Abstract
Parkinson’s disease (PD) is a movement disorder, mainly affecting population consisting of the aged. PD occurs chiefly due to progressive loss of dopaminergic neurons in nigrostriatal pathway. Largely, PD patients suffer from non-motor symptoms, such as depression, anxiety, fatigue, and sleep disorders, that needs further investigation and addressing during PD research. Depression in PD is a predominant and complex symptom, and its pathology exists extrinsic to the nigrostriatal system. This disease can ultimately be managed by a combination of regular physiotherapy and proper medication. Taking together the present scenario of PD, including the nature of disease, characteristics, treatment, diagnosis of the patients with PD, these outcomes were reviewed to be explored along with many speech-based solutions to PD in this study. This neurodegenerative disorder needs advancement in research and development which can help patients with PD to lead a normal life. (www.actabiomedica.it)
Keywords: Parkinson disease, symptom, diagnosis, treatment, physiotherapy, medication
Introduction
Parkinson’s disease (PD) is a chronic disorder of the central nervous system with symptoms appearing gradually with the increase in age. PD was first described by James Parkinson in 1817 and he explained in an essay titled as “shaking palsy”. In the late nineteenth century, the description of the disease was further refined by Charcot based on the cardinal clinical features (1).
Loss of nerve cells in the brain leading to PD is known as substantia nigra. These nerve cells make up the neurochemical messenger of dopamine, which is responsible for all messages that coordinate normal movement. The lack of dopamine in a PD patient’s brain cells, leads to motor complications and the progress turns out to be slow, gradually expanding over years. Commonly occurring cardinal motor symptoms in PD patients, includes resting Tremor, Rigidity Akinesia and Postural instability (TRAP) (4). Assessment of PD manifestations are done using foot pressure analysis, finger motion analysis and the Unified Parkinson’s Disease Rating Scale (UPDRS) are (5). Treatment options for PD patients are limited and primarily focussed to reduce the disease symptoms (6). PD is the second most common motor disorder next to Alzheimer’s disease (AD). In near future, this disease will in course occupy a dominant place in research, due to its treatment modality and medical expenditures involved (7). Recently, researchers have been focussing on the non-motor symptoms (NMS) of PD which are not documented and thereby ineffectively cured through physicians. Non-motor symptoms (NMS) include depression, social phobias, low blood pressure, apathy, loss of sense of smell, fear and anxiety, panic attacks, which are due to the mild lesions of the meso-limbic and meso-cortical pathways (8). PD patients generally are seen to have NMS, observed much before motor symptoms, and it would really help if we recognize these symptoms as a part of PD symptoms and address to it. It is hard to choose the right medication and treatment as PD patients with NMS do not respond to medication prescribed for NMS (9). Stress is considered to be another cause of PD, but the mechanism is still unknown (10). Various studies have concluded that stress induced neural effects are progressive to various neurodegenerative diseases, including Alzheimer’s disease, Huntington’s disease and Parkinson’s disease. Psychological stress in humans due to depression, anxiety and impaired cognition is the primary cause orf PD. About 40-50% of all PD cases are caused due to depression (11) and it has been reported that acute or chronic stress might lead to an earlier onset of this disease (12-14). Recently Hemmerle et al. (2014), demonstrated that chronic stress-induced depression potentially impairs the behavioral dysfunction and dopaminergic degeneration of nigrostriatal system, rather than neurotoxin-induced neurodegeneration.
Epidemiology
PD normally affects about 1-2% of the world population, where the estimated incidence rate is found to be 20/100,000 and approximate prevalence rate is about 150/100,000. The current estimation shows 1.5 million people being affected by this disease in US (2, 3). Men are one and half times more prone to have this disease. European survey reported the rate of prevalence between 100 and 200 per 100,000 inhabitants (59). In Norwegian Park West survey, they calculated the age-standardized incidence to be 12.6 per 100,000 (60). Most studies reported the incidence of PD to be more common in rural population (61). Overall male to female ratio was found to be 1.58 based on published prevalence studies, reported by the Norwegian Park West.
Etiology
Till date the etiology of PD is not well understood. Modern experimental models of PD involving neuropathologic investigations, genetic analysis and epidemiologic studies have tried to elucidate the detailed disease condition of PD (53-58). Interestingly, researchers are focussing on genes linked to PD consisting of 10 distinct loci which is responsible for the expression of this disease condition.
Risk factors
The two types of risk factors include genetic and non-genetic risk factors. There is no reverse linkage between smoking and this disease and also consumption of coffee was found to decrease the risk of PD. Even the dietary factors like fatty acids and antioxidants are under investigation. A presence of genetic mutation is stipulated to show a risk for developing PD (62). A recent study has also showed some results where risk of PD was increased after a stroke. Thus, ischemia plays a role in the development of cognitive decline (65).
Neuropathology
Gliosis and cell loss in nigrostriatal neurons are interestingly the gold standard for the diagnosis of PD. In 2003, Braak and colleagues hypothesized that disease concerned pathology develops in a logical sequence. The Primary stage consists of levels-I & II, where lesions occur in the anterior olfactory nucleus, the dorsal motor nucleus of the IX/X nerves, the raphe nuclei and the reticular formation. In later stages three and four, the pathology is restricted to the brainstem and anteromedial temporal mesocortex. The chief characteristic of this stage is that the substantia nigra gets affected. Stage five and six is consists of the acute involvement of the brain including most of the neocortical areas (98).
Clinical manifestations
The four cardinal signs of Parkinson’s disease are tremor, rigidity, bradykinesia and postural instability (15). Postural instability may be the most debilitating feature (16) and leads to further disability. Mostly it can be defined as the disability to maintain the body’s centre of gravity over the base of support during standing and also during movement (17) and is essential for any locomotor activity. Further, patients lack the coordination of biomechanical, sensory, motor and the central nervous systems (Fig. 1).
Figure 1.
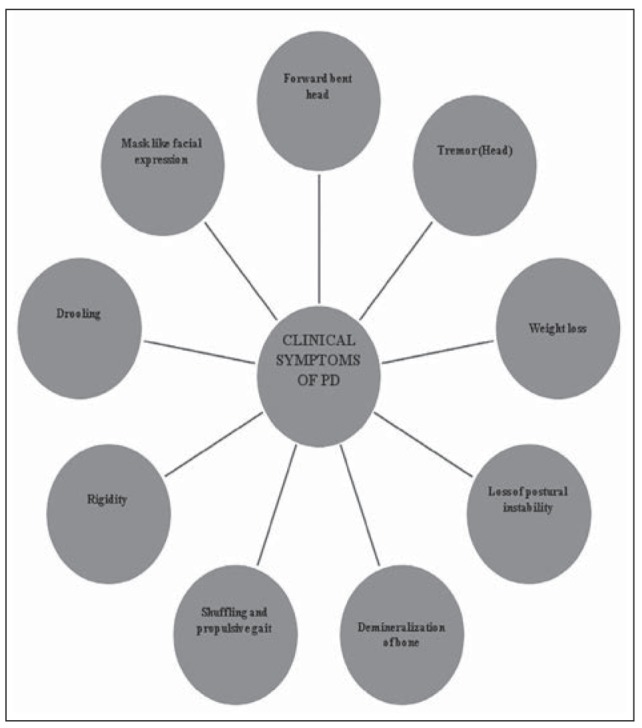
Clinical Symptoms of PD
Motor neuron and dopamine controls
The motor disability symptoms of Parkinson’s disease result from the loss of dopamine - secreting (dopaminergic) pigmented cells, in the pars compacta region of the substantia nigra (literally “black substance”). The substantia nigra is a very small area located deep within the brain and in PD patients these dopaminergic neural cells of substantia nigra degenerates and dies, only few live neurons in this region are observed in PD brain tissues than in the normal brain tissue. The loss of dopaminergic neurons leads to the loss of dopamine and dopamine is the major neurotransmitter which relays neuronal signals from the brain to other motor centers. The lack of dopamine in PD patients disturbs the movement control of the patients (motor symptoms) and mood, behavior, thinking and sensation of the patients (non – motor symptoms).
