Abstract
Human siglecs are a family of 14 sialic acid-binding proteins, most of which are expressed on subsets of immune cells where they regulate immune responses. Siglec-8 is expressed selectively on human allergic inflammatory cells—primarily eosinophils and mast cells—where engagement causes eosinophil apoptosis and inhibits mast cell mediator release. Evidence supports a model in which human eosinophils and mast cells bind to Siglec-8 sialoglycan ligands on inflammatory target tissues to resolve allergic inflammation and limit tissue damage. To identify Siglec-8-binding sialoglycans from human airways, proteins extracted from postmortem human trachea were resolved by size-exclusion chromatography and composite agarose–acrylamide gel electrophoresis, blotted and probed by Siglec-8-Fc blot overlay. Three size classes of Siglec-8 ligands were identified: 250 kDa, 600 kDa and 1 MDa, each of which was purified by affinity chromatography using a recombinant pentameric form of Siglec-8. Proteomic mass spectrometry identified all size classes as the proteoglycan aggrecan, a finding validated by immunoblotting. Glycan array studies demonstrated Siglec-8 binding to synthetic glycans with a terminal Neu5Acα2-3(6-sulfo)-Gal determinant, a quantitatively minor terminus on keratan sulfate (KS) chains of aggrecan. Treating human tracheal extracts with sialidase or keratanase eliminated Siglec-8 binding, indicating sialylated KS chains as Siglec-8-binding determinants. Treating human tracheal histological sections with keratanase also completely eliminated the binding of Siglec-8-Fc. Finally, Siglec-8 ligand purified from human trachea extracts induced increased apoptosis of freshly isolated human eosinophils in vitro. We conclude that sialylated KS proteoglycans are endogenous human airway ligands that bind Siglec-8 and may regulate allergic inflammation.
Keywords: apoptosis, eosinophil, sialic acid, siglec, trachea
Introduction
Inflammation is carefully tuned to maximize clearance of pathogens while limiting host tissue damage (Fullerton and Gilroy 2016; Barnig et al. 2018). While pro-inflammatory signaling is essential for host protection, timely resolution is required to prevent excessive or chronic inflammatory tissue damage. Among the regulators of inflammation are members of the siglec family, sialic acid-binding immunoglobulin-like lectins, most of which are expressed on overlapping sets of immune cells and many of which carry intracellular immunoreceptor tyrosine-based inhibitory motif (ITIM) domains that aid in resolution of immune responses (Macauley et al. 2014). The focus of the current study, Siglec-8, is unique in its selective expression on human eosinophils, mast cells and basophils, all of which are implicated in allergic inflammation (Bochner 2016; Schleimer et al. 2016). Siglec-8 is a transmembrane protein with its outermost N-terminal Ig-like domain mediating sialic acid glycan binding and its intracellular domain carrying ITIM and ITIM-like domains. Experimental antibody-induced cross-linking of Siglec-8 on the surface of eosinophils results in transmembrane signaling that induces eosinophil apoptosis (Kano et al. 2013), whereas cross-linking Siglec-8 on mast cells inhibits release of immune mediators (Yokoi et al. 2008). Notably, the effects of cross-linking Siglec-8 are greater when inflammatory cells are in the active rather than quiescent state (Nutku-Bilir et al. 2008), suggesting that Siglec-8-mediated downregulation of inflammatory cells is a natural step in resolving ongoing allergic inflammation to limit tissue damage. This hypothesis is supported by the discovery of human Siglec-8 gene polymorphisms associated with susceptibility to asthma (Gao et al. 2010). A current hypothesis consistent with the data is that Siglec-8 on allergic inflammatory cells engages polyvalent complementary binding sialoglycans on target tissues as an integral step in resolving and appropriately limiting allergic inflammation. The identity and regulation of the Siglec-8-binding sialoglycans on inflammatory target tissues is the focus of ongoing investigations.
Although endogenous sialoglycan ligands for Siglec-8 had not been identified, screening of synthetic sialoglycans revealed that Siglec-8 is among the most specific lectins tested, favoring a sialic acid α2-3 linked to a galactose that is also carrying a sulfate ester on its 6-hydroxyl group (e.g., Neu5Acα2-3[6 S]Galβ1-4GlcNAc-R) (Bochner et al. 2005; Yu et al. 2017). This finding was confirmed by structural elucidation of Siglec-8 bound to 6′-sulfo-siayl Lewis X (Neu5Acα2-3[6 S]Galβ1-4(Fucα1-3)GlcNAc-R) in which the sulfate on the galactose engages an arginine (R56) in the binding pocket (Propster et al. 2016). The results implicate sialylated 6′-sulfated glycans on target tissues as functional Siglec-8 ligands.
In prior studies (Jia et al. 2015; Yu et al. 2017), we used an expressed soluble Siglec-8 human Fc chimera (Siglec-8-Fc) to probe for endogenous Siglec-8 ligands on human upper and lower airways—both clinically relevant targets of allergic inflammation. Using lectin overlay histochemistry of postmortem lower human airways, we found that Siglec-8 ligands are strongly expressed in tracheal and bronchial submucosal glands and cartilage. Mouse airways failed to bind Siglec-8-Fc, consistent with the absence of both Siglec-8 and Siglec-8 ligands in mouse airways. Furthermore, we found Siglec-8 ligands were extracted from human airway tissues using guanidinium hydrochloride (GuHCl) (but not nonionic detergents), were resolved by composite agarose–acrylamide electrophoresis primarily as very large-molecular weight species and were O-linked glycans but did not co-migrate with any single major airway mucin (Yu et al. 2017). In the current study, we purified Siglec-8 ligands from human tracheal extracts by sequential size-exclusion chromatography and affinity chromatography, identified them by proteomic mass spectrometry, validated the candidate molecules and revealed that sialylated KS proteoglycan(s) are the primary carrier of Siglec-8 ligands in human airways.
Results
Pentavalent Siglec chimeras for enhanced ligand avidity
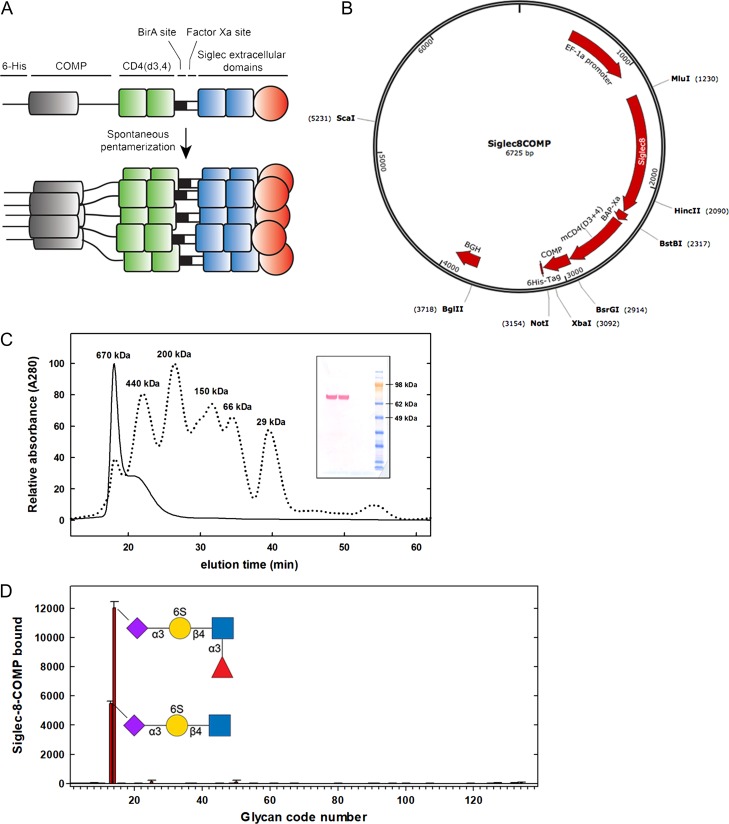
To provide sufficient multivalent avidity for high-stringency affinity purification of siglec ligands extracted from natural sources, a spontaneously pentamerizing soluble chimera of the extracellular domains of Siglec-8 was designed and created based on a previously published strategy (Voulgaraki et al. 2005). The three N-terminal extracellular Ig-like domains of human Siglec-8 were fused to a short spacer, two Ig domains from CD4, a spontaneously pentamerizing domain from cartilage oligomeric matrix protein and a 6-His C-terminal tag (Siglec-8-COMP, Figure 1A and B). On denaturing polyacrylamide gel electrophoresis, the expressed soluble protein product had a molecular weight of ~80 kDa as expected (Figure 1C, insert). Upon nondenaturing size-exclusion chromatography (Figure 1C), only multimeric species were detected, including an expected >400 kDa species and higher order aggregates that may be due to residual nickel cross-linking of the clustered His tag (Valenti et al. 2006).
