Abstract
Persistent infection or chronic inflammation contributes significantly to tumourigenesis and tumour progression. C-X-C motif ligand 8 (CXCL8) is a chemokine that acts as an important multifunctional cytokine to modulate tumour proliferation, invasion and migration in an autocrine or paracrine manner. Studies have suggested that CXCL8 and its cognate receptors, C-X-C chemokine receptor 1 (CXCR1) and CX-C chemokine receptor 2 (CXCR2), mediate the initiation and development of various cancers including breast cancer, prostate cancer, lung cancer, colorectal carcinoma and melanoma. CXCL8 also integrates with multiple intracellular signalling pathways to produce coordinated effects. Neovascularisation, which provides a basis for fostering tumour growth and metastasis, is now recognised as a critical function of CXCL8 in the tumour microenvironment. In this review, we summarize the biological functions and ficlinical significance of the CXCL8 signalling axis in cancer. We also propose that CXCL8 may be a potential therapeutic target for cancer treatment
Keywords: CXCL8, CXCR1, CXCR2, Cancer, Angiogenesis, Metastasis
1. Introduction
Long-lasting chronic infection is a hallmark of tumourigenesis. Inflammation caused by chemical and physical agents increases the risk of malignancy [1]. Inflammatory responses to numerous cytokines in the tumour microenvironment play a more crucial role in facilitating tumour growth, progression, and immunosuppression compared to rendering a potent anti-tumour effect [2].
CXCL8 which is recognised as a prototypical chemokine belonging to the CXC family is responsible for the recruitment and activation of neutrophils and granulocytes to the site of inflammation [3]. CXCL8 is almost undetectable in physiological states, but is rapidly induced by pro-inflammatory cytokines such as tumour necrosis factor a (TNFa) and interleukin-1b (IL-1b) [4]. The function of CXCL8 mainly relies on its interaction with specific cell surface G protein-coupled receptors (GPCR), CXCR1 and CXCR2 [5]. Ligation of CXCL8 with different receptors triggers signalling with distinct biological outcomes, even though CXCR2 is its primary functional receptor [5]. While CXCL8/CXCR1 mainly increases the proliferation of tumour cells, CXCL8/CXCR2 promotes angiogenesis in prostate cancer [6].
The mechanism of CXCL8-CXCR1/2 signalling in tumourigenesis and tumour progression has been explored extensively. CXCL8 is typically known to promote angiogenesis, but it also activates matrix metalloproteinase (MMP) that is involved in metastasisrelated tissue remodelling [7–9]. The CXCL8 signalling nexus directly influences the sensitivity of tumour cells to chemotherapies by altering pathways associated with apoptosis and multidrug resistance [10,11]. High levels of CXCL8 are indicative of an increased risk of cancer and poor disease prognosis [12,13].
In this review, we summarize our current understanding of CXCL8-CXCR1/2 signalling pathways and their role in initiation, immunosuppression, angiogenesis and metastasis of tumours. We discuss the implication of CXCL8 and its receptors as potential biomarkers for cancer diagnosis and prognosis as well as cancer therapeutic targets.
2. Structure of CXCL8 and CXCR1/2
CXCL8, also known as Interleukin-8 (IL-8), belongs to the elastin-like recombinamer (ELR)+ CXC chemokines family. It is produced by macrophages, epithelial cells, airway smooth muscle cells and endothelial cells [14]. CXCL8 is initially produced as a protein of 99 amino acids that undergoes cleavage to form active CXCL8 isoforms, a 77 amino acid peptide in non-immune cells or a 72 amino acid peptide in monocytes and macrophages [5]. The gene encoding CXCL8 is located on chromosome 4q13-q21 [15]. Dimerisation of CXCL8 forms the structural basis for receptor binding [16].
CXCR1 and CXCR2, known as Interleukin-8 receptor A (IL-8RA) and Interleukin-8 receptor B (IL-8RB), respectively, are members of the GPCR family which contains 7 transmembrane domains (Fig. 1A–D) [17]. IL-8RA, IL-8RB and IL8RBP (a pseudogene of IL8RB) form a gene cluster in a region located on chromosome 2q33-q36 [18]. CXCR1 interacts with CXCL6 and CXCL8, whereas CXCR2 binds to CXCL1, CXCL2, CXCL3, CXCL5, CXCL6, CXCL7 and CXCL8 with high affinity [19]. Both CXCR1 and CXCR2 are expressed on granulocytes, monocytes, mast cells and some natural killer cells [20]. CXCR1 interacts with CXCL8 through its N-terminal b strand [16]. The simulated tertiary structures of the interaction model between CXCL8-dimer and CXCR1 N-terminal are shown in Fig. 1E and 1F.
Fig. 1.
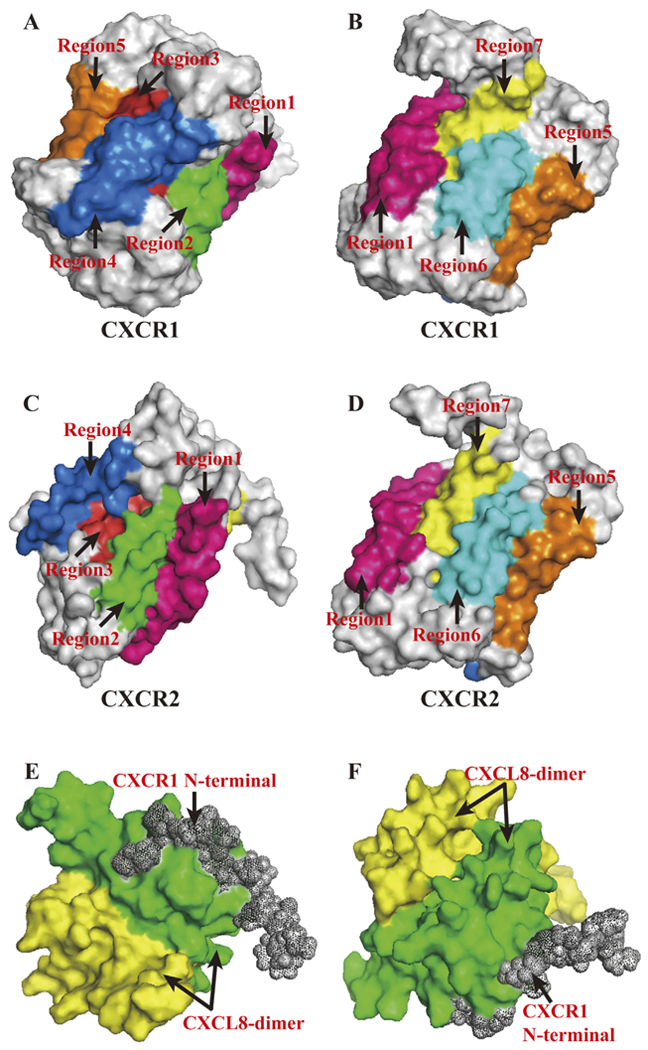
PyMOL Molecular Graphics System was used to present above structures. (A, B). Corresponding transmembrane regions within CXCR1 tertiary structure. Regions marked in different colours with arrows showed its seven transmembrane domains. Simulation of tertiary structure was constructed using PDB lefiof 2LNL produced by Park et al. [17]. (C, D). Corresponding transmembrane regions within CXCR2 tertiary structure. Regions marked in different colours with arrows showed its seven transmembrane domains. The amino acid sequence of CXCR2, NCBI RefSeq NP_0015481, were used to model CXCR2 tertiary structure in Swiss Model [155]. (E, F). Interaction model between CXCL8-dimer and CXCR1 N-terminal. Simulation of tertiary structure was constructed using PDB le of 1ILP produced by Skeltonfiet al. [16].
3. Intracellular signalling pathways of CXCL8
3.1. Activation of phospatidylinositol-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK)
PI3K acts as the major downstream intracellular signal of CXCL8 inducing phosphorylation of its substrate, Akt, which plays a critical role in modulating cell survival, angiogenesis, and migration [21,22]. CXCL8 also increases the expression of Akt in androgen-independent prostate cancer (AIPC) cell lines [3]. LY294002, GDC-0941 and BEZ235 are known potent inhibitors of PI3K.
MAPK signalling cascade consists of multiple serine/threonine kinases among which the best characterised is the Raf-1/MAP/Erk cascade. CXCL8 activates this classic signalling cascade in both neutrophils and cancer cells [23–26]. MAPK-targeted inhibitors such as vemurafenib, sorafenib, dabrafenib, trametinib, PD184352, SCH772984, and XMD8–92 may potentially interrupt the MAPK-associated signal transduction in tumours. Activation of p38 MAPK cascade downstream of CXCL8 has also been reported; however, its functional significance is yet to be determined [27]. CXCL8 activates MAPK signalling via PI3K in neutrophils [26], and via transactivation of epidermal growth factor receptor (EGFR) resulting in Ras-GTPase activation in ovarian and lung cancer cell lines [24,25]. A subsequent study suggests that PI3K is essential for CXCL8-induced migration of human neutrophils independent of MAPK signalling [21].
