SUMMARY
Mechanisms implicated in robust transplantation tolerance at the cellular level can be broadly categorized into those that inhibit alloreactive T cells intrinsically (clonal deletion and dysfunction) or extrinsically through regulation. Here, we investigated whether additional population-level mechanisms control T cells by examining whether therapeutically induced peripheral transplantation tolerance could influence T cell populations’ avidity for alloantigens. Whereas T cells with high avidity preferentially accumulated during acute rejection of allografts, the alloreactive T cells in tolerant recipients retained a low-avidity profile, comparable to naive mice despite evidence of activation. These contrasting avidity profiles upon productive versus tolerogenic stimulation were durable and persisted upon alloantigen reencounter in the absence of any immunosuppression. Thus, peripheral transplantation tolerance involves control of alloreactive T cells at the population level, in addition to the individual cell level. Controlling expansion or eliminating high-affinity, donorspecific T cells long term may be desirable to achieve robust transplantation tolerance in the clinic.
In Brief
The net strength of multiple TCR binding interactions determines a cell’s avidity for antigen. Miller et al. find that high-avidity T cells preferentially accumulate following alloantigen encounter. This T cell repertoire skewing is durably prevented by tolerance induction, where lower avidity cell populations persist even upon subsequent alloantigen rechallenge.
Graphical Abstract
INTRODUCTION
T cells express surface T cell receptors (TCRs) that recognize antigens with a range of affinities and/or avidities. T cell affinity refers to the binding strength of a single TCR and peptide-major histocompatibility complex (pMHC) interaction, whereas T cell avidity integrates interactions between multiple TCRs and coreceptors with pMHC molecules (van den Berg and Rand, 2007). TCR clustering and colocalization with coreceptors upon T cell activation can also affect T cell avidity (Demotte et al., 2008; Fahmy et al., 2001). The fluorescence intensity of T cells stained with pMHC multimers has been used as a proxy for the affinity and/or avidity of endogenous T cell populations, because pMHC multimer binding intensity has correlated with functional assays of T cell avidity (Crawford et al., 1998; Falta et al., 2005; Wang and Altman, 2003; Yee et al., 1999).
Several animal models have demonstrated that the avidity profile of peripheral T cell populations increases following foreign antigen encounter (Busch and Pamer, 1999; Savage et al., 1999) through the selective expansion of high-affinity T cells that may better compete for limited amounts of antigen and receive a stronger signal to proliferate and survive (Bos et al., 2012; Ozga et al., 2016). In addition, regulatory T cells may preferentially inhibit low-avidity T cells (Pace et al., 2012). This accumulation of high-affinity and/or avidity T cells, based on interactions with pMHC, has been termed T cell affinity maturation at the cellpopulation level (Busch and Pamer, 1999).
Following solid organ transplantation, recipient T cells are activated by donor alloantigens, either via direct recognition of donor MHC molecules or via indirect recognition of polymorphic donor proteins presented by host MHC. Short-term therapeutic blockade of costimulation induces transplantation tolerance, which is thought to depend on peripheral mechanisms of T cell tolerance. Many studies have examined these mechanisms using monoclonal allospecific TCR-transgenic T cells with fixed affinities (Iwakoshi et al., 2000; Miller et al., 2016a; Pinelli et al., 2013; Quezada et al., 2005). However, a polyclonal endogenous response to a given donor antigen comprises multiple T cell clones of various affinities and/or avidities competing for antigen (Honjo et al., 2000; Tsang et al., 2011), and the fates of T cells of differing affinities and/or avidities in rejection versus tolerance has not been analyzed. One possibility is that similar alloreactive clones either expand or die in rejection and tolerance, thus yielding similar avidity profiles in both post-transplant states, though in higher numbers in rejection. Alternatively, there may be a biased expansion or elimination of only some alloreactive clones of select avidities in rejection or tolerance, leading to a distinct avidity skewing in rejection versus tolerance.
Using mouse pMHC multimers to track endogenous graftreactive T cells specific for model donor antigens (Young et al., 2016), we here report that during productive alloimmunity, endogenous CD4+ and CD8+ T cells increased in avidity for alloantigen at the population level. In contrast, following therapeutically induced peripheral tolerance, the avidity profile of T cell populations remained low and comparable to that of naive populations, despite upregulation of CD44 expression by tolerant T cells. This low-avidity profile in tolerized mice was stable, even upon rechallenge with donor antigen in the absence of costimulation blockade, and was due, at least partly, to an inhibition of expansion of high-avidity clones. Thus, our study reveals that avidity maturation at the T cell-population level occurs not only following infections but also during unmodified alloreactivity. In addition, our data reveal that therapeutically induced peripheral tolerance can durably shape T cell repertoires and suggests that reducing the abundance of high-avidity, donor-specific effector T cell clones may be desirable to achieve long-term tolerance.
RESULTS
Endogenous Alloreactive Tolerant T Cells Become Antigen Experienced but Accumulate Less Than in Rejecting Mice
To understand the nature of the endogenous alloresponse during rejection and tolerance, we used C57BL/6 (B6) recipient mice, and F1 B6 3 BALB/c donor mice transgenic for the model fusion protein of the peptide 2W1S and the protein ovalbumin (OVA). The donor 2W1S (2W) peptide, a mutated form of a peptide derived from I-Ed, is presented on donor and recipient I-Ab, whereas the donor OVA peptide is presented on donor and recipient Kb. These ubiquitous model antigens are recognized by endogenous graft-reactive CD4+ and CD8+ T cells, respectively, that are detected by binding to pMHC multimers 2W: I-Ab and OVA:Kb. This experimental system models a haploidentical setting, in which donor and recipient share some major histocompatibility complex class I and II (MHC I and II) alleles and in which select donor-derived minor antigens (alloantigens) are presented to recipient T cells by donor cells or by recipient antigen-presenting cells (APCs). In some experiments, donor BALB/ c-OVA mice were used, providing a situation in which OVA is exclusively cross-presented by recipient APCs. Although these two models represent two physiologically different settings, the data were indistinguishable and therefore combined where noted. 2W- and OVA-reactive T cell populations are rare in naive, antigen-inexperienced mice (Moon et al., 2011), such that in all experiments, T cell enrichment by negative selection was first performed to increase the frequency of multimer-binding T cells during subsequent flow cytometric analysis. Transplantation tolerance was induced with a combination of anti-CD154 and donor splenocyte transfusion (DST) at the time of transplantation. This regimen results in permanent acceptance of cardiac allografts and in donor-specific transplantation tolerance, as tolerant mice accept secondary donor-matched cardiac allografts in the absence of immunosuppression but reject thirdparty allografts (Lee et al., 2005).
The numbers of 2W:I-Ab+ Foxp3+ and Foxp3− and OVA:Kb+ T cells in naive B6 mice, as well as in mice transplanted and left untreated (rejected) or tolerized with αCD154 + DST (tolerant), were enumerated after transplantation. As expected, the numbers of graft-reactive T cells recovered from spleens of rejected mice were greater than those from naive or tolerant mice, whereas the numbers of 2W:I-Ab+ Foxp3− and OVA:Kb+ T cells were similar in tolerant and naive mice (Figure S1A). As previously reported (Young et al., 2018), 2W:I-Ab+ T regulatory cells (Tregs) expanded in rejection and tolerance, but the percentage of 2W:I-Ab+ Tregs was higher in tolerance given the lack of expansion of 2W:I-Ab+ Foxp3− conventional T cells (Figure S1B). Graft-specific T cells were significantly more activated in tolerant than in naive mice, as measured by the percentage of CD44hi, albeit not as uniformly activated as in rejecting mice (Figure S1C). Thus, despite antigen experience, graft-specific T cells failed to accumulate in tolerant mice.
Endogenous Allospecific T Cell Populations Have Higher-Avidity Profiles in Rejection Than in Tolerance
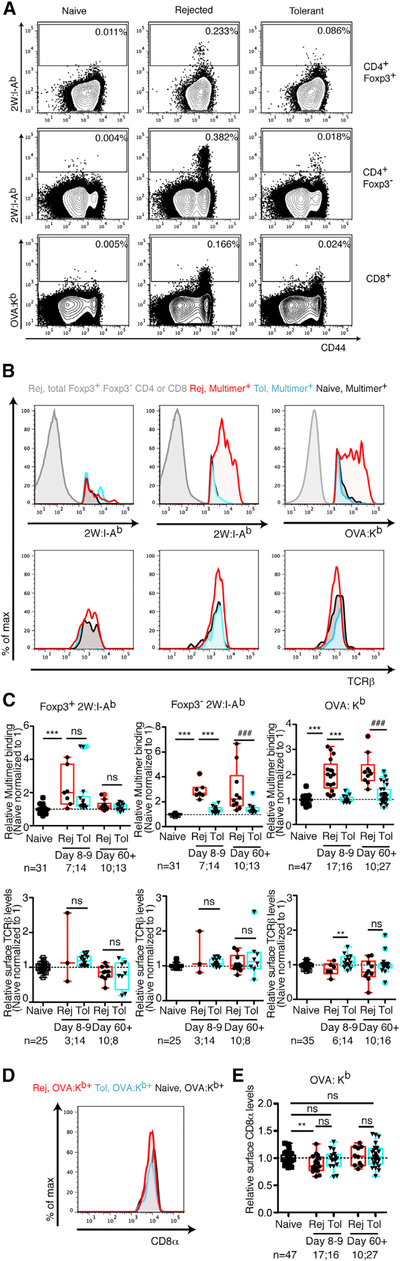
In addition to the quantity of alloreactive T cells, functional responsiveness per cell may affect the overall alloimmune response: a few high-avidity T cells may be as potent as numerous low-avidity cells. Analysis of pMHC multimer binding on splenic endogenous graft-reactive T cells revealed higher multimer binding in Foxp3− CD4+ and CD8+ T cells from rejecting than tolerant or naive mice (Figures 1A–1C). Tregs from rejecting and tolerant hosts displayed similar 2W:I-Ab binding (Figures 1A– 1C), though the number of events analyzed was low. Therefore, subsequent analyses of 2W:I-Ab-binding T cells gated out Foxp3+ T cells to focus on conventional T cells. The differences in multimer binding between rejecting and tolerant conventional T cells were not due to the greater proportion of antigen-inexperienced T cells in tolerant than in rejecting hosts, because pMHC multimer-binding differences remained when analyzing only CD44hi 2W:I-Ab+ and CD44hi OVA:Kb+ cells (Figure S2A). Moreover, they were not due to lower TCR expression on tolerant T cells, because staining with the anti-TCRβ clone H57–597 that does not interfere with TCR-pMHC binding (Wang et al., 1998) did not reveal lower TCRβ levels on multimer-binding T cells in tolerant than in rejecting mice (Figures 1B and 1C). CD8α has been shown to contribute to OVA:Kb multimer binding (Daniels and Jameson, 2000), but no significant decreases in CD8α levels were observed in tolerant mice (Figures 1D and 1E). Thus, T cell populations in acutely rejecting mice undergo avidity maturation in response to alloantigen, similar to T cells responding to a pathogen (Busch and Pamer, 1999), and this avidity maturation is reduced in tolerant mice.