Motor Symptoms
Tremor
Tremor is one of the common symptoms and moreover first symptom of Parkinson’s disease which is observed in >70% of the PD patients. Typically the tremor in PD patients starts at one limb and may spread to another on the same side of the body before proceeding to the other side. The progression of tremor is gradual which could affect the arms, legs, feet, lips and head. These tremors, or shakes most likely occur in the resting condition and this symptom could disappear when the patient is actually moving. For many PD patients tremor is one of the most distressing symptoms due to its psychological impact when exposed to the society and also this symptom can become worse if a patient is anxious or excited.
Tremors in PD patients are unilateral which normally occurs at a frequency between 4 and 6 Hz, and most of the time they are prominent in the distal part of the extremity. Some PD patients have a history of postural tremor for many years before the onset of parkinsonian tremor or other PD related features and this postural tremor is symptomatically identical to essential tremor (18) (Fig. 2).
Figure 2.
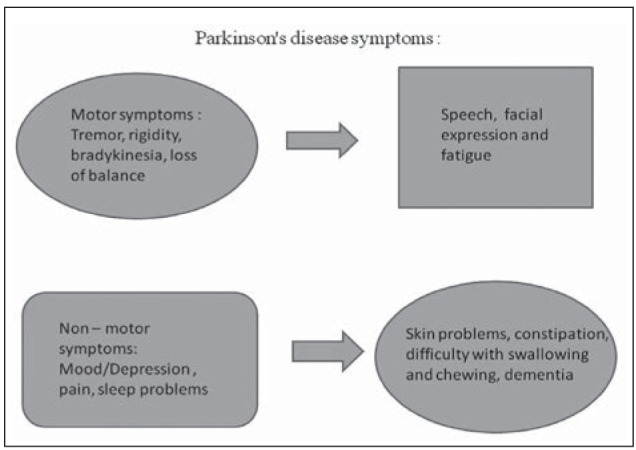
Parkinson disease symptoms
Stiffness (rigidity)
Rigidity, or resistance to movement affects most of the Parkinson’s disease patients. The principle behind the body movement is coordinated by two muscles where one will be an opposing muscle. Hence the movement is achieved by one muscle that becomes active and the opposing muscle relaxes. In Parkinson’s disease, the rigidity originates due to the disturbed response to signals from the brain, which leads to the disturbance of the balance of opposing muscle during the muscular coordination. The relaxation of the muscle during a movement is disturbed, which makes the muscles to remain constantly tensed and contracted and hence the patient arches or feels stiff or weak. The rigidity turns out to be a noticeable one when another person tries to move the patient’s arm, which will display a ratchet – like or short, jerky movement known as “cogwheel” rigidity.
Reinforcing maneuvers (e.g. Voluntary movements of the contralateral limb), known as the froments maneuver (19) usually increases rigidity and are particularly useful in detecting mild cases of rigidity. Rigidity may be associated with pain, and painful shoulder, although it is commonly misdiagnosed as arthritis, bursitis or rotator cuff injury (20).
Slowness of the movement (bradykinesia)
Due to the lack of Dopamine, the signals from the brain to the muscles slowdown, that leads to Bradykinesia (slowness of the movement) Bradykinesia slows down day to day activities of the patient, such as walking, bathing or dressing etc, and this is very disabling as it interferes routine life style. The patient may begin to shuffle (called festination) and their walking steps become shorter and shorter and more likely they will have problems like starting and stopping and turning while walking and some patients may feel to be falling forward. All these walking complications are known as “Parkinson’s gait.” Bradykinesia is considered to be a hallmark of basal ganglia disorders, and it includes difficulties with planning, initiating and executing movement and with performing sequential and simultaneous tasks (21).
Loss of balance (postural instability)
The posture and balance maintaining ability would be disturbed, which could lead to instability while walking, turning, standing or when performing actions such as rising from a chair or bending over. These unsteady movements lead to a fall, which is a major cause of injury in PD patients. Several other parkinsonian symptoms such as orthostatic hypotension, age related sensory changes and the ability to integrate visual, vestibular and proprioceptive sensory input (kinesthesia) (22, 23).
Speech and facial expression
PD patients have reduced facial expression that can lead to communication difficulties, disinterest, or as a lack of understanding, vocal change including speedy or rough speech.
Fatigue
Fatigue resulting from physical or mental tiredness is very common. Fatigue can be caused by one or more factors, including drug treatment, disturbed sleep or depression. Alternatively, fatigue may be caused directly by the chemical changes that occur in the brain of PD patients. If it is found to be associated with depression, the depression should be treated; if it is caused by sleeping problems, then that should be assessed and treated.
Non-motor symptoms
Mood/Depression
Mood change or depression is a natural feedback, commonly diagnosed due to lower level of signals in the PD brain that control the mood. Signs of depression include: a negative view of oneself, the environment and the future, loss of motivation, energy and interests (including social and sexual), poor sleep and memory, and a decreased hunger. Depression is one of the most common non-motor symptoms of PD, affecting over 40 percent of the patients (24). Depression is difficult to be characteristically identified in the clinical practice as it overlaps with many other signs and symptoms of PD. The depression in the PD patients, may cause major interference with the quality of life, but often might involve less severe symptoms with more understated features for which the clinician should be prepared.
Pain
Most of the patients develop muscle and joints pain. Mostly pain seems to increase with duration of disease, severity, depression or dopaminergic therapy. PD is directly related to pain (e.g. dystonia when ‘off1) or pain can also be unrelated to PD (e.g. osteoarthritis or neuropathic pain) (25).
Sleep problems
Sleep problems being a preclinical marker in PD (26) includes difficulty staying asleep at night, restless sleep, nightmares, emotional dreams and drowsiness or sudden sleep onset during the day. Rapid eye movement (REM) sleep behavior disorder is characterized by loss of muscle atonia allowing patients to physically act out their dreams. Injury to the patient or bed partner is not unknown.
Difficulty with swallowing and chewing
Muscles used in swallowing may work less efficiently and food and saliva may collect in the mouth and the back of the throat, which can result in choking or drooling. These chewing and swallowing difficulties may lead to malnutrition to the patient.
