Abstract
Taxanes, including paclitaxel and docetaxel, are one of the most active cytotoxic agents in breast cancer treatment including Her-2 positive subtype characterized by aggressive clinical and pathological features since the early stage. However, their use is sometimes limited by the occurrence of hypersensivity reactions (HSRs) characterized by erythematous rashes, bronchospasm, respiratory distress, hypotension, and pulmonary edema. Cross-reactions between paclitaxel and docetaxel are described in literature with a rate ranging from 49% to 90%. Abraxane (nab-paclitaxel), an albumin-bound form of paclitaxel, has a different toxicity profile from solvent-based paclitaxel and a lower rate of HSRs. Interestingly, several authors have recently reported cases of patients who developed HSRs to taxanes, principally paclitaxel, and were then safety treated with Abraxane, suggesting the absence of cross-reactivity between these drugs. Based on these considerations, we report our clinical experience and perform a literature review on this topic with the aim to investigate the cross-reactivity between nab-paclitaxel and other taxanes, in particular with docetaxel. (www.actabiomedica.it)
Keywords: hypersensivity reaction, breast cancer, nab-paclitaxel, neoadjuvant chemotherapy
Introduction
Taxanes, including paclitaxel and docetaxel, are one of the most active cytotoxic agents in breast cancer treatment (1-3). However their use is sometimes limited by the occurrence of hypersensivity reactions (HSRs) characterized by erythematous rashes, bronchospasm, respiratory distress, hypotension and pulmonary edema (4). The aetiology of HSRs to paclitaxel (P) and docetaxel (DOC) is poorly understood; some authors suggested it could be due to their solvents, Cremophor EL and polysorbate80, combined with paclitaxel and docetaxel, respectively (5). Hypersensitivity reactions occur in 10% of the patients receiving paclitaxel treatment with an incidence of severe events ranging from 2 to 5%, despite the use of premedication with dexamethasone and histamine receptor antagonists (6). According to Vasey et al (7), in a Taxane-naïive population, the incidence of allergic reactions to docetaxel is lower than 2%. Other authors suggested that HSR might be due to the direct effect of the taxane itself (8), not to its diluents, partially explaining the cross-reactivity between paclitaxel and docetaxel described in literature. Dizon et al. (9) performed a retrospective analysis of 10 patients who developed HSRs to paclitaxel; then they were treated with docetaxel and a cross-reactivity rate of 90% was registered. In a retrospective study conducted by Sánchez-Muñoz et al (10), 41% of patients had severe cross-HSRs (grade 3-4) between the two taxanes that led to a permanently discontinuation of the treatment, despite an adequate premedication and a prolonged infusion time. Abraxane, an albumin-bound form of paclitaxel, has a different toxicity profile from solvent-based paclitaxel and a lower rate of HSRs (11). This formulation delivers paclitaxel as a suspension of albumin nanoparticles in saline, avoiding the use of Cremophor EL, premedication and special infusion sets. Interestingly, several authors have recently reported cases of patients who had HSRs to taxanes, principally paclitaxel, and then were safety treated with Abraxane (1, 4, 5), suggesting the absence of cross-reactivity between these drugs. Based on these considerations, we report our clinical experience and perform a literature review on this topic with the aim to investigate the cross-reactivity between nab-paclitaxel and other taxanes, in particular with docetaxel.
Case Report
In March 2016, a 65-years-old woman was diagnosed with a left breast ductal carcinoma. On the 6th April 2016, she underwent left para-central quadrantectomy; the sentinel node biopsy was negative. The histological exam confirmed infiltrating ductal carcinoma G3, 1,8 cm of diameter, estrogen receptor (ER) negative, progesterone receptor (PgR) negative, Her-2 positive, ki67=35% without lymphvascular infiltration. The staging CT-scan showed multiple axillar lymphadenopathies; a fine needle biopsy confirmed they were Her-2 positive breast cancer metastases. On the 6th of May 2016, she started a neoadjuvant chemo-immunotherapic treatment with Herceptin 560 mg, cyclophosphamide 1000 mg and threeweekly docetaxel 130 mg; she further developed afebrile grade 4 prolonged neutropenia so prophylactic G-CSF and antibiotic prophylaxis were administered. On the 27th of May 2016, during her second infusion of docetaxel, she started to complain itchy hands and feet, generalized weakness and thoracic oppression; the infusion was suddenly discontinued with symptoms regression. Docetaxel was replaced by weekly nab-paclitaxel and the patient was planned to receive two cycles of Abraxane 100 mg/m2 IV on days 1, 8, and 15, Herceptin 6mg/kg every 21 days and cyclophosphamide 600 mg/m2 IV on day 1 every 21 days. After 3 cycles of treatment, she achieved a complete metabolic response and not allergic reaction nor neutropenia have been registered. At the end of the chemo-immunotherapic treatment, she underwent axillary dissection and a pathological complete response was recorded.
