Abstract
Complement factor H (FH), an elongated and substantially glycosylated 20-domain protein, is a soluble regulator of the complement alternative pathway (AP). It contains several glycan binding sites which mediate recognition of α2-3-linked sialic acid (FH domain 20) and glycosaminoglycans (domains 6–8 and 19–20). FH also binds the complement C3-activation product C3b, a powerful opsonin and focal point for the formation of C3-convertases of the AP feedback loop. In freely circulating FH the C3b binding site in domains 19–20 is occluded, a phenomenon that is not fully understood and could be mediated by an intramolecular interaction between FH’s intrinsic sialylated glycosylation and its own sialic acid binding site. In order to assess this possibility, we characterized FH’s sialylation with respect to glycosidic linkage type and searched for further potential, not yet characterized sialic acid binding sites in FH and its seven-domain spanning splice variant and fellow complement regulator FH like-1 (FHL-1). We also probed FH binding to the sialic acid variant Neu5Gc which is not expressed in humans but on heterologous erythrocytes that restrict the human AP and in FH transgenic mice. We find that FH contains mostly α2-6-linked sialic acid, making an intramolecular interaction with its α2-3-sialic acid specific binding site and an associated self-lock mechanism unlikely, substantiate that there is only a single sialic acid binding site in FH and none in FHL-1, and demonstrate direct binding of FH to the nonhuman sialic acid Neu5Gc, supporting the use of FH transgenic mouse models for studies of complement-related diseases.
Keywords: carbohydrate, complement, glycans, mass spectrometry, saturation transfer difference NMR
Introduction
Complement factor H (FH), a serum protein composed of 20 ellipsoid complement control protein repeats (CCPs) that are connected head-to-tail, is a negative regulator of the complement alternative pathway (AP), a branch of innate immunity that requires active down-regulation. FH contains eight N-glycosylation sites, each carrying single- to multiantennae sialoglycans, adding almost 18 kDa to its mass (Fenaille et al. 2007). Its regulatory action targets the complement component C3-activation product C3b, the central protein of a positive feedback loop in which all complement pathways converge. FH accelerates the decay of the AP C3 convertase (C3bBb) and serves as a co-factor to the serine protease factor I (FI) that degrades C3b (Xue et al. 2017). FH-mediated down-regulation of C3b activity is targeted to host tissue to which FH localizes via one or both of its two host glycan recognition sites, located in CCPs 6–8 and CCPs 19–20. These domains engage host-specific glycans, namely glycosaminoglycans (GAGs, CCPs 6–8 and CCPs 19–20) or sialic acid (Sia)-containing glycans (CCP 20). Both GAG sites prefer the heparan sulfate (HS)/heparin type over other GAG types but differ in their relative affinities for differentially (de-)sulfated forms of heparin (Clark et al. 2013). FH also binds sialylated glycans (Fearon 1978; Pangburn and Müller-Eberhard 1978; Michalek et al. 1988a), likely simultaneously with surface-bound C3b (Wu et al. 2009; Kajander et al. 2011; Morgan et al. 2011; Blaum et al. 2015). In full length FH the two binding sites for C3b (located in CCPs 1–4 and 19–20) are not equally accessible (Schmidt et al. 2013; Herbert et al. 2015). Instead, free FH adopts one or several conformation(s) in which the C-terminal C3b binding site is obscured (DiScipio 1992; Oppermann et al. 2006; Schmidt et al. 2010; Makou et al. 2012; Herbert et al. 2015; Baud et al. 2016). The structural basis for the FH conformational heterogeneity or for a possible regulatory mechanism that could adjust the equilibrium between conformations with accessible and obscured C-terminal C3b binding sites is unknown. One possibility could be a self-locking mechanism in which the FH sialylated glycan chains interact with the FH sialic acid binding site in domain 20.
In our past structural analysis of Sia recognition by FH (Blaum et al. 2015), we focused on the previously established Sia binding site in FH’s most C-terminal domain CCP 20 and the most common Sia variant in humans, α-N-acetylneuraminic acid (αNeu5Ac). We found that FH binds α2-3- but not α2-6- or α2-8-linked Neu5Ac, in agreement with functional investigations by other groups (Michalek et al. 1988b; Ram et al. 1998; Gulati et al. 2005; Hyvärinen et al. 2016). We now characterized the glycosidic linkage types present in the eight FH glycan chains. We further probed for the existence of additional, not yet characterized Neu5Ac binding sites in FH and its N-terminal splice variant FH-like 1 (FHL-1) (Schwaeble et al. 1987; Ripoche et al. 1988) to exclude that potential Sia-recognition by FHL-1, which is the main complement regulator on Bruch’s membrane, is implicated in the pathophysiology of age-related macular degeneration (AMD) (Clark et al. 2014). We also wanted to cross-validate the common approach of investigating (human) FH functions in FH transgenic mice models as well as on heterologous erythrocytes, which traditionally serve in complement activity assays (e.g., sheep erythrocytes). To this end we probed direct binding of FH to α-N-glycolylneuraminic acid (αNeu5Gc), a common mammalian Sia variant that is not expressed in humans but in the FH transgenic mice model and on the surface of heterologous erythrocytes.
Results
The glycan recognition site in FH CCPs 6–8 does not contribute to Sia-mediated erythrocyte protection from complement attack by FH
Sheep erythrocytes (RBCs) are non-activators of the human AP and are stable in normal human serum (NHS) supplemented with Mg-EGTA (which preserves activity of the AP but not the classical or mannose-binding lectin complement pathways). Hemolysis can be induced by neuraminidase treatment or by addition of recombinant FH CCPs 19–20 (FH19–20), which necessarily requires the presence of RBC sialylation in order to compete FH and induce lysis (Schmidt et al. 2013; Hyvärinen et al. 2016). The current model for these observations is direct competition of FH19–20 with serum FH for simultaneous binding of plasma-membrane Sia and small amounts of cell-bound C3b (Ferreira et al. 2006).