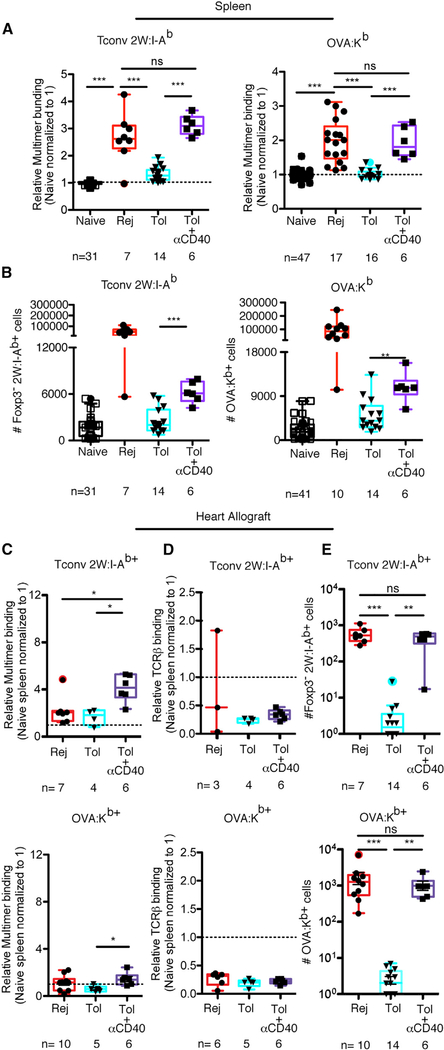
Figure 1. Graft-Specific T Cells from Tolerant Mice Bind Less pMHC Multimer Than Cells from Rejecting Mice.
(A) Representative flow cytometry plots of splenic Foxp3+ and Foxp3− T cell populations reactive to 2W:I-Ab and OVA:Kb isolated from naive untransplanted B6 mice, mice transplanted 8–9 days prior with a BALB/c × B6 F1 2W-mOVA or BALB/c-OVA heart and left untreated to undergo acute rejection (Rej), or mice transplanted and tolerized with anti-CD154 and DST (Tol). (B–E) Histograms and relative 2W:I-Ab and OVA:Kb multimer mean fluorescence intensity (MFI) reflecting multimer staining (B and C), TCRβ (B and C), and CD8α (D and E) levels of endogenous alloreactive T cells over time in naive, acute rejection, and tolerant recipients. Values were normalized, with the average of the MFI for the naive mice in each experiment set to 1. Data are pooled from 2–19 independent experiments. Box-and-whisker plots with Tukey whiskers are superimposed with the individual data points. Numbers of mice per group are listed below each panel. Data were analyzed by one-way ANOVA with Bonferroni correction for multiple testing. **p < 0.01, ***p < 0.001 (for comparisons of naive, day 8–9 Rej, and day 8–9 Tol), ###p < 0.001 (for comparisons of naive, day 60+ Rej, and day 60+ Tol). NS, not significant. See also Figures S1 and S2.
Differential TCR Avidity Affects T Cell Function
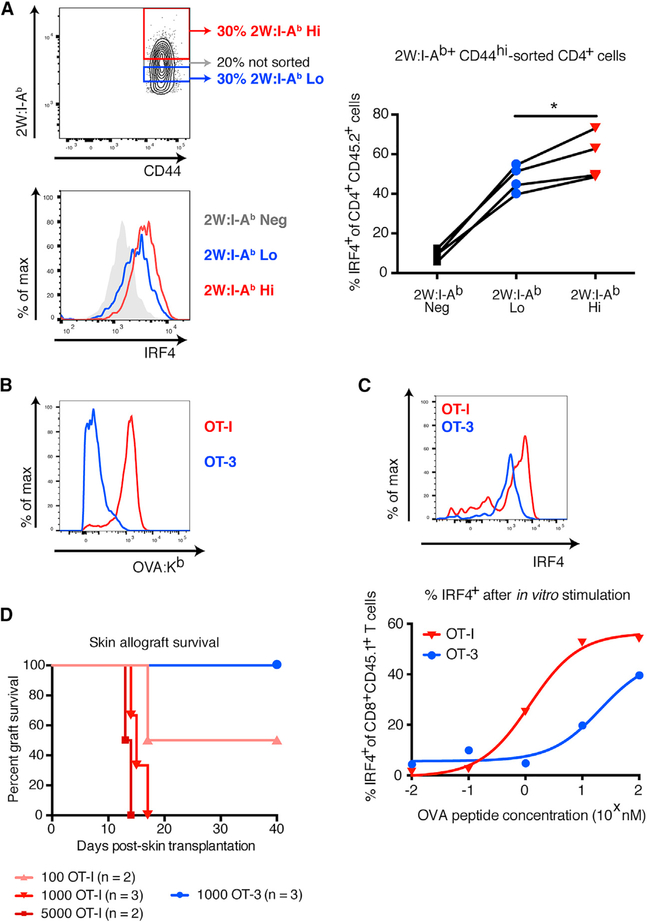
To investigate whether different pMHC binding avidity translated into distinct functional avidity, an injection of DST from 2W/OVA transgenic F1 mice into B6 hosts was used to expand donorreactive T cells. Seven days later, secondary lymphoid organ cells were stained with pMHC multimers and sorted by flow cytometry into high- and low-multimer binders (Figure 2A) before restimulation with peptide for 6 hr. A larger percentage of the high than the low 2W:I-Ab-binding T cells upregulated interferon regulatory factor 4 (IRF4) (Figure 2A), a quantifier of TCR signal strength (Krishnamoorthy et al., 2017), demonstrating more limited functional avidity of low 2W:IAb-binding T cells. A similar analysis with OVA:Kb binding T cells could not be performed, because cell sorting using OVA:Kb pentamers resulted in maximal IRF4 expression even without peptide restimulation, an artifact of pMHC multimers inducing TCR signaling during the sorting process. To circumvent these caveats, we obtained OT-I/recombination-activating gene-knockout (OT-I/RAG-KO) and OT-3/TCRα-KO mice whose T cells can be sorted with congenic markers, thus leaving their TCRs untouched. As shown by their differential binding to OVA:Kb (Figure 2B), OT-I and OT-3 T cells bear the extremes of high versus low TCR affinity, a component of TCR avidity. This difference in pMHC binding correlated with a difference in T cell signaling upon OVA peptide stimulation, as evidenced by reduced IRF4 (Figure 2C). To determine the impact on transplant outcome, we used an OVA transgenic skin allograft model. P14/RAG-KO mice we used as hosts of OT-I and OT-3 to prevent homeostatic proliferation of the transferred cells (Figure S3), while ensuring that only the transferred cells recognized the graft, as P14 T cells recognize an irrelevant antigen. Whereas 103 high-affinity OT-I T cells rejected OVA-expressing skin grafts readily, low-affinity OT-3 T cells failed to do so (Figure 2D), demonstrating the superior capacity of high-avidity T cells to reject allografts over that of low-avidity T cells.
Figure 2. Tetramer Binding Correlates with T Cell Functional Avidity and Capacity to Mediate Skin Allograft Rejection.
(A) Top left: flow cytometry plot of layout to sort populations with differential 2W:I-Ab binding. Bottom left: representative histogram showing levels of IRF4 staining in tetramer-sorted, 2W:I-Ab-specific T cells after in vitro stimulation with 2W peptide. Right: percentage of IRF4+ tetramer-sorted, 2W:I-Ab-specific T cells after in vitro stimulation with 2W peptide. (B) Histograms showing intensity of OVA:Kb pentamer binding in OT-I/RAG-KO and OT-3/TCRα-KO T cells 7 days after immunization with 2W-mOVA DST. (C) Top: representative histogram showing levels of IRF4 staining in OT-I/RAG-KO and OT-3/TCRα-KO T cells after in vitro stimulation with 10 nM SIINFEKL peptide. Bottom: percentage of IRF4+ cells within the population of CD8+ CD45.1+ OT-I/RAG-KO and OT-3/ TCRα-KO T cells after in vitro stimulation with a dose titration of SIINFEKL peptide. Lines indicate nonlinear regression analysis. (D)Survival of B6 2W-mOVA skin allografts in P14/RAG-KO recipients adoptively transferred with sorted OT-I/RAG-KO or OT-3/TCRα-KO T cells (p < 0.05 by log rank test). Data in (A) were analyzed by one-way paired ANOVA. *p < 0.05. See also Figure S3.