Discussion and literature review
Taxanes, including paclitaxel and docetaxel, are one of the most active drugs in breast cancer including Her-2 positive subtype (2), characterized by aggressive clinical and pathological features since the early stage (12). Unfortunately, their use is sometimes limited by the onset of HSR but Abraxane, an albumin-bound form of paclitaxel, seems to induce a lower rate of HRSs (11). Several studies explored the role of nab-paclitaxel in the neo-adjuvant setting for HER2-overexpressing breast cancer (13). A recent phase II study of preoperative nab-paclitaxel (260 mg/m2 q2w) followed by vinorelbine plus trastuzumab in HER2 positive breast cancer (N=27) reported a pathological Complete Response (pCR) of 48 % (14). Sub-analysis by Hormone Receptor (HR) status showed a pCR of 18% in patients with ER+/PgR+ disease and of 69 % in patients with ER-/PgR-disease. Six patients had grade 2/3 neuropathy, with no grade 4 neuropathy reported; no severe hypersensitivity reactions were recorded. Similarly, another phase II trial of neoadjuvant anthracycline followed by nabpaclitaxel (260 mg/m2 q3w) plus trastuzumab reported 49 % pCR in the group of operable HER2+ breast cancer (N=46) (15). A pCR of 71 % was achieved in cases with ER-disease compared with 36 % in ER + disease; no severe hypersensitivity reactions were recorded. The efficacy and safety of nab-paclitaxel in combination with HER2-targeted therapy were also evaluated in the adjuvant setting (16). In a pilot single-arm trial, Yardley et al. (17) evaluated the feasibility and toxicity of a nab-paclitaxel-containing adjuvant regimen in patients with early breast cancer. Sixty-three patients received nab-paclitaxel 100 mg/ m2 IV on days 1, 8, and 15 and cyclophosphamide 600 mg/m2 IV on day 1 every 21 days for 4 cycles. Trastuzumab was administered to patients with HER2+ tumors: 8 mg/kg on day 1, cycle 1, followed by 6 mg/ kg every 21 days for a total of 52 weeks. The regimen was well tolerated and full doses of all agents were administered in >90% of cycles. Thirty-three patients had grade 3/4 neutropenia; however only one patient developed febrile neutropenia. No severe hypersensitivity reactions were observed. With a median follow-up of 17.8 months, all 63 patients remained alive and no evidence of disease recurrence was observed. Standing the efficacy of Abraxane in this subset of patients, we performed a literature review, according to PRISMA guidelines (18), to explore the crossreactivity rate between nab-paclitaxel and docetaxel. The database searched was MEDLINE (2006 to 19th June 2016) and the research was complemented by additional sources, including Google Scholar and the original website of the journals where the papers were published. The search items were “nabpaclitaxel after taxane allergic reaction”; “nabpaclitaxel taxane allergic reaction”; “nab paclitaxel allergic reaction”; “nab paclitaxel allergy”; “abraxane after taxane allergic reaction”. Exclusion criteria were: articles not written in English, abstract only, not pertinent, review. The study population was limited to patients with previous hypersensivity reaction to docetaxel and HRSs to paclitaxel were excluded. Our search identified 32 abstracts and titles; 14 articles were excluded because not pertinent, 4 because not written in English and 11 because review articles. 4 articles with 9 patients’ reports were analysed: 7 patients were excluded because treated with paclitaxel and 1 because developed skin toxicity after docetaxel infusion and not HSR. Finally, only one patient met our inclusion criteria (Fig. 1). She was a 36-year-old female affected by locally advanced breast cancer (stage IIIC; T3N3bM0; ER-positive, PR-positive and HER2-negative) who underwent a pre-operative systemic therapy with 4 cycles of 5-fluorouracil (500 mg/m2), epirubicin (100 mg/m2) and cyclophosphamide (500 mg/m2) every 3 weeks, followed by 4 cycles of docetaxel (75 mg/ m2) every 3 weeks. Although dexamethasone (20 mg/ body) was used as premedication for docetaxel, she developed dyspnea and nausea 5 mins after the second administration. The infusion was suddenly stopped and, after 1 hour, the symptoms improved; subsequently, the regimen was adjusted to treatment with nab-paclitaxel. The patient was administered nab-paclitaxel (260 mg/m2) for 30 mins every 3 weeks for 3 cycles: she was premedicated with dexamethasone (8 mg) and she did not exhibit any HSRs. After this preoperative treatment, she experienced a clinically complete response and underwent a partial resection of the breast with axillary lymph node dissection. The case presented by Kimura et al (1) is similar to ours: both women were affected by locally advanced breast cancer and achieved a complete clinical response after the pre-operatory treatment. Conversely, our patient was affected by Her-2 positive breast cancer while Kimura’s one was affected by hormone-receptor positive, Her-2 negative breast cancer; the last was pretreated with anthracyclines and she did not receive Herceptin combined with docetaxel nor abraxane. Nab-paclitaxel was also administered with a different schedule: weekly and every three weeks, respectively. Both patients were pre-medicated with dexamethasone before the infusion of docetaxel and, interestingly, even if no symptoms were referred during and after their first administration, they developed a HRS during the second infusion of the taxane. Thus, these toxicities seemed to be correlated to an IgE-immune response against the specific drug (19), docetaxel, or one of its excipients or solvents, not to the mechanism of action of the taxane itself, partially explaining why patients did not react to abraxane.