3.2. Activation of phospholipase C (PLC)
CXCL8 stimulates PLC signalling which in turn induces the phosphorylation of protein kinase C (PKC). CXCL8 promotes migration of human cancer cells by activation of the PLC-dependent PKC signalling pathway which when coupled with an increase in Ca2+ concentration regulates the actin cytoskeleton[28]. CXCL8 also regulates cyclin D1 expression in AIPC cells by activation of an atypical isoform of PKC, PKCj [23]. Enzastaurin, sotrastaurin, Go 6983, staurosporine and quercetin have been established as highly effective inhibitors of PKC in vivo.
3.3. Activation of non-receptor tyrosine kinases and Rho-GTPases
Non-receptor tyrosine kinases including Src family members and focal adhesion kinase (FAK) are involved in CXCL8-induced signalling cascades. CXCL8 signalling is positively correlated with an increase in phosphorylation of Src-kinases and FAK in cancer cells, which contributes to cell proliferation, cell survival, and chemoresistance [29,30]. A putative pathway linking PI3K signalling to the activation of FAK-Src has been described [3].
CXCL8 promotes motility and invasion of cancer cells via Rho-GTPases-induced polymerisation of actin cytoskeleton [3]. While CXCR1 rapidly stimulates Rho-GTPase in endothelial cells, activation of CXCR2 induces a delayed effect [31]. Moreover, Rho-GTPase may promote phosphorylation of Src and FAK with further impact on downstream transcriptional factors [3].
4. CXCL8-CXCR1/2 axis in tumour immunosuppression
4.1. CXCL8 and myeloid-derived suppressor cells (MDSCs)
The functional importance of MDSCs in the immune response to tumours has been well described. MDSCs are identified as a highly heterogeneous population with myeloid progenitor cells and immature myeloid cells as two major components [32]. Based on their surface markers, MDSCs exhibit two distinct phenotypes and are defined as granulocytic MDSCs (GrMDSCs) and monocytic MDSCs (MoMDSCs) [33]. In 1995, human MDSCs were first proposed to infiltrate tumours and metastatic lymph nodes in head and neck cancer patients [34]. MDSCs suppress anti-tumour immune response mainly by inhibiting T cells via multiple molecular mechanisms [35–37].
In a recent investigation, CXCR1/2 were detected on the surface of tumour-derived MDSCs. CXCL8 was identified as a potent chemotactic stimulus for recruitment of MDSCs to tumour foci in a dose-dependent manner in a tumour engraftment mouse model [38]. Similar results were obtained from CXCL8-containing super-natants of HT29 colon carcinoma cells as well as from CXCL8-containing sera of patients [38]. In this study, only MoMDSCs from peripheral blood of cancer patients exhibited a suppressive effect on T-cells [38]. Interestingly, CXCL8 was found to induce GrMDSCs to release DNA to form Neutrophil Extracellular Traps (NETs), which were involved in thrombus formation and metastasis in cancer patients [38–40]. Moreover, the CXCR1/2 blocking agent, Reparisxin, abolished the above effects of CXCL8 in vivo [38].
4.2. The relevant mechanisms of tumour-associated neutrophils (TANs) and Epithelial–Mesenchymal Transition (EMT) in CXCL8-induced immune resistance
TANs are associated with poor clinical outcome and heavy tumour burden in most solid malignancies [41–49]. TANs exhibit two phenotypes that play diverse roles in the immune response to tumour. N1 TANs exert anti-tumour activity mainly via antibody-dependent cellular cytotoxicity and oxidative damage [50,51], as well as via enhancing immune surveillance by secreting multiple inflammation-associated cytokines [52]. In contrast, N2 TANs contribute to tumour neovascularisation and distant metastasis [53,54]. Arginase 1 secreted by N2 TANs was found to favour immunosuppression by restraining T-cell receptor expression, attenuating antigen-specific T-cell responses and recruiting T regulatory cells [35,55,56]. CXCL8 has been shown to chemoattract TANs to the tumour microenvironment in Ras-driven cancer [57–59]. It can be inferred that CXCL8-associated resistance to immune killing occurs mainly by attracting the N2 phenotype.
In addition to contributing to metastasis, EMT is proposed to confer tumour escape to immune destruction by inhibiting CTL lysis by inducing autophagy and reducing the formation of immunological synapse [60]. An autocrine feedback loop exists between CXCL8 and EMT. CXCL8 contributes to EMT and initiates the cytokines and/or growth factors cascade including CXCL8 itself [61]. However, the mechanism of CXCL8-induced immunosuppression via EMT is still unknown.
5. The role of CXCL8-CXCR1/2 pathway in various cancer types
5.1. Breast cancer
CXCL8 can enhance the immunoregulatory ability to defend against cancer, and can also modify the microenvironment to facilitate tumourigenesis. In the context of breast cancer, the latter role is more dominant compared to the former. All breast cancer cells express CXCR1 and CXCR2 [62]. CXCL8 is also associated with growth receptors expressed on the surface of breast cancer cells. Increased CXCL8 has been mostly detected in oestrogen receptor (ER)-negative, progesterone receptor (PR)-negative and human epidermal growth factor receptor-2 (HER-2)/neu-positive breast cancers [62,63]. Moreover, CXCL8 increases the activity of breast cancer stem-like cells (CSCs) by transactivation of HER2 [64,65].
Breast cancer cell-derived CXCL8 cooperates with vascular endothelial growth factor (VEGF) to establish and expand tumour neovasculature [66]. Glucose deprivation and endoplasmic reticulum stress are regarded as effective upregulating factors of VEGF and CXCL8 [67]. Downregulation of CXCL8 significantly reduces the microvessel density (MVD) in ER-negative breast tumours in vivo, while it does not affect proliferation and cell cycle of cancer cells[68]. Paradoxically, anti-CXCL8 therapy alone is ineffective in vitro owing to the other compensatory angiogenic factors in supernatant such as monocyte chemotactic protein-1 (MCP-1), growth-regulated protein (GRO), VEGF, and TGF-b1 [69,70].
Tumour neovascularisation not only contributes to the initiation and growth of breast cancer but also offers blood supply for distant metastasis. The ectopic expression of CXCL8 stimulated by IL-1b and TNF-a can enhance the metastatic potential of breast cancer, as high level of CXCL8 can promote angiogenesis and attract neutrophils to release enzymes involved in tissue remodelling and tumour establishment [71]. Atypical methylation of two deoxycytidylate-phosphate-deoxyguanylate (CpG) sites (−1241 and −1311) upstream of the CXCL8 promoter counterintuitively upregulate the expression of CXCL8 in high metastatic cell lines, MDA-231 and MDA-345 [72]. As bone is a common site for breast cancer metastasis, it was suggested that cyclooxygenase-2 (COX-2)-mediated production of CXCL8 in the ER-negative breast cancer cells might contribute to both human osteoclast formation and bone resorption [73]. A novel tumour suppressor, Dachshund 1 (DACH1), inhibits CXCL8-induced breast cancer cell migration and metastasis through binding to the AP-1 and NF-kB binding sites of CXCL8 promoter [74].
Considering the significance of CXCL8 in the initiation, progression, angiogenesis and metastasis of breast cancer, CXCL8 is defined as an unfavourable prognostic factor. Elevated serum level of CXCL8 is associated with an advanced clinical status, a severe tumour load, and earlier distant metastasis [75]. In lymph node-negative breast cancer, patients with higher CXCL8 levels (>102.27 pg/mg) suffer a poor prognosis including shorter survival time and distant metastasis [76]. With the development of genomic sequencing in recent years, the single nucleotide polymorphism (SNP) of CXCL8 and CXCR2 indicates the individual difference among different ethnic populations. The CXCL8 (−251) A allele and/or the CXCR2 (+1208) T allele are correlated with the increased risk and poor prognosis of breast cancer in Tunisian population [77]. Inversely, CXCL8 (−251) T allele (TT/TA) is associated with an increased risk in Asian population, but a decreased risk in African population [78]. However, the functional analysis of these SNPs in breast cancer has not been well explored. Influence of CXCL8 (−251) allele on its expression requires more in-depth research.
5.2. Prostate cancer
Increased CXCL8 secretion by prostate cancer (PCa) cells is associated with malignant biological behaviours of cancer cells. CXCL8/CXCR2 promote castration-resistant growth and proliferation of AIPC cells by activating cyclin D1 expression in a PI3K/Akt/mTOR and MAPK pathways-dependent manner [6,23]. In addition to binding to its receptors, CXCL8 also upregulates the expression of CXCR7, which directly interacts with EGFR to induce prostate cancer cell growth [79]. While CXCL8 promotes prostate cancer progression by recruiting adipose stromal cells (ASCs) to tumours, such chemotaxis is blocked by CXCR1/2-antibodies [80]. Phosphatase and tensin homologue (PTEN), a tumour suppressor gene, is frequently mutated in metastatic PCa [81]. PTEN-deficient prostate tumours may promote hypoxia-inducible factor-1 (HIF-1) and NF-kB, which in turn can upregulate the expression of CXCL8 resulting in sustained tumour development and accelerated tumour progression [82,83]. DACH1 inhibits CXCL8-mediated proliferation and migration of prostate epithelial cells by binding to its promoter and suppressing CXCL8 transcription in a tissue specific DACH1-knockdown model [84].