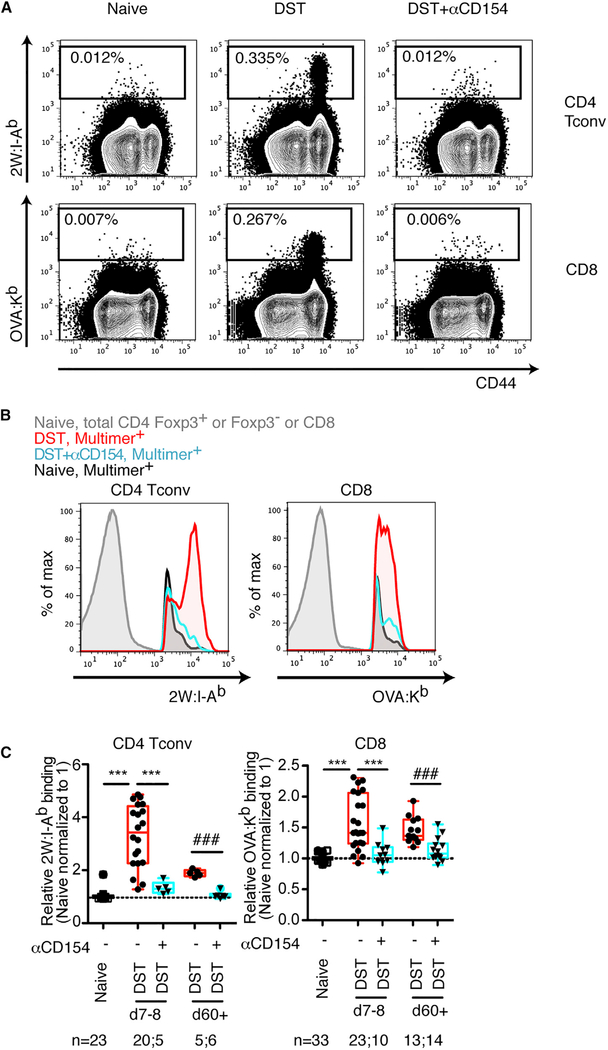
Costimulation Blockade Prevents Avidity Maturation of Allospecific T Cells Exposed to Alloantigen in the Absence of a Graft
To determine whether the presence of an allograft was required for the differences in T cell avidity observed between rejecting and tolerant mice, we exposed mice to DST in the absence (DST alone) or presence of costimulation blockade (DST+αCD154). As observed following cardiac transplantation, αCD154 reduced the increased pMHC multimer binding that occurred in response to DST (Figures 3A–3C). These differences were retained when gating only on antigen-experienced CD44hi T cells (Figure S2B). Thus, administration of anti-CD154 was sufficient to block avidity maturation following exposure to donor antigen in the presence or absence of a persistent allograft.
Figure 3. Exposure to Donor Antigen Alone without an Allograft also Promotes Avidity Maturation of Alloreactive T Cell Populations, which Is Blunted in the Absence of Costimulation.
(A) Representative flow cytometry plots of splenic T conventional cell populations stained with 2W:I-Ab and OVA:Kb multimers in naive mice, and mice immunized one week prior with BALB/c 3 B6 F1 2W-mOVA donor splenocytes in the absence (DST) or in the presence of anti-CD154 (DST+αCD154). (B) Histograms showing the levels of 2W:I-Ab and OVA:Kb multimer-binding. (C) 2W:I-Ab and OVA:Kb multimer mean fluorescence intensity (MFI) of endogenous alloreactive conventional T cells isolated from spleens on the days indicated. Values were normalized, with the average of the MFI for the naive mice in each experiment set to 1. Data are pooled from 2–11 independent experiments; numbers of mice per group are listed below each panel. Box-and-whisker plots with Tukey whiskers are superimposed with the individual data points. Data were analyzed by one-way ANOVA with Bonferroni post-tests for multiple comparisons. ***p < 0.001 (for comparisons of naive, day 7–8 DST, day 7–8 DST+αCD154). ###p < 0.001 (for comparisons of naive, day 60+ DST, day 60+ DST+αCD154). See also Figure S2.
Avidity Maturation Occurs at the Population Level
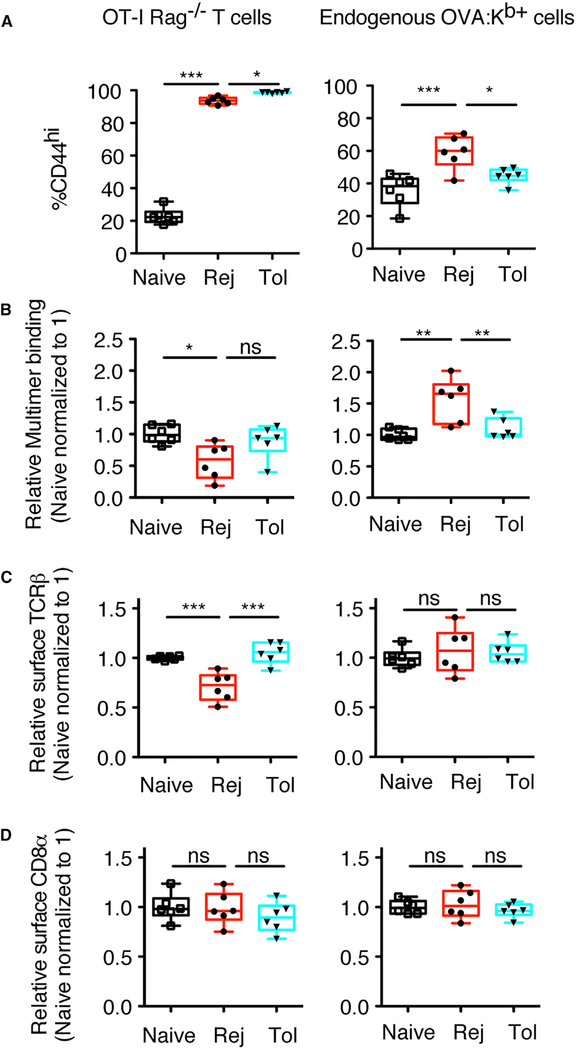
Changes to T cell avidity can occur cell intrinsically through posttranslational modifications such as glycosylation or nitration of the TCR (Daniels et al., 2001; Kuball et al., 2009; Nagaraj et al., 2007). To determine whether post-translational modifications of the tolerant TCRs contributed to the ability of costimulation blockade to restrain population avidity maturation, we adoptively transferred congenically marked OT-I/RAG-KO T cells into syngeneic B6 recipients before transplantation and, using OVA:Kb multimers, simultaneously tracked in the same mice the avidity of polyclonal endogenous and monoclonal transferred T cells. Most OT-I T cells became CD44hi in both rejection and tolerance (Figure 4A), but their transfer reduced the number of endogenous
Figure 4. Differences in T Cell Avidity between Rejecting and Tolerant Mice Result from Population-Level Changes Post-Alloantigen Encounter and Are Not Observed with TCRTg OT-I T Cells.
1 × 105 OT-I/RAG-KO T cells were adoptively transferred into lymphoreplete naive untransplanted mice, mice transplanted with a BALB/c-OVA heart and left untreated (Rej), or transplanted mice tolerized with anti-CD154 (Tol), and T cells were analyzed 7–8 days later. (A) Percentages of CD44hi OT-I/RAG-KO and endogenous OVA:Kb+ cells. (B–D) Normalized values of the geometric mean fluorescence intensity of multimer binding (B), surface TCRβ (C), and CD8α (D) for CD44hi OT-I/RAG-KO and endogenous OVA:Kb+ cells are shown. Values were normalized, with the average of the MFI for the naive mice in each experiment set to 1. Data are pooled from 2 independent experiments, with n = 6 mice total per group. Box-and-whisker plots with Tukey whiskers are superimposed with the individual data points. Data were analyzed by one-way ANOVA with Bonferroni correction for multiple testing. *p < 0.05, **p < 0.01, ***p < 0.001. NS, not significant.
OVA:Kb-binding CD8+ T cells that were activated (compare Figures 4A and S1C for rejecting mice), likely because of high-affinity competition for antigen. Thus, analysis of these experiments was performed on CD44hi-gated events to analyze comparable populations. In contrast to the increased avidity maturation of endogenous T cell populations in rejecting mice, monoclonal OT-I T cells in the same animals bound less pMHC multimer in rejecting than in tolerant or naive mice (Figure 4B). We speculate that this was most likely due to activationinduced downregulation of surface TCRβ, not CD8α, in OT-I T cells, but not endogenous OVA:Kb-binding T cells (Figures 4C and 4D). Thus, avidity maturation in rejecting mice occurs through population-level changes to the T cell repertoire, rather than post-translational modifications to the TCRs at the cell-intrinsic level. While post-translational modifications may also be occurring, the effect of population-level changes dominates. In addition, these experiments emphasize that monoclonal TCR-Tg (transgenic) T cells reflect a single TCR affinity and/or avidity for antigen and fail to capture the dynamic range of endogenous polyclonal T cell populations that encompass multiple pMHC affinities and/or avidities.