Fig. 1.
Pentavalent Siglec-8-COMP. (A) Chimeric Siglec-8-COMP schematic design includes the extracellular domain of Siglec-8, two spacer domains from CD4, spontaneously pentamerizing COMP and a 6-His tag. (B) Plasmid design for Siglec-8-COMP. (C) Superdex-200 size-exclusion chromatography of expressed Siglec-8-COMP with protein size standards indicated. Denaturing SDS-PAGE gel (insert) immunostained for the 6-His tag indicates a monomer molecular mass of ~80 kDa. (D) Glycan array screening of Siglec-8-COMP binding. A custom glycan array containing 10 neutral glycans, 69 α2,3-linked sialoglycans and 56 α2,6-linked sialoglycans (Supplementary data, Table I) was probed with Siglec-8-COMP and binding detected with anti-6-His antibody. The mean and standard error for binding to six spots of each glycan are presented. Glycans that supported Siglec-8-COMP binding are shown using symbol nomenclature (Varki et al. 2015).
A direct comparison of Siglec-8-Fc and Siglec-8-COMP binding to an immobilized synthetic target neoglycolipid (Neu5Acα2-3[6 S]Galβ1-4GlcNAc β ethylamine covalently linked to N-(succinimidyloxy-glutaryl)-L-α-phosphatidylethanolamine, (Yu et al. 2017)) revealed a half-life of dimeric Fc chimera of minutes, whereas pentavalent Siglec-8-COMP had a binding half-life of nearly 20 h (data not shown). A specialty sialoglycan microarray was used to test the specificity of Siglec-8-COMP binding (Figure 1D). On an array containing 10 neutral glycans, 69 α2,3-linked sialoglycans and 56 α2,6-linked sialoglycans (Supplementary data, Table I) only two structures bound Siglec-8-COMP, both of which contained the sialylated 6′-sulfo-LacNAc substructure (Neu5Acα2-3[6 S]Galβ1-4GlcNAc) previously shown to bind Siglec-8-Fc (Bochner et al. 2005; Campanero-Rhodes et al. 2006; Kiwamoto et al. 2015; Schnaar 2016; Yu et al. 2017). Together, these data validate the COMP construct as a high-avidity specific Siglec-8 chimera suitable for affinity purification of natural Siglec-8 ligands.
Purification of three size classes of Siglec-8 ligands extracted from human trachea
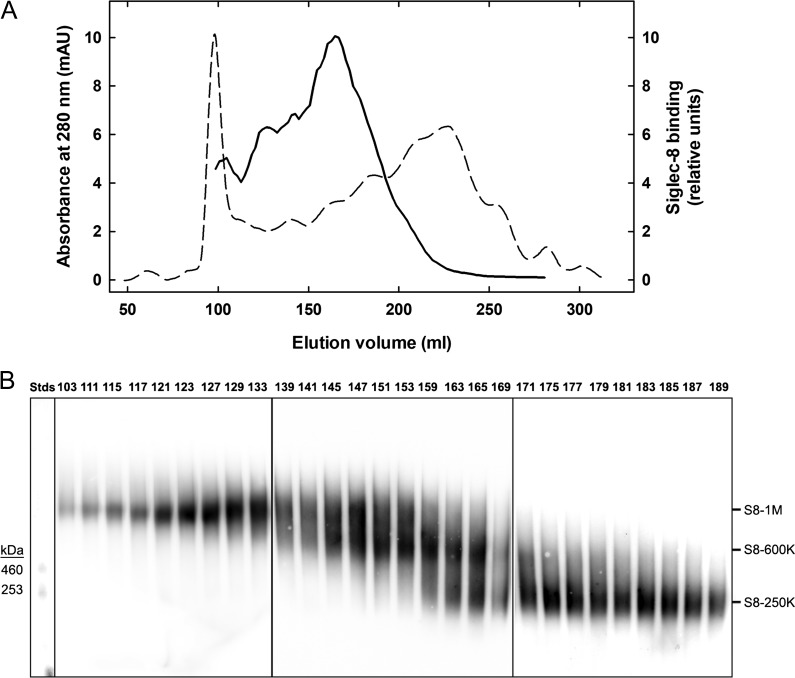
Our prior studies indicated that Siglec-8 ligands were preferentially extracted from postmortem human airways using GuHCl (Yu et al. 2017). When that extract was subjected to denaturing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), most of the Siglec-8 binding species were very large, hardly entering the gels. Electrophoresis in composite agarose–acrylamide gels designed for separation of mucins (Holden et al. 1971) revealed a heterogeneous mixture of proteins all in the high-molecular weight range (>200 kDa to ~1 MDa). Based on those findings, Siglec-8 ligands extracted from human trachea were further resolved by size-exclusion chromatography using Sephacryl S-500 for large size range molecules. The results (Figure 2) reveal a significant resolution of Siglec-8-binding components that eluted differently than the major protein peaks near the excluded or included volumes. Upon composite gel electrophoresis, size-exclusion chromatography fractions were resolved into three major size classes that, based on relative electrophoretic mobility, were designated based on their apparent molecular weights as S8-1M (~1 MDa), S8-600K (~600 kDa) and S8-250K (~250 kDa). Fractions highly enriched in S8-1M and S8-250K were obtained, with S8-600K fractions incompletely resolved from the other two size classes. When similar extracts from several different human tissue donors were subjected to the same size-exclusion chromatography and electrophoresis, the same three size classes were found with variable ratios among them (data not shown).
Fig. 2.
