.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyva-perez J, Jara-marini ME, Garcı L. Content and daily intake of copper, zinc, lead, cadmium and mercury from dietary supplements in Mexico. 2007;45:1599–1605. doi: 10.1016/j.fct.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Jarup L. Low level exposure to cadmium and early kidney damage: the OSCAR study. Occup Environ Med. 2000;57:668–672. doi: 10.1136/oem.57.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johri N, Jacquillet G, Unwin R. Heavy metal poisoning: the effects of cadmium on the kidney. Biometals. 2010;23:783–792. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- 19.Avula B, Wang Y, Duzgoren-Aydin NS, Khan IA. Inorganic elemental compositions of commercial multivitamin/mineral dietary supplements: Application of collision/reaction cell inductively coupled-mass spectroscopy. Food Chem. 2011;127:54–62. doi: 10.1016/j.foodchem.2010.12.083. [DOI] [Google Scholar]
- 20.Smichowski P, Londonio A. The role of analytical techniques in the determination of metals and metalloids in dietary supplements: A review. Microchem J. 2018;136:113–120. doi: 10.1016/j.microc.2016.11.007. [DOI] [Google Scholar]
- 21.Thompson RQ, Christopher SJ. Novel separation for the determination of cadmium by isotope dilution ICP-MS in samples containing high concentrations of molybdenum and tin. Anal Methods. 2013;5:1346–1351. doi: 10.1039/c2ay26212f. [DOI] [Google Scholar]
- 22.Christopher SJ, Thompson RQ. Determination of trace level cadmium in SRM 3280 Multivitamin/Multielement Tablets via isotope dilution inductively coupled plasma mass spectrometry. Talanta. 2013;116:18–25. doi: 10.1016/j.talanta.2013.04.068. [DOI] [PubMed] [Google Scholar]
- 23.Arslan Z. Analysis of fish otoliths by electrothermal vaporization inductively coupled plasma mass spectrometry: aspects of precipitating otolith calcium with hydrofluoric acid for trace element determination. Talanta. 2005;65:1326–1334. doi: 10.1016/j.talanta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Arslan Z, Oymak T, White J. Triethylamine-assisted Mg(OH)2 coprecipitation/preconcentration for determination of trace metals and rare earth elements in seawater by inductively coupled plasma mass spectrometry (ICP-MS) Anal Chim Acta. 2018;1008:18–28. doi: 10.1016/j.aca.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hatfield WE, Yoke JT. Complexes of the Ethylamines with the Halides of calcium, cobalt(II), and zinc. Inorg Chem. 1962;1:463–470. doi: 10.1021/ic50003a004. [DOI] [Google Scholar]
- 26.Mesaric SS, Hume DN. The formation constants of copper, cadmium, and zinc fluoride complexes. Inorg Chem. 1963;2:1063–1064. doi: 10.1021/ic50009a043. [DOI] [Google Scholar]
- 27.Bond AM. A study of the fluoride complexes of cadmium by a.c. and d.c. polarography. J Electroanal Chem Interfacial Electrochem. 1969;20:223–230. doi: 10.1016/S0022-0728(69)80123-3. [DOI] [Google Scholar]
- 28.Fassett JD, Paulsen PJ. Isotope dilution mass spectrometry for accurate elemental analysis. Anal Chem. 1989;61:643A–649A. doi: 10.1021/ac00185a715. [DOI] [Google Scholar]
- 29.Agency for Toxic Substances and Disease Registry (ATSDR) ToxGuide for Cadmium, Agency Toxic Subst Dis Regist. 2012. p. 2. [Google Scholar]