Abstract
To synchronize the onset of sexual maturity in the face of high BW variation, the age at photostimulation has been increasing in the broiler breeder industry. This experiment studied the effects of increased BW and earlier photostimulation on broiler breeder reproductive performance where within-treatment BW uniformity was very high. The experiment tested BW and age at photostimulation treatments in a 2 × 2 factorial arrangement. Hens (n = 120) were fed with a precision feeding system to allocate feed individually following the breeder-recommended target BW (Standard) or to a 22% heavier target BW curve reaching the Standard 21 wk BW at 18 wk (High). Hens were photostimulated at either 18 wk (18WK) or 21 wk (21WK) with a 16L:8D photoschedule. Age at first egg (AFE) and individual egg production to 55 wk were recorded. Differences were reported as significant if P ≤ 0.05. The AFE was decreased and maturation interval between photostimulation and AFE was shorter for hens on the High BW treatment compared to the Standard BW treatment (178.1 vs. 194.7 d and 41.8 vs. 58.2 d, respectively). Hens on the 21WK treatment had a decreased AFE compared to the 18WK treatment (177.0 d vs. 195.9 d) and their maturation interval was shorter (30.0 d vs. 69.9 d). The CV for AFE was higher in the 18WK treatment compared to the 21WK treatment (28.2% vs. 11.2%). Total egg production was higher for hens on the High BW treatment compared to the Standard BW treatment (129.4 vs. 92.8, respectively). Total egg production was higher for hens on the 21WK treatment compared to the 18WK treatment (138.4 vs. 83.8, respectively). Egg weight of Standard BW × 18WK hens was lower compared to High BW × 18WK hens. Current recommended breeder BW may be too low for optimal sexual maturation after photostimulation. It is concluded that even when BW variation is minimized, photostimulation at 18 wk of age is not recommended.
Keywords: chicken, egg production, reproduction, photorefractory, uniformity
INTRODUCTION
Broiler breeders are kept on a strict level of feed restriction to manage their reproductive performance. Every year the level of feed restriction becomes more severe, as genetic growth potential of broilers increases while the recommended broiler breeder BW profiles are not adjusted (Renema et al., 2007b). Especially during the rearing period when broiler breeders are most restricted, high competition for feed within broiler breeder flocks results in high BW variation. It is known that pullets that are underweight at photostimulation subsequently exhibit lower egg production (Robinson and Robinson, 1991; Melnychuk et al., 2004). Flocks with a high variation in BW exhibit low production efficiency as a high proportion of hens weigh less than the target at photostimulation. Therefore, it was previously recommended to delay the moment of photostimulation to 22 or 23 wk (Robinson et al., 1996; Renema et al., 2001a, 2007a). More recently, Pishnamazi et al. (2014) concluded that the beneficial effects of later photostimulation were only BW dependent, which would mean that accelerated growth could facilitate earlier photostimulation. Minimizing BW variation in hens photostimulated at wk 23 did not advance age at sexual maturity nor increase egg production (Romero et al., 2009a), yet it is unclear if at earlier photostimulation the same effect could be expected. If earlier photostimulation results in earlier age at sexual maturity, this would shorten the rearing period. If there would be no negative effects on settable egg production, shortening the rearing period would be economically beneficial as this shortens the period of no return or lengthen the productive period for hatching egg producers.
Several studies have suggested that there is a minimum age and a minimum BW for the ability to respond to photostimulation (photosensitivity) and sexually mature (Katanbaf et al., 1989; Lewis et al., 2007a). Lewis (2007a) showed that the minimum age after which broiler breeders can be photosensitive is 10 wk. Before this age, the onset of lay does not advance when hens are photostimulated and hens respond as if they are maintained on long days from hatch. After wk 24 broiler breeder pullets respond uniformly to photostimulation, irrespective of genetic line or feeding program (Melnychuk et al., 2004), indicating that all pullets have dissipated their photorefractory state. Accelerating growth advances the dissipation of the photorefractory state, so increasing target BW will result in earlier photosensitivity (Lewis et al., 2007b). As genetic selection for growth traits did not change time between photostimulation and age at first egg (Pishnamazi et al., 2014), advancing the age at photostimulation could be feasible if BW variation were to be controlled. Yuan et al. (1994) concluded that increased BW can facilitate advancing the onset of lay with earlier photostimulation; however, they noted that increased feed allowance would have to be continued during the laying period, to support the increased maintenance requirements of higher BW hens.
As recent developments in feeding technology have allowed group housed hens to be reared toward individual target BW with less than 2% CV for BW (Zuidhof et al., 2016, 2017), the aim of this research was to investigate the effects of BW and age at photostimulation on broiler breeder reproductive performance in group housed hens, when within-treatment variation in BW is minimized. It was hypothesized that hens following a higher BW profile would show faster dissipation of photorefractoriness at the same age at photostimulation and therefore show an increased egg production, due to a lengthened laying period because of an earlier onset of lay.
MATERIALS AND METHODS
Experimental Design
The animal protocol for the study was approved by the University of Alberta Animal Care and Use Committee for Livestock and followed principles established by the Canadian Council on Animal Care Guidelines and Policies (CCAC, 2009). The experiment was conducted as a 2 × 2 factorial arrangement of treatments with pullets being reared following the breeder-recommended target BW curve (Aviagen, 2016; Standard), or an accelerated target BW curve reaching the 21 wk BW at 18 wk (High), and photostimulated at either wk 18 (18WK) or wk 21 (21WK). As a result, the High target BW was 22% heavier than the Standard target BW at 21 wk of age. As birds were individually fed to achieve the defined BW treatments, each individual bird was considered to be one experimental unit.
Animals and Housing
The experimental protocol was similar to that previously described by van der Klein (2018). Briefly, Ross 708 broiler breeder chicks were provided by Aviagen (Huntsville, AL; n = 120) and were randomly allocated to one of 4 environmentally controlled rooms (30 chicks per room). Each room was equipped with a precision feeding (PF) station (Zuidhof et al., 2016, 2017), which controlled individual feed intake to achieve and adhere to the assigned target BW curves. Water was provided ad libitum during the entire experiment. From day 0 to 16, birds were trained to use the PF station and fed ad libitum. At day 16, birds were randomly assigned to either the Standard or High BW treatment, such that approximately half of the birds per room were assigned to either target BW curve. From day 16 onwards, all birds were fed individually and were allowed access to feed for a duration of 45 s when birds qualified to eat. Birds qualified to eat when their BW as measured by the PF station was lower than their treatment target BW. When their measured BW was equal to or higher than their treatment target, birds were ejected from the station and not provided access to feed. At the start of the experiment, pairs of rooms were randomly assigned to either a 18WK or 21WK photostimulation treatment. For the first 2 d, a 23L:1D photoschedule was used after which the light period was decreased by 2 h daily until 8L:16D and remained constant until photostimulation. Photostimulation was achieved in a single step to 16L:8D. The light source used was a 60% red, 20% green, and 20% blue LED light bulb (PGR-11, AgriLux, Cambridge, ON) set to provide 8 lux during the rearing phase and 25 lux during the production phase. For the first 3 wk, chicks received a standard wheat based starter diet (2,900 AME, 19% CP, 1.1% Ca). From wk 4 to 2 after photostimulation, pullets received a wheat and barley based grower diet (2,589 AME, 14.2% CP, and 0.9% Ca). From 2 wk after photostimulation to wk 34, hens received a wheat based peak layer diet (2,689 AME, 15.0% CP, and 3.3% Ca). From wk 35 to 55, hens received a wheat based post peak layer diet (2,682 AME 14.6% CP, and 3.3% Ca).