Figure 1.
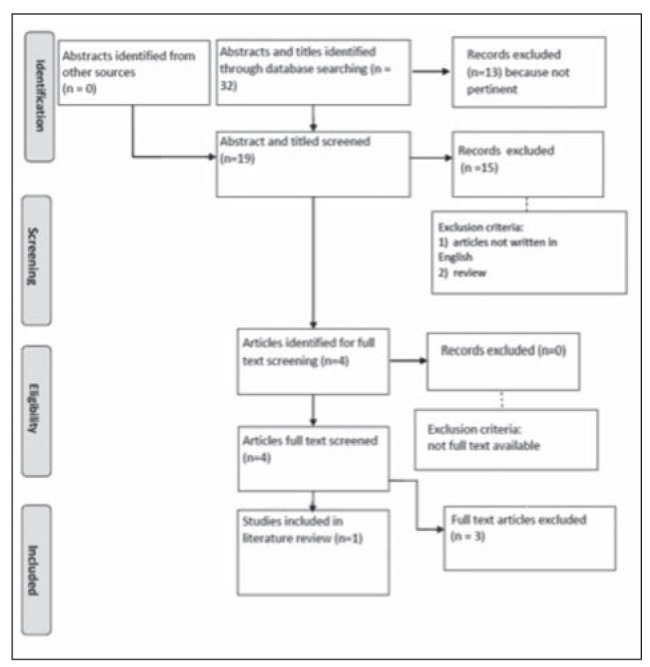
PRISMA flow diagram
Table 1.
Patients’ features
| Author | Kimura | Pellegrino |
| Age | 36 | 65 |
| Sex | Female | Female |
| Type of cancer | Breast | Breast |
| Stage of disease | III | III |
| Setting | Neoadjuvant | Neoadjuvant |
| Dose of docetaxel | 75 mg/m2 every 3 weeks | 75 mg/m2 every 3 weeks |
| Premedication | Dexamethasone 20 mg | Dexamethasone and antihistamines |
| Concomitant administration | None | Cyclophosphamide IV 1000 mg and Herceptin IV 560 mg |
| Number of previous cycles of docetaxel | 1 | 1 |
| Symptoms associated with HSR | Dyspnea and nausea | Itchy hands and feet, generalized weakness and thoracic oppression |
| Time from the allergic reaction to nab-paclitaxel infusion | 3 weeks | 3 weeks |
| Dose and schedule of nab-paclitaxel | 260 mg/m2 IV every 3 weeks | 100 mg/m2 IV on days 1, 8. and 15 |
| Premedication with nab-paclitaxel | Dexamethasone 8 mg | Dexamethasone 8 mg |
| Concomitant administration | None | Herceptin 6mg/kg IV every 21 days and cyclophosphamide 600 mg/m2 IV on day 1 every 21 days |
| Number of cycles of nab-paclitaxel administrated | 3 | 3 |
| Response to treatment | Complete clinical response | Complete clinical response |
Conclusion
Our clinical case suggests nab-paclitaxel can be a safe and efficient option in patients affected by Her-2 positive locally advanced breast cancer who developed a HRS to docetaxel; only one similar case is reported in literature and, for these reasons, further clinical trial should be encouraged to better investigate this topic.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Kimura K, Satoru T, Mitsuhiko I, et al. Safety of Nab-Ptx in breast cancer patients. Oncology Letters. 2013;6:881–4. doi: 10.3892/ol.2013.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Laurentiis M, Cancello G, D’Agostino D, et al. Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol. 2008;26:44–53. doi: 10.1200/JCO.2007.11.3787. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Burzykowski T, Buyse M, et al. Taxanes alone or in combination with anthracyclines as first-line therapy of patients with metastatic breast cancer. J Clin Oncol. 2008;26:1980–6. doi: 10.1200/JCO.2007.10.8399. [DOI] [PubMed] [Google Scholar]
- 4.Fader A, Rose P. Abraxane for the Treatment of Gynecologic Cancer Patients With Severe Hypersensitivity Reactions to Paclitaxel. Int J Gynecol Cancer. 2009;19:1281–3. doi: 10.1111/IGC.0b013e3181a38e2f. [DOI] [PubMed] [Google Scholar]