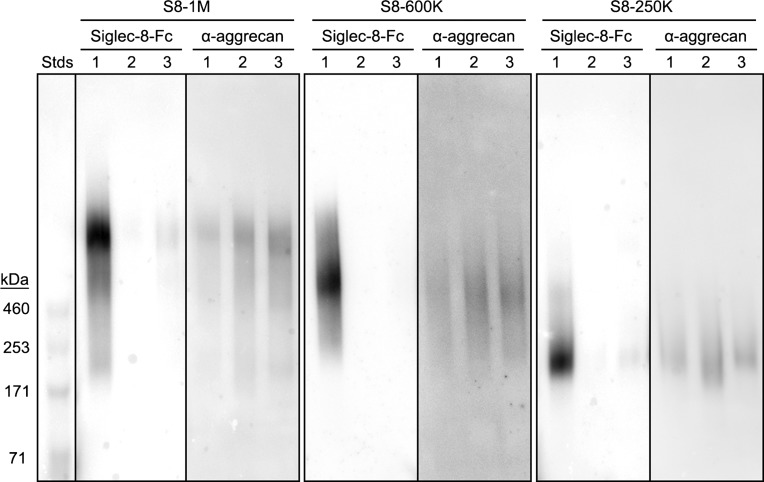
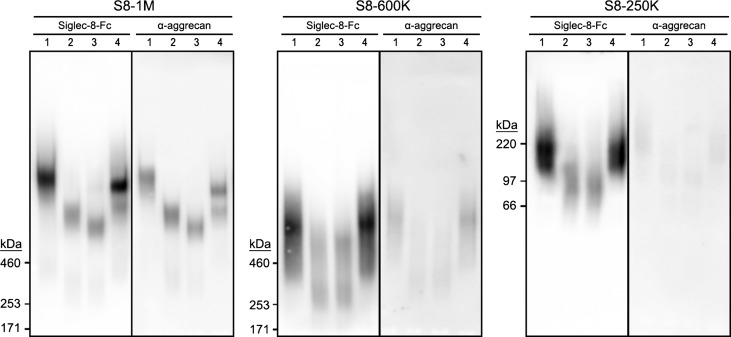
Chromatographic resolution of Siglec-8 ligands extracted from human airway. (A) Sephacryl S-500 column chromatography of Siglec-8 ligands extracted from human trachea. Elution of proteins (A280, dashed line) and Siglec-8 binding activity (solid line) determined by semiquantitative dot blot Siglec-8-Fc overlay are plotted against the elution volume. (B) Composite gel electrophoresis of active binding fractions. Equal aliquots of fractions with Siglec-8-Fc-binding activity detected by semiquantitative dot blot were resolved by 1.5% acrylamide, 2% agarose composite gel electrophoresis, blotted to PVDF membranes and probed for Siglec-8-Fc binding. Images of three replicate gels (boxed) used to accommodate fractions across the active elution fractions were stitched together by lining up common molecular weight standards (Stds, left). Fractions were pooled to represent three size classes of Siglec-8 ligands (designated by estimated molecular weight in daltons): S8-1M, S8-600K and S8-250K.
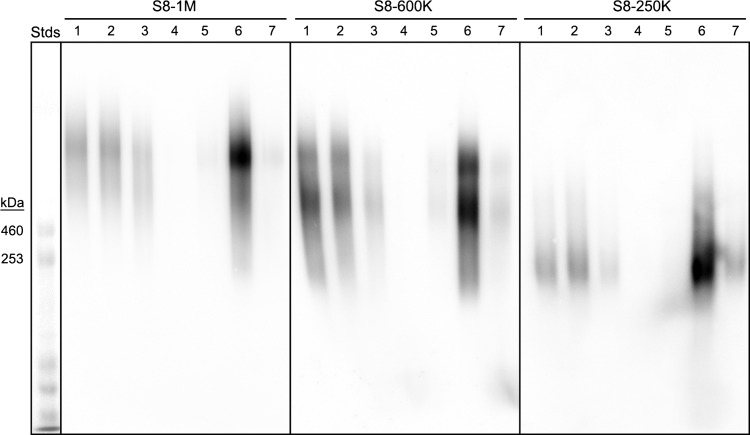
Further purification of pooled size classes of human tracheal Siglec-8 ligands was performed by affinity chromatography using Siglec-8-COMP immobilized on nickel Sepharose columns (Figure 3). For each size class, an aliquot of combined fractions from size-exclusion chromatography was precleared on a nickel Sepharose column and then passed through the Siglec-8-COMP column. In every case only a fraction of the Siglec-8-binding material was retained. After thorough washing, the majority of the bound Siglec-8 ligands were concentrated in the eluted fractions. Elution was accomplished by the release of Siglec-8-COMP using imidazole to compete with nickel binding, releasing Siglec-8 ligand and Siglec-8-COMP (data not shown). Subsequent studies revealed that each Siglec-8 ligand was efficiently eluted from the same column by increasing the salt concentration, leaving the Siglec-8-COMP bound to the nickel column. This not only improved downstream analyses of the purified ligands (by avoiding co-elution with Siglec-8-COMP) but also allowed repeated sequential affinity column loading of the unbound material (at least three times) until most of the size-separated ligand was affinity captured and eluted.
Fig. 3.
Siglec-8-COMP affinity purification of Siglec-8 ligands from human trachea. Siglec-8 ligands extracted from human trachea were resolved by Sephacryl S-500 size-exclusion chromatography (Figure 2) and fractions corresponding to S8-1M, S8-600K and S8-250K separately combined for affinity purification. Pooled fractions of each molecular size were loaded separately onto 1-mL nickel Sepharose affinity columns carrying 6-His-tagged pentameric Siglec-8-COMP. After loading and washing each column, bound Siglec-8 ligands were eluted with high salt. Equal aliquots of washes and eluates were subjected to 1.5% acrylamide, 2% agarose composite gel electrophoresis, blotted to PVDF membranes and probed with Siglec-8-Fc. Lanes: (1) pooled Sephacryl S-500 fraction; (2) affinity column flow-through; (3) wash 1; (4) wash 2; (5) elution 1; (6) elution 2; (7) elution 3. HiMark-prestained molecular weight standards are shown at the left (Stds).
Identification of aggrecan as a carrier of Siglec-8 sialoglycan ligands
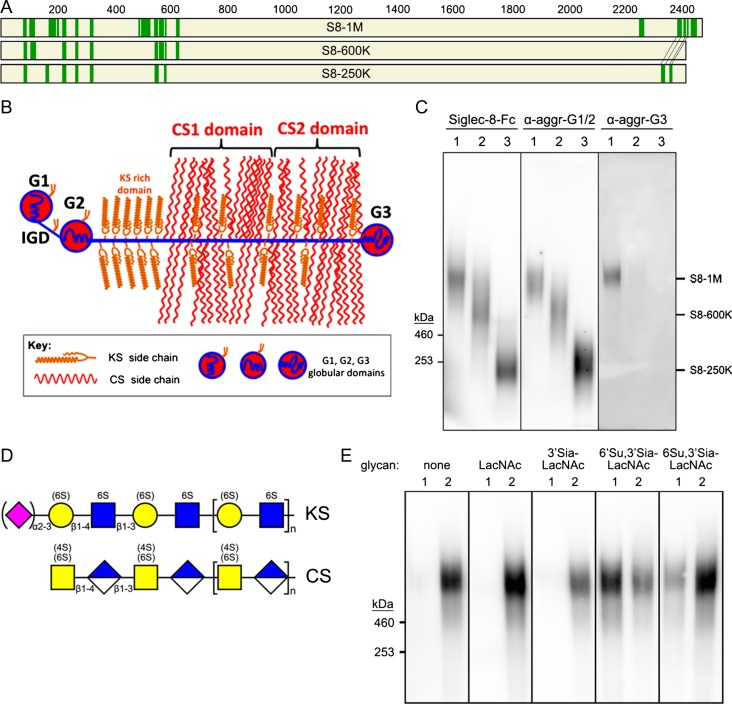
Affinity-purified Siglec-8-binding proteins of each size class were subjected to proteomic mass spectrometry. The protein with the highest confidence in every size class (excluding contaminating keratin) was aggrecan (Figure 4A). Eighteen separate aggrecan peptides were identified among the three size classes (Table I), distributed in three distinct regions of the protein. Aggrecan is a large (>2,000 amino acid) protein with three long proteoglycan domains—one keratan sulfate (KS)-rich and two chondroitin sulfate (CS)-rich domains—and three globular domains (Figure 4B). All of the identified peptides were in the globular domains, consistent with the proteolytic and proteomic identification methods used.