Positive Costimulation Downstream of CD40 Can Rescue T Cell-Population Avidity Maturation during Tolerance Induction
Reduced acquisition of avidity maturation in alloreactive T cell populations from tolerant hosts may be due to a lack of expansion of high-avidity clones because of their activation in the absence of costimulation. To test this possibility, we treated mice with agonistic anti-CD40 at the time of transplantation to restore signaling to APCs while concurrently blocking CD154 on activated T cells. Agonistic anti-CD40 rescued avidity maturation in transplanted mice treated with DST+αCD154 (Figure 5A). The observed increase in population avidity coincided with greater numbers of allospecific T cells, albeit less than in untreated transplanted hosts in the spleen (Figure 5B) but to an equivalent degree in the graft (Figure 5E). In contrast to the spleen, the avidity of T cell populations for pMHC could not be evaluated in the allograft (Figure 5C), because antigen load in the heart transplant resulted in significant TCR downregulation (Figure 5D) that precluded using pMHC binding as a proxy for T cell-population avidity. These data suggest that selective costimulation-dependent expansion of high-avidity T cells downstream of CD40 is important for allospecific T cell avidity maturation.
Figure 5. Concurrent Agonistic Anti-CD40 Treatment Allows Costimulation Blockade-Treated Alloreactive T Cell Populations to Undergo Avidity Maturation and Expansion.
(A) Normalized MFI of Foxp3− 2W:I-Ab and OVA:Kb multimer-reactive splenic T cells from naive, rejected, and tolerant mice. In addition, a group of transplanted mice received a single concurrent dose of agonistic anti-CD40 on day 0 at the same time as the DST and anti-CD154 used for tolerance induction. (B) Numbers of splenic T conventional cells binding 2W:I-Ab and OVA:Kb. All transplanted mice were analyzed 8–9 days post-transplantation. (C and D) Normalized multimer and TCRβ (D) MFI of Foxp3− 2W:I-Ab and OVA:Kb multimer-reactive graft-infiltrating T cells. (E) Numbers of graft-infiltrating T conventional cells binding 2W:I-Ab and OVA:Kb. Data points in naive, rejected, and tolerant groups were also presented in Figures S1 and 1C. Data are pooled from 2–5 independent experiments, including the Tol + anti-CD40 data that are pooled from two independent experiments, with n = 6 mice total per group. Box-and-whisker plots with Tukey whiskers are superimposed with the individual data points. Data were analyzed 8–9 days post-transplantation by one-way ANOVA with Bonferroni correction for multiple testing (A, C, and D), Kruskal-Wallis with Dunn’s test (E), or Student’s t test (B). **p < 0.01, ***p < 0.001. NS, not significant. See also Figures S4 and S5.
To determine whether avidity maturation requires T cell expansion, we treated mice with mycophenolate mofetil (MMF), an immunosuppressive drug commonly used in clinical transplantation that inhibits purine synthesis and therefore cell proliferation both directly and perhaps indirectly via modulation of APC activation (Cicinnati et al., 2009). In the mice in which MMF was effective at inhibiting proliferation, measured by Ki-67 staining, the DST-reactive T cell populations failed to undergo avidity maturation, instead resembling naive T cell populations. There was a strong correlation between the proportion of cells that had proliferated and the populations’ ability to avidity mature (Figure S4). To test whether increased T cell expansion could modulate T cell avidity, we used a model that provided enhanced T cell help and increased expansion of alloreactive CD8+ T cells (Miller et al., 2016b). At the time of transplantation, we adoptively transferred 105 CD4+ TCR75 cells, which recognize BALB/ c-derived Kd peptide presented on recipient I-Ab and could provide help to OVA:Kb-reactive CD8+ T cells. An increase in multimer binding on OVA:Kb-reactive CD8+ T cells (Figure S5A) paralleled the increase in the percentage (Figure S5B) and total number (Miller et al., 2016b) of splenic OVA:Kb-reactive CD8+ T cells, supporting the correlation between T cell expansion and avidity maturation.
Allosensitized Mice Have a More Skewed T Cell Repertoire Than Mice Treated with αCD154
To determine whether avidity maturation correlated with a distinct TCR repertoire following alloantigen exposure from that in tolerant hosts, the repertoires of sorted 2W:I-Ab- and OVA:Kb-binding cells from DST-treated and DST+αCD154-treated mice were analyzed through TCRβ spectratyping. Examples of spectratype traces of a previously described 2W:I-Ab-specific Vβ family member (Moon et al., 2007), Vβ8.2, are shown for 2W:I-Ab− and 2W:I-Ab+ T cells (Figure 6A). The DST-treated mice are exposed to numerous BALB/c antigens within the F1 background, in addition to 2W and OVA, such that the 2W: I-Ab− spectratypes revealed modest skewing. 2W:I-Ab+ T cells in DST-treated mice had a more restricted Vβ8.2 repertoire than their DST+αCD154 counterparts, suggesting lack of expansion of specific clones in the costimulation blockade-treated animals (Figure 6A).
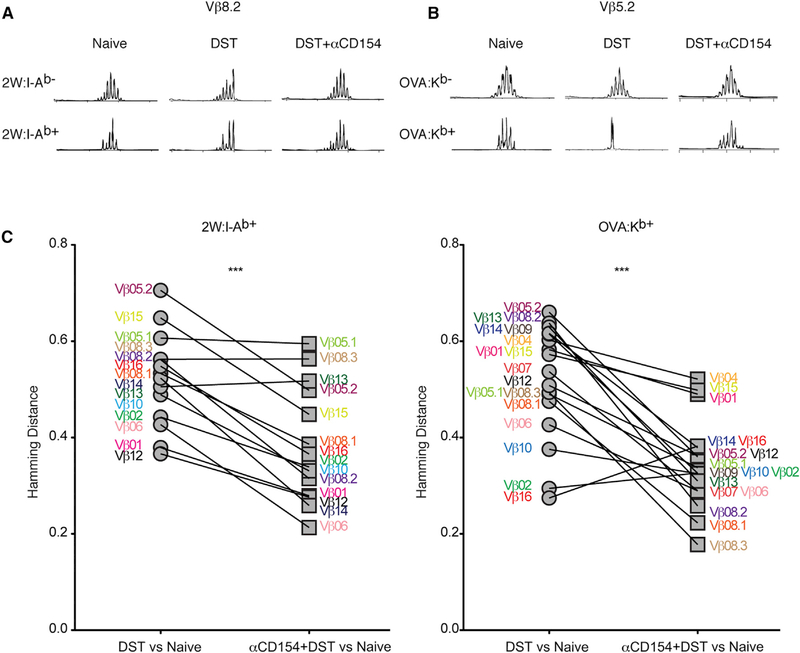
Figure 6. Costimulation Blockade Dampens Skewing of the T Cell Repertoire that Normally Occurs following Alloantigen Encounter.
(A) Representative TCR Vβ 8.2 spectratypes for CD4+ T cells that were non-multimer binding (2W:I-Ab−) or that bound multimer (2W:I-Ab+) from splenic T cells isolated from naive mice or one week after DST or DST+αCD154. (B) Representative TCR Vβ 5.2 spectratypes for CD8+ T cells that were non-multimer binding (OVA:Kb−) or that bound multimer (OVA:Kb+) from splenic T cells isolated from naive mice or one week after DST or DST+αCD154. Traces were normalized to the height of the tallest peak. (C) Hamming distances comparing the degree of similarity (= 0) or skewness (= 1) of populations of TCR Vβ lengths across all 18 Vβ families sampled between the multimer-positive samples in the DST and DST+αCD154 groups compared to the multimer-positive samples in the naive group. One trace each is shown out of 4–8 mice per group, pooled from two independent experiments (A and B). ***p < 0.001 by paired Student’s t test. See also Figure S6 and Table S1.
Immunization with OVA has been shown to expand T cells that express Vβ5.2, and Vβ5.2 transgenic mice have a high proportion of OVA-reactive T cells (Dillon et al., 1994). Similarly to the alloreactive CD4+ T cells, OVA:Kb+ CD8+ T cells from DST-treated mice had a more skewed Vβ5.2 repertoire than T cells from DST+αCD154-treated mice (Figure 6B). To measure the degree of skewness of each population of pMHC multimer-binding T cells, Hamming distances were calculated for each TCR Vβ family for each mouse using the relative areas under each peak in the spectratypes in treated mice relative to naive mice (Figure 6C). A value of zero for the Hamming distance indicates no difference in the populations compared, while a value of one signifies the two populations are distinct. TCR CDR3 lengths across all Vβ families were more skewed in DST-treated than in DST+αCD154-treated groups relative to multimer non-binding T cells in the same mice, as well as relative to multimer-binding T cells in naive mice. In combination with the individual spectratype traces showing more restricted repertoires, we infer that productive alloimmunity results in the preferential expansion of T cell clones with high avidity, and this leads to the overall avidity maturation of the allospecific T cell populations observed following alloantigen encounter. In contrast, the less skewed repertoire and the more Gaussian-like spectratypes in αCD154-treated mice reveal lack of selective expansion of particular T cell clones, correlating with the reduced ability of this population to undergo avidity maturation in the absence of positive costimulation.