Poor clinicopathological features including high Gleason score and advanced pathological stage of PCa are associated with an increased mRNA expression of CXCL8 [85]. A combination of serum CXCL8 levels and free/total PSA ratios may provide a substantial improvement in distinguishing benign prostatic hyperplasia (BPH) from PCa and predicting disease outcome [86].
5.3. Lung cancer
Elevated CXCL8 was detected in lung cancer, especially in non-small cell lung cancer (NSCLC) cell lines [87–89]. The mitogenic role of CXCL8 in lung cancer is mediated mainly through CXCR1 or via transactivation of EGFR [25]. The combined effect of VEGF and CXCL8 on intratumoural angiogenesis of lung cancer has been verified [90]. Autocrine CXCL8 and VEGF collaboratively mediate neovascularisation and EMT, which facilitates invasion in A549 cells [91]. CXCL8 also mediates osteoclastogenesis in lung cancer patients via PLD/PKC/Erk1/2 or PLD/Akt signalling [92,93].
Early diagnosis is critical for lung cancer. Circulating CXCL8 may predict the risk of lung cancer, since it is upregulated prior to clinical diagnosis [12,94]. The National Cancer Institute-Maryland (NCI-MD) case–control study and the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial showed that high expression of CXCL8 can raise the risk of lung cancer by 45%−86% [12,94]. High CXCL8 mRNA levels were strongly associated with advanced stages, distant lymph node metastasis, shortened survival time and early relapse of NSCLC [95,96]. As for SCLC, no statistical relevance was found between serum levels of CXCL8 and disease stage and tumour burden [97]. In conclusion, serum protein or tumour mRNA levels of CXCL8 can be an effective marker to monitor tumour occurrence and relapse in lung cancer patients.
5.4. Melanoma
CXCL8 is a predominant regulator of growth, angiogenesis and metastasis of melanoma in preclinical animal models [98–100]. The metastatic potential of CXCL8 relies on its ability to promote vascularisation, activate MMP-2, and enhance anoikis resistance [99,101]. Consistently, CXCL8 produced by melanoma cells correlates with tumour burden and poor prognosis [102]. However, controversies exist about whether both the receptors of CXCL8 impact melanoma progression. While CXCR1 alone is associated with CXCL8-mediated chemotaxis [103], CXCR2 upregulates CXCL8-mediated angiogenesis, invasion and migration of human melanoma cells independent of CXCR1 [104,105].
Anti-VEGF drug, bevacizumab, slightly increases CXCR2 expression in human umbilical vein endothelial cells and activates CXCL8 signalling as an alternative pro-angiogenic pathway in uveal melanoma [106]. Knockdown of host CXCR2 decreases growth, angiogenesis, and experimental lung metastasis [111]. Together, these studies indicate that inhibition of the CXCL8/CXCR2 axis with neutralizing antibodies may control melanoma neoangiogenesis and enhance the sensitivity to therapy.
5.5. Other cancers
Apart from the cancer types discussed above, CXCL8 signalling axis also plays an indispensable role in colorectal carcinoma [107,108], renal cell carcinoma [109], pancreatic cancer [110], thyroid tumours [111,112], gastric cancer [113–115], ovarian cancer [116], lymphomas [117], and haematologic malignancies [118–120]. As one of the markedly upregulated chemokines in colorectal carcinoma (CRC), CXCL8 has been demonstrated to induce CRC cell proliferation in an autocrine manner and enhance the resistance to anoikis [107,108]. Aberrant expression of CXCL8 is correlated with progression, VEGF-independent angiogenesis and chemoresistance of CRC in vitro and in vivo [121,122]. Demethylation of CXCL8 promoter region significantly increases its serum level, which correlates with distant metastasis, advanced Dukes stage and poor overall survival [123,124]. Notably, CXCL8 secreted by renal cancer cells induces the migration of mesenchymal stem cells (MSCs), which play a vital role in the development, metastasis, and drug resistance of cancers [125]. In clear cell renal cell cancer (ccRCC), resistance to kinase inhibitors such as sunitinib is accompanied with increased expression of tumour-derived CXCL8 [126]. Anti-CXCL8 could sensitize tumours to sunitinib treatment in a nude mouse model [126].
6. Targeted therapy research
6.1. Preclinical studies
Owing to the significant association between the CXCL8-CXCR1/2axis and certain types of tumours, targeted therapies against this axis are expected to have high clinical value in tumour treatment. Reparixin, a clinical grade CXCR1/2 inhibitor, was shown to block the binding of CXCL8 to CXCR1/2 in a non-competitive manner and inhibit CXCL8-induced T lymphocyte and NK cell chemotaxis and migration in previous study [127]. Reparixin or CXCR1-antibody can selectively deplete CSCs and tumour cells via FASL/FAS signalling in vitro and can inhibit tumour growth and metastasis in a tumour xenograft model in vivo [128]. While reparixin and paclitaxel exhibited a synergistic effect towards arresting cell cycle and inhibiting tumoursphere formation in vitro, they showed an additive effect towards reducing brain metastasis in vivo [129]. In addition to reparixin, other small-molecule antagonists of CXCR1/2 such as SCH479833 and SCH527123 exerted anti-tumour activity in xenograft models of breast cancer [128], colorectal cancer [130], melanoma [131] and spontaneous colon cancer liver metastasis [132]. SCH563705 has been demonstrated to robustly inhibit primary human breast CSC activity [133]. In preclinical colon cancer models, the combination of SCH527123 and oxaliplatin was more potent in controlling cell proliferation and angiogenesis and inducing apoptosis compared to single agents [130]. G31P, another CXCR1/2 inhibitor, significantly reduced the viability, adhesion and migration of PC-3 cells in vitro, and inhibited the growth of transplanted PCa xenografts in a nude mouse model [134].
CXCL8 neutralising antibodies, ABX-CXCL8 and HuMax-CXCL8, are mostly used to block CXCL8-CXCR1/2 pathway in preclinical studies. ABX-CXCL8 had no effect on proliferation of bladder cancer cells in vitro but significantly inhibited tumour growth in a mouse model [135]. ABX-CXCL8-treated mice exhibited a significant reduction in tumour growth, angiogenesis and metastasis of human melanoma cells [136]. Mechanistically, ABX-CXCL8 suppresses tumour metastasis by downregulation of MMP-2 and MMP-9 in vitro [135].
6.2. Clinical trials
Based on the preclinical studies, reparixin is a potential candidate for clinical trial in breast cancer. An open label phase I clinical trial including 33 female patients diagnosed with HER-2-negative metastatic breast cancer was conducted to determine the pharmacokinetic profile and evaluate safety and tolerability of orally administered reparixin in combination with a fixed dose of weekly paclitaxel (NCT02001974). Subsequently, a double-blind phase II study with 190 estimated enrolments is in progress to compare the progression free survival of metastatic TNBC patients receiving paclitaxel alone or with reparixin (NCT02370238). Reparixin has also been introduced to prevent graft dysfunction after islet transplantation (NCT01220856), kidney transplantation (NCT00248040) and lung transplantation (NCT00224406) in phase II clinical trials. A phase Ib pilot study to perform gradient trial with HuMax-CXCL8 is recruiting patients with metastatic or unresectable, locally advanced malignant solid tumours (NCT02536469).
7. Conclusions
To date, great endeavours have been made to identify the roles of the CXCL8-CXCR1/2 pathways in human cancers. CXCL8 exerts multiple effects on biological activities of tumour cells including proliferation, invasion and migration, all of which are essential for tumour growth and metastasis. PI3K, Akt and Erk signalling pathways have been identified to be involved in CXCL8-associated intracellular signals. A simplified signalling diagram is shown in Fig. 2 and detailed information is presented in Table 1. Interruption of the related signalling pathways may thus provide promising therapeutic avenues for tumours with high activity of CXCL8-CXCR1/2.
Fig. 2.