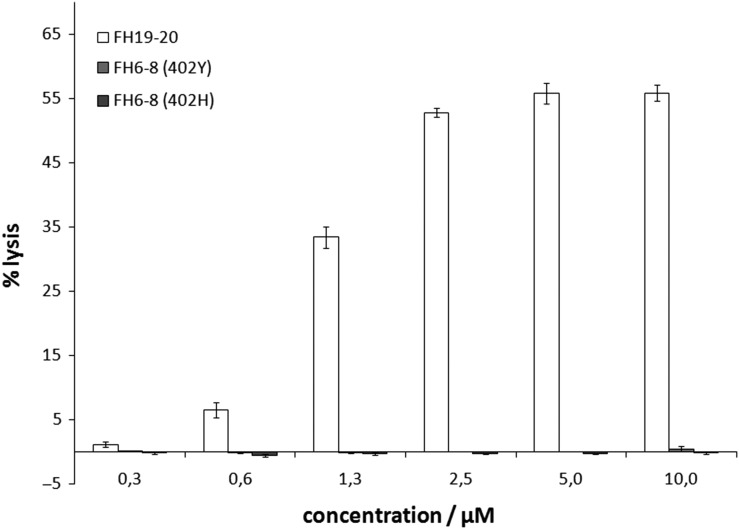
We employed this assay to assess the possibility that FH CCPs 6–8, which contain one of the two FH GAG binding sites, also bind to plasma membrane sialoglycans. To this end, we prepared washed sheep RBCs in NHS supplemented with Mg-EDTA and added recombinant FH6–8 (both the Y402 and H402 variant that is linked to AMD) or recombinant FH19–20 (for comparison). Even at a concentration of 10 μM FH6–8 virtually no hemolysis was observed, whereas 2.5 μM of FH19-20 induced well over 50% of hemolysis (where 100% equals complete osmotic hemolysis with distilled water) (Figure 1). This observation suggests that recombinant FH6–8, unlike FH19–20, does not compete with serum FH for Sia binding on sheep RBCs and hence that FH CCPs 6–8 do not contribute to Sia recognition by FH.
Fig. 1.
The glycan recognition site in FH CCPs 6–8 does not compete for erythrocyte binding by FH. Sheep erythrocytes were prepared in normal human serum supplemented with Mg-EDTA and incubated with varying amounts of recombinant FH fragments (white bars: FH19–20, light gray bars: FH6–8 402Y, dark gray bars: FH6–8 402H). Hemolysis was quantified via absorbance at 415 nm and is given as percentage relative to hemolysis in distilled water. Average values of four independent assays with SD are shown.
Neither FH6–8 nor FHL-1 bind directly to sialylated glycans
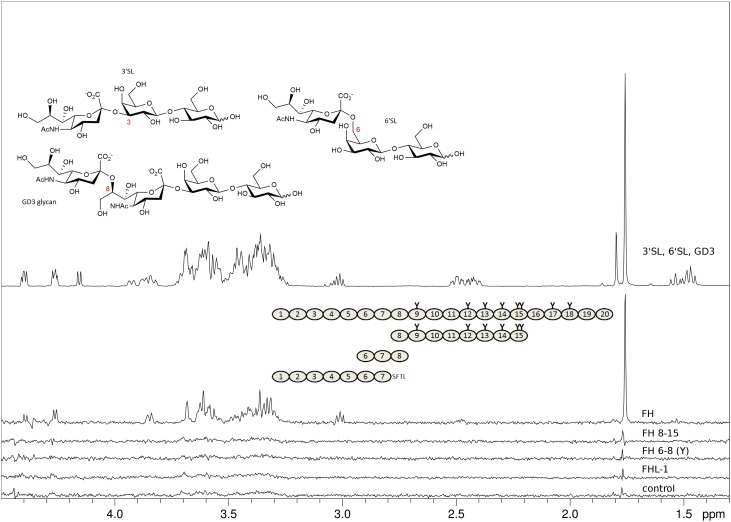
Because of the so-called AP “tick-over”, which constantly produces a small amount of C3-convertase and thus deposits C3b in a rather unselective manner, the hemolytic assay may assess FH’s capacity to simultaneously bind to Sia and cell-bound C3b rather than to Sia alone (Ferreira et al. 2006). While FH19–20 also contains a C3b binding site directly adjacent to the Sia binding site in CCP 20 (Blaum et al. 2015, 2016), it is not entirely clear if FH6–8 also engages C3b. A potential binding site in FH CCPs 6–8 for C3b was reported but the binding affinity was too low for an accurate determination (Schmidt et al. 2008). Thus, to complement the hemolytic assay with FH6–8 by an experiment that is entirely independent of FH’s interaction with C3 activation fragments, we conducted saturation transfer difference (STD) NMR experiments with two recombinant FH6–8 variants (Y402 and H402) and sialylated glycan representatives of the three glycosidic linkage types that are most commonly observed in human sialoglycans (i.e., with α2-3-, α2-6-, or α2-8-linked Neu5Ac caps, respectively). We also included FHL-1 and a FH construct containing CCP 13 (for which a potential carbohydrate recognition site has been proposed but could not be confirmed (Pangburn et al. 1991; Ormsby et al. 2006; Schmidt et al. 2008)) in the STD NMR experiments. Unlike FH and FH19–20, none of the other shortened FH constructs (FH6–8Y, FH6–8H and FH8–15) nor FHL–1 bound to any of the three soluble sialylated glycans (3′sialyllactose (3′SL), 6′sialyllactose (6′SL), and the GD3 glycan) (Figure 2A). Together with the hemolytic assay these experiments confirm the Sia binding site in CCP 20 as the only Sia binding site in FH and suggest that FHL-1 does not bind to Sia in vitro.
Fig. 2.
FHL-1 and recombinant fragments of FH devoid of CCPs 19–20 do not bind to sialylated glycans. Representatives of α2-3, α2-6, and α2-8-linked Neu5Ac (3’SL, 6’SL, and the GD3 glycan, respectively, chemical structures shown with Neu5Ac glycosidic linkage types in red) were measured at 2 mM each and STD NMR experiments were recorded at 283 K in PBS containing D2O instead of H2O with different proteins at around 10 μM present. Spectra from top to bottom: proton 1D reference spectrum of the sugar mix (top), STD NMR difference spectra with FH (second from top), with FH8-15 (third from top), with FH6–8 402Y (fourth from top), with FHL-1 (fifth from top), and, for comparison, without protein (bottom). Proteins used are shown schematically with naturally occurring glycosylation sites highlighted as “Y”. All recombinantly produced proteins (i.e., all apart from FH) were deglycosylated with Endo Hf during the purification procedure. The FHL-1 C-terminus (deviation from FH sequence) is spelled out as amino acid sequence. The STD NMR difference spectrum with FH is the only STD spectrum with noticeable magnetization transfer to one of the three sialoglycans (signals belong to 3’SL proton resonances).