Fig. 4.
Aggrecan carries Siglec-8-binding glycans. (A) Proteins extracted from human trachea were purified by sequential size-exclusion and Siglec-8 affinity chromatography, proteolyzed, and peptides identified by mass spectrometry. Maps of aggrecan protein sequences with identified peptides from three size classes of affinity-purified Siglec-8 ligands are shown. Green bars are peptides (see Table I) that exceed strict false discovery rates for each of the Siglec-8 ligand size classes as indicated. The smaller size classes (S8-600 K and S8-250K) are mapped on aggrecan Uniprot reference sequence P16112 (2415 amino acids); whereas the largest size class (S8-1M) is mapped on C-terminal alternatively spliced aggrecan sequence H0YM81 (2492 amino acids). (B) Schematic map of aggrecan. IGD, interglobular domain; CS1, CS2, chondroitin sulfate (CS)-rich domains. Modified from Caterson and Melrose (2018), with permission. (C) Co-migration of purified human Siglec-8 ligands and aggrecan immunoreactivity. Three purified size classes of Siglec-8 ligands from human trachea were resolved by electrophoresis on replicate 1.5% acrylamide, 2% agarose composite gels, blotted to PVDF membranes and probed with Siglec-8-Fc, anti-aggrecan antibody 7D4 (α-aggr-G1/2) or anti-aggrecan antibody PA1-1745 (α-aggr-G3). Migration positions of HiMark-prestained standards are indicated at the left. Lanes: (1) S81M; (2) S8-600K and (3) S8-250K. (D) Generalized schematic structures of KS and CS chains depicted using symbol nomenclature (Varki et al. 2015). Sialic acid and sulfates that are variable are shown in parentheses. (E) Elution of S8-1M from Siglec-8-COMP affinity chromatography with soluble glycans. Pooled size-exclusion fractions containing S8-1M (Figure 2) were captured on Siglec-8-COMP magnetic beads. The beads were thoroughly washed prior to eluting with β-azidoethylglycosides (lane 1) followed by 500 mM imidazole to elute the bound Siglec-8-COMP with any remaining ligand attached (lane 2). Eluates were resolved by composite gel electrophoresis, blotted and probed with Siglec-8-Fc. Elution was tested with the following β-azidoethylglycosides: none, N-acetyllactosamine (LacNAc, Galβ1-4GlcNAc), 3′-sialyl LacNac (3′Sia-LacNAc, Neu5Acα2-3Galβ1-4GlcNAc), 6′-sulfo-3′-sialyl-LacNAc (6′Su,3′Sia-LacNAc, Neu5Acα2-3[6 S]Galβ1-4GlcNAc) and 6-sulfo-3′-sialyl-LacNAc (6 Su,3′Sia-LacNAc, Neu5Acα2-3Galβ1-4[6S]GlcNAc).
Table I.
Aggrecan peptides identified by mass spectrometry proteomics in Siglec-8 ligands purified from human trachea
| Sequest HT Xcorr values | ||||
|---|---|---|---|---|
| aa# | Sequence | S8-1M | S8-600K | S8-250K |
| 81 | EKEVVLLVATEGR | 3.13 | 4.88 | 5.08 |
| 104 | VSLPNYPAIPSDATLEVQSLR | 4.03 | 5.88 | |
| 159 | AISTRYTLDFDR | 4.86b | ||
| 174 | ACLQNSAIIATPEQLQAAYEDGFHQCDAGWLADQTVR | 5.13 | ||
| 218 | EGCYGDKDEFPGVR | 2.91 | 3.66 | 3.94 |
| 264 | FTFQEAANECR | 3.38 | 4.32 | 4.47 |
| 314 | ARPNCGGNLLGVR | 3.67 | 5.44 | 4.14 |
| 489a | YSLTFEEAQQACLR | 4.62 | ||
| 503 | TGAVIASPEQLQAAYEAGYEQCDAGWLR | 6.04 | ||
| 543 | TPCVGDKDSSPGVR | 3.42 | 4.36 | 4.52 |
| 557 | TYGVRPSTETYDVYCFVDR | 3.66 | 6.82 | |
| 576 | LEGEVFFATR | 3.73 | 4.11 | 3.87 |
| 621/622c | CYAGWLADGSLR | 3.61 | 4.43 | |
| 2271a | ESESTAADQEVCEEGWNK | 4.17 | ||
| 2328/2405c | GTVACGEPPVVEHAR | 2.95 | 4.15b | |
| 2428 | YEINSLVR | 2.52 | ||
| 2359/2436c | YQCTEGFVQR | 3.01 | 3.40 | |
| 2452a | CQPSGHWEEPQITCTDPTTYK | 4.04 | ||
Peptide positions and sequences for the 18 peptides identified with high confidence from three size classes of human tracheal Siglec-8 ligands are shown. Peptides are matched to human reference sequence accession number P16112 (S8-600K and S8-250K) or alternatively spliced version accession number H0YM81 (S8-1M). Amino acid numbers for the two forms are identical except as noted. Xcorr values are for collision-induced dissociation except as noted.
aFound only in H0YM81.
bValue for electron transfer dissociation mode.
To confirm the proteomic identification, replicate composite gel blots of three size classes were probed with Siglec-8-Fc to reveal Siglec-8-binding and anti-aggrecan antibodies (Figure 4C). Anti-aggrecan antibody blotting was found at the same relative electrophoretic migration positions of each of the affinity-purified Siglec-8 ligand size classes when using an antibody that binds to the first two globular domains of aggrecan (G1/IGD/G2, Figure 4B). Purified S8-1M also bound to an anti-aggrecan G3-specific antibody, whereas the other size classes did not, whereas proteomics data for S8-600K lacked peptides from the G3 region, perhaps indicating truncation, high-confidence peptides from that region were found in S8-250 K (Table 1), indicating that the loss of G3 antibody-reactive protein was not exclusively due to lack of the C-terminus. All nine of the high-confidence peptides identified in S8-600K proteomics were also found in S8-1M, as were eight of the nine high-confidence peptides in S8-250K.
Identification of sialylated KS chains as Siglec-8 sialoglycan ligands
Aggrecan typically carries significant amounts of two major types of proteoglycan chains, KS and CS (Figure 4D), whereas CS chains are generally not sialylated, a small proportion of KS chains can carry a terminal α2-3-linked sialic acid. Furthermore, a portion of galactose residues in KS may carry a 6-sulfate. Together, this can generate the terminal sialylated sulfated disaccharide (Neu5Acα2-3[6 S]Gal) common to the synthetic glycans discovered as Siglec-8 ligands by multiple glycan array studies (Bochner et al. 2005; Campanero-Rhodes et al. 2006; Kiwamoto et al. 2015; Yu et al. 2017), and in Figure 1D. To test whether affinity binding to Siglec-8-COMP immobilized on beads was via this glycan binding site, different glycans were tested for their ability to elute one of the ligands (S8-1M) once bound to the beads (Figure 4E). Incubating with buffer alone failed to elute any of the ligand. Likewise, incubation with β-azidoethylglycosides of N-acetyllactosamine (LacNAc) or 3′sialyl-LacNAc failed to elute ligand. In contrast, incubation with 6′sulfo-3′-sialyl-LacNAc released the majority of ligand from Siglec-8-COMP beads, and a small amount was released with 6-sulfo-3′-sialyl-LacNAc. These data suggest that binding of the purified ligand to the affinity beads was via the Siglec-8 sialoglycan-binding site.