Skin problems
In PD, improper functioning of autonomic nervous system, causes oily skin specifically on the T region surrounding forehead, and nose and also causes dandruff as well.
Urinary problems or constipation
Due to the improper functioning of the nervous system, bladder and bowel problems can occur in some patients and also experience problems with urinating and others might become incontinent. As the intestinal tract operates more slowly, poor diet or less fluid intake are the major factors for constipation. Sometimes medications are also used to treat PD and contribute to constipation. If the problem is serious and persistent, then the patients require hospitalization in rare cases. Constipating patients are encouraged to consume more fluid and fiber intake for stool softness, macrogel 3350 and electrolytes for consumption (27).
Dementia or other cognitive problems
Dementia is the largest predictor of quality of life in PD with a six fold increased risk that leads to PD (28).
Diagnosis
Diagnosis of PD is the main challenge for the scientists and the clinicians. The disease is sometime underdiagnosed (29) while misdiagnosis occurs owing to drugs, Wilson’s disease and other neurological disorders. Due to misdiagnosis, approximately 10-20% of people suffer from PD. The data for the diagnosis of PD is usually made with the help of the patient’s history and physical examination of the patient. Early onset of Parkinson’s disease in patients may include slowness in walking, tremor, imbalance even when the neurological examination is normal (30). The infrequent occurrence of tremor at rest is 4-6 Hz. but it is also absent in up to one quarter of cases (31). Initially 90% of patients have a response to levodopa drug, whereas the remaining 10%, serve to be the main lead to opt for alternative diagnosis. To date, there are no biological markers available to confirm the diagnosis of the PD. The presymptomatic patients are undergoing irrelevant treatment because of inaccurate diagnosis of PD. To overcome this problem, it is of prime importance to find biomarkers, imaging techniques and laboratory based clinical assays. Routine imaging studies of brain, PET, single photon emission tomography (SPECT) and functional imaging techniques are helpful in differentiating Parkinsonism with 95% accuracy (32).
One of the potent feature of the diagnosis is dementia and its presence shows that survival rate lessens in PD patient. Based on the large population based survey in Norway population, 28% of the patients encountered dementia (33). These results have high sensitivity, but care should be taken while distinguishing the idiopathic PD. In another study, 65% of the surviving cohorts experienced dementia. The diagnosis was also strengthened by the assessment of The Unified Parkinson’s Disease Rating Scale assessment which in turn strengthened the PD diagnosis. The physical and mental symptoms are the major criteria for diagnosis, which have an influence on the quality of life (QoL) of patients with PD (34). One of the physical symptoms of PD is also called “Parkinson’s mask” (35).
Differential diagnosis
There are several factors that positively differentiate PD from other diseases and that includes response to levodopa. Contemporary science has stated many neuroimaging techniques for differentiating PD. A new positron emission tomography imaging study resulted in downstream changes which was indicative of a possible mechanism for the lack of response against PD as the study conducted was also used in relative preservation of dopamine receptors in PSP (37). Potential imaging studies include high field strength (1.5 T) heavily T2 weighted MRI, [18F]-fluorodopa positron emission tomography, (38) [11C] raclopride imaging of dopamine D2 receptors (39) and single photon emission computed tomography of striatal dopamine reuptake sites (40). When compared to nuclear imaging, MRI is the best structural imaging technique which provides no ionizing radiation. Most of the standard MRI techniques had failed to identify disease specific abnormalities in early stage of PD. Recently, brain parenchyma sonography which is a heavily tapped testing resource for diagnostic evaluation of PD (41), showed abnormal hyperechogenicity not only in PD but in essential tremor as well (42). Levodopa has been an important drug to improve the effects of PD as there has been a recent finding where a study revealed that 77% of patients responded well to levodopa initially (43). The researchers reported that it is also to be noted that clinicians have claimed that levodopa hinders prognosis as it not definitive of PD alone (44). Subcutaneous injection of apomorphine has been used to differentiate between PD and other parkinsonian disorders; however, this test is not superior to levodopa therapy and meagerly contributes to PD diagnosis (45).
Treatment
Pharmacotherapy
The standard treatment approach is oral based pharmacotherapy and recently they focussed on the surgical alteration of the brain region associated with PD. Surgical treatments are proceeds only on selected patients with approximately 8-10% of success (66). Generally, pharmacotherapies are related to monoamine neurotransmitter imbalances. There are several drugs available to treat motor impairments in PD like, carbidopa/levodopa and pramipexole and ropinirole (67, 68). These drugs are able to modify the imbalances in dopamine producing neurons in PD patients. Pharmacotherapies are very effective within short course time, along with side effect like levodopa induced dyskinesias (LIDs) (69) and additionally optimized dosage of individual patient might lead to increasing motor fluctuations resulting in “wearing off” periods (70). Upper and lower limbs will respond to the dopomeric treatment where there is limited response from axial symptoms. There are significant responses to levodopa for axial and appendicular rigidity in PD patients. Besides levodopa executes significant effect on appendicular system (knees, arms, wrists) and on the other hand exerted insignificant effect on rigidity in the axial system (trunk, torso) (72, 73).
During the early stage of disease progression, appendicular symptoms and non-dopaminergic axial symptoms occur. In extended stages non-dopaminergic systems (frontal cortex and cerebellum) are further affected (74-76). For better understanding novel interventions and replacement of oral medication for betterment of quality of life are needed. One of the studies reported 21 PD participants who underwent STN-DBS surgery, established that there were major improvements in the energy levels, and possible relief for a yearpost-operation, mainly due to the lesser dose of medication with neurostimulation (77).
Drugs and the treatments
Currently, no permanent treatment against PD is available. Only medication and surgery will provide relief from the PD symptoms. Thirty years ago, approximately about 50,000 people were diagnosed with PD per year. The only drug that was available at that time was Levodopa – a chemical compound that the body can convert into dopamine. This helped many of the PD patients to survive, but the long term use of this drug resulted in the uncontrolled movements. Brain surgery of the destroyed regions also is an alternate mode of available treatment other than the drug therapy. Recently several new drugs have been introduced other than the Levodopa as a treatment outcome for PD patients. PD medications fall under three distinct categories that help in controlling the disease and ease the effects of PD. Drugs that work directly or indirectly to increase the level of dopamine in the brain that include dopamine precursors like levodopa make up the first genre of PD drugs. It crosses the blood brain barrier and triggers dopamine secretion. The second type of PD drugs affects other neurotransmitters in the body in order to control the disease. Drugs like anticholinergic agents are an excellent example that interferes with the production or uptake of the neurotransmitter acetylcholine. These drugs help to reduce tremors and muscle stiffness, which can result from having more acetylcholine than dopamine in the system. The third type of drugs prescribed for PD includes medications that help control the non–motor symptomatic effects of the disease. Other drugs mimic dopamine or prevent or slow its breakdown (Fig. 3).
Figure 3.