FH binds the nonhuman Sia variant Neu5Gc
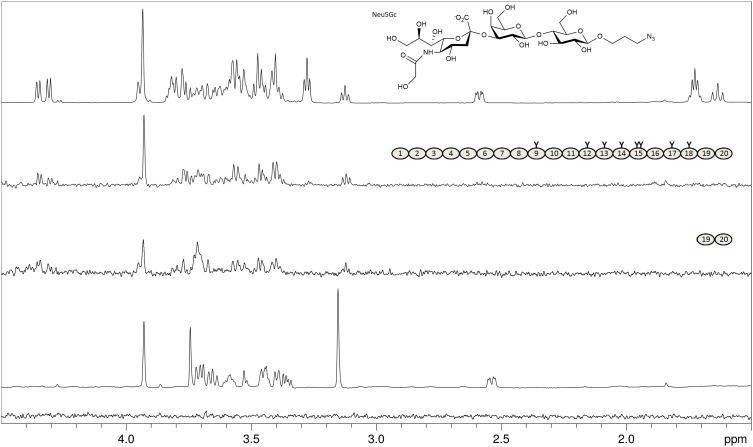
Using STD NMR experiments, we analyzed whether serum-derived FH recognizes Neu5Gc, which is not expressed in humans but in other mammals, including sheep and mice (Chou et al. 1998; Hedlund et al. 2007). First, we used the monosaccharide 2-O-methyl-α-N-glycolylneuraminic acid (Neu5GcαOMe, 2-O-methylated in order to prevent mutarotation and formation of the non-physiological β-anomer) but did not observe any interaction with FH (Figure 3). However, with a larger Neu5Gcα-containing glycan, namely the trisaccharide Neu5Gcα2-3Galβ1-4Glcβ-propyl-N3 (glycolyl-3′SL-ProN3), good magnetization transfer from FH and from FH19–20 was detected (Figure 3). This experiment, together with indirect evidence from biochemical studies (Gulati et al. 2005), demonstrates that nonhuman Neu5Gc is recognized by human FH just as well as Neu5Ac. Our observation that a single Sia ring (i.e., Neu5GcαOMe) is not capable of FH binding is in agreement with our previous crystallographic and NMR spectroscopic analysis, which showed that further pyranoses attached to the specifically recognized Sia ring (for example in sialylated trisaccharides such as 3′SL) entertain so-called CH–π interactions with the W1183 side chain in FH and also receive substantial magnetization transfer in STD NMR experiments (Blaum et al. 2015, 2016). For glycolyl-3′SL-N3 such transfer of magnetization to the non-Sia rings Gal and Glc (but, notably, not to the non-physiological propargyl linker) is also observed (Figure 3), suggesting that Neu5Gc-capped glycans are bound by FH in much the same manner as Neu5Ac-capped glycans.
Fig. 3.
FH binds the nonhuman Neu5Gc variant of sialic acid. From top: Proton 1D reference spectrum of Neu5Gcα2-3Galβ1-4Glcβ-propyl-N3 (glycolyl-3’SL-ProN3, chemical structure shown; Neu5Gc differs only by one additional OH-group in the acetyl side chain from the Neu5Ac Sia-variant in 3’SL); STD NMR difference spectrum of 2 mM glycolyl-3’SL-ProN3 with 8 μM of FH; STD NMR difference spectrum of 2 mM glycolyl-3’SL-ProN3 with 50 μM of FH19–20; Proton 1D reference spectrum of Neu5GcαOMe; STD NMR difference spectrum of 2 mM Neu5GcαOMe with 8 μM of FH. The structures of FH and FH19–20 are shown schematically with glycosylation sites highlighted as “Y”. Magnetization transfer from the Neu5Gc-trisaccharide is observed for all three pyranose rings but not for the propyl linker protons (resonances at 1.7 ppm and 3.25 ppm), while no transfer is observed for the monosaccharide (bottom spectrum).
FH is primarily α2-6-sialylated
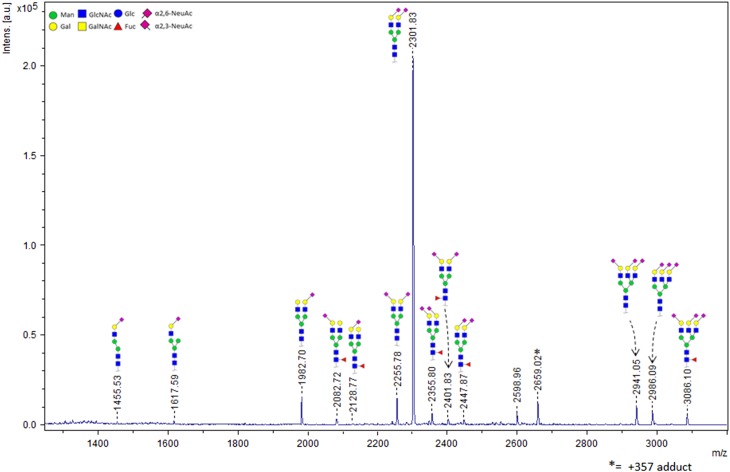
The FH sequence contains nine theoretical N-glycosylation sites, eight of which are in fact glycosylated (Fenaille et al. 2007). Glycosylation is restricted to domains 9 to 18. The most common non-reducing end oligosaccharide sequences of human complex N-glycans are Neu5Acα2-3Galβ1-4GlcNAc and Neu5Acα2-6Galβ1-4GlcNAc. We have previously shown that FH specifically binds to trisaccharides which terminate in the Neu5Acα2-3Gal disaccharide (Blaum et al. 2015). Thus, FH’s own glycosylation may resemble the sialylated host markers on which it relies for self-recognition purposes. In order to clarify in how far FH itself is α2-3- or α2-6-sialylated, we undertook mass spectrometry-based characterization of the individual glycosidic linkage types. We employed a chemical modification strategy with which α2-6-linked Neu5Ac is ethyl esterified while the α2-3-linked variant is lactonized, introducing a mass difference between both Sia types and achieving charge neutralization and sialic acid stabilization at the same time (Reiding et al. 2014). The glycans were enzymatically released, derivatized, and subsequently analyzed using matrix-assisted laser desorption/ionization time-of-flight tandem mass spectrometry (MALDI-TOF-MS). We identified 15 different glycan compositions in FH from human serum, with the A2S2 type (two-antennary disialylated) being the most abundant type by far (79.5% ± 0.1%). In total, 74.8% ± 0.3% of the FH glycans are diantennary with both Sia caps in the α2-6 linkage variant. Only 4.7% ± 0.2% of the diantennary glycans have a single α2-3-linked Sia cap, and no diantennary glycans with two α2-3-linked Sia caps are observed (Figure 4 and Table I). The total percentage of α2-6 and α2-3 linked Sia, with respect to the total level of sialylation and taking mixed species into account, was found to be 91.5% and 8.5%, respectively. Consistent with the absence of O-glycosylation, treatment with a deglycosylation mix containing PNGase F and O-glycosidase did not reduce the apparent molecular weight of FH (as observed by SDS-PAGE) further than treatment with PNGase F only (data not shown).