At wk 18 a nest box equipped with radio frequency identification (RFID) readers was installed in each room, which assigned eggs to individual hens. The day before photostimulation 3 roosters were added per room. Roosters were reared in a separate location under an 8L:16D photoschedule and fed toward the recommended target BW curve (Aviagen, 2016) using a PF station.
Data Collection
A detailed description of data collection methods can be found in van der Klein (2018). Briefly, the PF station recorded BW and feed intake individually on a per visit basis after individual feeding started. Because it would not be possible for floor eggs to be linked with individual hens, and hens on different BW treatments were housed in the same room, all hens were palpated daily via the cloaca to detect hard-shelled eggs in the shell gland. This was essential to measure age at first egg (AFE) and individual egg production from wk 20 to 36. As the majority of the birds on the 21WK treatment had entered lay by wk 36, from 36 wk onward, daily palpation was performed every second week. Eggs laid in the radio frequency identification-equipped nest boxes that could be traced to specific hens were weighed daily. Eggs between 40 and 90 g were included in statistical analysis for egg weight. Eggs weighing more than 90 g were considered double-yolked eggs and they were analyzed separately. The incidence of mortality (including culls) was recorded throughout the experiment. At wk 55, 16 hens per BW × photostimulation treatment were killed by cervical dislocation directly after lights turned on and dissected. The abdominal fat pad, full gastrointestinal tract (GIT), breast muscle (total weight of pectoralis major and pectoralis minor), heart, liver, oviduct (without content), and ovary weight were recorded. In addition, the number of yellow follicles larger than 10 mm (LYF) was recorded.
Statistical Analysis
All analysis of variance were conducted using the MIXED procedure of SAS (Version 9.4. SAS Institute Inc., Cary, NC, 2012). Pairwise differences between means were determined with the PDIFF option of the LSMEANS statement and were considered significant at P ≤ 0.05. Tukey's range test was used to compare treatment means. Hen was the experimental unit, except for cumulative hens in lay and percentage of hens that did not commence egg production before wk 55. For the latter, hens within each BW treatment within each chamber were randomly assigned to 1 of 3 groups, after which the parameters were calculated per group and group was used as experimental unit. The model used for the CV for BW, egg production, cumulative hens in lay, rate of lay, and egg weight data included BW treatment, age at photostimulation, and age as fixed effects and all 2- and 3-way interactions. Additional analysis for egg weight included BW at AFE as a covariate within the statistical model. Random variation due to hen was accounted for in all serial measurements. Rate of lay was calculated as the hen day egg production of those hens that had reached their AFE. Due to insufficient data points prior to 30 wk of age, egg weight was analyzed from wk 30 onward. The model used for cumulative feed intake (CFI), AFE, maturation interval, percentage of hens that did not commence egg production before wk 55, CV for AFE, CV for BW at AFE, and cumulative egg production data included BW treatment and age at photostimulation as fixed effects, and their interaction. Dissection data were reported as percentage of live BW to correct for BW variation within the BW treatment. The model used for the dissection data included BW treatment and age at photostimulation as fixed effects, and their interaction and a binary random effect, whether or not the hen had laid her first egg.
RESULTS AND DISCUSSION
BW, BW Variation, and CFI
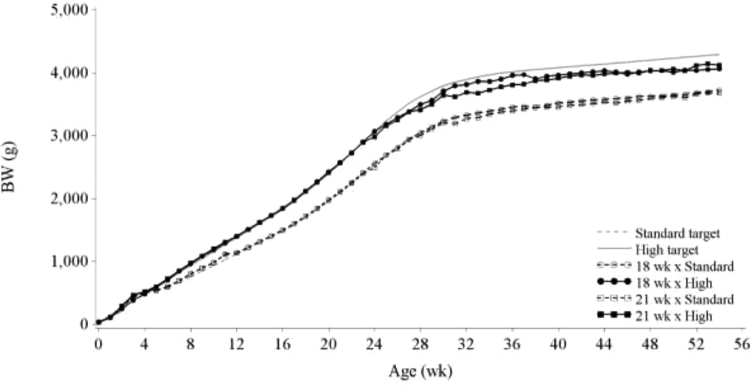
Actual Standard and High BW profiles closely matched their target profiles up to 24 wk of age (Figure 1). The CV in BW throughout the experiment is reported in Table 1. According to the analysis of variance, the CV in BW was dependent on age (P = 0.012) and on BW treatment (P < 0.001). As no significant pair-wise differences were indicated after Tukey's range test to compare LSMeans, Table 1 shows result of the least significant difference test. At both wk 18 and 21, the CV in BW in all treatments was less than 1%, which confirmed that the PF stations were able to minimize variation in BW at photostimulation. All High BW hens had reached the 21 wk breeder recommended target BW at wk 18. At wk 20, the BW of High BW hens was higher than Standard BW hens (P < 0.001) and there was no effect of age at photostimulation on BW. At wk 20, the High BW hens were 2,423 and 2,417 g, and Standard BW hens were 1,978 and 1,975 g (±21 g) on the 18WK and 21WK treatments, respectively. The CV in BW of the High BW treatment increased after photostimulation compared to the Standard BW treatment. As previously reported by van der Klein et al. (2017), the High BW treatment hens started laying earlier compared to hens on the Standard BW treatment and some High BW treatment hens sexually matured at a BW below their target BW (Table 3 and Figure 1). Some of the earlier laying hens remained at a BW lower than their target throughout the study, increasing the BW variability in the High BW treatment. The interaction between effect of age at photostimulation and BW on CFI was not significant during the rearing phase (P = 0.181), which means that there was no difference in CFI between hens reared toward the 21 wk BW at 18 wk or at 21 wk. As CFI was calculated from day 16 to photostimulation during rearing, CFI was lower in the 18WK compared to 21WK treatment (Table 2). During rearing, CFI was lower in the Standard treatment compared to the High BW treatment (P < 0.001), due to lower ME requirements for growth and maintenance. Although CFI during the laying phase was calculated over a 3-wk longer period for the 18WK treatment, CFI was 2,349 g lower in the 18WK treatment compared to the 21WK treatment. Presumably, the lower egg production in the 18WK treatments (see section below) compared to the 21WK treatment and the associated lower ME requirements for egg production accounted for this difference (Romero et al., 2009b). Mortality from day 16 to the end of the trial averaged 7.95%, and did not differ significantly between treatment groups (data not shown).