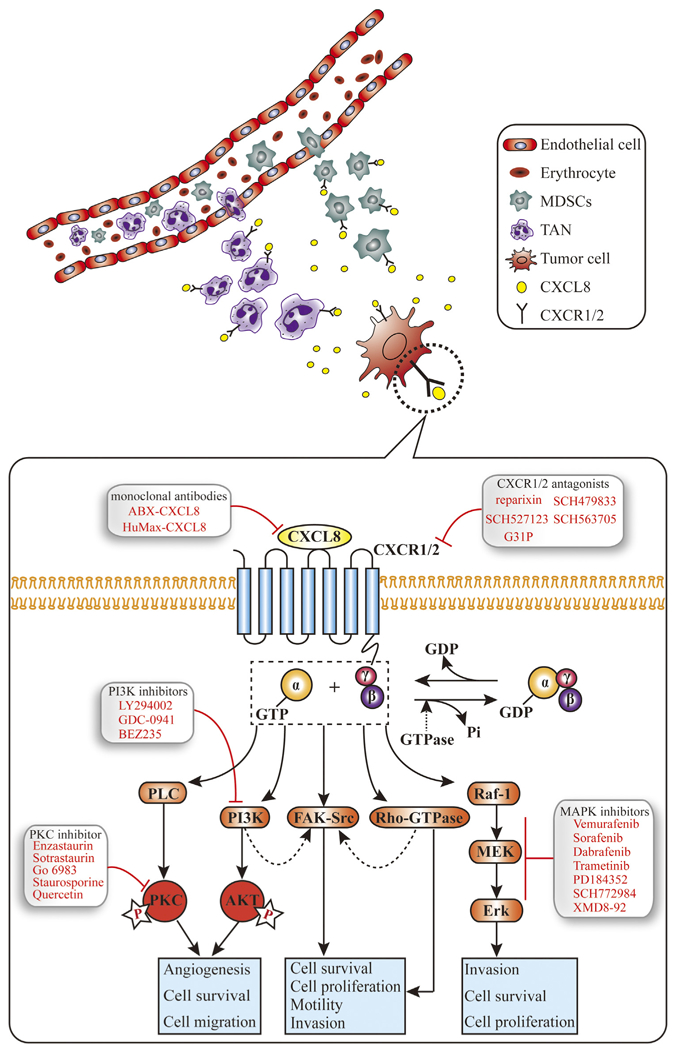
The diagram summarizing the major signalling pathways of CXCL8 in cancers. CXCL8 chemoattractant myeloid-derived suppressor cells (MDSCs) and tumour-associated neutrophils (TAN) to tumour microenvironment which are associated with immune suppression. At the celluar level, CXCL8 binds to G protein-coupled receptors (GPCRs), namely CXCR1 or CXCR2, leading to the activation of G protein. Heterotrimeric Ga and bg subunits stimulate the main effectors PLC and PI3K to induce phosphorylation of PKC and Akt, respectively. The two signalling pathways have been reported to activate respective transcription factors associated to survival, angiogenesis and migration of tumour cells. In addition, CXCL8 activates non-receptor tyrosine kinases (e.g., Src and FAK) and members of the RhoGTPase family, which promote cell proliferation, survival, motility and invasion. Activated Raf-1/MAP/Erk signalling cascade contributes to cell proliferation and survival. Dashed arrows, uncon rmed pathways involved in CXCL8 signalling axis.
Table 1.
The role of CXCL8-CXCR1/2 pathway in common cancers.
| Cancer type | Function | Associated factors | Ref. |
|---|---|---|---|
| Breast cancer | Proliferation | cyclin D1, p27Kip21 | [137] |
| Angiogenesis | MVD | [7,63,66] | |
| Metastasis | integrin 3β | [137,138] | |
| Chemoresistance | MRP | [10] | |
| CSCs activation | HER2 | [64,65] | |
| Prostate cancer | Proliferation | cyclin D1, AR, CXCR7, p53 | [23,79,139,140] |
| Angiogenesis | VEGF | [8,9] | |
| Metastasis | MMP-2/9, E-cadherin | [9,141] | |
| Chemoresistance | src, NF-κB, c-FLIP, Akt | [6,11,142] | |
| Lung cancer | Proliferation | EGFR | [25] |
| Angiogenesis | VEGF, MVD | [88,90,143,144] | |
| Metastasis | PLD, Akt, PKC, MMP-2/9, | [92,93,145,146] | |
| Colorectal cancer | Proliferation | EGFR, MAPK | [107,147,148] |
| Angiogenesis | CD31, MVD | [107,121] | |
| Metastasis | PI3K, Akt, Erk, integrin αvβ6 | [108,149,150] | |
| Chemoresistance | NF-κB, Bcl-2, survivin | [151,152] | |
| Melanoma | Proliferation | Akt, Erk | [105] |
| Angiogenesis | MMP-2/9, VEGF | [99,153,154] | |
| Metastasis | MMP-2 | [99] |
MRP, Multidrug resistance protein; AR, androgen receptor; Bcl-2, B-cell CLL/lymphoma 2.
Given that high expression of CXCL8 and its receptors is associated with tumourigenesis and progression of certain types of tumours, these factors may serve as biomarkers in screening patients and evaluating prognosis. The CXCL8–CXCR1/2 pathways play a confirmed role in resistance to chemotherapy in breast cancer, prostate cancer and colorectal carcinoma. CXCL8-upregulated expressions of anti-apoptotic protein and thymidylate synthase (TS) may attenuate the efficacy of chemotherapies. Therefore, targeted-inhibition of CXCL8 may be an attractive therapeutic strategy to sensitise tumour cells to chemotherapeutic agents and eventually increase the survival of patients with end-stage disease. CXCL8 or CXCR1/2 may offer effective approaches for the development of targeted molecular therapeutics for tumours. Nevertheless, substantial investigations are warranted before practically applying the predictive, prognostic, and therapeutic value of CXCL8 signalling in human cancers.
Acknowledgments
This review was supported by National Natural Science Foundation of China (Grant No. 81572608) and the National High Technology Research and Development Program of China (No. 2015AA020301).
Abbreviations:
- CXCL8
C-X-C motif ligand 8
- CXCR1
C-X-C chemokine receptor 1
- CXCR2
C-X-C chemokine receptor 2
- TNFα
tumour necrosis factor α
- IL-1β
Interleukin-1β
- GPCR
G protein-coupled receptors
- MMP
matrix metalloproteinase
- IL-8
Interleukin-8
- ELR
elastin-like recombinamer
- IL-8RA
Interleukin-8 receptors A
- IL-8RB
Interleukin-8 receptors B
- PI3K
phospatidylinositol-3-kinase
- MAPK
mitogen-activated protein kinase
- AIPC
androgen-independent prostate cancer
- EGFR
epidermal growth factor receptor
- PLC
phospholipase C
- PKC
protein kinase C
- FAK
focal adhesion kinase
- MDSCs
myeloid-derived suppressor cells
- GrMDSCs
granulocytic MDSCs
- MoMDSCs
monocytic MDSCs
- NETs
Neutrophil Extracellular Traps
- TAN
tumour-associated neutrophils
- EMT
epithelial–mesenchymal transition
- CSCs
cancer stem-like cells
- HER2
human epidermal growth factor receptor 2
- ER
oestrogen receptor
- PR
progesterone receptor
- SNP
single nucleotide polymorphism
- VEGF
vascular endothelial growth factor
- NF-κB
nuclear factor kappa B
- MVD
microvessel density
- MCP-1
monocyte chemotactic protein-1
- GRO
growth-regulated protein
- CpG
deoxycytidylate-phosphate-deoxyguanylate
- COX-2
cyclooxygenase-2
- TNFβ
tumour necrosis factor β
- DACH1
Dachshund 1
- TNBC
triple negative breast cancers
- MDR
multidrug resistance
- PCa
prostate cancer
- PTEN
phosphatase and tensin homolog
- NE
neuroendocrine
- PSA
prostate-speci c antigen
- BPH
benign prostatic hyperplasia
- 1α, 25-(OH)2 D3
1α, 25-dihydroxyvitamin D3
- NSCLC
non-small cell lung cancer
- SCLC
small cell lung cancer
- ADC
lung adenocarcinoma
- DFS
disease-free survival
- OS
overall survival
- NCI-MD
National Cancer Institute-Maryland
- PLCO
Prostate, Lung, Colorectal, and Ovarian
- CRC
colorectal carcinoma
- ccRCC
clear cell renal cell cancer
Biographies

Qian Liu, MD. She was graduated from Chongqin Medical University and currently a graduate student at the Department of Oncology, Tongji Hospital of Huazhong University of Science and Technology. Her research focuses on growth factor signaling in cancer.

Anping Li, MD. Graduated from Henan Medical University and performed cancer biology study at Georgetown University, Thomas Jefferson University and The Wistar Institute. Her research focuses on tumor biology.

Yijun Tian, MD. He was graduated from Huazhong University of Science and Technology, and currently a graduate student at the Department of Oncology, Tongji Hospital of Huazhong University of Science and Technology. His study focuses on molecular oncology.

Jennifer Wu, Ph.D. Received PhD from the University of British Columbia in Canada, post-doc trained at Fred Hutchinson Cancer Research Center. She is an Associate Professor in Immunology, Medical University of South Carolina. Her research focuses on tumor immunology.

Yu Liu, MD. She is a second year PhD student at the Department of Geriatrics, Tongji Hospital of Huazhong University of Science and Technology. Her study focuses on the field of signaling transduction and tumor biology.

Tengfei Li, MD. Graduated from Zhengzhou University. He is currently Associate Professor at the Department of Interventional Radiology, The First Affiliated Hospital of Zhengzhou University. His research focuses on interventional therapy of tumor.