Fig. 4.
FH itself is primarily α2-6-sialylated. MALDI-TOF-MS spectrum and structural assignment of glycans released from FH. Mostly disialylated diantennary (A2S2 and A2S2F) but also trisialylated triantennary (A3S3 and A3S3F) and traces of monosialylated diantennary glycans (A2S1 and A2S1F) are observed. The chemical composition, including the Sia glycosidic linkage type(s) of each species, is depicted schematically. Linkage types were assigned based on mass differences that were introduced via chemical derivatization prior to MALDI-TOF-MS analysis.
Table I.
Relative abundances of ethyl esterified glycans compared to HPCE-LIF analyzed APTS derived glycansa
| Ethyl esterified N-glycansa | Mean (±SD) | Mean (±SD) | Relative proportiona | APTS derived N-glycansb |
|---|---|---|---|---|
| H5N4E1 | 3.8 (0.28) | 3.8 (0.28) | 9.2 | A2S1 |
| H5N4F1L1 | 1.0 (0.06) | 1.3 (0.09) | 2.0 | A2S1F |
| H5N4F1E1 | 0.3 (0.03) | |||
| H5N4E1L1 | 4.7 (0.16) | 79.5 (0.10) | 67.4 | A2S2 |
| H5N4E2 | 74.8 (0.26) | |||
| H5N4F1L2 | 2.3 (0.06) | 4.7 (0.03) | 8.9 | A2S2F |
| H5N4F1E1L1 | 1.4 (0.02) | |||
| H5N4F1E2 | 1.0 (0.01) | |||
| H6N5E2L1 | 4.2 (0.15) | 7.0 (0.18) | 3.9 | A3S3 |
| H6N5E3 | 2.8 (0.04) | |||
| H6N5F1E2L1 | 2.0 (0.07) | 2.0 (0.07) | 4.0 | A3S3F |
| H3N3E1 | 0.3 (0.03) | 1.6 (0.14) | 4.6 | Other |
| H4N3E1 | 0.4 (0.06) | |||
| H6N5E1 | 0.8 (0.13) | |||
| H6N5E1 | 0.1 (0.02) |
“H” represents hexose, “N” N-acetyl hexosamine, “F” fucose, “E” ethylated N-acetyl neuraminic acid and finally “L” represents lactonized N-acetyl neuraminic acid. aReiding et al. (2014); bFenaille et al. (2007).
Discussion
Carbohydrate recognition by FH is linked via genetic disposition to tissue-specific complement-mediated diseases, in particular AMD (on Bruch’s membrane) and atypical hemolytic uremic syndrome (aHUS, in the kidney glomerulus) (Carroll and Sim 2011; Kavanagh et al. 2013). Due to the low affinities with which oligosaccharides bind to FH in vitro and the chemical complexity of mammalian glycosylation a comprehensive understanding of the role that glycans play in AP regulation is still lacking. It is, however, certain that both types of FH self-markers (i.e., HS-proteoglycans and sialylated glycans) are not functionally redundant with respect to their interaction with FH. Instead, it seems that both glycan types act as tissue-specific self-markers to FH, i.e. proteoglycans appear to be the dominant self-markers in Bruch’s membrane and sialylated glycans in the glomerulus (possibly with additional contribution by proteoglycans whose GAG chains are structurally distinct from those on Bruch’s membrane) (Clark et al. 2013; Blaum et al. 2015; Langford-Smith et al. 2015). Our finding that FHL-1, the main AP regulator on Bruch’s membrane, and FH CCPs 6–8, previously shown to bind stronger to Bruch’s membrane than FH CCPs 19–20, do not bind to sialylated oligosaccharides further supports the hypothesis that GAGs act as the major or sole self-markers in Bruch’s membrane (Clark et al. 2013). It is, however, possible that recruitment of FH by Sia plays a role in complement protection in other parts of the eye where full length FH may be present, for example in the retinal interphotoreceptor matrix for which a prevalence of α2-3-linked Neu5Ac was previously suggested on the basis of lectin binding experiments (Bishop et al. 1993).
Like many serum proteins, FH itself is heavily glycosylated, and it is possible that the intrinsic glycosylation plays a direct or indirect role in FH’s activity – including the reported conformational heterogeneity of FH, which impacts C3b binding. A comparison of N-glycosylation consensus sequence sites across different mammalian FH sequences, which could give hints as to which glycan chains are functionally important, shows that the N-glycosylation sites in FH are not subject to strict conservation but nevertheless tend to cluster in certain regions, namely those that are not directly implicated in ligand binding. Most glycosylation sites are located between CCPs 12 and 15, in CCP 17 and 18, and in the linker region between CCPs 8 and 9 (Supplementary data, Figure S1). Thus far, none of these regions have been convincingly implicated in direct interactions with FH’s principal ligands. A possible interpretation of this observation is that the glycan chains in FH serve predominantly indirect purposes, such as solubility or resistance to proteolytic degradation. In order to experimentally evaluate if the FH glycan chains may directly mediate a back-bending mechanism in which the FH Sia binding site in CCP 20 would bind to its own glycan chains and modulate accessibility of the C-terminal C3b binding site, we characterized the FH glycan chains with respect to the Neu5Ac glycosidic linkage types and demonstrate that the vast majority of glycan chains in pooled serum-derived FH is decorated with α2-6-linked Neu5Ac. This finding is in line with a reported excess of α2-6-over α2-3-linked Neu5Ac in pooled human plasma proteins (Gagneux et al. 2003; Reiding et al. 2014). Because FH itself has a clear preference for binding α2-3-linked Sia (Blaum et al. 2015; Hyvärinen et al. 2016) this finding makes a scenario in which the FH Sia binding site interacts with its own glycan chains (or those of other FH molecules) unlikely. Since sialylation levels and Sia-linkage types change with age and disease, such a regulatory mechanism would also be highly unlikely from a theoretical point of view as FH conformational equilibria would otherwise shift with age (Garcia et al. 2005; Pousset 1997). Of note, desialylation and further deglycosylation of plasma-purified FH with bacterial neuraminidase and fungal endo-N-acetyl-glucosaminidase did not decrease the protein’s ability to decay cell-bound AP C3-convertase nor its ability to distinguish between activators and non-activators of the AP (Jouvin et al. 1984). The same is true for full-length FH produced in Pichia pastoris and subsequently deglycosylated with Endo Hf (Schmidt et al. 2011). Together, these observations suggest that the glycan chains are not critical for FH’s structural architecture – although they could still influence the protein’s conformational dynamics.