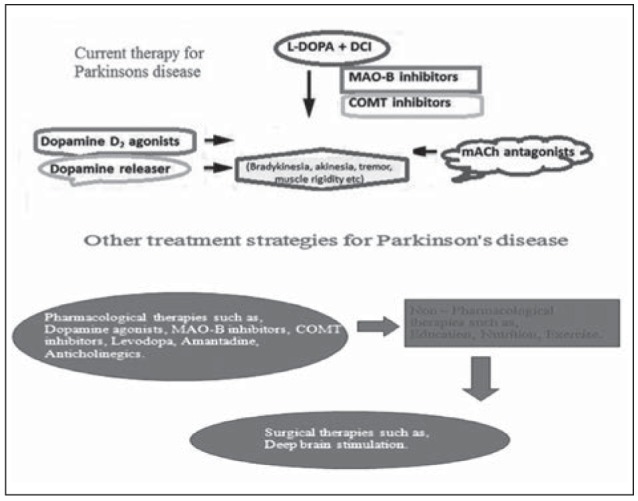
Current therapy for PD
Drugs that increase brain levels of the dopamine
Levodopa
Levodopa (L-3, 4-dihydroxyphenylalanine) is derived from both plants and animals. Though it is a very effective drug against early stage symptoms of PD, but cannot be considered to be a cure for PD. Patient are prescribed levodopa along with carbidopa. This combination therapy slows down the transformation of levodopa into dopamine until it reaches the brain and also inhibits levodopa from exerting related side effects. Carbidopa also reduces the amount of levodopa dosage. This combination may arrest the nausea and vomiting in patients. Long term usage with highest dose of levodopa may cause involuntary movements. Surgical procedure is the only option for severe dyskinesias. Anticholinergic agents were the most widely used drug for treatment of PD, before discovery of levodopa. This agent is very effective against tremor. Their symptomatic effect, however, is limited and side-effects such as blurred vision, urinary retention, constipation and impaired cognitive function limit their utility (78, 79). To prevent its peripheral conversion to dopamine, usually it was administered in combination with peripheral dopa decarboxylase inhibitors (carbidopa or benserazide) (80, 81). But the in-vitro and invivo experimental study results are not clearly reported about levodopa toxicity (82).
Drugs that mimic dopamine
Dopamine agonists
Dopamine agonists (DA), displays an anti-parkinson effect, which can act also in combination with levodopa in early stages of the disease. When compared to levodopa, these drugs are very less effective in controlling rigidity and bradykinesia. Drowsiness, sudden sleep onset, hallucinations, confusions, dyskinesias, edema, nightmares, and vomiting are side effects associated with the use of levodopa. Two main classes of dopamine receptor agonists are ergot derivatives and the non-ergot derivatives. DAs are treated as monotherapy in PD and they can successfully delay the need for levodopa. It is a replacement or adjunct treatment to control their PD symptom. Due to the risk of pleuropulmonary/retroperitoneal fibrosis and of fibrotic heart-valve reactions, ergot derivatives are limitedly used (83).
Drugs that inhibit dopamine breakdown
MAO-B inhibitors
MAO-B inhibitors can inhibit the activity of monoamine oxidase B, or MOA-B. Usually MOA-B inhibitors reduce the symptoms of PD through accumulation of dopamine on the a live nerve cells. Selegiline or deprenyl is one of the inhibitor of MOA-B, and is very active against PD along with levodopa and also reduce the side effects. It was harmful when combined with the antidepressant fluoxetine or the sedative meperidine. Tolcapone also reduce requirement of levodopa to patients but it will induce severe hepatotoxicity (83). Amantadine an antiviral agent, which is thought to block N-methyl-D-aspartate (NMDA) glutamate receptors reduces the levodopa-induced dyskinesias (84).
COMT inhibitors
Catechol-o-methyltransferase helps to break down the dopamine. There are two types of COMT inhibitors being entacapone and tolcapone. COMT inhibitors are used to reduce the person’s dose of levodopa. These types of inhibitors may cause other side effects including nausea, sleep disturbances, dizziness, urine discoloration, abdominal pain, low blood pressure, or hallucinations (Fig. 4).
Figure 4.
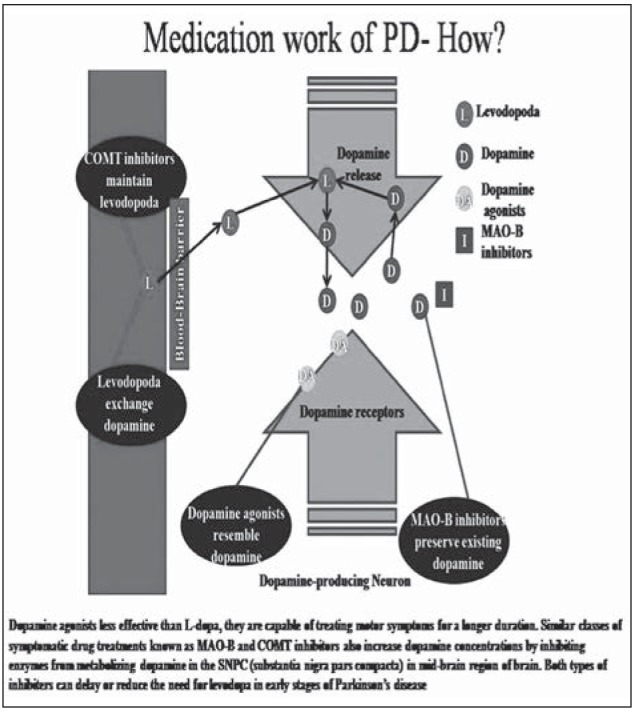
Mechanism of action of drugs in PD
Drugs with an unknown mechanism of action
Amantadine
Usually early stage of PD, an antiviral drug amantadine is used to reduce symptoms of PD and levodopa-induced dyskinesia. This drug can be used alone or in combination with anticholinergic drug or levodopa, Side effects such as insomnia, mottled skin, edema, agitation, or hallucinations are possible side effects of Amantadine.
Drugs that decrease the action of acetylcholine
Anticholinergics
Trihexyphenidyl, benztropine and ethopropazine are used to reduce the action of acetylcholine and tremors, muscle rigidity dry mouth, constipation, urinary retention, hallucinations, memory loss, blurred vision, and confusion are side effects of these drugs.
Drugs for non-motor symptoms
Depression and anxiety are the frequent non motor symptoms of PD. This symptom is treated with amitriptyline or fluoxetine and benzodiazepines for respective treatment. Some PD drugs are causing side effects like hallucinations, delusions, and other psychotic symptoms. Alleviating psychosis may help to reduce or stop the PD medication. If not effective treatments like atypical antipsychotics, for which clozapine and quetiapine are recommended.
Diet
Several studies have investigated, that nutrition displays a predominant role in PD. Despite of that inclusion or exclusion of food classes is necessary to improve the neurodegeneration because some of the food classes promote or exacerbate neuroprotection in PD (85, 86). Promisingly, Cruciferous vegetables with high content antioxidants activity might improve the neuroprotection. This food groups include cauliflower, cabbage, and broccoli. Presence of dopaminergic neurotoxins, including pesticides and polychlorinated biphenyls in any food products are high risk to PD patients (87, 88). A recent study addressed that amount of beer consumption is a relatively lower risk to PD, whereas liquor consumption increases highly the risk of PD (89). Epidemiological studies found that high intake of caffeine is associated with a reduced risk of PD and tea is associated with increased risk of PD. Dairy product intake also enhances the risk of PD (90).