To further test the hypothesis that sialylated termini on KS chains are responsible for Siglec-8 binding to human airway ligands, a series of directed depolymerization experiments were performed. First, each size class of purified human tracheal Siglec-8 ligand was subjected to treatment with sialidase or keratanase II. For each size class, treatment with either enzyme eliminated or greatly diminished Siglec-8 binding upon subsequent electrophoretic resolution, blotting and Siglec-8-Fc overlay (Figure 5). Treatment with the same enzymes left the protein carrier, aggrecan, intact as evidenced by binding of anti-aggrecan antibody (G1/G2 specific). Together, these data indicate that enzymatic removal of sialylated KS chains are responsible for Siglec-8 binding to each ligand size class. Consistent with prior findings (Yu et al. 2017), treatment with PNGase F to remove N-linked glycans had little effect, whereas mild base treatment (β-elimination) resulted in disappearance of Siglec-8 binding consistent with the conclusion that Siglec-8-binding KS chains are O-linked (Supplementary data, Figure 1).
Fig. 5.
Keratanase and sialidase pretreatments diminish Siglec-8 binding to purified Siglec-8 ligands. Siglec-8 ligands were extracted from human trachea, purified by sequential size-exclusion and Siglec-8 affinity chromatography and subjected to keratanase or sialidase treatments. Samples were resolved on 1.5% acrylamide, 2% agarose composite gels, blotted to PVDF membranes and probed with precomplexed Siglec-8-Fc or anti-aggrecan antibody (7D4) as indicated. Lanes: (1) incubation without enzyme; (2) sialidase (67 mU/mL, 90 min); (3) keratanase II (6 mU/mL, 16 h). HiMark molecular weight standards are shown at the left (Stds).
As further confirmation of the role of sialylated KS chains as Siglec-8 ligands from human airways, two different classes and sources of keratanase were compared. Both greatly diminished or eliminated Siglec-8 binding to purified Siglec-8 ligands. Keratanase II (Figure 5, lanes 3) is an endo-N-acetylglucosaminidase that prefers areas of high sulfation, whereas keratanase I (see Figure 7, lanes 3) is an endo-galactosidase that requires areas of low sulfation. Keratanase activities were independently determined chemically (by production of reducing ends from commercial KS) and immunochemically: Keratanase I activity on Siglec-8 ligands was confirmed by gain of binding of monoclonal antibody BKS-1 (Akhtar et al. 2008) that detects keratanase I-generated KS stubs (GlcNAc-6-sulfate termini). Keratanase II activity was confirmed by loss of binding of monoclonal antibody 5D4 (Caterson et al. 1983) that detects highly sulfated KS. Both enzyme preparations tested negative for contaminating sialidase activity (data not shown).
Fig. 7.
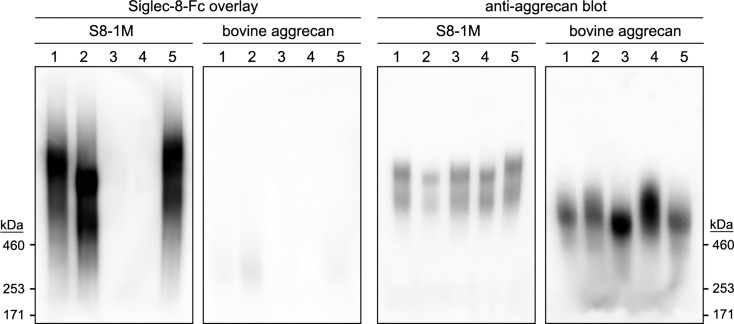
Chondroitinase ABC (ChABC) and keratanase treatments of a human tracheal Siglec-8 ligand reveal a chondroitin sulfate (CS) proteoglycan with Siglec-8-binding keratan sulfate (KS) chains. Siglec-8 ligands were extracted from human trachea and S8-1M purified by sequential size-exclusion and Siglec-8 affinity chromatography. Equal aliquots containing the isolated ligand (S8-1M) or commercial bovine articular cartilage aggrecan (bovine aggrecan) were treated with buffer alone (no enzyme), ChABC, keratanase I or both enzymes for 20 h at 37°C. Samples were denatured and resolved on 1.5% acrylamide, 2% agarose composite gels, blotted to PVDF membranes and probed with precomplexed Siglec-8-Fc overlay or anti-aggrecan antibody. Lanes (all enzyme treatments 20 h at 37°C): (1) no incubation; (2) ChABC (500 mU/mL); (3) keratanase I (21 mU/mL); (4) ChABC (500 mU/mL) plus keratanase I (21 mU/mL); (5) incubation without enzymes. Migration positions of HiMark molecular weight markers are shown.
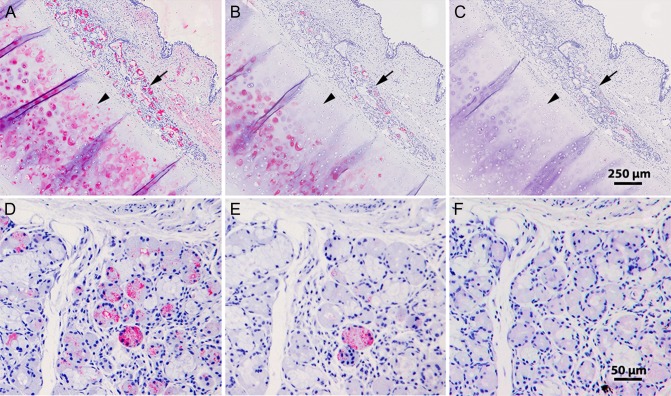
Siglec-8-Fc overlay histochemistry revealed Siglec-8 binding to cross sections of fixed human trachea but not mouse trachea (Jia et al. 2015; Yu et al. 2017). In human trachea, strong binding was seen in submucosal glands and underlying cartilage. All binding of Siglec-8 to human tracheal cross sections was completely eliminated by sialidase pretreatment. Consistent with the hypothesis that human tracheal Siglec-8 ligands are sialylated KS chains, similar reductions in binding were obtained when histological sections of human trachea were pretreated with keratanases (Figure 6). Keratanase I pretreatment significantly reduced subsequent Siglec-8-Fc binding to both cartilage and submucosal glands, and keratanase II treatment completely eliminated binding. We conclude that all human airway Siglec-8 ligands detectable by lectin Siglec-8-Fc overlay are sialylated KS proteoglycans.
Fig. 6.
Keratanase treatment of human trachea tissue sections diminishes Siglec-8-Fc binding. Cross sections of human trachea were incubated with Siglec-8-Fc precomplexed with AP-conjugated anti-human-Fc (A–C) or Siglec-8-Fc followed with the same secondary antibody (D–F) as described in the text. Lectin binding was detected using Vector Red stain and sections counterstained using hematoxylin QS. (A–C) Low-power images of serial sections of human trachea preincubated for 24 h at 37°C with buffer alone (A), 0.2 mU/mL keratanase I (B), or 5 mU/mL keratanase II (C) prior to Siglec-8-Fc overlay. Arrows, submucosal glands; arrowheads, cartilage. (D–F) Higher power images of human tracheal submucosal glands from sections preincubated for 25 h at 37°C with buffer alone (D), 21 mU/mL keratanase I (E), or 10 mU/mL keratanase II (F).