Yuan Chen, MD. Graduated from Tongji Medical University and received training at The MD Anderson Cancer Center, USA. He is currently full Professor at the Department of Oncology, Tongji Hospital of Huazhong University of Science and Technology. He is specialized in chemotherapy and radiotherapy of various solid tumors.

Xinwei Han, Ph.D MD. Graduated with Ph.D degree from Huazhong University of Science and Technology. He is a full Professor at the Department of Interventional Radiology, The First Affiliated Hospital of Zhengzhou University. His research focuses on interventional therapy of tumor.

Kongming Wu, Ph.D., MD. Graduated with Ph.D degree from Chinese Academy of Medical Science, then received postdoctoral training at Albert Einstein Medical College and Georgetown University, and acquired a faculty position as an Associate Professor at Thomas Jefferson University. Currently, he is a full Professor at the Department of Oncology, Tongji Hospital of Huazhong University of Science and Technology. His research focuses on signaling transduction and tumor biology.
Footnotes
Competing financial interests: The authors declare no competing financial interests.
References
- [1].Gulumian M, The role of oxidative stress in diseases caused by mineral dusts and fibres: current status and future of prophylaxis and treatment, Mol. Cell. Biochem 196 (1999) 69–77. [PubMed] [Google Scholar]
- [2].Balkwill F, Mantovani A, In ammation and cancer: back to Virchow, Lancet 357 (2001) 539–545. [DOI] [PubMed] [Google Scholar]
- [3].Waugh DJ, Wilson C, The interleukin-8 pathway in cancer, Clin. Cancer Res 14 (2008) 6735–6741. [DOI] [PubMed] [Google Scholar]
- [4].Hoffmann E, Dittrich-Breiholz O, Holtmann H, Kracht M, Multiple control of interleukin-8 gene expression, J. Leukoc. Biol 72 (2002) 847–855. [PubMed] [Google Scholar]
- [5].Brat DJ, Bellail AC, Van Meir EG, The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis, Neuro Oncol 7 (2005) 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, et al. , Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer, Cancer Res 67 (2007) 6854–6862. [DOI] [PubMed] [Google Scholar]
- [7].Azenshtein E, Meshel T, Shina S, Barak N, Keydar I, Ben-Baruch A, The angiogenic factors CXCL8 and VEGF in breast cancer: regulation by an array of pro-malignancy factors, Cancer Lett 217 (2005) 73–86. [DOI] [PubMed] [Google Scholar]
- [8].Ferrer FA, Miller LJ, Andrawis RI, Kurtzman SH, Albertsen PC, Laudone VP, et al. , Angiogenesis and prostate cancer: in vivo and in vitro expression of angiogenesis factors by prostate cancer cells, Urology 51 (1998) 161–167. [DOI] [PubMed] [Google Scholar]
- [9].Kim SJ, Uehara H, Karashima T, McCarty M, Shih N, Fidler IJ, Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice, Neoplasia 3 (2001) 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shi Z, Yang WM, Chen LP, Yang DH, Zhou Q, Zhu J, et al. , Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production, Breast Cancer Res. Treat 135 (2012) 737–747. [DOI] [PubMed] [Google Scholar]
- [11].Wilson C, Wilson T, Johnston PG, Longley DB, Waugh DJ, Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells, Mol. Cancer Ther 7 (2008) 2649–2661. [DOI] [PubMed] [Google Scholar]
- [12].Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. , Increased levels of circulating interleukin 6, interleukin 8, C-reactive protein, and risk of lung cancer, J. Natl. Cancer Inst 103 (2011) 1112–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Balasoiu M, Balasoiu AT, Mogoanta SS, Barbalan A, Stepan AE, Ciurea RN, et al. , Serum and tumor microenvironment IL-8 values in different stages of colorectal cancer, Rom. J. Morphol. Embryol 55 (2014) 575–578. [PubMed] [Google Scholar]
- [14].Matsushima K, Baldwin ET, Mukaida N, Interleukin-8 and MCAF: novel leukocyte recruitment and activating cytokines, Chem. Immunol 51 (1992) 236–265. [PubMed] [Google Scholar]
- [15].Modi WS, Dean M, Seuanez HN, Mukaida N, Matsushima K, O’Brien SJ, Monocyte-derived neutrophil chemotactic factor (MDNCF/IL-8) resides in a gene cluster along with several other members of the platelet factor 4 gene superfamily, Hum. Genet 84 (1990) 185–187. [DOI] [PubMed] [Google Scholar]
- [16].Skelton NJ, Quan C, Reilly D, Lowman H, Structure of a CXC chemokine-receptor fragment in complex with interleukin-8, Structure 7 (1999) 157–168. [DOI] [PubMed] [Google Scholar]
- [17].Park SH, Das BB, Casagrande F, Tian Y, Nothnagel HJ, Chu M, et al. , Structure of the chemokine receptor CXCR1 in phospholipid bilayers, Nature 491 (2012) 779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ahuja SK, Ozcelik T, Milatovitch A, Francke U, Murphy PM, Molecular evolution of the human interleukin-8 receptor gene cluster, Nat. Genet 2 (1992) 31–36. [DOI] [PubMed] [Google Scholar]
- [19].Matsuo Y, Raimondo M, Woodward TA, Wallace MB, Gill KR, Tong Z, et al. , CXC-chemokine/CXCR2 biological axis promotes angiogenesis in vitro and in vivo in pancreatic cancer, Int. J. Cancer 125 (2009) 1027–1037. [DOI] [PubMed] [Google Scholar]
- [20].Chuntharapai A, Lee J, Hebert CA, Kim KJ, Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes, J. Immunol 153 (1994) 5682–5688. [PubMed] [Google Scholar]
- [21].Knall C, Worthen GS, Johnson GL, Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases, Proc. Natl. Acad. Sci. U. S. A 94 (1997) 3052–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cheng GZ, Park S, Shu S, He L, Kong W, Zhang W, et al. , Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery, Curr. Cancer Drug Targets 8 (2008) 2–6. [PubMed] [Google Scholar]
- [23].MacManus CF, Pettigrew J, Seaton A, Wilson C, Maxwell PJ, Berlingeri S, et al. , Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells, Mol. Cancer Res 5 (2007) 737–748. [DOI] [PubMed] [Google Scholar]
- [24].Venkatakrishnan G, Salgia R, Groopman JE, Chemokine receptors CXCR-1/2 activate mitogen-activated protein kinase via the epidermal growth factor receptor in ovarian cancer cells, J. Biol. Chem 275 (2000) 6868–6875. [DOI] [PubMed] [Google Scholar]
- [25].Luppi F, Longo AM, de Boer WI, Rabe KF, Hiemstra PS, Interleukin-8 stimulates cell proliferation in non-small cell lung cancer through epidermal growth factor receptor transactivation, Lung Cancer 56 (2007) 25–33. [DOI] [PubMed] [Google Scholar]
- [26].Knall C, Young S, Nick JA, Buhl AM, Worthen GS, Johnson GL, Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils, J. Biol. Chem 271 (1996) 2832–2838. [DOI] [PubMed] [Google Scholar]
- [27].Murphy C, McGurk M, Pettigrew J, Santinelli A, Mazzucchelli R, Johnston PG, et al. , Nonapical and cytoplasmic expression of interleukin-8, CXCR1, and CXCR2 correlates with cell proliferation and microvessel density in prostate cancer, Clin Cancer Res 11 (2005) 4117–4127. [DOI] [PubMed] [Google Scholar]
- [28].Lang K, Niggemann B, Zanker KS, Entschladen F, Signal processing in migrating T24 human bladder carcinoma cells: role of the autocrine interleukin-8 loop, Int. J. Cancer 99 (2002) 673–680. [DOI] [PubMed] [Google Scholar]
- [29].Kopetz S, Shah AN, Gallick GE, Src continues aging: current and future clinical directions, Clin Cancer Res 13 (2007) 7232–7236. [DOI] [PubMed] [Google Scholar]
- [30].Siesser PM, Hanks SK, The signaling and biological implications of FAK overexpression in cancer, Clin. Cancer Res 12 (2006) 3233–3237. [DOI] [PubMed] [Google Scholar]
- [31].Schraufstatter IU, Chung J, Burger M, IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways, Am. J. Physiol. Lung Cell. Mol. Physiol 280 (2001) L1094–103. [DOI] [PubMed] [Google Scholar]
- [32].Youn JI, Nagaraj S, Collazo M, Gabrilovich DI, Subsets of myeloid-derived suppressor cells in tumor-bearing mice, J. Immunol 181 (2008) 5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Poschke I, Kiessling R, On the armament and appearances of human myeloid-derived suppressor cells, Clin. Immunol 144 (2012) 250–268. [DOI] [PubMed] [Google Scholar]
- [34].Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR, Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor, Clin. Cancer Res 1 (1995) 95–103. [PubMed] [Google Scholar]
- [35].Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. , Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses, Cancer Res 64 (2004) 5839–5849. [DOI] [PubMed] [Google Scholar]
- [36].Yu J, Du W, Yan F, Wang Y, Li H, Cao S, et al. , Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer, J. Immunol 190 (2013) 3783–3797. [DOI] [PubMed] [Google Scholar]
- [37].Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S, Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine, Cancer Res 70 (2010) 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP, et al. , Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs), Clin. Cancer Res (2016). [DOI] [PubMed] [Google Scholar]