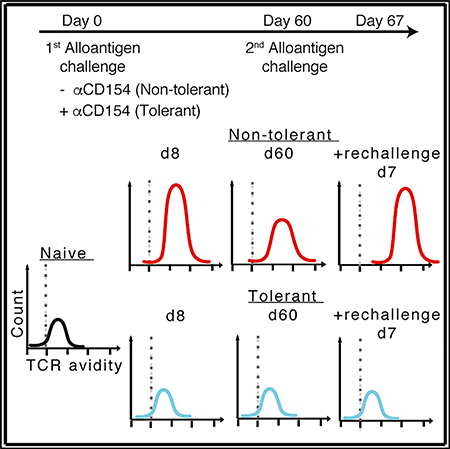
The Low-Avidity Profile of Allospecific T Cells in Tolerance Resists Alloantigen Rechallenge
To determine the long-term stability of alloreactive T cell populations’ low avidity in αCD154-treated mice, we rechallenged mice 30 to 60 days following initial DST or DST+αCD154 immunization with donor antigen (DST) in the absence of any immunosuppression (Figure 7A). Similar to the increased population avidity reported upon secondary response to infections (Busch and Pamer, 1999), rechallenge of DST-immunized mice with donor splenocytes resulted in a further increase in pMHC multimer binding, characteristic of a memory T cell response (Figure 7B). In contrast, DST rechallenge did not increase the T cell avidity profile in mice initially exposed to DST+αCD154 (Figure 7B). No significant difference was observed in 2W:I-Ab+ regulatory T cell percentages upon DST rechallenge of the tolerant hosts (Figure 7C). These results suggest the T cell-population avidity profile is altered long term upon antigen exposure in the presence of initial costimulation blockade, despite the potential input of new donor-reactive thymic emigrants.
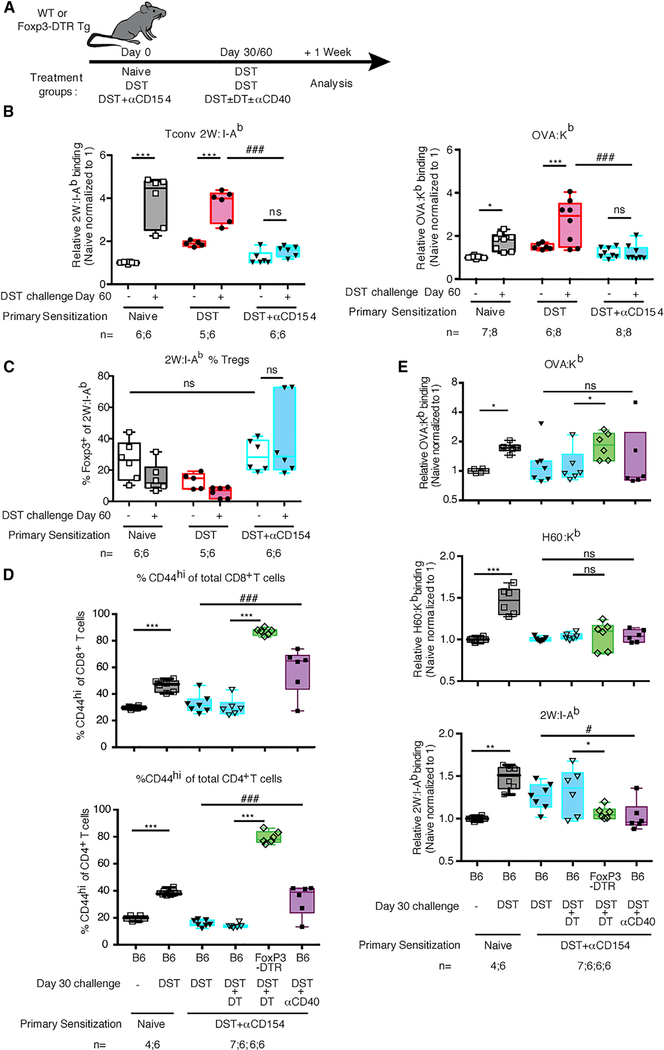
Figure 7. Low-Avidity Profile of Alloreactive T Cells Primed in the Presence of Costimulation Blockade Persists upon Antigen Rechallenge.
(A) Experimental design. (B) Normalized MFI of Foxp3− 2W:I-Ab and OVA:Kb multimer binding of endogenous alloreactive splenic T cells isolated from mice one week post-challenge with DST. Values were normalized, with the average of the MFI for the naive mice in each experiment set to 1. Data from non-rechallenged groups are the same as those displayed in Figure 3C. (C) Percentages of Foxp3+ 2W:I-Ab multimer-binding splenic T cells isolated from mice one week post-challenge with DST. (D) Percentage of total CD8+ or CD4+ splenocytes that are CD44hi one week post-challenge with DST. (E) Normalized MFI of CD44hi-gated OVA:Kb, H60:Kb, and 2W:I-Ab multimer binding of endogenous alloreactive splenic T cells isolated from mice one week postchallenge with DST. Values were normalized, with the average of the MFI for the naive mice in each experiment set to 1. Data are pooled from 2–3 independent experiments; numbers of mice per group are listed below each panel. Box-and-whisker plots with Tukey whiskers are superimposed with the individual data points.Data were analyzed by two-way ANOVA with Bonferroni post-tests for multiple pairwise comparisons (B) or one-way ANOVA with Bonferroni post-tests for multiple pairwise comparisons (D and E). By two-way ANOVA, *p < 0.05; **p < 0.01, ***p < 0.001, and by one-way ANOVA, #p < 0.05, ###p < 0.001. NS, not significant.
To investigate whether long-term resistance to avidity maturation upon antigen rechallenge was mediated by Tregs or could be overcome with additional costimulation, Foxp3-DTR (Diphteria toxin receptor) transgenic mice or wild-type mice initially immunized with DST or DST+αCD154 were restimulated with DST at day 30, following diphtheria toxin (DT) injection to deplete Tregs, or with agonistic anti-CD40 to enhance costimulation. Post-DT, Tregs in the blood were reduced from 4.3% ± 0.6% to 0.3% ± 0.1% (n = 6) before DST rechallenge. The efficacy of Treg depletion and anti-CD40 was also verified at sacrifice, because both interventions significantly increased the proportion of CD44hi T cells (Figure 7D). Treg depletion, but not CD40 costimulation, at the time of DST rechallenge significantly restored avidity maturation of OVA:Kb-reactive T cells in mice initially exposed to DST+αCD154 (Figure 7E). To extend these results to an additional antigen that is not a model antigen, we also stained endogenous CD8 population with H60-Kb multimer, because H60 is a natural antigen present in BALB/c cells (Malarkannan et al., 1998). H60-reactive CD8+ T cell population in animals initially treated with DST+αCD154 also failed to avidity mature following DST rechallenge at day 30 (Figure 7E). However, neither depletion of Tregs nor increased costimulation restored avidity maturation of the H60:Kb-binding population at the maintenance phase of tolerance (Figure 7E). Similarly, neither Treg depletion nor CD40 costimulation restored avidity maturation of 2W:I-Ab-binding CD4+ T cells upon DST rechallenge (Figure 7E). These results suggest that at the maintenance phase of tolerance, Tregs can play a role in constraining the avidity of alloreactive T cell populations for certain TCR specificities, while Treg-independent mechanisms are responsible for the long-term control of T cell-population avidity for other specificities.
DISCUSSION
In this study, we investigated the avidity profile of alloreactive T cells following rejection and induction of peripheral T cell tolerance with costimulation blockade. In animal models, costimulation blockade therapy has been shown to induce robust donor-specific tolerance through peripheral T cell mechanisms (Markees et al., 1998), including clonal deletion and anergy and/or exhaustion at a cell-intrinsic level and suppression by regulatory cells at a cell-extrinsic level (Pinelli et al., 2013; Quezada et al., 2005). Here, we observed that peripheral transplantation tolerance induced with costimulation blockade was also associated with the persistence of graft-reactive CD4+ and CD8+ T cells with low avidity for alloantigens at the T cellpopulation level. Low avidity depended on limiting the selective expansion of higher-avidity T cell clones and was stable and resistant to population avidity maturation upon subsequent encounter with alloantigen, and Tregs played a role in its maintenance for certain TCR specificities. These findings highlight a previously unknown element of peripheral transplantation tolerance, in which alloreactivity is shaped at the T cell-population level via control of the TCR repertoires within each given peptide specificity.
The successful induction of peripheral transplantation tolerance is thought to require T cell deletion through passive apoptosis (Wells et al., 1999). In addition, it has been shown that peripheral tolerance to self-antigens and the induction of oral tolerance can result in abortive proliferation of antigen-specific T cells (Sun et al., 2003; Townsend and Goodnow, 1998). Studies using carboxyfluorescein succinimidyl ester (CFSE)labeled TCR-Tg T cells transferred into tolerant recipients have shown that tolerance induction does not inhibit proliferation of T cells (Chai et al., 2015; Miller et al., 2016a) but does prevent their accumulation. Although studies using TCR-Tg T cells suggest there was no defect in proliferation in a tolerant environment, those studies only compared proliferation of T cells of a single specificity and affinity and thus did not fully recapitulate the endogenous response to alloantigens where interclonal competition occurs. Our use of OT-I T cells emphasizes that results from a single TCR sequence cannot be extrapolated to the behavior of the whole polyclonal population reactive to OVA. The monoclonal OT-I cells exhibited less OVA:Kb binding in the rejecting group primarily because of a reduction in TCR expression levels on the cell surface, as a result of its particularly high TCR affinity for OVA. Surface TCR levels have been shown to be dynamically tuned by the strength and duration of antigen signaling and presence of costimulation (Schrum et al., 2000). Thus, at the polyclonal level, pMHC staining may miss detecting the highest-affinity T cells, which may downregulate their TCR like OT-I cells, but our results show that most splenic T cells don’t downregulate their TCR during rejection or tolerance, and the dominant phenomenon is that rejection results in accumulation of higher-avidity endogenous T cells than tolerance. This observation also highlights the importance of analyzing expression levels of the TCR when measuring T cell avidity by pMHC multimers. Whereas TCR expression levels were not affected in secondary lymphoid organs of rejecting and tolerant mice at the time points analyzed and enabled us to use pMHC binding as a proxy for TCR avidity, this was not the case in the graft in which TCR downregulation was profound.