The sialic acid family consists of more than 50 different α-keto sugars. Most of these differ from the prevalent Sia type in humans, the nine-carbon pyranose αNeu5Ac, through additional acetyl and/or hydroxyl groups (Varki et al. 2017). Absolute and relative expression levels of individual Sia variants are species- and tissues specific, with the Neu5Ac and Neu5Gc being the overall most common mammalian variants. Humans lost the ability to hydroxylate Neu5Ac to yield Neu5Gc but can incorporate Neu5Gc directly from dietary sources (Tangvoranuntakul et al. 2003; Ng et al. 2014; Springer et al. 2014). Sheep RBCs, on the other hand, contain Neu5Gc (Klimas et al. 1982). The observation that sheep RBCs are non-activators of the human AP, therefore, hints to a certain promiscuity of human FH with respect to this nonhuman Sia variant. Notably, wild type mice and thus human FH transgenic mouse models that are used to study complement diseases express Neu5Gc in multiple organs including the kidney, making the question if human FH binds Neu5Gc highly relevant for the interpretation of data obtained with these animal models (Hedlund et al. 2007; Pickering et al. 2007). From a structural point of view, the Sia binding site in FH CCP 20 should be able to accommodate an additional hydroxyl group positioned at the Neu5Ac acetyl function, i.e., Neu5Gc (Blaum et al. 2015), and our STD NMR experiment with a Neu5Gc-containing trisaccharide confirms this assumption. Potential physiological benefits of FH’s incapacity to discriminate between Neu5Ac and nonhuman Neu5Gc, however, remain enigmatic. Presentation of Sia and related nonulosonate sugars on microbial lipooligosaccharide (LOS) is an AP evasion strategy (Ram et al. 1998). For Neisseria gonorrhoeae (Ng) the specificity of glycosylation-mediated serum resistance is well documented and it was shown that both Neu5Ac- and Neu5Gc-modification of Ng LOS convey human AP-resistance (Gulati et al. 2015). However, in a physiological setting Ng scavenges CMP-activated sugars for LOS-decoration from its host, and humans provide only traces of CMP-Neu5Gc (possibly from dietary intake). Therefore, no evolutionary disadvantages appear associated with the lack of discrimination between the two Sia types by FH. Nevertheless, the finding that FH directly binds to Neu5Gc-containing glycans has medical implications as human FH transgenic mice (which produce Neu5Gc) are used as animal models for infection with serum-resistant Ng strains and complement-specific diseases such as aHUS (Pickering et al. 2007; Gulati et al. 2015). Since both Ng serum resistance and aHUS predisposition are linked to FH-Sia recognition (Blaum et al. 2015; Gulati et al. 2015; Hyvärinen et al. 2016) such animal models would be highly questionable if FH was not promiscuous with respect to Neu5Ac hydroxylation.
In the context of Neu5Gc we would like to note that the aHUS-linked FH variant S1191L/V1197A, which reflects also the only sequence difference between the C-terminal domains of FH and its antagonist FH related-1 (FHR-1), may (unlike FH) bind weaker to Neu5Gc- than to Neu5Ac-containing glycans because the 1191L side chain may interfere with the additional hydroxyl group (for crystallographic images of the binding site including the S1191 side chain see (Blaum et al. 2015)). It was, however, already shown that the double mutation impairs FH19–20 binding also to human erythrocytes (Ferreira et al. 2009). A reason could be steric hindrance by the larger Leu side chain in position 1191 (which is close to the Neu5Ac methyl group in the complex) or reduced hydrophobic contacts between the smaller Ala side chain at position 1197 and the Sia glycerol chain (Blaum et al. 2015). It was proposed that the S1191L/V1197A double mutation could alter sialic acid specificity (de Jorge et al. 2018). We do not think this likely (with the exception of Neu5Gc) since the mutations are both well away from the Sia glycosidic linkage and the preceding pyranoses in the crystal structure of the complex. It is, however, possible that the conformational dynamics of the hypervariable loop (FH residues 1182–1189) are affected by the mutations. Since the double mutant reduces C3b binding by FH19–20 (Ferreira et al. 2009), and since C3b binding may promote the open, Sia-binding conformation of this loop (Blaum 2017) altered structural dynamics in this region of mutated FH and FHR-1 could also be the reason for impaired Sia binding by FHR-1.
Materials and methods
Proteins, recombinant production and purification
In terms of full length FH, a commercial source (CompTech, USA) of FH purified from human plasma was used. FHL-1 (402Y) and FH fragments FH6–8, FH19–20 and FH8–15 were produced as secreted proteins in Pichia pastoris as described previously (Schmidt et al. 2008; Clark et al. 2014). In brief, P. pastoris cells (strain KM71H, Invitrogen) that had been stably transformed with the expression cassette (pPICZαB, Invitrogen) containing the relevant coding DNA were grown in a fermenter and protein expression was induced by supplementation with methanol (according to standard procedure described by the supplier’s manual and as published in detail in Schmidt et al. (2011)). The recombinant proteins were purified by consecutive cation- and/or anion-exchange chromatography steps. The construct FHL-1 (corresponding to FH1–7), FH6–8 and FH19–20 are not glycosylated in P. pastoris, in contrast to FH8-15. However, the N-linked glycans of FH8–15 were removed during the purification process with the endoglycosidase Endo Hf (New England Biolabs). Optionally a size-exclusion chromatography was employed to further increase purity if necessary.
AP hemolytic assay
This assay is based on the findings that sialic acid moieties on sheep erythrocytes are crucial to restrict the alternative complement pathway (Fearon 1978; Pangburn and Müller-Eberhard 1978; Michalek et al. 1988a). 1 mL of defibrinated sheep blood (TCS Biosciences) was diluted with 10 mL precooled buffer (20 mM HEPES, 145 mM NaCl, 0.1% (w/v) gelatin (from pork skin, Fluka) and 10 mM EDTA, pH 7.3 at 25°C). The cell suspension was mixed gently and spun for 10 min at 500 × g, at 4°C. The supernatant and a layer of leukocytes were aspirated off and the procedure was repeated two more times with buffer and subsequently three times with an identical buffer without EDTA but with 5 mM MgCl2 and 5 mM EGTA (Mg-EGTA). 2.5 μL of the concentrated cell suspension was then mixed with 20 μL of normal human serum and 1 μL of 100 mM Mg-EGTA. Recombinant FH fragments (FH19–20, FH6–8 402Y or FH6–8 402 H) were added from 30 μM stocks prepared in HEPES/NaCl buffer without further supplements. Final recombinant protein concentrations were 0.3 μM, 0.6 μM, 1.3 μM, 2.5 μM, 5.0 μM and 10 μM. Individual reaction volumes were adjusted to 40 μL with HEPES/NaCl buffer without supplements. Individual reactions were incubated for 20 min at 37°C, stopped by addition of 150 μL cold buffer without gelatin but with 10 mM EDTA, and spun at 1500 × g for 5 min at 4°C. A control experiment was conducted without recombinant FH fragments and heat-inactivated human serum (56°C for 30 min). Hemolysis was determined as absorbance of the supernatant at 415 nm (A415) and compared to the A415 value for the same amount of cells lysed with distilled water. Four independent experiments were conducted and A415 values averaged for each data point.