- [39].Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. , Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome, Arthritis Rheumatol 67 (2015) 2990–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. , Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis, J. Clin. Invest (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, et al. , Hepatocyte growth factor production by neutrophils in ltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death, Cancer Res 63 (2003) 1405–1412. [PubMed] [Google Scholar]
- [42].Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, et al. , Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis, PLoS One 7 (2012) e30806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wang J, Jia Y, Wang N, Zhang X, Tan B, Zhang G, et al. , The clinical significance of tumor-infiltrating neutrophils and neutrophil-to-CD8+ lymphocyte ratio in patients with resectable esophageal squamous cell carcinoma, J. Transl. Med 12 (2014) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Trellakis S, Farjah H, Bruderek K, Dumitru CA, Hoffmann TK, Lang S, et al. , Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors, Int. J. Immunopathol. Pharmacol 24 (2011) 683–693. [DOI] [PubMed] [Google Scholar]
- [45].Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, et al. , Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma, J. Hepatol 54 (2011) 948–955. [DOI] [PubMed] [Google Scholar]
- [46].Jensen TO, Schmidt H, Moller HJ, Donskov F, Hoyer M, Sjoegren P, et al. , Intratumoral neutrophils and plasmacytoid dendritic cells indicate poor prognosis and are associated with pSTAT3 expression in AJCC stage I/II melanoma, Cancer 118 (2012) 2476–2485. [DOI] [PubMed] [Google Scholar]
- [47].Donskov F, von der Maase H, Impact of immune parameters on long-term survival in metastatic renal cell carcinoma, J. Clin. Oncol 24 (2006) 1997–2005. [DOI] [PubMed] [Google Scholar]
- [48].Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H, Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma, J. Clin. Oncol 27 (2009) 4709–4717. [DOI] [PubMed] [Google Scholar]
- [49].Fridlender ZG, Sun J, Mishalian I, Singhal S, Cheng G, Kapoor V, et al. , Transcriptomic analysis comparing tumor-associated neutrophils with granulocytic myeloid-derived suppressor cells and normal neutrophils, PLoS One 7 (2012) e31524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, et al. , Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy, Cancer Res 71 (2011) 5134–5143. [DOI] [PubMed] [Google Scholar]
- [51].Zivkovic M, Poljak-Blazi M, Zarkovic K, Mihaljevic D, Schaur RJ, Zarkovic N, Oxidative burst of neutrophils against melanoma B16-F10, Cancer Lett 246 (2007) 100–108. [DOI] [PubMed] [Google Scholar]
- [52].Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. , Polarization of tumor-associated neutrophil phenotype by TGF-beta: N1 versus N2 TAN, Cancer Cell 16 (2009) 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nozawa H, Chiu C, Hanahan D, In ltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 12493–12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sun Z, Yang P, Role of imbalance between neutrophil elastase and alpha 1-antitrypsin in cancer development and progression, Lancet Oncol 5 (2004) 182–190. [DOI] [PubMed] [Google Scholar]
- [55].Rotondo R, Barisione G, Mastracci L, Grossi F, Orengo AM, Costa R, et al. , IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer, Int. J. Cancer 125 (2009) 887–893. [DOI] [PubMed] [Google Scholar]
- [56].Mishalian I, Bayuh R, Eruslanov E, Michaeli J, Levy L, Zolotarov L, et al. , Neutrophils recruit regulatory T-cells into tumors via secretion of CCL17-a new mechanism of impaired antitumor immunity, Int. J. Cancer 135 (2014) 1178–1186. [DOI] [PubMed] [Google Scholar]
- [57].Sparmann A, Bar-Sagi D, Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis, Cancer Cell 6 (2004) 447–458. [DOI] [PubMed] [Google Scholar]
- [58].Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, et al. , K-ras activation generates an inflammatory response in lung tumors, Oncogene 25 (2006) 2105–2112. [DOI] [PubMed] [Google Scholar]
- [59].Freisinger CM, Huttenlocher A, Live imaging and gene expression analysis in zebrafish identifies a link between neutrophils and epithelial to mesenchymal transition, PLoS One 9 (2014) e112183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, et al. , Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis, Cancer Res 73 (2013) 2418–2427. [DOI] [PubMed] [Google Scholar]
- [61].David JM, Dominguez C, Hamilton DH, Palena C, The IL-8/IL-8R Axis: A Double Agent in Tumor Immune Resistance, Vaccines (Basel) 4 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Zuccari DA, Leonel C, Castro R, Gelaleti GB, Jardim BV, Moscheta MG, et al. , An immunohistochemical study of interleukin-8 (IL-8) in breast cancer, Acta Histochem 114 (2012) 571–576. [DOI] [PubMed] [Google Scholar]
- [63].Lin Y, Huang R, Chen L, Li S, Shi Q, Jordan C, et al. , Identification of interleukin-8 as estrogen receptor-regulated factor involved in breast cancer invasion and angiogenesis by protein arrays, Int. J. Cancer 109 (2004) 507–515. [DOI] [PubMed] [Google Scholar]
- [64].Singh JK, Simoes BM, Clarke RB, Bundred NJ, Targeting IL-8 signalling to inhibit breast cancer stem cell activity, Expert Opin. Ther. Targets 17 (2013) 1235–1241. [DOI] [PubMed] [Google Scholar]
- [65].Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB, Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells, Breast Cancer Res 15 (2013) 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chelouche-Lev D, Miller CP, Tellez C, Ruiz M, Bar-Eli M, Price JE, Different signalling pathways regulate VEGF and IL-8 expression in breast cancer: implications for therapy, Eur. J. Cancer 40 (2004) 2509–2518. [DOI] [PubMed] [Google Scholar]
- [67].Marjon PL, Bobrovnikova-Marjon EV, Abcouwer SF, Expression of the proangiogenic factors vascular endothelial growth factor and interleukin-8/CXCL8 by human breast carcinomas is responsive to nutrient deprivation and endoplasmic reticulum stress, Mol. Cancer 3 (2004) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yao C, Lin Y, Chua MS, Ye CS, Bi J, Li W, et al. , Interleukin-8 modulates growth and invasiveness of estrogen receptor-negative breast cancer cells, Int. J. Cancer 121 (2007) 1949–1957. [DOI] [PubMed] [Google Scholar]
- [69].Kerbel RS, Yu J, Tran J, Man S, Viloria-Petit A, Klement G, et al. , Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches, Cancer Metastasis Rev 20 (2001) 79–86. [DOI] [PubMed] [Google Scholar]
- [70].Salcedo R, Martins-Green M, Gertz B, Oppenheim JJ, Murphy WJ, Combined administration of antibodies to human interleukin 8 and epidermal growth factor receptor results in increased antimetastatic effects on human breast carcinoma xenografts, Clin. Cancer Res 8 (2002) 2655–2665. [PubMed] [Google Scholar]
- [71].De Larco JE, Wuertz BR, Rosner KA, Erickson SA, Gamache DE, Manivel JC, et al. , A potential role for interleukin-8 in the metastatic phenotype of breast carcinoma cells, Am. J. Pathol 158 (2001) 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].De Larco JE, Wuertz BR, Yee D, Rickert BL, Furcht LT, Atypical methylation of the interleukin-8 gene correlates strongly with the metastatic potential of breast carcinoma cells, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 13988–13993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Singh B, Berry JA, Vincent LE, Lucci A, Involvement of IL-8 in COX-2-mediated bone metastases from breast cancer, J. Surg. Res 134 (2006) 44–51. [DOI] [PubMed] [Google Scholar]
- [74].Wu K, Katiyar S, Li A, Liu M, Ju X, Popov VM, et al. , Dachshund inhibits oncogene-induced breast cancer cellular migration and invasion through suppression of interleukin-8, Proc. Natl. Acad. Sci. U. S. A 105 (2008) 6924–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, et al. , Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival, Clin. Cancer Res 10 (2004) 7157–7162. [DOI] [PubMed] [Google Scholar]
- [76].Milovanovic J, Todorovic-Rakovic N, Abu Rabi Z, The prognostic role of interleukin-8 (IL-8) and matrix metalloproteinases [C0]2 and 9 in lymph node-negative untreated breast cancer patients, J. Buon 18 (2013) [C0] 866–873. [PubMed] [Google Scholar]