Recent therapy
Gene therapy
Gene therapy is one of treatment options where human gene therapy is implemented in somatic cells. Generally with gene modifications by either overexpressing or inhibiting particular target genes can restore the normal function of these genes. Currently, there are two types of vectors are used in gene therapy, such as viral mediated vectors, and nonviral systems. In viral vectors, it can transport the genetic material to target cells. Non-viral vector delivers the genes to the CNS by physical and chemical methods like a gene gun or electrophoresis. Various kinds of vectors have been constructed with differing by their packaging capacity, tropism, and immunogenicity. Adeno-associated virus (AAV ) and lentivirus derived vectors are under CNS gene therapy clinical trials.
Stem cell therapy
Dopamine modulates transmission of signals in the highly specialized areas of the brain, like the basal ganglia, concerned with the body and limb movements, which leads to tremors, rigidity, freezing and slurring of speech.
Recent advances in stem cell research involves administration of genetically modified stem cells which are able to produce dopamine and also can convert dopamine producing cells to treat PD patients. Furthermore, in stem cell research, the mesenchymal cells are infused into the part of the brain, where these cells are multiplied into healthy cells in substantia nigra, resuming normal production of dopamine that helps in retrieving much of the normal functions.
Biotechnology drugs
Initially atremorine showed the potent neuroprotective activity at the hippocampal level during deprivation of oxygen and glucose deprivation in human neuroblastoma SH-SY5Y cells. A recent concern has been initiated by E-PodoFavalin-15999 (Atremorine®), a novel compound, obtained through a nondenaturing biotechnological non-GMO manipulation of Vicia faba L. moieties. When tested in experimental animals, this compound exerted a significant protection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) induced dopaminergic neurodegeneration while also inhibiting MPTP-induced microglia activation and neurotoxicity in substantia nigra. The above effect showed notable changes in motor functions of mice (91, 92). In a most recent clinical trial, a single dose of atremorine administered to patients exhibited the tolerable increased level of DA to be up to 4556.61±678.95 pg/mL (p<0.001) after an hour wards, irrespective of the gender. Interestingly, all naïve patients showed a dopamine level increase from 0.29 to 2041.24±249.12 pg/mL (p<0.001) but also 98% of those under chronic treatment showed an increase of 2139.2 ±804.72 to 9168.11±1657.27 pg/ mL (p<0.001). Plasma DA response to Atremorine was in part associated with the APOE genotype where APOE-carriers showed a stronger response than APOE-3>APOE-4 carriers (93).
Ultrasound treatment
Nowadays, neurodegenerative diseases exist as an increasing challenge for people related to ageing. The pharmacological interventions are routinely followed for neurological diseases, unlike for cancers where they carry out non-pharmaceutical procedures. The use of ultrasound treatment for this Parkinson disease proved to be useful for instigating focused lesions, regulating neuronal function, eliminating protein aggregates, etc. (94).
Active immunization therapy
Vaccination is being scrutinized to be the prospective or possible treatment for Parkinson disease. This vaccination found to be a better option for these neurological diseases because of the unusual administration, less production costs for the huge amount of people, etc. In preclinical animal models of previous decade, there was progress in the active immunization against alpha-synuclein (95).
Rehabilitation
Other than pharmacological and surgery treatments, rehabilitation act as an adjuvant for less complications and maximize functional ability in Parkinson disease. When compared to physiotherapy, virtual reality technology leads to much improvement. It is a new rehabilitation tool where it revives the movement by computer based in a virtual reality environment. (97) A recent meta-analysis report identified that rehabilitation could instigate short-lasting, but significant benefits for gait and balance. But rehabilitation program should be organized as goal-based, where number of variables has to be identified and program should be made according to the individual’s characteristics (96) (Fig. 5).
Figure 5.
Administration of drug and management of PD
Conclusion
This review highlights that there is a paucity of information about PD worldwide. There are very few research groups working on neurodegenerative type of disorders. Diagnosis is of paramount importance for clinical manifestation and treatment strategies for PD. Medication and routine exercise, is primary to treatment strategies for this neurodegenerative disease. The social and psychological issues in PD affected patients should also be considered and might vary in individual patients. Therapies, such as deep brain stimulation and surgical lesioning ought to be explored. Further research should be encouraged for the better understanding of the disease involving its characteristics and etiology. Future scientific research involving Parkinson’s disease might enlighten our knowledge of disease onset and progression and can deliver some added aspects/components to help find more effective therapies to improve quality of life of patients with PD.
Acknowledgments
The authors are thankful to Chettinad Academy of Research and Education (CARE), Chennai, India.
Funding Sources:
This study was financially supported by grants Chettinad Academy of Research and Education, Chennai, India.
Authors’ Contributions: The study was designed by SP, RM and FM. JAJ, MM, VS, VPM, AK, AB, AR, SS, MG wrote the manuscript. All authors have read and approved the final manuscript.