Figure 1.
BW of hens fed toward either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High) and photostimulated at wk 18 or 21.
Table 1.
Coefficient of variation for BW (BW CV) at various ages of hens fed with a precision feeding station toward a High and Standard BW1 curve and photostimulated (PS) at wk 18 or 21.
| –––––––––––––––––––– BW CV (%) –––––––––––––––––––––– | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | PS | Week 4 | Week 7 | Week 14 | Week 18 | Week 21 | Week 27 | Week 40 | Week 54 | Pooled SEM | |
| Wk2 | 5.7x | 2.4y,z | 0.3z | 0.4z | 0.9y,z | 2.8x-z | 3.5x,y | 3.7x,y | 1.0 | ||
| BW × wk | High | 7.9a | 2.1 | 0.3 | 0.4a | 0.9 | 4.4a | 5.4a | 6.0a | 1.1 | |
| Standard | 3.4b | 2.6 | 0.4 | 0.4a | 0.8 | 1.3b | 1.6b | 1.3b | |||
| PS × wk | 18 | 7.1 | 2.1 | 0.4 | 0.5 | 0.7 | 3.0 | 3.0 | 4.0 | 1.3 | |
| 21 | 4.2 | 2.7 | 0.3 | 0.3 | 1.0 | 2.7 | 4.0 | 3.3 | |||
| BW × PS × wk | High | 18 | 11.0a | 1.8 | 0.4 | 0.5 | 1.0 | 4.6 | 5.5a | 7.5a | 1.6 |
| 21 | 4.9b | 2.5 | 0.2 | 0.3 | 0.8 | 4.1 | 5.4a,b | 4.6a,b | |||
| Standard | 18 | 3.3b | 2.3 | 0.3 | 0.5 | 0.4 | 1.3 | 0.5b | 0.5b | ||
| 21 | 3.6b | 2.8 | 0.4 | 0.4 | 1.2 | 1.2 | 2.6a,b | 2.0b | |||
a,bLSMeans within a column and treatment group lacking a common superscript differ (P ≤ 0.05).
x–zLSMeans within a row lacking a common superscript differ (P ≤ 0.05).
1Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
2 P-values for the different sources of variation were as follows: wk, P = 0.012; BW, P < 0.001; PS, P = 0.686; BW × PS, P = 0.044; week × BW, P = 0.014; week × PS P = 0.895; week × BW × PS, P = 0.448.
Table 3.
Age at first egg (AFE)1, maturation interval (MI)1, percentage of hens that did not commence egg production before 55 wk (not laid), BW at AFE, coefficient of variation (CV) for AFE1, and coefficient of variation for BW at AFE1 of hens fed toward a High and Standard BW2 curve and photostimulated (PS) at wk 18 or 21.
| BW | PS | AFE (d) | SEM | MI (d) | SEM | Not laid (%) | SEM | BW at AFE (g) | SEM | AFE CV (%) | SEM | BW at AFE CV (%) | SEM | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | High | 178.1b | 5.3 | 41.8b | 5.3 | 5.8 | 5.15 | 3,157a | 54 | 16.2 | 3.46 | 11.7 | 0.90 | ||
| Standard | 194.7a | 5.8 | 58.2a | 5.8 | 17.6 | 2,824b | 59 | 23.2 | 13.2 | ||||||
| PS | 18 | 195.9a | 6.1 | 69.9a | 6.1 | 21.8a | 5.15 | 3046 | 62 | 28.2a | 3.46 | 13.3 | 0.90 | ||
| 21 | 177.0b | 4.9 | 30.0b | 4.9 | 1.7b | 2935 | 50 | 11.2b | 11.6 | ||||||
| BW × PS | High | 18 | 182.8 | 8.0 | 56.8 | 8.0 | 11.7 | 7.28 | 3176 | 81 | 25.2 | 4.89 | 14.4a | 1.27 | |
| 21 | 173.5 | 6.9 | 26.5 | 6.9 | 0.0 | 3137 | 70 | 7.2 | 9.0b | ||||||
| Standard | 18 | 209.0 | 9.1 | 83.0 | 9.1 | 31.9 | 2915 | 93 | 31.2 | 12.3a,b | |||||
| 21 | 180.4 | 7.0 | 33.4 | 7.0 | 3.3 | 2732 | 72 | 15.1 | 14.2a | ||||||
| Source of variation | ––––––––––––––––––––––––––––––– P-value ––––––––––––––––––––––––––––––– | ||||||||||||||
| BW | 0.036 | 0.036 | 0.121 | <0.001 | 0.225 | 0.290 | |||||||||
| PS | 0.017 | <0.001 | 0.012 | 0.166 | 0.025 | 0.237 | |||||||||
| BW × PS | 0.220 | 0.220 | 0.258 | 0.366 | 0.853 | 0.044 | |||||||||
a,bLSMeans within a column and treatment group lacking a common superscript differ (P < 0.05).
1Hens that did not commence egg production before wk 55 were excluded from the analysis.
2Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
Table 2.
Cumulative feed intake (CFI) of broiler breeder hens from day 16 to photostimulation (rearing phase) and from photostimulation to wk 55 (laying phase), fed toward a High and Standard BW1 curve and photostimulated (PS) at wk 18 or 21.
| Rearing phase | Laying phase | |||||
|---|---|---|---|---|---|---|
| BW | PS | CFI (g) | SEM | CFI (g) | SEM | |
| BW | High | 7,985a | 38 | 34,295a | 700 | |
| Standard | 6,510b | 40 | 28,004b | 728 | ||
| PS | 18 | 6,404b | 41 | 29,975b | 755 | |
| 21 | 8,091a | 37 | 32,324a | 672 | ||
| BW × PS | High | 18 | 7105 | 56 | 33,939 | 1029 |
| 21 | 8865 | 52 | 34,652 | 950 | ||
| Standard | 18 | 5704 | 60 | 26,011 | 1104 | |
| 21 | 7316 | 52 | 29,996 | 950 | ||
| Source of variation | –––––––––– P value –––––––––– | |||||
| BW | <0.001 | <0.001 | ||||
| PS | <0.001 | 0.022 | ||||
| BW × PS | 0.181 | 0.109 | ||||
a,bLSMeans within a column and treatment group lacking a common superscript differ (P < 0.05).
1Hens followed either the breeder-recommended target BW curve (Standard) or an accelerated target BW curve reaching the 21 wk BW at 18 wk (High).