Human trachea aggrecan but not bovine articular cartilage aggrecan expresses Siglec-8-binding sialylated KS chains
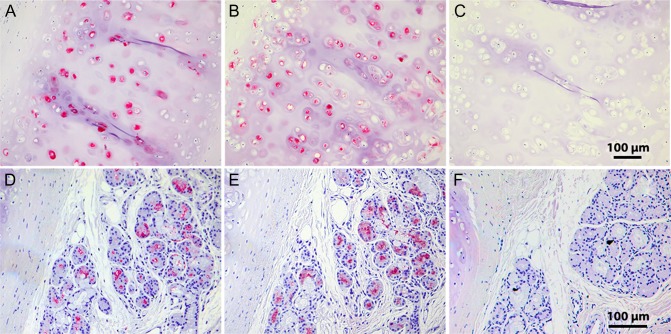
Based on the requirement of sialic acid α2-3 linked to a 6-sulfated galactose for binding (Propster et al. 2016), and the requirement for GlcNAc-6-sulfation for KS chain elongation (Funderburgh 2002), we propose that Siglec-8-binding determinants on aggrecan are KS chains with the terminal disulfated trisaccharide structure Neu5Acα2-3[6 S]Galβ1-4[6 S]GlcNAc. This is a minor but well-established structural KS terminus in vertebrates including KS from abundant sources such as human and bovine cornea and articular cartilage (Brown et al. 1994, 1998; Lauder et al. 1995, 1997; Funderburgh 2000). To test the distribution of Siglec-8-binding determinants on other sources of aggrecan, the purified human Siglec-8 ligand S8-1M was compared with commercially obtained bovine articular cartilage. For comparable amounts of anti-aggrecan immunoreactivity human S8-1M robustly bound Siglec-8-Fc, whereas binding to bovine aggrecan was essentially absent (Figure 7, lane 1). To test the potential of CS chains on aggrecan to support or block Siglec-8 binding, S8-1M and bovine aggrecan were treated with chondroitinase ABC (ChABC) prior to electrophoretic resolution and blotting. ChABC treatment shifted the migration position of S8-1M and increased Siglec-8 binding (Figure 7, lane 2). In contrast, ChABC had little effect on commercial bovine aggrecan but revealed a very minor Siglec-8-binding component that did not co-migrate with anti-aggrecan binding. Siglec-8 binding was eliminated by treatment with keratanase, either alone or after treatment of ChABC. Consistent with these findings, pretreatment of tissue sections of human trachea with ChABC resulted in equal (cartilage) or increased (submucosal glands) binding of Siglec-8-Fc (Figure 8). After ChABC treatment, subsequent treatment with keratanase completely eliminated Siglec-8 binding.
Fig. 8.
Chondroitinase ABC (ChABC) treatment of human trachea tissue sections enhances Siglec-8-Fc binding. Cross sections of human trachea were incubated with Siglec-8-Fc precomplexed with AP-conjugated anti-human-Fc. Lectin binding was detected using Vector Red stain and sections counterstained using hematoxylin QS. (A–C) Human tracheal cartilage from sections treated: (A) for 44 h with buffer alone; (B) for 18 h with 150 mU/mL ChABC followed by 25 h with buffer alone, or (C) for 18 h with 150 mU/mL ChABC followed by a 1-h wash and further incubation for 25 h with 10 mU/mL keratanase II. (D–F) Human trachea submucosal glands from the same experiment: (D), buffer incubation; (E) ChABC followed by buffer incubation; (F) ChABC followed by keratanase II.
Additional validation of the identity of the Siglec-8 ligands extracted from human trachea was obtained by treating each purified size class with the protease ADAMTS-4 (also known as aggrecanase-1) that cleaves selected sites in aggrecan (Figure 9). For each size class of Siglec-8 ligand, ADAMTS-4 treatment resulted in a shift of molecular size accompanied by reduction (but not elimination) of Siglec-8 binding. In each case, the remaining Siglec-8-Fc binding co-migrated on electrophoresis with antibody reactivity for the G1/IGD/G2 region of aggrecan, suggesting that Siglec-8-binding proteolytic fragments were from the N-terminal (KS-rich) regions of aggrecan.
Fig. 9.
ADAMTS-4 (aggrecanase-1) treatment of human airway Siglec-8 ligands shifts their electrophoretic migration. Siglec-8 ligands were extracted from human trachea, purified by sequential size exclusion followed by Siglec-8 affinity chromatography and subjected to ChABC, aggrecanase or both enzymes. Samples were resolved on 1.5% acrylamide, 2% agarose composite gels, blotted to PVDF membranes and probed with precomplexed Siglec-8-Fc or anti-aggrecan antibody (7D4) as indicated. Lanes (all enzyme treatments 16 h at 37°C): (1) incubation without enzyme; (2) aggrecanase (0.3 mU/mL); (3) aggrecanase (0.3 mU/mL) plus ChABC (0.5 U/mL); (4) ChABC (0.5 U/mL). S8-1M and S8-600K were resolved on 1.5% acrylamide, 2% agarose composite gels with migration positions of HiMark molecular weight markers shown; S8-250K was resolved on 3% acrylamide, 2% agarose composite gels with migration positions of SeeBlue Plus2 molecular weight markers shown.
Human tracheal Siglec-8 ligand increases apoptosis of primary human eosinophils
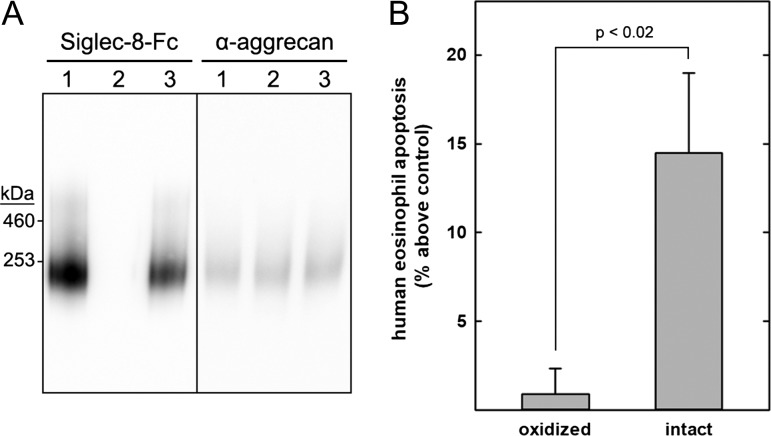
S8-250K, an abundant human tracheal Siglec-8 ligand with good chromatographic resolution (Figure 2), was purified from a single donor for combined structural and functional studies. Pooled S8-250K fractions from three consecutive Sephacryl S-500 size-exclusion runs were loaded repeatedly onto a Siglec-8-COMP affinity column, the column was washed each time and the bound ligand was salt-eluted as in Figure 3. After reloading the flow-through four times, most of the S8-250K in the sample had been affinity purified. Eluted fractions from the affinity chromatography runs were pooled, a portion of the combined fractions was subjected to mass spectrometric proteomics (Figure 4) and another portion was used to test biological activity. Matched experimental and control samples were prepared by treating equal portions of purified S8-250K on ice with or without addition of 6 mM sodium periodate for 30 min, then both samples with excess glycerol (300 mM) followed by 10 mM sodium borohydride on ice for 30 min. Cold mild periodate treatment results in selective oxidation of sialic acid glycerol (C7-C9) side chains and completely abrogates Siglec-8 binding (Figure 10A).