NMR spectroscopy
NMR spectra were recorded at 283 K using 3 mm tubes (200 μL sample volume) and a Bruker AVIII-600 spectrometer equipped with a room temperature probe head and processed with TOPSPIN 3.0 (Bruker). Samples for STD NMR spectra with Neu5Ac-containing glycans contained 2 mM of each of the three glycans (3′SL, 6′SL and the GD3 glycan) and varying concentrations (10 μM ± 2 μM) of either full-length FH (Complement Technology, Inc.), recombinant FHL-1 μM, recombinant FH6–8 402Y, or recombinant FH8–15. Proteins were buffer-exchanged prior to NMR experiments in 10 kDa MWCO centrifugal concentrators to 20 mM potassium phosphate pH 7.4, 150 mM NaCl in D2O. Individual glycans were added to the protein samples from 40 mM stock solutions prepared in D2O. A sample with 2 mM of each of the three glycans but without protein was used to verify that no direct excitation of the glycans took place during the on-resonance irradiation step of the STD pulse program, and also to record a proton 1D reference spectrum of the sugar mix.
Off- and on-resonance irradiation frequencies were set to −30 ppm and 7.3 ppm, respectively. The irradiation power of the selective pulses was 57 Hz, the saturation time was 2 s, and the total relaxation delay was 3 s. A 50 ms continuous-wave spin-lock pulse with a strength of 3.2 kHz was employed in order to suppress residual protein signals. A total number of 512 scans were recorded. A total of 12 k points were collected, and spectra were multiplied with a Gaussian window function prior to Fourier transformation. The sample with glycolyl-3′SL-ProN3 contained 8 μM FH and 2 mM glycolyl-3′SL-ProN3 and was recorded at 288 K for technical reasons with otherwise identical settings.
Enzymatic deglycosylation
FH at 1 μg/μL in PBS (Complement Technologies, Texas) was denatured for 10 min at 95°C with the NEB protein denaturation buffer supplied with the NEB deglycosylation mix, and subsequently centrifuged for 5 min at 16,900 ×g. One microgram of denatured FH was then mixed 1:0.25 with 4-fold reducing SDS-PAGE buffer (29% (v/v) glycerol, 290 mM Tris pH 6.8, 1.5% (w/v) SDS, 12 mM EDTA, 3 M 2-mercaptoethanol) and left to incubate at room temperature for 10 min while another μg was treated with PNGase F (NEB, glycerol-free, from Elizabethkingia miricola) and 1 μg was treated with the NEB deglycosylation mix (containing PNGase F from Elizabethkingia miricola, O-glycosidase from Enterococcus faecalis, α2-3,6,8,9 neuraminidase A from Arthrobacter ureafaciens, β1-4 galactosidase S from Streptococcus pneumoniae, and β-N-acetylhexosaminidasef from Streptomyces plicatus). The reactions were left to incubate at 37°C for 4 h, centrifuged for 10 min at 16,900 rpm (g), incubated with reducing SDS-PAGE buffer for 10 min at room temperature, centrifuged again and subsequently subjected to SDS-PAGE. Bovine fetuin (which contains O- and N-glycosylation) was used as a positive control for PNGase F and the deglycosylation mix.
N-glyan release and linkage-specific sialic acid derivatisation
Five microgram of the protein was denatured with 10 μL 2% sodium dodecyl sulfate (SDS; Merck, Darmstadt, Germany) by incubation for 10 min at 60°C. Subsequently the release step was performed by adding 10 μL of a mixture containing 2% Nonidet P-40 (NP-40) and 1 U recombinant peptide-N-glycosidase F (PNGase F; Roche Diagnostics, Mannheim, Germany) in 2.5× PBS, followed by overnight incubation at 37°C. The released glycans were derivatized by ethyl esterification as described by elsewhere (Reiding et al. 2014). Briefly, the derivatization reagent mixture contains 0.25 M 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide ((EDC); Fluorochem, Hadfield, UK) and 0.25 M 1-hydroxybenzotriazole ((HOBt); Sigma-Aldrich, Steinheim, Germany), in ethanol (Merck). Two microliter of released N-glycans were added to 20 μL of derivatization reagent and incubated 1 h at 37°C. Allowing to cool down to room temperature, the derivatized glycans were purified by hydrophilic interaction chromatography (HILIC) using pipette tips filled with cotton as stationary phase (Selman et al. 2011). Briefly, 20 μL of acetonitrile ((ACN); Biosolve, Valkenswaard, The Netherlands) was added to the reaction mixture. The cotton inserted tips were washed with three-times 20 μL of MQ water and three-times 20 μL of 85% ACN followed by sample loading by pipetting 20 times up and down. Finally, cotton tips were washed by pipetting three-times 20 μL 85% ACN containing 1% trifluoroacetic acid ((TFA); Merck) and three-times 20 μL 85% ACN prior to the elution of the derivatized glycan sample using 10 μL MQ.
MALDI-TOF-MS-(/MS) analysis of released N-glycans
Two microliter of purified derivatized glycans were spotted on a MTP AnchorChip 800/384 TF MALDI target plate (Bruker Daltonics) together with 1 μL of matrix (5 mg/mL super-DHB, 1 mM NaOH in 50% ACN) and left to dry at room temperature. Mass spectrometry was performed in reflectron positive mode on an UltrafleXtreme mass spectrometer with a Smartbeam-II laser (Bruker Daltonics) controlled by flexControl 3.4 (Build 135). Within a window of m/z 1000–5000, spectra were recorded with 10,000 laser shots at a frequency of 1000 Hz, employing a full sample random walking pattern of 100 shots per raster spot. Tandem mass spectrometry (MALDI-TOF/TOF-MS/MS) was performed on the most abundant peaks via laser-induced dissociation to confirm glycan composition (data not shown).