Onset of Sexual Maturity and Egg Production
Results for the onset of sexual maturity are reported in Table 3. As expected, there was a lower AFE for the High BW treatment compared to the Standard BW treatment (178.1 vs. 194.7 d, P = 0.036). This is in line with the conclusion in previous literature that heavier broiler breeders mature earlier compared to lighter weight birds (Lewis and Morris, 2005; Lewis et al., 2005, 2007b; Lewis and Gous, 2006). AFE was not different for the High BW × 18WK birds compared to the Standard BW × 21WK birds (182.8 vs. 180.4 d), where it was expected that the High BW × 18WK treatment would have matured earlier, as it was anticipated that they would have reached the minimum BW target for sexual maturition. AFE of the 21WK treatment was similar to the most recent report of broiler breeders photostimulated at 21 wk (Pishnamazi et al., 2014), where hens in the current study matured at 177.0 d and in the previously mentioned study at 179.5 d. Renema et al. (2007a) found an interaction between BW and age at photostimulation. They found that when photostimulation occurred at 18 wk of age, hens with a BW 25% below the recommended target at wk 12 came into production 17.4 d after hens with a BW 200% of the recommended target at wk 12. However, when they delayed photostimulation until wk 22, BW profile did not affect the timing of sexual maturation. In the current experiment, no such interaction was found. It is suggested that the larger difference between BW profiles and age at photostimulation, and greater BW variation within treatment in the study by Renema et al. (2007a) increased the ability to detect an interaction compared to the current study.
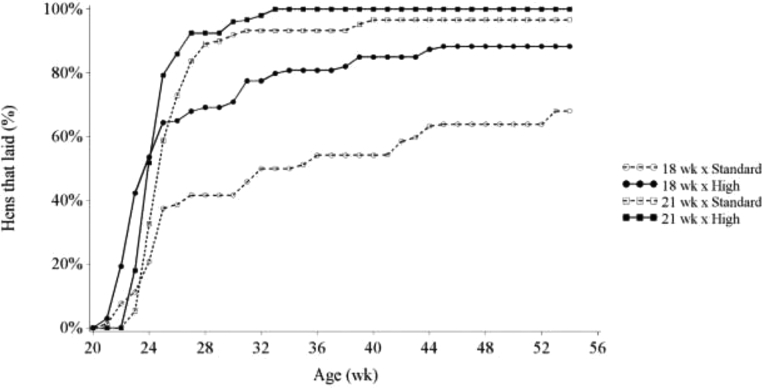
Looking at the rate at which birds started laying (Figure 3), from the rapid increase at wk 22 and the flattening of the curve after wk 24, it can be estimated that approximately 40% of the Standard BW birds and approximately 60% of the High BW birds were responsive to photostimulation at wk 18. These birds responded uniformly to photostimulation by sexually maturing, thus indicating that all 3 levels of the reproductive axis (hypothalamus, pituitary, and ovary) were in a ready state. Hens that came into production after this point matured spontaneously and not uniformly suggesting that one or more component of the axis was not responsive at the time of photostimulation. Comparing this to the responsiveness to photostimulation of birds on the 21WK treatment, approximately 90% of the birds were responsive to photostimulation, irrespective of BW treatment. The observation that not all High BW birds had dissipated their photorefractory state at wk 18 could be explained in 2 ways. First, it could indicate that the breeder recommended 21-wk BW target (Aviagen, 2016) was below the actual required minimum BW for onset of sexual maturity after photostimulation. Second, it could indicate that some hens at wk 18 had not reached the age required to sexually mature, irrespective of their BW. For both factors, BW and age, it is hypothesized that there is most likely not a fixed threshold but rather a mean with some level of variation around it. This was previously concluded by Lewis et al. (2007b); however, none of the described trials in their paper entailed a comparison with the exact same BW at 2 different photostimulation ages. In addition, studies in the past have always dealt with high CV for BW. A model proposed by Lewis et al. (2007b) predicting the age at 50% production in broiler breeders given a single increment in photoperiod from BW at wk 20 did not accurately estimate the current results. Only for the High BW × 21WK treatment their model estimated the mean age at 50% production close to the current result with an estimated mean of 195.4 d, compared to the current calculated mean of 194.3. Estimated means of the other treatments were over 22 d lower than current calculated means. In addition, estimated CV of age at 50% production did not compare with the current results. Therefore, it was concluded that the current results do not fit the models as described by Lewis et al. (2007b). All hens on the High BW × 21WK treatment laid their first egg before the end of the experiment, but 11.7% of the hens on the High BW × 18WK treatment never commenced egg production (Table 3). For the Standard BW hens, 31.9 and 3.3% never commenced egg production on the 18WK and 21WK treatment, respectively. It is hypothesized that hens which never commenced egg production either did not meet their individual required BW for sexual maturation or were missing a different metabolic incentive to sexually mature, such as a sharp increase in feed intake (discussed later). Lewis et al. (2007b) acknowledged that faster growth increased the rate of dissipation of juvenile photorefractoriness. Although not analyzed in the current experiment, body composition may have played an additional role. Over the past decades, abdominal fat pad weight as a percentage of BW has been decreasing from 4.9 ± 0.2% in a 1978 selected line to 2.9 ± 0.2% in a 2015 selected line at 21 wk of age (Reimer et al., 2017). This is hypothesized to be related to the delay in AFE in modern breeder lines, as Lewis et al. (2003) previously showed that hens from a leaner male breeder line had a delayed sexual maturity compared to a female breeder line. In addition, there is a growing body of literature in human medicine that describes that an early onset of sexual maturity coincides with an increased body fat percentage in women (Walvoord, 2010). De Beer and Coon (2007) concluded that total lean protein mass was a threshold for the onset of sexual maturity. However, dissection results at the end of the current experiment did not indicate a difference in the proportion of breast muscle between birds on the Standard and High BW treatment. Still, hens on the Standard BW treatment had lower proportional fat pad weight compared to hens on the High BW treatment (Table 5, 1.6 vs. 2.2%, P < 0.018). In addition, birds that had commenced egg production before wk 55 had a 3.2% (of BW) greater proportion of breast muscle compared to birds that had not commenced egg production before wk 55, while proportion of fat pad tended to be smaller (P = 0.083). Logistical constraints on bird numbers did not allow us to assess body composition around sexual maturation; hence, a direct relationship between body composition and AFE in the current experiment could not be studied. However, the observations at wk 55 indicate that a required fat threshold mass may be critical for the onset of lay. The novel feeding method the current study used may have altered body composition, as pullets were fed multiple times a day in small meals, instead of one meal every 1 or 2 d. This could have changed both their total and proportional lean and fat tissue mass, and therefore affected reproductive performance, as compared to conventional feeding methods (Carneiro, 2016). Further studies are needed to reveal the extent to which this is the case. In addition, as previously mentioned, every year breeding companies have been recommending similar BW profiles for broiler breeders while increasing growth potential of broilers (Renema et al., 2007b). Data from the current experiment support the statement that breeding companies have now approached the limit of the ability of broiler breeders to reach their mature BW within the recommended BW profiles; therefore, it is hypothesized that the current BW recommendations from the primary breeder are too low.