Fig. 10.
Purified human tracheal Siglec-8 ligand induces human eosinophil apoptosis. Siglec-8 ligands were extracted from human trachea and purified by size-exclusion chromatography followed by Siglec-8 affinity chromatography. A portion of purified S8-250K was treated with cold periodate to selectively oxidize the glycerol sidearm of its sialic acid followed by sodium borohydride reduction (negative control). An equal portion was incubated without periodate and treated with sodium borohydride (intact ligand). Oxidized and intact S8-250K were dialyzed against RPMI medium and added at equal concentrations to freshly isolated primary human eosinophils. After 24 h in culture, eosinophil apoptosis was quantified by flow cytometry. (A) Oxidized and intact S8-250 K were electrophoretically resolved on a composite agarose–acrylamide gel, blotted to PVDF membranes. Replicate blots were probed with Siglec-8-Fc to reveal Siglec-8 ligands or anti-aggrecan antibody (7D4). Lanes: (1) untreated S8-250K; (2) periodate oxidized and reduced S8-250K; (3) reduced S8-250K (intact ligand). (B) After isolation and overnight interleukin 5 (IL-5) priming, human eosinophils were incubated with equal portions of oxidized and intact S8-250K and apoptosis was assessed 18–24 h later. Results are expressed relative to untreated eosinophils, which had average apoptosis of 42 ± 5% (SEM). Data are displayed as mean and SEM of six replicates performed on three separate primary human eosinophil preparations.
Freshly isolated human peripheral blood eosinophils were cultured overnight with interleukin 5 (IL-5) to activate them, then for an additional 24 h with equal portions of intact or oxidized S8-250K. The oxidized ligand had no effect, whereas the intact ligand significantly increased human eosinophil apoptosis (Figure 10B). Although other siglecs are expressed on eosinophils, the ability of a ligand purified by Siglec-8 affinity chromatography to induce eosinophil apoptosis suggests the response is via Siglec-8 binding.
Discussion
Most of the members of the siglec family of sialic acid-binding proteins (13 of 14 in humans) are expressed on subsets of immune cells and are believed to be immune regulatory, either inhibiting or activating depending on the particular siglec and cellular context (Macauley et al. 2014; Varki et al. 2017). Many siglecs carry ITIM and/or ITIM-like domain(s) on their intracellular C-terminus, implicating a role in inhibitory immune regulation. This implication is supported by experiments using human immune cells and animal models (Nutku et al. 2003; von Gunten et al. 2005; Yokoi et al. 2008; Cho et al. 2010; McMillan et al. 2013), indicating that engagement of certain siglecs results in immune cell apoptosis or other events associated with immune inhibition (e.g., blocking of immune mediator release). Presumably, immune cells that carry siglecs on their surface come into contact with optimal sialoglycan ligands on immune target tissues resulting in transmembrane signals that initiate immune inhibition (Schleimer et al. 2016). This scenario is particularly well established in the case of Siglec-8 on the surface of eosinophils and mast cells, where experimental treatment with anti-Siglec-8 antibodies or synthetic multivalent 6′-sulfated sialoglycan polymers results in β2-integrin and reactive oxygen species-dependent apoptosis of activated eosinophils and/or inhibition of immune mediator release by activated mast cells (Nutku-Bilir et al. 2008; Yokoi et al. 2008; Hudson et al. 2009; Carroll et al. 2017). This inhibition is believed to be a component of the natural resolution of the immune response, and these findings are a potential basis for anti-inflammatory therapy (Bochner 2016; Kiwamoto et al. 2012).
The identities of endogenous sialoglycan ligands that engage Siglec-8 in vivo have yet to be established. This cannot be addressed using most animal models, since Siglec-8 is found in Hominidae (human, chimpanzee and orangutan) but not monkeys, dogs or rodents (Angata et al. 2004; Hudson et al. 2011). Although mice have a functional paralog, Siglec-F, on their eosinophils, Siglec-8-binding ligands are absent from mouse airways (Yu et al. 2017) and Siglec-F-dependent apoptosis of mouse eosinophils is quite modest in comparison to that in humans (Mao et al. 2013). These observations make it imperative to use human airways as the tissue source for isolating and identifying human airway Siglec-8 ligands.
The data presented here when considered with prior published data indicate that sialylated KS with the terminal sequence “Neu5Acα2-3[6 S]Galβ1-4[6 S]GlcNAc” (perhaps in conjunction with other determinants) are endogenous Siglec-8 ligands in human airways. Prior glycan array binding (Bochner et al. 2005; Schnaar 2016) and the custom sialoglycan array data reported here (Figure 1D) indicate that Siglec-8 is among the most specific lectins known, binding only to an α2-3 sialic acid when it is bound to a 6-sulfated galactose. On the 610-glycan array of the Consortium for Functional Glycomics (CFG, http://www.functionalglycomics.org) and the 125-sialoglycan array tested here (Figure 1D), Siglec-8 bound only to 6′-sulfo sialyl LacNAc (Neu5Acα2-3[6 S]Galβ1-4GlcNAc) and 6′-sulfo sialyl Lewis X (Neu5Acα2-3[6 S]Galβ1-4[Fucα1-3]GlcNAc). It failed to bind at all to the same structures missing the sialic acid ([6 S]Galβ1-4GlcNAc), structures missing the sulfate (Neu5Acα2-3Galβ1-4GlcNAc; Neu5Acα2-3Galβ1-4[Fucα1-3]GlcNAc), or when the sulfate was on the 6-hydroxyl of GlcNAc instead of Gal (Neu5Acα2-3Galβ1-4[6 S]GlcNAc; Neu5Acα2-3Galβ1-4[Fucα1-3][6 S]GlcNAc).
The molecular basis for this Siglec-8 specificity was determined by NMR structural analysis (Propster et al. 2016). The sialic acid-binding site, with the sialic acid carboxylate making a salt bridge to R109, is flanked by a secondary binding site (R56, Q59) that makes salt bridge and/or hydrogen bonds to the 6-sulfate on the galactose. Together, these clamp the terminal structure (Neu5Acα2-3[6 S]Gal) in place. We propose that since GlcNAc residues in KS disaccharides are uniformly 6-sulfated (Lauder et al. 1995; Brown et al. 1998; Seko et al. 2003), that the sialylated disulfated trisaccharide (Neu5Acα2-3[6 S]Galβ1-4[6 S]GlcNAc) on KS is a key binding component for Siglec-8. Comparisons of Siglec-8 binding to the 6′-sulfated and 6,6′-disulfated forms of sialyl Lewis X (Neu5Acα2-3[6 S]Galβ1-4[Fucα1-3]GlcNAc; Neu5Acα2-3[6 S]Galβ1-4[Fucα1-3][6 S]GlcNAc) by array binding (Campanero-Rhodes et al. 2006) and NMR titration (Propster et al. 2016) revealed only a modest effect of the additional sulfate on the GlcNAc residue. Isolation of intact KS chains from human airways and comparison of Siglec-8-binding and nonbinding species may reveal further subtlety or complexity in endogenous Siglec-8-binding determinants.