The population-level changes in endogenous T cell avidity between rejection and tolerance were directly observed by analyzing the repertoires of individual TCR Vβ families using TCRβ spectratyping. CD8+ Vβ5.2+ cells were clonally restricted upon immunization with DST expressing OVA, which confirms that this Vβ family harbors T cells containing greater affinity for recognizing OVA:Kb (Dillon et al., 1994). Mice treated with DST+αCD154 exhibited a less restricted Vβ5.2 repertoire. These results were extended across all TCR Vβ families examined, and both CD4+ and CD8+ T cells in DST+αCD154-treated mice had less skewed T cell repertoires than those from DST-treated mice. Thus, costimulation blockade reduces population-level avidity maturation by limiting expansion of high-avidity T cells.
When given at the time of transplantation and tolerance induction, anti-CD40 could trigger avidity maturation, with a concurrent increase in T cell numbers. Our results complement other studies in models of cancer, vaccines, or tolerance to self-antigens, showing that costimulation has a positive impact on T cell-population avidity maturation (Black et al., 2014; Hodge et al., 2005; Yang et al., 2005). Providing enhanced CD4+ T cell help increased the avidity of CD8+ T cell populations in our model. How APCs program the proliferation and selection of high-avidity alloreactive T cells during rejection remains incompletely understood. One possibility may be through Program Death-1/Program Death Ligand 1 (PD-1/PD-L1) signaling, because populations of T cells deficient in PD-1 expand more and have higher-avidity TCRs (Jiang et al., 2016). Another factor may be levels of interleukin (IL) 27, a cytokine that is produced by APCs and has been shown to play a role in the generation of high-avidity T cells (Pennock et al., 2014). Similarly, IL-12 produced by dendritic cells may also contribute to the generation of these cells (DeBenedette et al., 2008).
The low-avidity profile after costimulation blockade was remarkably stable, because avidity remained low upon antigen rechallenge. Although deletion of high-avidity clones could explain this phenomenon, we did not find evidence of deletion in our spectratyping data, because there was no reduction in the total number of peaks detected in tolerant versus naive mice (Figure S6). Instead, resistance to population-level changes depended on the presence of Tregs for OVA:Kb-binding populations, but not for H60:Kb- or 2W:I-Ab-binding ones. For these other specificities, resistance to avidity maturation may be epigenetically imprinted, similar to T cell-intrinsic hyporesponsiveness, which makes cells resistant to secondary encounter of antigen (Philip et al., 2017; Schietinger et al., 2012), or may be constrained by regulatory cells distinct from classical Tregs. Studies of T cells in tumor microenvironments that are deemed dysfunctional and of self-specific T cells (Black et al., 2014; Kuball et al., 2009; Malhotra et al., 2016; Moon et al., 2011; Soong et al., 2014; Souders et al., 2007; Wong et al., 2008; Yu et al., 2015) have also revealed that many of these cells are low avidity at the population level, supporting the idea that tolerance, whether spontaneously acquired in the tumor microenvironment or upon self-antigen encounter or, as we show in this study, therapeutically induced by costimulation blockade therapy, may be simultaneously enforced through multiple mechanisms, one of which being the preservation of low-avidity repertoire for the relevant antigen.
There are strong correlations between accumulation of highavidity T cells and clearance of infections (Busch and Pamer, 1999), elimination of tumors (Black et al., 2014; Kuball et al., 2009; Soong et al., 2014), better memory responses (Turner et al., 2008; Zehn et al., 2009), and autoimmunity (Maeda et al., 2014). Low-avidity T cells, however, which are correlated with less effector functionality (Falta et al., 2005; Kuball et al., 2009; Tsang et al., 2011), would be beneficial for transplantation. Our study demonstrates that a lower population avidity can be achieved therapeutically via costimulation blockade, correlating with robust transplantation tolerance, while unmodified transplant rejection correlates with population-avidity maturation. Therapeutic approaches that durably constrain T cells with high avidity for donor antigens may be desirable to achieve robust transplantation tolerance in the clinic and may be considered to prevent or treat autoimmunity.
EXPERIMENTAL PROCEDURES
Mice
B6 and BALB/c mice were purchased from Envigo RMS (Indianapolis, Indiana), CD45.1+ mice and OT-I/RAG-KO mice were purchased from Jackson Laboratory (Bar Harbor, Maine), and P14/RAG-KO mice were purchased from Taconic Biosciences (Rensselaer, New York). OT-3/TCRα-KO mice were obtained from Stephen Schoenberger (La Jolla Institute), TCR75 TCR-Tg mice were obtained from R. Pat Bucy (University of Alabama), BALB/c-OVA mice were obtained from Elizabeth Ingulli (when at the University of Minnesota), and Foxp3-DTR mice from Peter Savage (University of Chicago). 2W-mOVA (membrane OVA) (2W-mOVA) mice on a B6 background, as previously described (Moon et al., 2011), were crossed to BALB/c mice to generate F1 progeny. Both male and female mice at least 6–8 weeks of age were used for the experiments. In some experiments, mice were treated with one-sixth to one-quarter spleen for DST (intravenous [i.v.]) on day 0 ± 0.6 mg αCD154 (i.v.) or daily gavage with 300 mg/kg/day of MMF in 5% dextrose. In some experiments, mice were rechallenged with a second DST injection i.v. 30 or 60 days after primary immunization in the presence or absence of a single 100 μg dose of agonistic anti-CD40 (FGK4.5) or 1 μg of DT (Sigma) on days 28, 29, and 31. Mice were housed under specific pathogen-free conditions.
Heart Transplantation
Cardiac allografts were transplanted, adapting a technique from Corry et al. (1973), by anastomosing the aorta and pulmonary artery of the graft end to side to the recipient’s aorta and vena cava, respectively. Tolerant mice were treated with three 0.6 mg doses of αCD154 (MR1) on days 0, 7, and 14 post-transplantation, as well as one-quarter spleen for DST i.v. on day 0. In certain experiments, mice were treated with 100 mg of agonistic αCD40 (FGK4.5) on day 0 post-transplantation.
Skin Transplantation
Tail skin from F1 2W-mOVA mice was transplanted onto the flank of P14/ RAG-KO recipients as previously described (Kellersmann and Zhong, 1998). Sorted OT-I or OT-3 T cells were adoptively transferred on the day of transplantation. Bandages were removed 7 days post-transplantation. Rejection was reported when <20% of the transplanted skin was viable.
Magnetic Enrichment
T cells were magnetically enriched by negative selection from spleens of recipient mice following staining with anti-CD19-biotin (Fitch Monoclonal Facility), anti-Ter119-biotin (eBioscience), and anti-CD11b-biotin (eBioscience) and incubation with streptavidin magnetic beads (Pierce, Thermo Fisher Scientific). APCs were magnetically enriched by negative selection of splenocytes from CD45.1+ B6 mice following staining with anti-Thy1.2-biotin (eBioscience) and incubation with streptavidin magnetic beads (Pierce).
Flow Cytometry
Five million T-enriched cells were stained with a fixable live/dead stain (Aqua, Invitrogen) followed by phycoerythrin (PE)-coupled-2W:I-Ab tetramers for 1 hr in a room temperature (RT) water bath, washed, and then stained with PE-coupled-OVA:Kb pentamers (ProImmune, Oxford, UK) or PE-coupled-H60:Kb tetramers (NIH Tetramer Core Facility) for 20 min in a RT water bath. Cells were then stained with anti-CD4 (L3T4), anti-CD8 (Ly2), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD44 (IM7), anti-TCRβ (H57–597), and anti-B220 (RA3–6B2). Surface-stained cells were then fixed with the Foxp3 fixation permeabilization buffer kit (eBioscience, San Diego, California) for 15 min at RT and washed with 1 × permeabilization buffer. Some samples were intracellularly stained with anti-Ki-67 (SolA15), anti-IRF4 (3E4), or anti-Foxp3 (FJK-16 s) for 30 min at RT; washed with permeabilization buffer; and analyzed by flow cytometry. CFSE labeling was performed by labeling cells with CellTrace CFSE (Invitrogen) for 20 min at 37°C. All monoclonal antibodies (mAbs) were from BD Biosciences, eBioscience, or Invitrogen.
Adoptive Cell Transfer
Spleen and lymph node cells were isolated into single-cell suspensions and counted with an Accuri C6 flow cytometer (BD Biosciences, San Jose, California). The percentage of CD8α+ Va2+ CD45.2+ CD44lo cells was determined by flow cytometry and used to calculate the total number of OT-I/RAG-KO cells for the adoptive transfer. In other experiments, the percentage of CD4+ Vb8.3+ CD45.1+ CD44lo cells was determined by flow cytometry and used to calculate the total number of TCR75 cells for the adoptive transfer. 105 cells were injected retro-orbitally in 200 μL of PBS on the day before or the day of transplantation. For direct comparison of skin allograft rejection or homeostatic proliferation by OT-I/RAG-KO and OT-3/TCRα cells, CD8+ CD44lo transgenic T cells were sorted before adoptive transfer in 200 mL of PBS. For comparison of homeostatic proliferation of OT-I versus OT-3 cells, 1.5 × 105 cells were injected.