- [77].Snoussi K, Mahfoudh W, Bouaouina N, Fekih M, Khairi H, Helal AN, et al. , Combined effects of IL-8 and CXCR2 gene polymorphisms on breast cancer susceptibility and aggressiveness, BMC Cancer 10 (2010) 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Huang Q, Wang C, Qiu LJ, Shao F, Yu JH, IL-8–251A > T polymorphism is associated with breast cancer risk: a meta-analysis, J. Cancer Res. Clin. Oncol 137 (2011) 1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Singh RK, Lokeshwar BL, The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth, Cancer Res 71 (2011) 3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zhang T, Tseng C, Zhang Y, Sirin O, Corn PG, Li-Ning-Tapia EM, et al. , CXCL1 mediates obesity-associated adipose stromal cell trafficking and function in the tumour microenvironment, Nat. Commun 7 (2016) 11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Zhao H, Dupont J, Yakar S, Karas M, LeRoith D, PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells, Oncogene 23 (2004) 786–794. [DOI] [PubMed] [Google Scholar]
- [82].Maxwell PJ, Coulter J, Walker SM, McKechnie M, Neisen J, McCabe N, et al. , Potentiation of inflammatory CXCL8 signalling sustains cell survival in PTEN-deficient prostate carcinoma, Eur. Urol 64 (2013) 177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Armstrong CW, Maxwell PJ, Ong CW, Redmond KM, McCann C, Neisen J, et al. , PTEN deficiency promotes macrophage in ltration and hypersensitivity of prostate cancer to IAP antagonist/radiation combination therapy, Oncotarget (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chen K, Wu K, Jiao X, Wang L, Ju X, Wang M, et al. , The endogenous cell-fate factor dachshund restrains prostate epithelial cell migration via repression of cytokine secretion via a cxcl signaling module, Cancer Res 75 (2015) 1992–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Uehara H, Troncoso P, Johnston D, Bucana CD, Dinney C, Dong Z, et al. , Expression of interleukin-8 gene in radical prostatectomy specimens is associated with advanced pathologic stage, Prostate 64 (2005) 40–49. [DOI] [PubMed] [Google Scholar]
- [86].Veltri RW, Miller MC, Zhao G, Ng A, Marley GM, Wright GL Jr., et al. , Interleukin-8 serum levels in patients with benign prostatic hyperplasia and prostate cancer, Urology 53 (1999) 139–147. [DOI] [PubMed] [Google Scholar]
- [87].Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, et al. , Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma, J. Exp. Med 179 (1994) 1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Arenberg DA, Kunkel SL, Polverini PJ, Glass M, Burdick MD, Strieter RM, Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice, J. Clin. Invest 97 (1996) 2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Li X, Wang S, Zhu R, Li H, Han Q, Zhao RC, Lung tumor exosomes induce a pro-inflammatory phenotype in mesenchymal stem cells via NFkappaB-TLR signaling pathway, J. Hematol. Oncol 9 (2016) 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Masuya D, Huang C, Liu D, Kameyama K, Hayashi E, Yamauchi A, et al. , The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients, Cancer 92 (2001) 2628–2638. [DOI] [PubMed] [Google Scholar]
- [91].Desai S, Laskar S, Pandey BN, Autocrine IL-8 and VEGF mediate epithelialmesenchymal transition and invasiveness via p38/JNK-ATF-2 signalling in A549 lung cancer cells, Cell Signal 25 (2013) 1780–1791. [DOI] [PubMed] [Google Scholar]
- [92].Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, et al. , Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway, Cancer Res 65 (2005) 11001–11009. [DOI] [PubMed] [Google Scholar]
- [93].Hsu YL, Hung JY, Ko YC, Hung CH, Huang MS, Kuo PL, Phospholipase D, signaling pathway is involved in lung cancer-derived IL-8 increased osteoclastogenesis, Carcinogenesis 31 (2010) 587–596. [DOI] [PubMed] [Google Scholar]
- [94].Lagiou P, Trichopoulos D, In ammatory biomarkers and risk of lung cancer, J. Natl. Cancer Inst 103 (2011) 1073–1075. [DOI] [PubMed] [Google Scholar]
- [95].Yuan A, Yu CJ, Luh KT, Kuo SH, Lee YC, Yang PC, Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small-cell lung cancer, J. Clin. Oncol 20 (2002) 900–910. [DOI] [PubMed] [Google Scholar]
- [96].Sunaga N, Kaira K, Tomizawa Y, Shimizu K, Imai H, Takahashi G, et al. , Clinicopathological and prognostic signi cance of interleukin-8 expression and its relationship to KRAS mutation in lung adenocarcinoma, Br. J. Cancer 110 (2014) 2047–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E, Serum vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) levels in small cell lung cancer, Cancer Invest 24 (2006) 492–496. [DOI] [PubMed] [Google Scholar]
- [98].Singh RK, Varney ML, Regulation of interleukin 8 expression in human malignant melanoma cells, Cancer Res 58 (1998) 1532–1537. [PubMed] [Google Scholar]
- [99].Luca M, Huang S, Gershenwald JE, Singh RK, Reich R, Bar-Eli M, Expression of interleukin-8 by human melanoma cells up-regulates MMP-2 activity and increases tumor growth and metastasis, Am. J. Pathol 151 (1997) 1105–1113. [PMC free article] [PubMed] [Google Scholar]
- [100].Kunz M, Hartmann A, Flory E, Toksoy A, Koczan D, Thiesen HJ, et al. , Anoxia-induced up-regulation of interleukin-8 in human malignant melanoma: a potential mechanism for high tumor aggressiveness, Am. J. Pathol 155 (1999) 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Uen WC, Hsieh CH, Tseng TT, Jiang SS, Tseng JC, Lee SC, Anchorage independency promoted tumor malignancy of melanoma cells under reattachment through elevated interleukin-8 and CXC chemokine receptor 1 expression, Melanoma Res 25 (2015) 35–46. [DOI] [PubMed] [Google Scholar]
- [102].Scheibenbogen C, Mohler T, Haefele J, Hunstein W, Keilholz U, Serum interleukin-8 (IL-8) is elevated in patients with metastatic melanoma and correlates with tumour load, Melanoma Res 5 (1995) 179–181. [DOI] [PubMed] [Google Scholar]
- [103].Ramjeesingh R, Leung R, Siu CH, Interleukin-8 secreted by endothelial cells induces chemotaxis of melanoma cells through the chemokine receptor CXCR1, FASEB J 17 (2003) 1292–1294. [DOI] [PubMed] [Google Scholar]
- [104].Singh S, Varney M, Singh RK, Host CXCR2-dependent regulation of melanoma growth, angiogenesis, and experimental lung metastasis, Cancer Res 69 (2009) 411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gabellini C, Trisciuoglio D, Desideri M, Candiloro A, Ragazzoni Y, Orlandi A, et al. , Functional activity of CXCL8 receptors, CXCR1 and CXCR2, on human malignant melanoma progression, Eur. J. Cancer 45 (2009) 2618–2627. [DOI] [PubMed] [Google Scholar]
- [106].Lattanzio L, Tonissi F, Torta I, Gianello L, Russi E, Milano G, et al. , Role of IL-8 induced angiogenesis in uveal melanoma, Invest. New Drugs 31 (2013) 1107–1114. [DOI] [PubMed] [Google Scholar]
- [107].Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE, Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro, Cytokine 12 (2000) 78–85. [DOI] [PubMed] [Google Scholar]
- [108].Xiao YC, Yang ZB, Cheng XS, Fang XB, Shen T, Xia CF, et al. , CXCL8, overexpressed in colorectal cancer, enhances the resistance of colorectal cancer cells to anoikis, Cancer Lett 361 (2015) 22–32. [DOI] [PubMed] [Google Scholar]
- [109].Konig B, Steinbach F, Janocha B, Drynda A, Stumm M, Philipp C, et al. , The differential expression of proin ammatory cytokines IL-6, IL-8 and TNF-alpha in renal cell carcinoma, Anticancer Res 19 (1999) 1519–1524. [PubMed] [Google Scholar]
- [110].Kamohara H, Takahashi M, Ishiko T, Ogawa M, Baba H, Induction of interleukin-8 (CXCL-8) by tumor necrosis factor-alpha and leukemia inhibitory factor in pancreatic carcinoma cells: impact of CXCL-8 as an autocrine growth factor, Int. J. Oncol 31 (2007) 627–632. [PubMed] [Google Scholar]
- [111].Yoshida M, Matsuzaki H, Sakata K, Takeya M, Kato K, Mizushima S, et al. , Neutrophil chemotactic factors produced by a cell line from thyroid carcinoma, Cancer Res 52 (1992) 464–469. [PubMed] [Google Scholar]
- [112].Weetman AP, Bennett GL, Wong WL, Thyroid follicular cells produce interleukin-8, J. Clin. Endocrinol. Metab 75 (1992) 328–330. [DOI] [PubMed] [Google Scholar]
- [113].Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K, Tumor necrosis factor alpha and interferon gamma synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-kB-like binding sites of the interleukin 8 gene, J. Biol. Chem 267 (1992) 22506–22511. [PubMed] [Google Scholar]
- [114].Kitadai Y, Haruma K, Sumii K, Yamamoto S, Ue T, Yokozaki H, et al. , Expression of interleukin-8 correlates with vascularity in human gastric carcinomas, Am. J. Pathol 152 (1998) 93–100. [PMC free article] [PubMed] [Google Scholar]
- [115].Kitadai Y, Takahashi Y, Haruma K, Naka K, Sumii K, Yokozaki H, et al. , Transfection of interleukin-8 increases angiogenesis and tumorigenesis of human gastric carcinoma cells in nude mice, Br. J. Cancer 81 (1999) 647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Lee LF, Hellendall RP, Wang Y, Haskill JS, Mukaida N, Matsushima K, et al. , IL-8 reduced tumorigenicity of human ovarian cancer in vivo due to neutrophil infiltration, J. Immunol 164 (2000) 2769–2775. [DOI] [PubMed] [Google Scholar]