Figure 3.
Percentage of hens that had laid their first egg fed toward either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High) and photostimulated at wk 18 or 21.
Table 5.
Breast, fat pad, liver, heart, gastro intestinal tract (GIT), ovary, and oviduct weight as percentage of live BW, and number of large yellow follicles (LYF) of hens at 55 wk fed toward a High and Standard BW1 target and photostimulated (PS) at wk 18 or 21 and that either commenced egg production (laid) or did not commence egg production (not laid) before wk 552.
| BW | PS (wk) | Breast (%) | SEM | Fat pad (%) | SEM | Liver (%) | SEM | Heart (%) | SEM | GIT (%) | SEM | Ovary (%) | SEM | Oviduct (%) | SEM | LYF | SEM | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BW | High | 26.1 | 0.74 | 2.2a | 0.26 | 1.8a | 0.09 | 0.35 | 0.016 | 4.5 | 0.17 | 1.2 | 0.18 | 1.0 | 0.16 | 3.8 | 0.58 | |
| Standard | 26.7 | 0.62 | 1.6b | 0.22 | 1.5b | 0.08 | 0.34 | 0.013 | 4.6 | 0.14 | 0.9 | 0.15 | 0.8 | 0.13 | 3.0 | 0.54 | ||
| PS | 18 | 27.0 | 0.61 | 1.7 | 0.21 | 1.5b | 0.08 | 0.33 | 0.013 | 4.4b | 0.14 | 1.0 | 0.15 | 0.8 | 0.13 | 3.2 | 0.52 | |
| 21 | 25.8 | 0.76 | 2.1 | 0.27 | 1.8a | 0.09 | 0.35 | 0.016 | 4.7a | 0.17 | 1.2 | 0.18 | 1.0 | 0.16 | 3.6 | 0.60 | ||
| BW × PS | High | 18 | 26.9 | 0.88 | 2.0 | 0.31 | 1.6 | 0.11 | 0.34 | 0.019 | 4.3 | 0.20 | 1.0 | 0.21 | 0.9 | 0.19 | 3.1 | 0.68 |
| 21 | 25.3 | 0.95 | 2.5 | 0.33 | 1.9 | 0.12 | 0.35 | 0.020 | 4.7 | 0.21 | 1.5 | 0.23 | 1.1 | 0.21 | 4.4 | 0.73 | ||
| Standard | 18 | 27.1 | 0.76 | 1.5 | 0.26 | 1.4 | 0.10 | 0.32 | 0.016 | 4.4 | 0.17 | 0.9 | 0.18 | 0.7 | 0.16 | 3.3 | 0.67 | |
| 21 | 26.4 | 0.91 | 1.7 | 0.32 | 1.7 | 0.11 | 0.35 | 0.019 | 4.8 | 0.20 | 0.9 | 0.22 | 0.9 | 0.20 | 2.7 | 0.69 | ||
| Laid | 24.8b | 0.41 | 2.3 | 0.14 | 2.0a | 0.05 | 0.39a | 0.009 | 4.7 | 0.09 | 1.7a | 0.10 | 1.4a | 0.09 | 5.6a | 0.31 | ||
| Not laid | 28.0a | 1.07 | 1.6 | 0.38 | 1.3b | 0.14 | 0.30b | 0.023 | 4.3 | 0.24 | 0.4b | 0.26 | 0.4b | 0.23 | 1.2b | 0.92 | ||
| Source of variation | –––––––––––––––––––––––––––––––– P-value ––––––––––––––––––––––––––––– | |||||||||||||||||
| BW | 0.410 | 0.018 | 0.021 | 0.545 | 0.542 | 0.134 | 0.274 | 0.223 | ||||||||||
| PS | 0.155 | 0.170 | 0.007 | 0.156 | 0.043 | 0.208 | 0.259 | 0.559 | ||||||||||
| BW × PS | 0.594 | 0.568 | 0.985 | 0.572 | 0.953 | 0.181 | 0.853 | 0.104 | ||||||||||
| Laid | 0.008 | 0.083 | <0.001 | 0.001 | 0.116 | <0.001 | <0.001 | <0.001 | ||||||||||
a,bLSMeans within a column and treatment group lacking a common superscript differ (P < 0.05).
1Hens followed either the breeder-recommended target BW curve (Standard) or an accelerated target BW curve reaching the 21 wk BW at 18 wk (High).
2The effect of whether hens had commenced egg production or not before wk 55 on body composition did not depend on age at photostimulation.
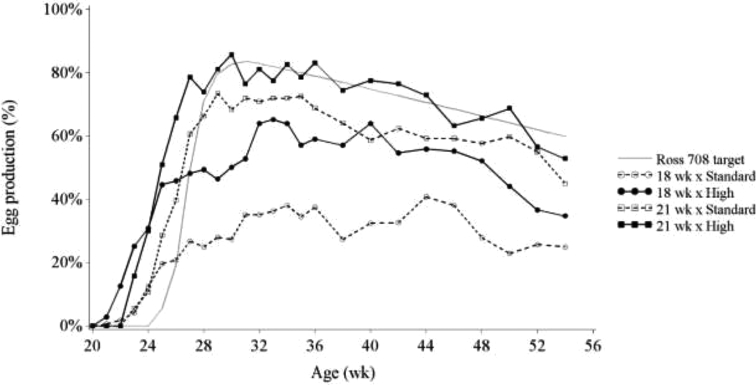
Hen day egg production as observed in Figure 2 is a combination of the number of hens that are in production and the rate of lay of the individual hens. To illustrate this, a 50% hen day egg production could mean that 50% of the hens are in production and laying 100% of the days. Alternatively, it could mean that 100% of the hens are in production, but they only lay at a 50% rate. To separate these 2 interpretations of the same parameter, the rate of lay was calculated in the current study as the hen day egg production for the subgroup of hens that were in production, i.e., had reached AFE. There was a significant effect of BW treatment, photostimulation treatment, and age on rate of lay (for all effects P < 0.001). Mean rate of lay was 69.9 ± 0.8 and 59.9 ± 1.0% for High and Standard BW treatment, and 61.3 ± 1.1 and 68.4 ± 0.8% for the 18WK and 21WK treatment, respectively. The rate of lay is assumed to be associated with laying sequence analysis, as both reproductive parameters give an indication of the reproductive performance of hens that have laid their first egg. Prime sequence length and mean sequence length are positively related to increased rate of lay. Previously, it was found that an increase in BW profile during the rearing phase reduced prime sequence and mean sequence length, which would mean a reduced rate of lay (Zuidhof et al., 2007), which is in contrast with the current results. Also in contrast with the current results, it was found that there was no effect of age at photostimulation on laying sequence traits (Joseph et al., 2002; Zuidhof et al., 2007).