Tetramer Sorting
Spleens were isolated from B6 mice 7 days after immunization with F1 2W-mOVA splenocytes, pooled in groups of three spleens, homogenized, and magnetically enriched for T cells. Cells were stained with APC-coupled-2W:I-Ab tetramers for 1 hr in a RT water bath, washed, and then stained with PE-coupled-OVA:Kb pentamers for 20 min in a RT water bath. Cells were then stained with anti-CD4 (L3T4), anti-CD8 (Ly2), anti-CD44 (IM7), and anti-B220 (RA3–6B2). Within the population of CD4+ CD44+ 2W:I-Ab+ cells, 30% of cells with the brightest 2W:I-Ab staining were sorted as 2W:I-Ab Hi. The 20% next brightest were not sorted. The following 30% brightest were sorted as 2W:I-Ab Lo. The dimmest 20% of 2W:I-Ab+ cells were not sorted. CD4+ CD44+ 2W:I-Ab− cells were sorted as 2W:I-Ab Neg. Gates were adjusted between samples before sorting so that the percentage of cells in each gate remained the same across samples.
In vitro Stimulation
5 × 103 sorted 2W:I-Ab Hi, 2W:I-Ab Lo, or 2W:I-Ab Neg cells were co-cultured with 2 × 104 APC-enriched CD45.1+ B6 splenocytes and 25 μg/mL 2W peptide for 6 hr at 37C and 5% CO2. For peptide stimulation of OT-I and OT-3 T cells, splenocytes were isolated from CD45.1+ OT-I/RAG-KO and CD45.1+ OT-3/ TCRα-KO mice 7 days after immunization with 2W-mOVA DST and then magnetically enriched for T cells. 1 × 104 T cell-enriched OT-I or OT-3 T cells were then co-cultured with 4 × 104 CD45.1− APC-enriched B6 splenocytes, and the indicated dose of SIINFEKL peptide for 6 hr at 37°C and 5% CO2. Stimulated cells were then stained with a fixable live/dead stain (Aqua, Invitrogen), washed, and stained with anti-CD4 (L3T4), anti-CD8 (Ly2), anti-CD45.1 (A20), and anti-CD45.2 (104). Using the Foxp3 fixation buffer set as mentioned earlier, surface-stained cells were fixed and intracellularly stained with anti-IRF4 (3E4) for 30 min at RT and then were analyzed by flow cytometry.
TCRβ Spectratyping
Multimer-positive and multimer-negative T cells were sorted from T cellenriched spleens into QIAGEN’s RLT Plus lysis buffer and frozen before isolating RNA using RNEasy mini and micro kits (QIAGEN, Valencia, California). cDNA was synthesized using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, California). CDR3β regions were amplified by PCR with 21 Vβ 5’ primers (Table S1) paired with a fluorescein amidite-Cβ1.1 primer. The Vβ11 PCR reactions did not reach significant amplification for analysis and were removed from the analysis. CDR3 peaks were measured on an Applied Biosystems 3130XL or 3730XL machine and aligned using the Liz500 ladder.
Data Analysis
Flow cytometry data were analyzed using FlowJo (Tree Star, Ashland, Oregon). For TCR spectratyping data, the areas under each peak were analyzed using PeakStudio software (McCafferty et al., 2012). Hamming distances were calculated as previously described (Currier and Robinson, 2001). A minimum area of 2,000 units was used as a threshold before including samples in the Hamming distance calculations.
Statistics
With 3 mice per group per experiment, power calculations indicated 80% power at α = 0.05 to detect differences in avidity of 90% or greater with each group’s SD ≤ 30% of each group’s respective mean. Results from such individual experiments were pooled using a common group within each experiment for normalization to show all data from multiple experiments in single graphs. Statistical analyses were performed where appropriate using GraphPad Prism (GraphPad, La Jolla, California). Each test is listed in the figure legends.
Study Approval
The studies were performed in agreement with the University of Chicago’s Institutional Animal Care and Use Committee, according to the NIH guidelines for animal use.
Supplementary Material
Highlights.
Alloantigen encounter results in preferential expansion of high-avidity T cells
Costimulation blockade prevents skewing of T cell populations toward higher avidity
Low-avidity alloreactive T cell populations typify transplant tolerance long term
Tolerant mice retain low-avidity T cell populations despite alloantigen rechallenge
ACKNOWLEDGMENTS
We would like to thank Jessalynn Holman for breeding and genotyping all animals, Doug Kline for providing anti-CD40, Peter Savage for providing Foxp3-DTR transgenic mice, and Justin Kline for providing DT. We thank the Fitch Monoclonal Facility, the Flow Cytometry Facility, and the DNA Sequencing and Genotyping Facility of the University of Chicago. M.L.M. was funded by American Heart Association predoctoral fellowships (13PRE14550022 and 15PRE22180007), a Cardiovascular Pathophysiology and Biochemistry Training Grant (T32 HL07237), and an HHMI Med-into-Grad Program training grant (56006772). C.M.M. was funded by the Growth, Development, and Disabilities Training Program (T32 HD007009). The work was also supported by a National Institute of Allergy and Infectious Diseases grant (P01AI-97113) to A.S.C. and M.-L.A.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and one table and can be found with this article online at https://doi.org/10.1016/j.celrep.2018.07.067.
REFERENCES
- Black CM, Armstrong TD, and Jaffee EM (2014). Apoptosis-regulated low-avidity cancer-specific CD8(+) T cells can be rescued to eliminate HER2/neu-expressing tumors by costimulatory agonists in tolerized mice. Cancer Immunol. Res 2, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, Marquardt KL, Cheung J, and Sherman LA (2012). Functional differences between low- and high-affinity CD8(+) T cells in the tumor environment. OncoImmunology 1, 1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch DH, and Pamer EG (1999). T cell affinity maturation by selective expansion during infection. J. Exp. Med 189, 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai J-G, Ratnasothy K, Bucy RP, Noelle RJ, Lechler R, and Lombardi G (2015). Allospecific CD4(+) T cells retain effector function and are actively regulated by Treg cells in the context of transplantation tolerance. Eur. J. Immunol 45, 2017–2027. [DOI] [PubMed] [Google Scholar]
- Cicinnati VR, Hou J, Lindemann M, Horn PA, Sotiropoulos GC, Paul A, Gerken G, and Beckebaum S (2009). Mycophenolic acid impedes the antigen presenting and lymph node homing capacities of human blood myeloid dendritic cells. Transplantation 88, 504–513. [DOI] [PubMed] [Google Scholar]
- Corry RJ, Winn HJ, and Russell PS (1973). Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation 16, 343–350. [DOI] [PubMed] [Google Scholar]
- Crawford F, Kozono H, White J, Marrack P, and Kappler J (1998). Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity 8, 675–682. [DOI] [PubMed] [Google Scholar]
- Currier JR, and Robinson MA (2001). Spectratype/immunoscope analysis of the expressed TCR repertoire. Curr. Protoc. Immunol 38, 10.28.1–10.28.24. [DOI] [PubMed] [Google Scholar]
- Daniels MA, and Jameson SC (2000). Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J. Exp. Med 191, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, Devine L, Miller JD, Moser JM, Lukacher AE, Altman JD, Kavathas P, Hogquist KA, and Jameson SC (2001). CD8 binding to MHC class I molecules is influenced by T cell maturation and glycosylation. Immunity 15, 1051–1061. [DOI] [PubMed] [Google Scholar]
- DeBenedette MA, Calderhead DM, Ketteringham H, Gamble AH, Horvatinovich JM, Tcherepanova IY, Nicolette CA, and Healey DG (2008). Priming of a novel subset of CD28+ rapidly expanding high-avidity effector memory CTL by post maturation electroporation-CD40L dendritic cells is IL-12 dependent. J. Immunol 181, 5296–5305. [DOI] [PubMed] [Google Scholar]
- Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, Hivroz C, Nicaise J, Squifflet J-L, Mourad M, et al. (2008). Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity 28, 414–424. [DOI] [PubMed] [Google Scholar]
- Dillon SR, Jameson SC, and Fink PJ (1994). V beta 5+ T cell receptors skew toward OVA+H-2Kb recognition. J. Immunol. 152, 1790–1801. [PubMed] [Google Scholar]
- Fahmy TM, Bieler JG, Edidin M, and Schneck JP (2001). Increased TCR avidity after T cell activation: a mechanism for sensing low-density antigen. Immunity 14, 135–143. [PubMed] [Google Scholar]
- Falta MT, Fontenot AP, Rosloniec EF, Crawford F, Roark CL, Bill J, Marrack P, Kappler J, and Kotzin BL (2005). Class II major histocompatibility complex-peptide tetramer staining in relation to functional avidity and T cell receptor diversity in the mouse CD4(+) T cell response to a rheumatoid arthritis-associated antigen. Arthritis Rheum. 52, 1885–1896. [DOI] [PubMed] [Google Scholar]
- Hodge JW, Chakraborty M, Kudo-Saito C, Garnett CT, and Schlom J (2005). Multiple costimulatory modalities enhance CTL avidity. J. Immunol 174, 5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo K, Xu XY, and Bucy RP (2000). Heterogeneity of T cell clones specific for a single indirect alloantigenic epitope (I-Ab/H-2Kd54–68) that mediate transplant rejection. Transplantation 70, 1516–1524. [DOI] [PubMed] [Google Scholar]
- Iwakoshi NN, Mordes JP, Markees TG, Phillips NE, Rossini AA, and Greiner DL (2000). Treatment of allograft recipients with donor-specific transfusion and anti-CD154 antibody leads to deletion of alloreactive CD8+ T cells and prolonged graft survival in a CTLA4-dependent manner. J. Immunol 164, 512–521. [DOI] [PubMed] [Google Scholar]