References
- 1.Josephs KA, Matsumoto JY, Ahlskog JE. Benign tremulous Parkinsonism. Archives of Neurology. 2006;63(3):354–357. doi: 10.1001/archneur.63.3.354. [DOI] [PubMed] [Google Scholar]
- 2.Booby Beal. Parkinson’s diseases. Human molecular genetics. 2007;16(2):183–194. [Google Scholar]
- 3.Schapira AH V. Science, medicine, and the future: Parkinson’s disease. British Medical Journal. 1999;318(7179):311. doi: 10.1136/bmj.318.7179.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ene M. Neural network-based approach to discriminate healthy people from those with Parkinson’s disease. Annals of the University of Craiova-Mathematics and Computer Science Series. 2008;35:112–116. [Google Scholar]
- 5.Kostek B, Kaszuba K, Zwan P, Robowski P, Slawek J. Automatic assessment of the motor state of the Parkinson’s disease patient-a case study. Diagnostic pathology. 2012;7(1):1. doi: 10.1186/1746-1596-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian XW, Lim JS, Zhang ZX, Kim YG, Lee HY, Lee SH. Minimum Feature Selection for Telemonitoring of Parkinson’s Disease. Int Conf on Computer Science and Information Technology, Pattaya. 2011:33–36. [Google Scholar]
- 7.Gil D, Johnson M. Diagnosing Parkinson by using artificial neural networks and support vector machines. Global Journal of Computer Science and Technology. 2009;9(4):63–71. [Google Scholar]
- 8.Janakiraman U, Manivasagam T, Thenmozhi AJ, et al. Influences of Chronic Mild Stress Exposure on Motor, Non-Motor Impairments and Neurochemical Variables in Specific Brain Areas of MPTP/Probenecid Induced Neurotoxicity in Mice. PloS one. 2016;11(1):e0146671. doi: 10.1371/journal.pone.0146671. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Modugno N, Lena , Di Biasio F, Cerrone G, Ruggieri S, Fornai F. A clinical overview of non-motor symptoms in Parkinson’s disease. Archives italiennes de biologie. 2013;151(4):148–168. [PubMed] [Google Scholar]
- 10.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing research reviews. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Movement Disorders. 2009;24(15):2175–2186. doi: 10.1002/mds.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz CG, Tanner CM, Penn RD, et al. Adrenal medullary transplant to the striatum of patients with advanced Parkinson’s disease 1-year motor and psychomotor data. Neurology. 1990;40(2):273–273. doi: 10.1212/wnl.40.2.273. [DOI] [PubMed] [Google Scholar]
- 13.Treves TA, Rabey JM, Korczyn AD. Case-control study, with use of temporal approach, for evaluation of risk factors for Parkinson’s disease. Movement Dis. 1990;5(11) [Google Scholar]
- 14.Smith AD, Castro SL, Zigmond MJ. Stress-induced Parkinson’s disease: a working hypothesis. Physiol Behav. 2002;77:527–531. doi: 10.1016/s0031-9384(02)00939-3. PMID: 12526994. [DOI] [PubMed] [Google Scholar]
- 15.Smith LK, Jadavji NM, Colwell KL, Katrina Perehudoff S, Metz G A. Stress accelerates neural degeneration and exaggerates motor symptoms in a rat model of Parkinson’s disease. European Journal of Neuroscience. 2008;27(8):2133–2146. doi: 10.1111/j.1460-9568.2008.06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tien I, Glaserj SD, Aminoff MJ. Characterization of gait abnormalities in Parkinson’s disease using a wireless inertial sensor system. In 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. 2010:3353–3356. doi: 10.1109/IEMBS.2010.5627904. IEEE. [DOI] [PubMed] [Google Scholar]
- 17.Nocera J, Horvat M, Ray CT. Effects of home-based exercise on postural control and sensory organization in individuals with Parkinson disease. Parkinsonism and related disorders. 2009;15(10):742–745. doi: 10.1016/j.parkreldis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kara B, Genc A, Colakoglu BD, Cakmur R. The effect of supervised exercises on static and dynamic balance in Parkinson’s disease patients. NeuroRehabilitation. 2012;30(4):351–357. doi: 10.3233/NRE-2012-0766. [DOI] [PubMed] [Google Scholar]
- 19.Shahed J, Jankovic J. Exploring the relationship between essential tremor and Parkinson’s disease. Parkinsonism and related disorders. 2007;13(2):67–76. doi: 10.1016/j.parkreldis.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 20.Broussolle E, Krack P, Thobois S, et al. Contribution ofJules Froment to the study of parkinsonian rigidity. Movement Disorders. 2007;22(7):909–914. doi: 10.1002/mds.21484. [DOI] [PubMed] [Google Scholar]
- 21.Riley D, Lang AE, Blair RD, Birnbaum A, Reid B. Frozen shoulder and other shoulder disturbances in Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1989;52(1):63–66. doi: 10.1136/jnnp.52.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson’s disease. Brain. 2001;124(11):2131–2146. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- 23.Bronte-Stewart HM, Minn AY, Rodrigues K, Buckley EL, Nashner LM. Postural instability in idiopathic Parkinson’s disease: the role of medication and unilateral pallidotomy. Brain. 2002;125(9):2100–2114. doi: 10.1093/brain/awf207. [DOI] [PubMed] [Google Scholar]
- 24.Adkin AL, Frank JS, Jog MS. Fear of falling and postural control in Parkinson’s disease. Movement Disorders. 2003;18(5):496–502. doi: 10.1002/mds.10396. [DOI] [PubMed] [Google Scholar]
- 25.Shulman LM, Taback RL, Rabinstein AA, Weiner WJ. Non-recognition of depression and other non-motor symptoms in Parkinson’s disease. Parkinsonism and related disorders. 2002;8(3):193–197. doi: 10.1016/s1353-8020(01)00015-3. [DOI] [PubMed] [Google Scholar]
- 26.Ha AD, Jankovic J. Pain in Parkinson’s disease. Movement Disorder. 2012;27(4):485–491. doi: 10.1002/mds.23959. [DOI] [PubMed] [Google Scholar]
- 27.Chaudhuri KR. Nocturnal symptom complex in PD and its management. Neurology. 2003;61(6 suppl 3):S17–S23. doi: 10.1212/wnl.61.6_suppl_3.s17. [DOI] [PubMed] [Google Scholar]
- 28.Eichhorn TE, Oertel WH. Macrogol. 3350/electrolyte improves constipation in Parkinson’s disease and multiple system atrophy. Movement Disorders. 2001;16(6):1176–1177. doi: 10.1002/mds.1211. [DOI] [PubMed] [Google Scholar]
- 29.Emre M. Dementia associated with Parkinson’s disease. The Lancet Neurology. 2003;2(4):229–237. doi: 10.1016/s1474-4422(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 30.de Rijk MD, Tzourio C, Breteler MM, et al. Prevalence of parkinsonism and Parkinson’s disease in Europe: the EURO PARKINSON Collaborative Study. European Community Concerted Action on the Epidemiology of Parkinson’s disease. Journal of Neurology, Neurosurgery & Psychiatry. 1997;62(1):10–15. doi: 10.1136/jnnp.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hughes AJ, Daniel SE, Blankson S, Lees A J. A clinicopathologic study of 100 cases of Parkinson’s disease. Archives of Neurology. 1993;50(2):140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 32.Van Laar AD, Jain S. Non-motor symptoms of Parkinson disease: Update on the diagnosis and treatment. The neurologist. 2004;10(4):185. [Google Scholar]