- 5.De Leon M, Bolla S, Greene B, et al. Successful treatment with nab-paclitaxel after hypersensitivity reaction to paclitaxel and docetaxel. Gynecologic Oncology Reports. 2013;5:70–1. doi: 10.1016/j.gynor.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon C, Verschraegen CF, Bevers M, et al. Use of docetaxel (Taxotere) in patients with paclitaxel (Taxol) hypersensitivity. Anticancer Drugs. 2000;11:565–8. doi: 10.1097/00001813-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Vasey PA, Atkinson R, Coleman R, et al. Docetaxel-carboplatin as first line chemotherapy for epithelial ovarian cancer. Br J Cancer. 2001;84:170–8. doi: 10.1054/bjoc.2000.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essayan DM, Kagey-Sobatka A, Colarusso PJ, et al. Successful parenteral desensitization to paclitaxel. J Allergy Clin Immunol. 1996;97:42–46. doi: 10.1016/s0091-6749(96)70281-6. [DOI] [PubMed] [Google Scholar]
- 9.Dizon DS, Schwartz J, Rojan A, et al. Cross-sensitivity between paclitaxel and docetaxel in a women’s cancers program. Gynecol Oncol. 2006;100:149–51. doi: 10.1016/j.ygyno.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Muñoz A, Jiménez B, García-Tapiador A, et al. Cross-sensitivity between taxanes in patients with breast cancer. Clin Transl Oncol. 2011;13:904–6. doi: 10.1007/s12094-011-0753-3. DOI 10.1007/ s12094-011-0753-3. [DOI] [PubMed] [Google Scholar]
- 11.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 12.Musolino A, Michiara M, Conti GM, et al. Human Epidermal Growth Factor Receptor 2 Status and Interval Breast Cancer in a Population-Based Cancer Registry Study. JCO. 2012;30 no. 192362-2368 doi: 10.1200/JCO.2011.37.6434. [DOI] [PubMed] [Google Scholar]
- 13.Ueno N, Mamounas E. Neoadjuvant nab-paclitaxel in the treatment of breast cancer. Breast Cancer Res Treat. 2016;156:427–40. doi: 10.1007/s10549-016-3778-z. DOI 10.1007/s10549-016-3778- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelnak AB, Leyland-Jones B, Gabram SG, et al. High pathologic complete response (pCR) in HER2-positive breast cancer to novel non-anthracycline neoadjuvant chemotherapy. Presented at American Association for Cancer Research [abstract 2703] 2012 [Google Scholar]
- 15.Tanaka S, Iwamoto M, Kimura K, et al. Phase II study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin Breast Cancer. 2015;15:191–6. doi: 10.1016/j.clbc.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Megerdichian C, Olimpiadi Y, Hurvitz S. Nab-Paclitaxel in combination with biologically targeted agents for early and metastatic breast cancer. Cancer Treatment Reviews. 2014;40:614–25. doi: 10.1016/j.ctrv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Yardley D, Burris 3rd H, Peacock N, et al. A pilot study of adjuvant nanoparticle albumin-bound (nab) paclitaxel and cyclophosphamide, with trastuzumab in HER2-positive patients, in the treatment of early-stage breast cancer. Breast Cancer Res Treat. 2010;123(2):471–5. doi: 10.1007/s10549-010-1047-0. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 19.Joerger M. Prevention and handling of acute allergic and infusion reactions in oncology. Ann Oncol. 2012;23(suppl 10) doi: 10.1093/annonc/mds314. 313-9.doi: 10.1093/annonc/mds314. [DOI] [PubMed] [Google Scholar]