Figure 2.
Hen day egg production of hens fed toward either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High) and photostimulated at wk 18 or 21.
The maturation interval was increased in the 18WK treatment compared to the 21WK treatment (69.9 vs. 30.0 d, Table 3). Robinson et al. (1996) reported maturation intervals between 50.6 and 24.2 d, when broiler breeders were photostimulated at ages ranging from 120 to 160 d. Renema et al. (2007a) reported maturation intervals of 41.5 and 29.9 d for the hens photostimulated at wk 18 and 22, respectively. The increased maturation interval in the current results could have been the result of genetic changes over the years; however, Pishnamazi et al. (2014) concluded that genetic selection for growth traits did not change the maturation interval. They reported maturation intervals of 49.2, 41.2, 32.5, and 24.0 d for hens photostimulated at 17, 19, 21, and 23 wk of age, respectively. The maturation interval for the 21WK treatment in the current study is comparable to these results, but the maturation interval of the 18WK treatment is much larger. Previously, hypotheses were proposed that the rate of sexual maturation after photostimulation increases, when photostimulation occurred later, such that for every day that photostimulation was delayed, AFE was delayed between 0.21 and 0.40 d (Yuan et al., 1994; Robinson et al., 1996; Renema et al., 2001b; Joseph et al., 2002; Ciacciariello and Gous, 2005; Pishnamazi et al., 2014). However, in the current study, AFE was advanced by 19 d when photostimulation was delayed by 21 d, resulting in an advance of 0.90 d for every day that photostimulation was delayed. The counterintuitive result that delaying photostimulation actually advanced the AFE could be related to the alternative feeding method in the current study. As previously mentioned, the PF station provided a reduced meal size and an increased frequency of meals time separated over the day as compared to conventional feeding methods. In addition, there was an altered feed allocation strategy after photostimulation. As compared to conventional methods, there was no overall increase in feed allocation on the flock level, only once an individual hen had laid her first egg, there was a production-related feed increase for this individual, as losing the weight of the egg resulted in access to feed. This is important, as feeding program just before and right after photostimulation can affect AFE (Melnychuk et al., 2004; Ciacciariello and Gous, 2005; Renema et al., 2007a). Melnychuk et al. (2004) compared broiler breeders that were either restricted fed or ad libitum fed after photostimulation. They showed that the maturation interval was shorter for ad libitum fed birds compared to restricted birds photostimulated at wk 21 (36.4 vs. 49.9 d, respectively), but that there was no difference in maturation interval for the restricted and full-fed birds photostimulated at wk 24 (28.2 d). At photostimulation, BW in the 21WK treatment was higher compared to the 18WK treatment and birds were fed more in the 21WK treatment to sustain their growth and maintenance requirements. The higher feed allocation could possibly also have provided a stimulus for contributed to incentive to start sexual maturation. Therefore, in addition to the possibility of not having reached the minimum BW or the required body composition, the absence of the metabolic signal related to feed intake could have increased maturation interval as compared to previous research (Robinson et al., 1996; Renema et al., 2007a).
The CV for AFE was higher in birds photostimulated at wk 18 compared to birds photostimulated at wk 21 (28.2 vs. 11.2%, P = 0.025, Table 3). Renema et al. (2001a) reported a CV for AFE of 5.02% and 4.02% for 19 and 21 wk photostimulated broiler breeders, respectively. Pishnamazi et al. (2014) also showed no significant difference in SD of age at sexual maturity for hens photostimulated at 17, 19, 21, or 23 wk. However, in line with the current results, Robinson et al. (1996) reported that birds photostimulated at the older ages (up to 23 wk) reached sexual maturity with less variation for BW at first egg and in AFE. In addition, the novel LED light source could have affected dissipation of the photorefractory state. Bédécarrats et al. (2016) hypothesized that the hypothalamus receives both metabolic cues and cues from photoreceptors to dissipate the photorefractory state, and that light from the red spectrum is required for hypothalamic stimulation (Mobarkey et al., 2010). The current trial used an LED light source that included 60% red, 20% green, and 20% blue light, as compared to conventionally used incandescent light, which mostly consists of red spectrum light. However, it was previously found that there was no difference in AFE between hens reared under 60% red or 60% green light (Rodriguez, 2017). Therefore, it was not expected that the novel light source would have influenced the current results.
No interaction was found between BW and age at photostimulation for egg production (Figure 2). Cumulative egg production was higher in the High BW treatment and 21WK treatment compared to the Standard BW treatment and 18WK treatment, and the effect of photostimulation and BW were independent (Table 4). Previously, Robinson et al. (1996) discussed that total egg production did not differ between hens photostimulated between 120 and 160 d and reared toward the same target BW curve (159.7 eggs until wk 60). Also Joseph et al. (2002) did not find a difference in total egg production between hens photostimulated at wk 21 or 23 (131.1 to wk 48). Cumulative egg production until wk 55 for the 21WK treatment was 138.4 eggs, which is comparable to previous reports; however, in the 18WK treatment egg production was decreased to 83.8 eggs. Gibson et al. (2008) reported that total egg production was greater for hens fed every day after photostimulation, compared to hens on a skip-a-day feeding treatment until 8% production (172 vs. 155 to wk 65). This is a further indication, as previously discussed, that the alternative PF method after photostimulation could have had an important influence on total egg production in this study.
Table 4.
Cumulative egg production (eggs) from wk 23 to 55 and mean egg weight of hens fed toward a High and Standard BW1 curve and photostimulated (PS) at wk 18 or 21.
| BW | PS (wk) | Eggs | SEM | Egg weight (g) | SEM | |
|---|---|---|---|---|---|---|
| BW | High | 129.4a | 7.3 | 63.9a | 0.2 | |
| Standard | 92.8b | 7.3 | 62.6b | 0.2 | ||
| PS | 18 | 83.8b | 7.5 | 62.3b | 0.3 | |
| 21 | 138.4a | 7.0 | 64.1a | 0.2 | ||
| BW × PS | High | 18 | 106.8 | 10.5 | 64.0a | 0.4 |
| 21 | 152.0 | 10.0 | 63.8a | 0.2 | ||
| Standard | 18 | 60.8 | 10.8 | 60.6b | 0.4 | |
| 21 | 124.7 | 10.0 | 64.5a | 0.2 | ||
| Source of variation | ––––––P-value –––––– | |||||
| BW | 0.001 | <0.001 | ||||
| PS | <0.001 | <0.001 | ||||
| BW × PS | 0.368 | <0.001 | ||||
a,bLSMeans within a column and treatment group lacking a common superscript differ (P < 0.05).