- 33.Suchowersky O, Reich S, Perlmutter J, Zesiewicz T, Gronseth G, Weiner WJ. Practice Parameter: Diagnosis and prognosis of new onset Parkinson disease (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;66(7):968–975. doi: 10.1212/01.wnl.0000215437.80053.d0. [DOI] [PubMed] [Google Scholar]
- 34.Marek K, Jennings D, Seibyl J. Imaging the dopamine system to assess disease-modifying drugs Studies comparing dopamine agonists and levodopa. Neurology. 2003;61(6):S43–S48. doi: 10.1212/wnl.61.6_suppl_3.s43. [DOI] [PubMed] [Google Scholar]
- 35.Mayeux R, Chen J, Mirabello E, Marder K, Bell K, Dooneief G, Stern Y. An estimate of the incidence of dementia in idiopathic Parkinson’s disease. Neurology. 1990;40(10):1513–1513. doi: 10.1212/wnl.40.10.1513. [DOI] [PubMed] [Google Scholar]
- 36.Marzano C, Ferrara M, Mauro F, Moroni F, Gorgoni M, Tempesta D, De Gennaro L. Recalling and forgetting dreams: theta and alpha oscillations during sleep predict subsequent dream recall. Official Journal of the Society for Neuroscience. 2011;31(18):6674–6683. doi: 10.1523/JNEUROSCI.0412-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aarsland D, Tandberg E, Larsen JP, Cummings JL. Frequency of dementia in Parkinson disease. Archives of Neurology. 1996;53(6):538–542. doi: 10.1001/archneur.1996.00550060082020. [DOI] [PubMed] [Google Scholar]
- 38.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Archives of Neurology. 1993;50(2):140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 39.Clarke CE, Davies P. Systematic review of acute levodopa and apomorphine challenge tests in the diagnosis of idiopathic Parkinson’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2000;69(5):590–594. doi: 10.1136/jnnp.69.5.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piccini P, Brooks DJ. New developments of brain imaging for Parkinson’s disease and related disorders. Movement Disorders. 2006;21(12):2035–2041. doi: 10.1002/mds.20845. [DOI] [PubMed] [Google Scholar]
- 41.Brooks DJ, Ibanez V, Sawle GV, et al. Striatal D2 receptor status in patients with Parkinson’s disease, striatonigral degeneration, and progressive supranuclear palsy, measured with 11 C-raclopride and positron emission tomography. Annals of neurology. 1992;31(2):184–192. doi: 10.1002/ana.410310209. [DOI] [PubMed] [Google Scholar]
- 42.Marek KL, Seibyl JP, Zoghbi SS, et al. [123I] beta-CIT/ SPECT imaging demonstrates bilateral loss of dopamine transporters in hemi-Parkinson’s disease. Neurology. 1996;46:231–7. doi: 10.1212/wnl.46.1.231. [DOI] [PubMed] [Google Scholar]
- 43.Simons G, Thompson SB, Pasqualini MCS. An innovative education programme for people with Parkinson’s disease and their carers. Parkinsonism and related disorders. 2006;12(8):478–485. doi: 10.1016/j.parkreldis.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Damiano AM, Snyder C, Strausser B, Willian MK. A review of health-related quality-of-life concepts and measures for Parkinson’s disease. Quality of Life Research. 1999;8(3):235–243. doi: 10.1023/a:1008823222574. [DOI] [PubMed] [Google Scholar]
- 45.Warren NM, Piggott MA, Greally E, Lake M, Lees AJ, Burn DJ. Basal ganglia cholinergic and dopaminergic function in progressive supranuclear palsy. Movement Disorders. 2007;22(11):1594–1600. doi: 10.1002/mds.21573. [DOI] [PubMed] [Google Scholar]
- 46.Walter U, Niehaus L, Probst T, et al. Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology. 2003;60:74–7. doi: 10.1212/wnl.60.1.74. [DOI] [PubMed] [Google Scholar]
- 47.Stockner H, Sojer M, et al. Midbrain sonography in patients with essential tremor. Mov Disord. 2007;22:414–7. doi: 10.1002/mds.21344. [DOI] [PubMed] [Google Scholar]
- 48.De Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. The Lancet Neurology. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 49.Burke RE, O’Malley K. Axon degeneration in Parkinson’s disease. Experimental Neurology. 2013;246:72–83. doi: 10.1016/j.expneurol.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little S, Brown P. The functional role of beta oscillations in Parkinson’s disease. Parkinsonism and Related Disorders. 2014;20(1):44–8. doi: 10.1016/S1353-8020(13)70013-0. [DOI] [PubMed] [Google Scholar]
- 51.Florin E, Erasmi R, Reck C, Maarouf M, Schnitzler A, Fink GR, Timmermann L. Does increased gamma activity in patients suffering from Parkinson’s disease counteract the movement inhibiting beta activity? Neuroscience. 2013;23:42–50. doi: 10.1016/j.neuroscience.2013.01.051. [DOI] [PubMed] [Google Scholar]
- 52.Weinberger M, Mahant N, Hutchison WD, et al. Beta oscillatory activity in the subthalamic nucleus and its relation to dopaminergic response in Parkinson’s disease. Journal of Neurophysiology. 2006;96(6):3248–3256. doi: 10.1152/jn.00697.2006. [DOI] [PubMed] [Google Scholar]
- 53.Pahwa R, Lyons KE. Early diagnosis of Parkinson’s disease: Recommendations from diagnostic clinical guidelines. The American Journal of Managed Care. 2010;16(I):S94–S99. [PubMed] [Google Scholar]
- 54.Gunn DG, Naismith SL, Lewis SJG. Sleep disturbances in Parkinson disease and their potential role in heterogeneity. Journal of Geriatric Psychiatry and Neurology. 2010;23(2):131–137. doi: 10.1177/0891988709358591. [DOI] [PubMed] [Google Scholar]
- 55.Lindgren HS, Dunnett SB. Cognitive dysfunction and depression in Parkinson’s disease: what can be learned from rodent models? The European Journal of Neuroscience. 2012;35(12):1894–1907. doi: 10.1111/j.1460-9568.2012.08162.x. [DOI] [PubMed] [Google Scholar]
- 56.Bonnet AM, Jutras MF, Czernecki V, Corvol JC, Vidailhet M. Nonmotor symptoms in Parkinson’s disease in 2012: Relevant clinical aspects. Parkinson ’s disease. 2012:1–15. doi: 10.1155/2012/198316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siderowf A. Update on pakinsons disease. Ann Intern med. 2003;138(8):651–8. doi: 10.7326/0003-4819-138-8-200304150-00013. [DOI] [PubMed] [Google Scholar]
- 58.Stern M. Update on pakinsons disease. Ann Intern med. 2003;138(8):651–8. doi: 10.7326/0003-4819-138-8-200304150-00013. [DOI] [PubMed] [Google Scholar]
- 59.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302(5646):819–22. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 60.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 61.Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Annals of neurology. 2003;53(S3):S16–S25. doi: 10.1002/ana.10487. [DOI] [PubMed] [Google Scholar]
- 62.De Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. The Lancet Neurology. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 63.Alves G, Müller B, Herlofson K, et al. Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80(8):851–857. doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- 64.Tanner CM, Aston DA. Epidemiology of Parkinson’s disease and a kinetic syndromes. Current opinion in neurology. 2000;13(4):427–430. doi: 10.1097/00019052-200008000-00010. [DOI] [PubMed] [Google Scholar]
- 65.De Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. The Lancet Neurology. 2006;5(6):525–535. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 66.Thacker EL, Ascherio A. Familial aggregation of Parkinson’s disease: A meta-analysis. Movement Disorders. 2008;23(8):1174–1183. doi: 10.1002/mds.22067. [DOI] [PubMed] [Google Scholar]