Data analysis of released N-glycans
For relative quantification and quality control of the spectra, the raw spectra were exported from flexAnalysis 3.4 (Build 76; Bruker Daltonics) as.txt file (x,y), and further processed by MassyTools (version 0.1.8.0) (Jansen et al. 2015). Internal calibration based on a predefined list of analytes was applied, and 15 visually detected glycan compositions were extracted by integrating the areas of 95% of the theoretical isotopic envelope and subtracting from these the background found within a window of 20 m/z. The areas were normalized to the sum of all areas, and derived traits were calculated based on the compositional features. Analyses were performed in duplicate.
Supplementary Material
Abbreviations
- ACN, acetonitrile; AMD, age-related macular degeneration; APTS, (3-Aminopropyl)triethoxysilane); CCP, complement control protein; GAG, glycosaminoglycans; LOS, lipooligosaccharide NHS, normal human serum; STD, saturation transfer difference; TFA, trifluoroacetic acid.
Funding
This work was supported by the German Research Council (DFG, BL 1294/2-1, to B.S.B.; SCHM 3018/2-2, to C.Q.S.).
Conflict of interest statement
None declared.
References
- Baud A, Gonnet F, Salard I, Le Mignon M, Giuliani A, Mercère P, Sclavi B, Daniel R. 2016. Probing the solution structure of factor H using hydroxyl radical protein footprinting and cross-linking. Biochem J. 473:1805–1819. [DOI] [PubMed] [Google Scholar]
- Bishop PN, Boulton M, McLeod D, Stoddart RW. 1993. Glycan localization within the human interphotoreceptor matrix and photoreceptor inner and outer segments. Glycobiology. 3:403–412. [DOI] [PubMed] [Google Scholar]
- Blaum BS. 2017. The lectin self of complement factor H. Curr Opin Struct Biol. 44:111–118. [DOI] [PubMed] [Google Scholar]
- Blaum BS, Frank M, Walker RC, Neu U, Stehle T. 2016. Complement Factor H and Simian Virus 40 bind the GM1 ganglioside in distinct conformations. Glycobiology. 26:532–539. [DOI] [PubMed] [Google Scholar]
- Blaum BS, Hannan JP, Herbert AP, Kavanagh D, Uhrín D, Stehle T. 2015. Structural basis for sialic acid-mediated self-recognition by complement factor H. Nat Chem Biol. 11:77–82. [DOI] [PubMed] [Google Scholar]
- Chou HH, Takematsu H, Diaz S, Iber J, Nickerson E, Wright KL, Muchmore EA, Nelson DL, Warren ST, Varki A. 1998. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 95:11751–11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Ridge LA, Herbert AP, Hakobyan S, Mulloy B, Lennon R, Würzner R, Morgan BP, Uhrín D, Bishop PN, Day AJ. 2013. Tissue-specific host recognition by complement factor H is mediated by differential activities of its glycosaminoglycan-binding regions. J Immunol. 190:2049–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SJ, Schmidt CQ, White AM, Hakobyan S, Morgan BP, Bishop PN. 2014. Identification of Factor H–like Protein 1 as the Predominant Complement Regulator in Bruch’s Membrane: Implications for Age-Related Macular Degeneration. J Immunol. 193:4962–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll MV, Sim RB. 2011. Complement in health and disease. Adv Drug Deliv Rev. 63:965–975. [DOI] [PubMed] [Google Scholar]
- de Jorge EG, Yebenes H, Serna M, Tortajada A, Llorca O, de Córdoba SR. 2018. How novel structures inform understanding of complement function. Semin Immunopathol. 40:3–14. [DOI] [PubMed] [Google Scholar]
- DiScipio RG. 1992. Ultrastructures and interactions of complement factors H and I. J Immunol. 149:2592–2599. [PubMed] [Google Scholar]
- Fearon DT. 1978. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci USA. 75:1971–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaille F, Le Mignon M, Groseil C, Ramon C, Riandé S, Siret L, Bihoreau N. 2007. Site-specific N-glycan characterization of human complement factor H. Glycobiology. 17:932–944. [DOI] [PubMed] [Google Scholar]
- Ferreira VP, Herbert AP, Cortés C, McKee KA, Blaum BS, Esswein ST, Uhrín D, Barlow PN, Pangburn MK, Kavanagh D. 2009. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J Immunol. 182:7009–7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. 2006. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J Immunol. 177:6308–6316. [DOI] [PubMed] [Google Scholar]
- Gagneux P, Cheriyan M, Hurtado-Ziola N, van der Linden ECMB, Anderson D, McClure H, Varki A, Varki NM. 2003. Human-specific regulation of α2–6-linked sialic acids. J Biol Chem. 278:48245–48250. [DOI] [PubMed] [Google Scholar]
- Garcia GG, Berger SB, Akha AAAS, Miller RA. 2005. Age-associated changes in glycosylation of CD43 and CD45 on mouse CD4 T cells. Eur J Immunol. 35:622–631. [DOI] [PubMed] [Google Scholar]
- Gulati S, Cox A, Lewis LA, Michael FS, Li J, Boden R, Ram S, Rice PA. 2005. Enhanced Factor H binding to sialylated gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: Implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in gonococci. Infect Immun. 73:7390–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati S, Schoenhofen IC, Whitfield DM, Cox AD, Li J, St. Michael F, Vinogradov EV, Stupak J, Zheng B, Ohnishi M et al. 2015. Utilizing CMP-sialic acid analogs to unravel Neisseria gonorrhoeae lipooligosaccharide-mediated complement resistance and design novel therapeutics. PLoS Pathog. 11:e1005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Tangvoranuntakul P, Takematsu H, Long JM, Housley GD, Kozutsumi Y, Suzuki A, Wynshaw-Boris A, Ryan AF, Gallo RL et al. 2007. N-glycolylneuraminic acid deficiency in mice: Implications for human biology and evolution. Mol Cell Biol. 27:4340–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert AP, Makou E, Chen ZA, Kerr H, Richards A, Rappsilber J, Barlow PN. 2015. Complement evasion mediated by enhancement of captured Factor H: Implications for protection of self-surfaces from complement. J Immunol. 195:4986–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen S, Meri S, Jokiranta TS. 2016. Disturbed sialic acid recognition on endothelial cells and platelets in complement attack causes atypical hemolytic uremic syndrome. Blood. 127:2701–2710. [DOI] [PubMed] [Google Scholar]
- Jansen BC, Reiding KR, Bondt A, Hipgrave Ederveen AL, Palmblad M, Falck D, Wuhrer M. 2015. MassyTools: A high-throughput targeted data processing tool for relative quantitation and quality control developed for glycomic and glycoproteomic MALDI-MS. J Proteome Res. 14:5088–5098. [DOI] [PubMed] [Google Scholar]
- Jouvin MH, Kazatchkine MD, Cahour A, Bernard N. 1984. Lysine residues, but not carbohydrates, are required for the regulatory function of H on the amplification C3 convertase of complement. J Immunol. 133:3250–3254. [PubMed] [Google Scholar]