- [117].Isaza-Correa JM, Liang Z, van den Berg A, Diepstra A, Visser L, Toll-like receptors in the pathogenesis of human B cell malignancies, J. Hematol. Oncol 7 (2014) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Schonbohn H, Schuler M, Kolbe K, Peschel C, Huber C, Bemb W, et al. , Plasma levels of IL-1, TNF alpha, IL-6, IL-8, G-CSF, and IL1-RA during febrile neutropenia: results of a prospective study in patients undergoing chemotherapy for acute myelogenous leukemia, Ann. Hematol 71 (1995) 161–168. [DOI] [PubMed] [Google Scholar]
- [119].di Celle PF, Carbone A, Marchis D, Zhou D, Sozzani S, Zupo S, et al. , Cytokine gene expression in B-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) mRNA expression and secretion of biologically active IL-8 protein, Blood 84 (1994) 220–228. [PubMed] [Google Scholar]
- [120].Gruss HJ, Brach MA, Drexler HG, Bonifer R, Mertelsmann RH, Herrmann F, Expression of cytokine genes, cytokine receptor genes, and transcription factors in cultured Hodgkin and Reed-Sternberg cells, Cancer Res 52 (1992) 3353–3360. [PubMed] [Google Scholar]
- [121].Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, et al. , Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models, Int. J. Cancer 128 (2011) 2038–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ueda T, Shimada E, Urakawa T, Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis, J. Gastroenterol 29 (1994) 423–429. [DOI] [PubMed] [Google Scholar]
- [123].Dimberg J, Strom K, Lofgren S, Zar N, Lindh M, Matussek A, DNA promoter methylation status and protein expression of interleukin-8 in human colorectal adenocarcinomas, Int. J. Colorectal. Dis 27 (2012) 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Rubie C, Frick VO, Pfeil S, Wagner M, Kollmar O, Kopp B, et al. , Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer, World J. Gastroenterol 13 (2007) 4996–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Bi LK, Zhou N, Liu C, Lu FD, Lin TX, Xuan XJ, et al. , Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells, Urol. Oncol 32 (2014) 607–612. [DOI] [PubMed] [Google Scholar]
- [126].Huang D, Ding Y, Zhou M, Rini BI, Petillo D, Qian CN, et al. , Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma, Cancer Res 70 (2010) 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Casilli F, Bianchini A, Gloaguen I, Biordi L, Alesse E, Festuccia C, et al. , Inhibition of interleukin-8 (CXCL8/IL-8) responses by repertaxin, a new inhibitor of the chemokine receptors CXCR1 and CXCR2, Biochem. Pharmacol 69 (2005) 385–394. [DOI] [PubMed] [Google Scholar]
- [128].Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, et al. , CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts, J. Clin. Invest 120 (2010) 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Brandolini L, Cristiano L, Fidoamore A, De Pizzol M, Di Giacomo E, Florio TM, et al. , Targeting CXCR1 on breast cancer stem cells: signaling pathways and clinical application modelling, Oncotarget 6 (2015) 43375–43394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, et al. , The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models, Mol. Cancer Ther 11 (2012) 1353–1364. [DOI] [PubMed] [Google Scholar]
- [131].Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, et al. , Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis, Clin Cancer Res 15 (2009) 2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK, Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases, Cancer Lett 300 (2011) 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G, et al. , Targeting CXCR1/2 signicantly reduces breast cancer stem cell activity and increases the ef cacy of inhibiting HER2 via HER2-dependent and [C0] independent mechanisms, Clin. Cancer Res 19 (2013) 643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Liu X, Peng J, Sun W, Yang S, Deng G, Li F, et al. , G31P, an antagonist against CXC chemokine receptors 1 and 2, inhibits growth of human prostate cancer cells in nude mice, Tohoku J. Exp. Med 228 (2012) 147–156. [DOI] [PubMed] [Google Scholar]
- [135].Mian BM, Dinney CP, Bermejo CE, Sweeney P, Tellez C, Yang XD, et al. , Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB, Clin. Cancer Res 9 (2003) 3167–3175. [PubMed] [Google Scholar]
- [136].Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang XD, et al. , Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma, Am. J. Pathol 161 (2002) 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Shao N, Chen LH, Ye RY, Lin Y, Wang SM, The depletion of interleukin-8 causes cell cycle arrest and increases the ef cacy of docetaxel in breast cancer cells, Biochem. Biophys. Res. Commun 431 (2013) 535–541. [DOI] [PubMed] [Google Scholar]
- [138].Kamalakar A, Bendre MS, Washam CL, Fowler TW, Carver A, Dilley JD, et al. , Circulating interleukin-8 levels explain breast cancer osteolysis in mice and humans, Bone 61 (2014) 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Seaton A, Scullin P, Maxwell PJ, Wilson C, Pettigrew J, Gallagher R, et al. , Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation, Carcinogenesis 29 (2008) 1148–1156. [DOI] [PubMed] [Google Scholar]
- [140].Chen H, Sun Y, Wu C, Magyar CE, Li X, Cheng L, et al. , Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway, Endocr. Relat. Cancer 19 (2012) 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Inoue K, Slaton JW, Eve BY, Kim SJ, Perrotte P, Balbay MD, et al. , Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer, Clin Cancer Res 6 (2000) 2104–2119. [PubMed] [Google Scholar]
- [142].Singh RK, Lokeshwar BL, Depletion of intrinsic expression of Interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs, Mol. Cancer 8 (2009) 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Pold M, Zhu LX, Sharma S, Burdick MD, Lin Y, Lee PP, et al. , Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer, Cancer Res 64 (2004) 1853–1860. [DOI] [PubMed] [Google Scholar]
- [144].Boldrini L, Gisfredi S, Ursino S, Lucchi M, Mussi A, Basolo F, et al. , Interleukin-8 in non-small cell lung carcinoma: relation with angiogenic pattern and p53 alterations, Lung Cancer 50 (2005) 309–317. [DOI] [PubMed] [Google Scholar]
- [145].Iguchi H, Ono M, Matsushima K, Kuwano M, Overproduction of IL-8 results in suppression of bone metastasis by lung cancer cells in vivo, Int. J. Oncol 17 (2000) 329–333. [DOI] [PubMed] [Google Scholar]
- [146].Shiau MY, Fan LC, Yang SC, Tsao CH, Lee H, Cheng YW, et al. , Human papillomavirus up-regulates MMP-2 and MMP-9 expression and activity by inducing interleukin-8 in lung adenocarcinomas, PLoS One 8 (2013) e54423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Brew R, Erikson JS, West DC, Flanagan BF, Christmas SE, Interleukin-8 as a growth factor for human colorectal carcinoma cells in vitro, Biochem. Soc. Trans 25 (1997) 264s. [DOI] [PubMed] [Google Scholar]
- [148].Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, et al. , IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells, Cytokine 29 (2005) 275–282. [DOI] [PubMed] [Google Scholar]
- [149].Cheng XS, Li YF, Tan J, Sun B, Xiao YC, Fang XB, et al. , CCL20 and CXCL8 synergize to promote progression and poor survival outcome in patients with colorectal cancer by collaborative induction of the epithelial-mesenchymal transition, Cancer Lett 348 (2014) 77–87. [DOI] [PubMed] [Google Scholar]
- [150].Sun Q, Sun F, Wang B, Liu S, Niu W, Liu E, et al. , Interleukin-8 promotes cell migration through integrin alphavbeta6 upregulation in colorectal cancer, Cancer Lett 354 (2014) 245–253. [DOI] [PubMed] [Google Scholar]
- [151].Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O’Sullivan JM, et al. , Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis, J. Pharmacol. Exp. Ther 327 (2008) 746–759. [DOI] [PubMed] [Google Scholar]
- [152].Dabkeviciene D, Jonusiene V, Zitkute V, Zalyte E, Grigaitis P, Kirveliene V, et al. , The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116, Med. Oncol 32 (2015) 258. [DOI] [PubMed] [Google Scholar]
- [153].Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK, Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis, Angiogenesis 8 (2005) 63–71. [DOI] [PubMed] [Google Scholar]
- [154].van Hinsbergh VW, Koolwijk P, Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead, Cardiovasc. Res 78 (2008) 203–212. [DOI] [PubMed] [Google Scholar]
- [155].Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, et al. , SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information, Nucleic Acids Res 42 (2014) W252–W258. [DOI] [PMC free article] [PubMed] [Google Scholar]