- 67.Weiner WJ. There is no Parkinson disease. Archives of Neurology. 2008;65(6):705–708. doi: 10.1001/archneur.65.6.705. [DOI] [PubMed] [Google Scholar]
- 68.Stephenson R, Siderowf A, Stern MB. Premotor Parkinson’s disease: clinical features and detection strategies. Movement Disorders. 2009;24((S2)):S665–S670. doi: 10.1002/mds.22403. [DOI] [PubMed] [Google Scholar]
- 69.Tarazi FI, Sahli ZT, Wolny M, Mousa SA. Emerging therapies for Parkinson’s disease: From bench to bedside. Pharmacology and Therapeutics. 2014;144(2):123–133. doi: 10.1016/j.pharmthera.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Kalinderi K, Fidani L, Katsarou Z, Bostantjopoulou S. Pharmacological treatment and the prospect of pharmacogenetics in Parkinson’s disease. International Journal of Clinical Practice. 2011;65(12):1289–1294. doi: 10.1111/j.1742-1241.2011.02793.x. [DOI] [PubMed] [Google Scholar]
- 71.Aquino CC, Fox SH. Clinical spectrum of levodopa-induced complications. Movement Disorders. 2015;30(1):80–89. doi: 10.1002/mds.26125. [DOI] [PubMed] [Google Scholar]
- 72.Poewe W, Mahlknecht P. The clinical progression of Parkinson’s disease. Parkinsonism & Related Disorders. 2009;15(4):S28–S32. doi: 10.1016/S1353-8020(09)70831-4. [DOI] [PubMed] [Google Scholar]
- 73.Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson’s disease: Direct measurements of trunk and hip torque. Experimental Neurology. 2007;208(1):38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA: The Journal of the American Medical Association. 2014;311(16):1670–83. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 75.Maillet A, Pollak P, Debu B. Imaging gait disorders in parkinsonism: A review. Journal of Neurology, Neurosurgery and Psychiatry. 2012;83(10):986–993. doi: 10.1136/jnnp-2012-302461. [DOI] [PubMed] [Google Scholar]
- 76.Ferrara J, Diamond A, Hunter C, Davidson A, Almaguer M, Jankovic J. Impact of STN-DBS on life and health satisfaction in patients with Parkinson’s disease. Journal of Neurology, Neurosurgery, and Psychiatry. 2010;81(3):315–319. doi: 10.1136/jnnp.2009.184127. [DOI] [PubMed] [Google Scholar]
- 77.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: late (complicated) Parkinson’s disease. European Journal of Neurology. 2006;13(11):1186–1202. doi: 10.1111/j.1468-1331.2006.01548.x. [DOI] [PubMed] [Google Scholar]
- 78.Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Olanow CW, Agid Y, Mizuno Y, et al. Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord. 2004;19:997–1005. doi: 10.1002/mds.20243. [DOI] [PubMed] [Google Scholar]
- 80.Van Camp G, Flamez A, Cosyns B, et al. Treatment of Parkinson’s disease with pergolide and relation to restrictive valvular heart disease. Lancet. 2004;363:1179–1183. doi: 10.1016/S0140-6736(04)15945-X. [DOI] [PubMed] [Google Scholar]
- 81.Ives NJ, Stowe RL, Marro J, et al. Monoamine oxidase type B inhibitors in early Parkinson’s disease: metaanalysis of 17 randomised trials involving 3525 patients. BMJ. 2004;329:593. doi: 10.1136/bmj.38184.606169.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ben-Shlomo Y, Churchyard A, Head J, et al. Investigation by Parkinson’s Disease Research Group of United Kingdom into excess mortality seen with combined levodopa and selegiline treatment in patients with early, mild Parkinson’s disease: further results of randomised trial and confidential inquiry. BMJ. 1998;316:1191–1196. doi: 10.1136/bmj.316.7139.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brooks DJ, Leinonen M, Kuoppamaki M, Nissinen H. Five-year efficacy and safety of levodopa/DDCI and entacapone in patients with Parkinson’s disease. J Neural Transm. 2008;115:843–849. doi: 10.1007/s00702-008-0025-8. [DOI] [PubMed] [Google Scholar]
- 84.Leegwater-Kim J, Waters C. Tolcapone in the management of Parkinson’s disease. Expert Opin Pharmacother. 2006;7:2263–2270. doi: 10.1517/14656566.7.16.2263. [DOI] [PubMed] [Google Scholar]
- 85.Searles Nielsen S, Franklin GM, Longstreth WT, Swanson PD, Checkoway H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann. Neurol. 2013;74:472–477. doi: 10.1002/ana.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shaltiel-Karyo R, Frenkel-Pinter M, Rockenstein E, Patrick C, Levy-Sakin M, Schiller A. A blood-brain barrier (BBB) disrupter is also a potent alpha-synuclein (alpha-syn) aggregation inhibitor: a novel dual mechanism of mannitol for the treatment of Parkinson disease (PD) J. Biol. Chem. 2013;288:17579–17588. doi: 10.1074/jbc.M112.434787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanaka K, Miyake Y, Fukushima W, Sasaki S, Kiyohara C, Tsuboi Y. Intake of Japanese and Chinese teas reduces risk of Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:446–450. doi: 10.1016/j.parkreldis.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Chan DK, Woo J, Ho SC, Pang CP, Law LK, Ng PW. Genetic and environmental risk factors for Parkinson’s disease in a Chinese population. J Neurol Neurosurg Psychiatry. 1998;65:781–784. doi: 10.1136/jnnp.65.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 90.Kyrozis A, Ghika A, Stathopoulos P, Vassilopoulos D, Trichopoulos D, Trichopoulou A. Dietary and lifestyle variables in relation to incidence of Parkinson’s disease in Greece. Eur J Epidemiol. 2013;28:67–77. doi: 10.1007/s10654-012-9760-0. [DOI] [PubMed] [Google Scholar]
- 91.Cacabelos R. Bioactive extract obtained from Viciafaba and its use in the treatment and/or prevention of neurodegenerative diseases. European Patent. 2016:EP16382138. [Google Scholar]
- 92.Carrera I, Fernández-Novoa L, Sampedro C, Aliev G, Cacabelos R. Dopaminergic neuroprotection with atremorine in Parkinson’s disease. Current Medicinal Chemistry. 2016;2:36–44. doi: 10.2174/0929867325666180410100559. [DOI] [PubMed] [Google Scholar]
- 93.Cacabelos R, Fernández-Novoa L, Alejo R, et al. E-PodoFavalin-15999 (Atremorine®)-Induced Dopamine Response in Parkinson’s Disease: Pharmacogenetics-Related Effect. J Genomic Med Pharmacogenomics. 2016;1:1–26. [Google Scholar]
- 94.Leinenga, et al. Ultrasound treatment of neurological diseases-current and emerging applications. Nat Rev Neurol. 2016;12(3):161–74. doi: 10.1038/nrneurol.2016.13. [DOI] [PubMed] [Google Scholar]
- 95.Schneeberger A, et al. Active immunization therapies for Parkinson’s disease and multiple system atropy. Mov Disord. 2016;31(2):214–24. doi: 10.1002/mds.26377. [DOI] [PubMed] [Google Scholar]
- 96.Abbruzzese G, et al. Rehabilitation for Parkinson’s disease: Current outlook and future challenges. Parkinsonism Relat Disord. 2016;1:S60–4. doi: 10.1016/j.parkreldis.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Kim Dockx, et al. Virtual reality for rehabilitation in Parkinson’s disease Cochrane Database of Systematic Reviews. 2016 doi: 10.1002/14651858.CD010760.pub2. Issue 12. Art. No.: CD010760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]