- Kajander T, Lehtinen MJ, Hyvärinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. 2011. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proc Natl Acad Sci USA. 108:2897–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh D, Goodship TH, Richards A. 2013. Atypical hemolytic uremic syndrome. Semin Nephrol. 33:508–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimas NG, Caldwell KE, Whitney PL, Fletcher MA. 1982. Comparison of receptor properties of erythrocyte membrane glycoproteins. Dev Comp Immunol. 6:765–774. [PubMed] [Google Scholar]
- Langford-Smith A, Day AJ, Bishop PN, Clark SJ. 2015. Complementing the Sugar Code: Role of GAGs and sialic acid in complement regulation. Front Immunol. 6:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makou E, Mertens HDT, Maciejewski M, Soares DC, Matis I, Schmidt CQ, Herbert AP, Svergun DI, Barlow PN. 2012. Solution structure of CCP modules 10–12 illuminates functional architecture of the complement regulator, factor H. J Mol Biol. 424:295–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek MT, Bremer EG, Mold C. 1988. b. Effect of gangliosides on activation of the alternative pathway of human complement. J Immunol. 140:1581–1587. [PubMed] [Google Scholar]
- Michalek MT, Mold C, Bremer EG. 1988. a. Inhibition of the alternative pathway of human complement by structural analogues of sialic acid. J Immunol. 140:1588–1594. [PubMed] [Google Scholar]
- Morgan HP, Schmidt CQ, Guariento M, Blaum BS, Gillespie D, Herbert AP, Kavanagh D, Mertens HDT, Svergun DI, Johansson CM et al. 2011. Structural basis for engagement by complement factor H of C3b on a self surface. Nat Struct Mol Biol. 18:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng PSK, Böhm R, Hartley-Tassell LE, Steen JA, Wang H, Lukowski SW, Hawthorne PL, Trezise AEO, Coloe PJ, Grimmond SM et al. 2014. Ferrets exclusively synthesize Neu5Ac and express naturally humanized influenza A virus receptors. Nat Commun. 5:5750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann M, Manuelian T, Józsi M, Brandt E, Jokiranta TS, Heinen S, Meri S, Skerka C, Götze O, Zipfel PF. 2006. The C-terminus of complement regulator Factor H mediates target recognition: Evidence for a compact conformation of the native protein. Clin Exp Immunol. 144:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormsby RJ, Jokiranta TS, Duthy TG, Griggs KM, Sadlon TA, Giannakis E, Gordon DL. 2006. Localization of the third heparin-binding site in the human complement regulator factor H1. Mol Immunol. 43:1624–1632. [DOI] [PubMed] [Google Scholar]
- Pangburn MK, Atkinson MA, Meri S. 1991. Localization of the heparin-binding site on complement factor H. J Biol Chem. 266:16847–16853. [PubMed] [Google Scholar]
- Pangburn MK, Müller-Eberhard HJ. 1978. Complement C3 convertase: Cell surface restriction of beta1H control and generation of restriction on neuraminidase-treated cells. Proc Natl Acad Sci USA. 75:2416–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering MC, de Jorge EG, Martinez-Barricarte R, Recalde S, Garcia-Layana A, Rose KL, Moss J, Walport MJ, Cook HT, de Córdoba SR et al. 2007. Spontaneous hemolytic uremic syndrome triggered by complement factor H lacking surface recognition domains. J Exp Med. 204:1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pousset D. 1997. Increased α2,6 sialylation of N-glycans in a transgenic mouse model of hepatocellular carcinoma. Cancer Res. 57:4249–4256. [PubMed] [Google Scholar]
- Ram S, Sharma AK, Simpson SD, Gulati S, McQuillen DP, Pangburn MK, Rice PA. 1998. A novel sialic acid binding site on Factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 187:743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiding KR, Blank D, Kuijper DM, Deelder AM, Wuhrer M. 2014. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal Chem. 86:5784–5793. [DOI] [PubMed] [Google Scholar]
- Ripoche J, Day AJ, Harris TJ, Sim RB. 1988. The complete amino acid sequence of human complement factor H. Biochem J. 249:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Bai H, Lin Z, Risitano AM, Barlow PN, Ricklin D, Lambris JD. 2013. Rational engineering of a minimized immune inhibitor with unique triple-targeting properties. J Immunol. 190:5712–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrín D, Barlow PN. 2008. A new map of glycosaminoglycan and C3b binding sites on factor H. J Immunol. 181:2610–2619. [DOI] [PubMed] [Google Scholar]
- Schmidt CQ, Herbert AP, Mertens HDT, Guariento M, Soares DC, Uhrín D, Rowe AJ, Svergun DI, Barlow PN. 2010. The central portion of factor H (modules 10–15) is compact and contains a structurally deviant CCP module. J Mol Biol. 395:105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CQ, Slingsby FC, Richards A, Barlow PN. 2011. Production of biologically active complement factor H in therapeutically useful quantities. Protein Expr Purif. 76:254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaeble W, Zwirner J, Schulz TF, Linke RP, Dierich MP, Weiss EH. 1987. Human complement factor H: expression of an additional truncated gene product of 43 kDa in human liver. Eur J Immunol. 17:1485–1489. [DOI] [PubMed] [Google Scholar]
- Selman MHJ, Hemayatkar M, Deelder AM, Wuhrer M. 2011. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal Chem. 83:2492–2499. [DOI] [PubMed] [Google Scholar]
- Springer SA, Diaz SL, Gagneux P. 2014. Parallel evolution of a self-signal: humans and new world monkeys independently lost the cell surface sugar Neu5Gc. Immunogenetics. 66:671–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangvoranuntakul P, Gagneux P, Diaz S, Bardor M, Varki N, Varki A, Muchmore E. 2003. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 100:12045–12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schnaar RL, Schauer R. 2017. Sialic acids and other nonulosonic acids In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Essentials of Glycobiology, 3rd ed.Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press. [PubMed] [Google Scholar]
- Wu J, Wu Y-Q, Ricklin D, Janssen BJC, Lambris JD, Gros P. 2009. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat Immunol. 10:728–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Wu J, Ricklin D, Forneris F, Di Crescenzio P, Schmidt CQ, Granneman J, Sharp TH, Lambris JD, Gros P. 2017. Regulator-dependent mechanisms of C3b processing by factor I allow differentiation of immune responses. Nat Struct Mol Biol. 24:643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.