1Hens followed either the breeder-recommended BW curve (Standard) or an accelerated BW curve reaching the 21 wk BW at 18 wk (High).
Egg Weight
Egg weight increased with age for all treatments (P < 0.001), independent of BW treatment or age at photostimulation (data not shown). The number of eggs > 90 g was not different between treatments (data not shown). There was an interaction between the effect of BW and age at photostimulation on egg weight (P < 0.001). Egg weight was not different between High BW and Standard BW birds on the 21WK treatment, but egg weight of Standard BW × 18WK hens was 3.4 g lower compared to High BW × 18WK hens (Table 4). The general understanding is that egg weight is positively correlated with hen weight or hen weight at sexual maturity (McDaniel et al., 1981). However, the difference in hen weight was the same between Standard and High BW hens within the 18WK and 21WK treatments throughout the experiment (Figure 1). In addition, after including BW at sexual maturity as a covariate in the egg weight analysis, the interaction between BW and photostimulation remained significant (P < 0.001, data not shown). Previously, Pishnamazi et al. (2014) concluded that egg weight differences between hens photostimulated at different ages were attributed solely to the difference in BW at sexual maturity, not by the effect of photostimulation. In other studies, delayed photostimulation resulted in a difference in BW without subsequent differences in egg weight (Yuan et al., 1994; Robinson et al., 1996). Joseph et al. (2002) reported that hens photostimulated at wk 23 laid heavier eggs compared to hens photostimulated at wk 21 (60.8 vs 59.7 g, respectively), although it was unclear if this difference could be attributed to the higher BW at sexual maturity for hens photostimulated at wk 23 compared to hens photostimulated at 21 wk (3,105 vs. 2,966 g). Possibly BW is not the only factor playing a role in determining egg weight. Another factor could be feeding strategy around time of sexual maturity, as increased feeding levels during the onset of lay have been reported to result in higher egg weights (Zuidhof et al., 2007). However, in the current study all birds were fed according to a predefined PF strategy. The rate of lay could also have affected egg weight, as hens that lay less eggs may have a higher rate of yolk deposition per follicle, leading to higher egg weight (McLeod et al., 2014; Tůmová et al., 2017). However, this hypothesis is not supported by the current results, as the rate of lay was lower in the Standard BW × 18WK treatment compared to the High × 18WK treatment (55.5 vs. 67.1%, P < 0.001), whereas the eggs of the Standard BW × 18WK treatment weighed less compared to the High × 18WK treatment.
Body Conformation
Results for body conformation are summarized in Table 5. As previously referred to, logistical constraints on bird numbers did not allow us to assess body conformation around sexual maturation. Pishnamazi et al., (2014) reported that differences in body conformation at sexual maturity were only related to BW at sexual maturity. They suggested that the results of previous studies indicating that frame size, fatness, and proportion of breast would be increased by later photostimulation (Renema et al., 2001a, 2007a) could be explained by BW differences alone, without an additional effect of the later photostimulation. The current results show that at wk 55, proportional liver weight and GIT weight increased in the 21WK treatment compared to the 18WK treatment. In addition, fat pad and liver weight as a percentage of live BW were higher in the High BW treatment compared to the Standard BW treatment (2.2 vs. 1.6%, and 1.8 vs. 1.5%, respectively). It is hypothesized that the increased liver and GIT weight resulted from a higher metabolic rate and increased feed intake to support increased egg production in the 21WK treatment and the High BW treatment.
CONCLUSIONS
Even when within-treatment variation in BW was minimized, decreasing the age at photostimulation from wk 21 to 18 increased the variability in age at sexual maturity and decreased reproductive performance of broiler breeders. The current results indicate that the recommended breeder BW at wk 21 is below the optimal target for maturation after photostimulation. It is hypothesized that the hypothalamic responsiveness and dissipation of the photorefractory state might also be influenced by additional metabolic triggers resulting from a difference in feeding frequency and feed allocation.
Acknowledgements
Financial support from the Alberta Livestock and Meat Agency (Edmonton, Alberta), the Ontario Ministry of Agriculture, Food and Rural Affairs (Guelph, Ontario), the Canadian Poultry Research Council (Ottawa, Ontario), and the Alberta Chicken Producers (Edmonton, Alberta) is gratefully acknowledged. Broiler breeder chicks were donated by Aviagen (Huntsville, Alabama). Lights were donated by Thies Electrical Distributing Co. (Cambridge, Ontario). The authors would like to acknowledge all volunteering students for their help during collection of the presented data. Special thanks to K. L. Lovely and C. O. Ouellette for their excellent support throughout the experiment. Thanks to the staff of the Poultry Research Centre (Edmonton, Alberta) for their technical support. Poultry Research Centre stakeholder contributions, which made this research possible, are gratefully acknowledged.
REFERENCES
- Aviagen. 2016. Ross 708 Parent Stock: Performance Objectives. Aviagen Huntsville, AL. [Google Scholar]
- Bédécarrats G. Y., Baxter M., Sparling B.. 2016. An updated model to describe the neuroendocrine control of reproduction in chickens. Gen. Comp. Endocrinol. 227:58–63. [DOI] [PubMed] [Google Scholar]
- Carneiro P. R. O. 2016. Implications of precision feeding of broiler breeder pullets. MSc thesis. University of Alberta, Edmonton, AB. [Google Scholar]
- CCAC. 2009. CCAC Guidelines on: The Care and Use of Farm Animals in Research, Teaching and Testing. Canadian Council on Animal Care, Ottawa, ON, Canada. [Google Scholar]
- Ciacciariello M., Gous R. M.. 2005. A comparison of the effects of feeding treatments and lighting on age at first egg and subsequent laying performance and carcase composition of broiler breeder hens. Br. Poult. Sci. 46:246–254. [DOI] [PubMed] [Google Scholar]
- de Beer M., Coon C. N.. 2007. The effect of different feed restriction programs on reproductive performance, efficiency, frame size, and uniformity in broiler breeder hens. Poult. Sci. 86:1927–1939. [DOI] [PubMed] [Google Scholar]
- Gibson L. C., Wilson J. L., Davis A. J.. 2008. Impact of feeding program after light stimulation through early lay on the reproductive performance of broiler breeder hens. Poult. Sci. 87:2098–2106. [DOI] [PubMed] [Google Scholar]