- Jiang TT, Martinov T, Xin L, Kinder JM, Spanier JA, Fife BT, and Way SS (2016). Programmed death-1 culls peripheral accumulation of high-affinity autoreactive CD4 T cells to protect against autoimmunity. Cell Rep. 17, 1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellersmann R, and Zhong R (1998). Surgical technique for skin transplantation in mice In Organtransplantation in Rats and Mice, Timmermann W, Gassel H-J, Ulrichs K, Zhong R, and Thiede A, eds. (Springer; ), pp. 151–154. [Google Scholar]
- Krishnamoorthy V, Kannanganat S, Maienschein-Cline M, Cook SL, Chen J, Bahroos N, Sievert E, Corse E, Chong A, and Sciammas R (2017). The IRF4 gene regulatory module functions as a read-write integrator to dynamically coordinate T helper cell fate. Immunity 47, 481–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuball J, Hauptrock B, Malina V, Antunes E, Voss R-H, Wolfl M, Strong R, Theobald M, and Greenberg PD (2009). Increasing functional avidity of TCR-redirected T cells by removing defined N-glycosylation sites in the TCR constant domain. J. Exp. Med 206, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, and Hancock WW (2005). Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J. Exp. Med 201, 1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Nishikawa H, Sugiyama D, Ha D, Hamaguchi M, Saito T, Nishioka M, Wing JB, Adeegbe D, Katayama I, and Sakaguchi S (2014). Detection of self-reactive CD8+ T cells with an anergic phenotype in healthy individuals. Science 346, 1536–1540. [DOI] [PubMed] [Google Scholar]
- Malarkannan S, Shih PP, Eden PA, Horng T, Zuberi AR, Christianson G, Roopenian D, and Shastri N (1998). The molecular and functional characterization of a dominant minor H antigen, H60. J. Immunol 161, 3501–3509. [PubMed] [Google Scholar]
- Malhotra D, Linehan JL, Dileepan T, Lee YJ, Purtha WE, Lu JV, Nelson RW, Fife BT, Orr HT, Anderson MS, et al. (2016). Tolerance is established in polyclonal CD4(+) T cells by distinct mechanisms, according to self-peptide expression patterns. Nat. Immunol 17, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markees TG, Phillips NE, Gordon EJ, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, and Rossini AA (1998). Long-term survival of skin allografts induced by donor splenocytes and anti-CD154 antibody in thymectomized mice requires CD4(+) T cells, interferon-gamma, and CTLA4. J. Clin. Invest 101, 2446–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCafferty J, Reid R, Spencer M, Hamp T, and Fodor A (2012). Peak Studio: a tool for the visualization and analysis of fragment analysis files. Environ. Microbiol. Rep 4, 556–561. [DOI] [PubMed] [Google Scholar]
- Miller ML, Daniels MD, Wang T, Wang Y, Xu J, Yin D, Chong AS, and Alegre ML (2016a). Tracking of TCR-transgenic T cells reveals that multiple mechanisms maintain cardiac transplant tolerance in mice. Am. J. Transplant. 16, 2854–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Chen J, Daniels MD, McKeague MG, Wang Y, Yin D, Vu V, Chong AS, and Alegre ML (2016b). Adoptive transfer of tracer-alloreactive CD4+ T cell receptor transgenic T cells alters the endogenous immune response to an allograft. Am. J. Transplant 16, 2842–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, and Jenkins MK (2007). Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon JJ, Dash P, Oguin TH 3rd, McClaren JL, Chu HH, Thomas PG, and Jenkins MK (2011). Quantitative impact of thymic selection on Foxp3+ and Foxp3– subsets of self-peptide/MHC class II-specific CD4+ T cells. Proc. Natl. Acad. Sci. USA 108, 14602–14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, and Gabrilovich DI (2007). Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat. Med 13, 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozga AJ, Moalli F, Abe J, Swoger J, Sharpe J, Zehn D, Kreutzfeldt M, Merkler D, Ripoll J, and Stein JV (2016). pMHC affinity controls duration of CD8+ T cell-DC interactions and imprints timing of effector differentiation versus expansion. J. Exp. Med 213, 2811–2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace L, Tempez A, Arnold-Schrauf C, Lemaitre F, Bousso P, Fetler L, Sparwasser T, and Amigorena S (2012). Regulatory T cells increase the avidity of primary CD8+ T cell responses and promote memory. Science 338, 532–536. [DOI] [PubMed] [Google Scholar]
- Pennock ND, Gapin L, and Kedl RM (2014). IL-27 is required for shaping the magnitude, affinity distribution, and memory of T cells responding to subunit immunization. Proc. Natl. Acad. Sci. USA 111, 16472–16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, et al. (2017). Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinelli DF, Wagener ME, Liu D, Yamniuk A, Tamura J, Grant S, Larsen CP, Suri A, Nadler SG, and Ford ML (2013). An anti-CD154 domain antibody prolongs graft survival and induces Foxp3(+) iTreg in the absence and presence of CTLA-4 Ig. Am. J. Transplant 13, 3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, and Noelle RJ (2005). Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J. Immunol 175, 771–779. [DOI] [PubMed] [Google Scholar]
- Savage PA, Boniface JJ, and Davis MM (1999). A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10, 485–492. [DOI] [PubMed] [Google Scholar]
- Schietinger A, Delrow JJ, Basom RS, Blattman JN, and Greenberg PD (2012). Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335, 723–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrum AG, Wells AD, and Turka LA (2000). Enhanced surface TCR replenishment mediated by CD28 leads to greater TCR engagement during primary stimulation. Int. Immunol. 12, 833–842. [DOI] [PubMed] [Google Scholar]
- Soong R-S, Song L, Trieu J, Lee SY, He L, Tsai Y-C, Wu T-C, and Hung C-F (2014). Direct T cell activation via CD40 ligand generates high avidity CD8+ T cells capable of breaking immunological tolerance for the control of tumors. PLoS ONE 9, e93162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souders NC, Sewell DA, Pan Z-K, Hussain SF, Rodriguez A, Wallecha A, and Paterson Y (2007). Listeria-based vaccines can overcome tolerance by expanding low avidity CD8+ T cells capable of eradicating a solid tumor in a transgenic mouse model of cancer. Cancer Immun. 7, 2. [PMC free article] [PubMed] [Google Scholar]
- Sun J, Alison Stalls M, Thompson KL, and Fisher Van Houten N (2003). Cell cycle block in anergic T cells during tolerance induction. Cell. Immunol 225, 33–41. [DOI] [PubMed] [Google Scholar]
- Townsend SE, and Goodnow CC (1998). Abortive proliferation of rare T cells induced by direct or indirect antigen presentation by rare B cells in vivo. J. Exp. Med 187, 1611–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang JYS, Ratnasothy K, Li D, Chen Y, Bucy RP, Lau KF, Smyth L, Lombardi G, Lechler R, and Tam PKH (2011). The potency of allospecific Tregs cells appears to correlate with T cell receptor functional avidity. Am. J. Transplant 11, 1610–1620. [DOI] [PubMed] [Google Scholar]
- Turner MJ, Jellison ER, Lingenheld EG, Puddington L, and Lefranois L (2008). Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J. Exp. Med 205, 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg HA, and Rand DA (2007). Quantitative theories of T-cell responsiveness. Immunol. Rev 216, 81–92. [DOI] [PubMed] [Google Scholar]
- Wang XL, and Altman JD (2003). Caveats in the design of MHC class I tetramer/antigen-specific T lymphocytes dissociation assays. J. Immunol. Methods 280, 25–35. [DOI] [PubMed] [Google Scholar]
- Wang J, Lim K, Smolyar A, Teng M, Liu J, Tse AG, Liu J, Hussey RE, Chishti Y, Thomson CT, et al. (1998). Atomic structure of an alphabeta T cell receptor (TCR) heterodimer in complex with an anti-TCR fab fragment derived from a mitogenic antibody. EMBO J. 17, 10–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, Nuñez G, Tang A, Sayegh M, Hancock WW, et al. (1999). Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat. Med 5, 1303–1307. [DOI] [PubMed] [Google Scholar]
- Wong SBJ, Bos R, and Sherman LA (2008). Tumor-specific CD4+ T cells render the tumor environment permissive for infiltration by low-avidity CD8+ T cells. J. Immunol 180, 3122–3131. [DOI] [PubMed] [Google Scholar]
- Yang S, Hodge JW, Grosenbach DW, and Schlom J (2005). Vaccines with enhanced costimulation maintain high avidity memory CTL. J. Immunol 175, 3715–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Savage PA, Lee PP, Davis MM, and Greenberg PD (1999). Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol 162, 2227–2234. [PubMed] [Google Scholar]
- Young JS, Chen J, Miller ML, Vu V, Tian C, Moon JJ, Alegre M-L, Sciammas R, and Chong AS (2016). Delayed cytotoxic T lymphocyte-associated protein 4-immunoglobulin treatment reverses ongoing alloantibody responses and rescues allografts from acute rejection. Am. J. Transplant 16, 2312–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JS, Yin D, Vannier AGL, Alegre M-L, and Chong AS (2018). Equal expansion of endogenous transplant-specific regulatory T cell and recruitment into the allograft during rejection and tolerance. Front. Immunol 9, 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Jiang N, Ebert PJR, Kidd BA, Müller S, Lund PJ, Juang J, Adachi K, Tse T, Birnbaum ME, et al. (2015). Clonal deletion prunes but does not eliminate self-specific αβ CD8(+) T lymphocytes. Immunity 42, 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, and Bevan MJ (2009). Complete but curtailed T-cell response to very low-affinity antigen. Nature 458, 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.