- Joseph N. S., Dulaney A. A., Robinson F. E., Renema R. A., Zuidhof M. J.. 2002. The effects of age at photostimulation and dietary protein intake on reproductive efficiency in three strains of broiler breeders varying in breast yield. Poult. Sci. 81:597–607. [DOI] [PubMed] [Google Scholar]
- Katanbaf M. N., Dunnington E. A., Siegel P. B.. 1989. Restricted feeding in early and late-feathering chickens: 2. Reproductive responses. Poult. Sci. 68:352–358. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Backhouse D., Gous R. M.. 2005. Effect of constant photoperiods on the laying performance of broiler breeders allowed conventional or accelerated growth. J. Agric. Sci. 143:97–108. [Google Scholar]
- Lewis P. D., Ciacciariello M., Backhouse D., Gous R. M.. 2007a. Effect of age and body weight at photostimulation on the sexual maturation of broiler breeder pullets transferred from 8L:16D to 16L:8D. Br. Poult. Sci. 48:601–608. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Ciacciariello M., Gous R. M.. 2003. Photorefractoriness in broiler breeders: sexual maturity and egg production evidence. Br. Poult. Sci. 44:634–642. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Gous R. M.. 2006. Constant and changing photoperiods in the laying period for broiler breeders allowed [corrected] normal or accelerated growth during the rearing period. Poult. Sci. 85:321–325. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Gous R. M., Morris T. R.. 2007b. Model to predict age at sexual maturity in broiler breeders given a single increment in photoperiod. Br. Poult. Sci. 48:625–634. [DOI] [PubMed] [Google Scholar]
- Lewis P. D., Morris T. R.. 2005. Change in the effect of constant photoperiods on the rate of sexual maturation in modern genotypes of domestic pullet. Br. Poult. Sci. 46:584–586. [DOI] [PubMed] [Google Scholar]
- McDaniel G. R., Brake J., Eckman M. K.. 1981. Factors affecting broiler breeder performance.: 4. The interrelationship of some reproductive traits. Poult. Sci. 60:1792–1797. [Google Scholar]
- McLeod E. S., Jalal M. A., Zuidhof M. J.. 2014. Modeling ovarian follicle growth in commercial and heritage Single Comb White Leghorn hens. Poult. Sci. 93:2932–2940. [DOI] [PubMed] [Google Scholar]
- Melnychuk V. L., Kirby J. D., Kirby Y. K., Emmerson D. A., Anthony N. B.. 2004. Effect of strain, feed allocation program, and age at photostimulation on reproductive development and carcass characteristics of broiler breeder hens. Poult. Sci. 83:1861–1867. [DOI] [PubMed] [Google Scholar]
- Mobarkey N., Avital N., Heiblum R., Rozenboim I.. 2010. The role of retinal and extra-retinal photostimulation in reproductive activity in broiler breeder hens. Domest. Anim. Endocrinol. 38:235–243. [DOI] [PubMed] [Google Scholar]
- Pishnamazi A., Renema R. A., Zuidhof M. J., Robinson F.. 2014. Effect of age at photostimulation on sexual maturation in broiler breeder pullets. Poult. Sci. 93:1274–1281. [DOI] [PubMed] [Google Scholar]
- Reimer B., Carney V., Zuidhof M. J., Korver D. R., Robinson F. E., Anthony N. B.. 2017. Sexual maturation status at 21 weeks in 1957, 1978, 1997, and 2015 broiler breeder pullets.in Poultry Science Association 106th Annual Meeting Abstracts. Orlando. [Google Scholar]
- Renema R. A., Robinson F. E., Goerzen P. R.. 2001a. Effects of altering growth curve and age at photostimulation in female broiler breeders. 1. Reproductive development. Can. J. Anim. Sci. 81:467–476. [Google Scholar]
- Renema R. A., Robinson F. E., Goerzen P. R., Zuidhof M. J.. 2001b. Effects of altering growth curve and age at photostimulation in female broiler breeders. 2. Egg production parameters. Can. J. Anim. Sci. 81:477–486. [Google Scholar]
- Renema R. A., Robinson F. E., Zuidhof M. J.. 2007a. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 2. Sexual maturation. Poult. Sci. 86:2267–2277. [DOI] [PubMed] [Google Scholar]
- Renema R. A., Rustad M. E., Robinson F. E.. 2007b. Implications of changes to commercial broiler and broiler breeder body weight targets over the past 30 years. Worlds Poult. Sci. J. 63:457–472. [Google Scholar]
- Robinson F. E., Robinson N. A.. 1991. Reproductive performance, growth rate and body composition of broiler breeder hens differing in body weight at 21 weeks of age. Can. J. Anim. Sci. 71:1223–1231. [Google Scholar]
- Robinson F. E., Wautier T. A., Hardin R. T., Robinson N. A., Wilson J. L., Newcombe M., McKay R. I.. 1996. Effects of age at photostimulation on reproductive efficiency and carcass characteristics. 1. Broiler breeder hens. Can. J. Anim. Sci. 76:275–282. [Google Scholar]
- Rodriguez A. 2017. Effects of daytime and supplemental light spectrum on broiler breeder growth and sexual maturation. MSc thesis. University of Guelph, Guelph, ON. [Google Scholar]
- Romero L. F., Renema R. A., Naeima A., Zuidhof M. J., Robinson F.. 2009a. Effect of reducing body weight variability on the sexual maturation and reproductive performance of broiler breeder females. Poult. Sci. 88:445–452. [DOI] [PubMed] [Google Scholar]
- Romero L. F., Zuidhof M. J., Renema R. A., Robinson F. E., Naeima A.. 2009b. Nonlinear mixed models to study metabolizable energy utilization in broiler breeder hens. Poult. Sci. 88:1310–1320. [DOI] [PubMed] [Google Scholar]
- Tůmová E., Uhlířová L., Tůma R., Chodová D., Máchal L.. 2017. Age related changes in laying pattern and egg weight of different laying hen genotypes. Anim. Reprod. Sci. 183:21–26. [DOI] [PubMed] [Google Scholar]
- van der Klein S. A. S., Bédécarrats G. Y., Zuidhof M. J.. 2018. The effect of rearing photoperiod on broiler breeder reproductive performance depended on body weight. Poult. Sci. 10.3382/ps/pey199 (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walvoord E. C. 2010. The timing of puberty: Is it changing? Does it matter? J. Adolesc. Health 47:433–439. [DOI] [PubMed] [Google Scholar]
- Yuan T., Lien R. J., McDaniel G. R.. 1994. Effects of increased rearing period body weights and early photostimulation on broiler breeder egg production. Poult. Sci. 73:792–800. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Fedorak M. V., Kirchen C. C., Lou E. H. M., Ouellette C. A., Wenger I. I.. 2016. System and method for feeding animals. PrecisionZX, Inc, assignee.:Pat. No. United States Patent Application No. 15/283,125. [Google Scholar]
- Zuidhof M. J., Fedorak M. V., Ouellette C. A., Wenger I. I.. 2017. Precision feeding: innovative management of broiler breeder feed intake and flock uniformity. Poult. Sci. 96:2254–2263. [DOI] [PubMed] [Google Scholar]
- Zuidhof M. J., Renema R. A., Robinson F. E.. 2007. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 3. Reproductive efficiency. Poult. Sci. 86:2278–2286. [DOI] [PubMed] [Google Scholar]