In addition to sialylation and sulfation, KS chains can also be fucosylated. The role of fucose in binding to endogenous Siglec-8 ligands isolated from human airways has not been determined. However, glycan array binding has consistently shown that fucosylated and nonfucosylated versions of the same glycan (having a terminal sialylated 6′-sulfated galactose) bind Siglec-8. On the current array (Figure 1D), Siglec-8-COMP bound somewhat better to the fucosylated form, whereas on the CFG array the reverse was true (Yu et al. 2017). Isolation of endogenous Siglec-8-binding KS chains will be required to resolve the potential presence and role of fucose in Siglec-8 binding.
The previously reported sialidase-sensitive submucosal gland and cartilage-binding determinants for Siglec-8 on human airways (Jia et al. 2015; Yu et al. 2017) all disappear after keratanase treatment (Figures 6 and 8), indicating that they are all carried on KS chains. Nonetheless, the discovery of aggrecan, a KS (and CS) proteoglycan as the major detected protein carrier of Siglec ligands remains surprising, even though aggrecan genetic polymorphism has been associated with asthma (Vaillancourt et al. 2012). Although aggrecan is abundant in cartilage, its presence in submucosal glands is not well established, and exposure of eosinophils and mast cells to endogenous Siglec-8 ligands likely occurs on the airway surface rather than within the cartilage layer. The hypothesis that an unusual isoform of human aggrecan binds Siglec-8 is supported by the near absence of binding to purified bovine articular cartilage aggrecan (Figure 7). Furthermore, during enrichment of Siglec-8-binding ligands by affinity chromatography, aggrecan was not similarly enriched as detected by anti-aggrecan binding (data not shown). We conclude that there is a subpopulation of aggrecan glycoforms capable of binding Siglec-8, whereas other glycoforms from human airway and bovine articular cartilage fail to bind Siglec-8. In accordance with terminology we proposed to distinguish lectin-binding glycoforms of common proteins (Taylor et al. 2017), the aggrecan glycoforms that bind Siglec-8 may be designated aggrecanS8L to distinguish them from aggrecan glycoforms that fail to bind Siglec-8.
The relationship of the three molecular weight aggrecanS8L isoforms (S8-1M, S8-600K, and S8-250K) has yet to be determined. Although the smaller proteoglycans may include proteolytic fragments, the high-confidence C-terminal peptides identified on S8-250K (Figure 4, Table 1) suggest that may not be the case. Interestingly, whereas S8-1M migration on composite gel electrophoresis increased after ChABC treatment (without losing Siglec-8 binding, Figure 7), the migration of S8-250K was unchanged (data not shown). It is possible that migration on composite gel electrophoresis reflects differentially spliced, differentially glycosylated and/or proteolytically cleaved forms, as has been proposed for brain aggrecan isoforms (Virgintino et al. 2009). A major remaining question is whether aggrecanS8L in any form is expressed in submucosal glands and on the airway, or whether some other sialylated KS proteoglycan is produced in the submucosal glands. In either case, we conclude that unusual subclasses of sialylated KS proteoglycans are major Siglec-8-binding components on human airways and may function in the control of eosinophilic and mast cell-mediated inflammation.
Materials and Methods
Reagents
Siglec-8-(human) Fc was produced as described (Kikly et al. 2000). Keratanase I (Pseudomonas sp.) and Chondroitinase ABC (Proteus vulgaris) were from Amsbio (Cambridge, MA). A recombinant truncated form of Keratanase II (Bacillus circulans) was produced in Escherichia coli using an expression plasmid kindly provided by Dr. Andrew Muscroft-Taylor, GlycoSyn, New Zealand and expressed and purified essentially as described (Steward et al. 2015). Recombinant Vibrio cholerae sialidase was kindly provided by Dr Garry Taylor, University of St. Andrews, and purified produced in E. coli from an expression plasmid as described (Moustafa et al. 2004; Mountney et al. 2010). Recombinant human ADAMTS-4 (aggrecanase) was from R&D Systems (Minneapolis, MN). PNGase F was from New England Biolabs (Ipswich, MA). β-Azidoethylglycosides of LacNAc, 3-sialyl LacNAc, 6-sulfo-3-sialyl LacNAc and 6′-sulfo-3-sialyl LacNAc were synthesized as described (Chernyak et al. 1992; Cheng et al. 2015). Commercial aggrecan (A1960, MilliporeSigma, Burlington, MA) was extracted from bovine articular cartilage, chromatographically purified, dialyzed against water, sterile-filtered and lyophilized.
Siglec-8-COMP
Construction of the pentavalent Siglec-8-COMP (Figure 1) was based on a published concept (Voulgaraki et al. 2005). A plasmid containing the extracellular domain of Siglec-8 followed by a biotin acceptor peptide (BAP), a factor Xa cleavage site, Ig-like domains 3 and 4 from rat CD4, a COMP pentamerization domain and a His-6 C-terminal tag behind an EF1a promoter was created as follows. The extracellular domain of Siglec-8 was amplified by polymerase chain reaction (PCR) using 5′-TCACGCGTATGCTGCTGCTGCTGCTGCTGCTGCCCCT-3′ and 5′-GAGCTAGCCTGCAGGGAGAGGCTCAGGG-3′ as primers. Complementary synthetic DNA oligonucleotides 5′-AATTCACGCGTAGAGCTAGCTCTGGTAC-3 and 5′-GTGCGCATCTCGATCGAGAC-3 were annealed and the double-strand fragment containing MluI and NheI restriction sites flanked with EcoRI and KpnI sites was inserted into pEF-GFP (Addgene plasmid #11154), a gift of Dr Connie Cepko, (Matsuda and Cepko 2004). The Siglec-8 PCR product was cloned into the modified pEF-GFP between MluI and NheI sites to create pEF-Siglec-8-GFP. Complementary synthetic DNA oligonucleotides encoding BAP and Factor Xa cleavage sites (5′-CTAGCGGATCCGCCGGAGGCTCTGGAGGCCTGAACGATATTTTCGAAGCTCAGAAAATCGAATGGCACGAAATCGAGGGAAGGTCGGTAC-3′ and 5′-CGACCTTCCCTCGATTTCGTGCCATTCGATTTTCTGAGCTTCGAAAATATCGTTCAGGCCTCCAGAGCCTCCGGCGGATCCG-3′) were annealed and cloned into pEF-Siglec8-GFP between NheI and KpnI sites, creating pEF-Siglec8-BAP/X-GFP. Finally, a KpnI/NotI fragment from vector BasiginCD4d3+4-Comp-His-Stop (Addgene plasmid #36147), a gift of Gavin Wright (Crosnier et al. 2011) was cloned into pEF-Siglec8-BAP/X-GFP to create the expression vector shown in Figure 1 (pEF-Siglec8-COMP).
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. At 90–95% confluence the cells were transfected using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA). Culture supernatant was collected after 3 days and loaded onto nickel Sepharose beads for ligand affinity chromatography (see below) or purified via its C-terminal His tag for size and binding studies (Figure 1) as follows. Culture supernatant was loaded onto an Ni-NTA Superflow cartridge (Qiagen, Germantown, MD), which was then washed with 0.5 M NaCl, 20 mM imidazole, 10 mM β-mercaptoethanol, 50 mM sodium phosphate pH 8. The column was further washed with the same buffer containing 1 M NaCl, and then the product was eluted with the same buffer containing 600 mM imidazole. Purified protein was dialyzed against Dulbecco’s phosphate-buffered saline (PBS).
Siglec-8-COMP was characterized by size-exclusion chromatography, SDS-PAGE gel electrophoresis, immunoblotting and glycan binding on a custom sialoglycan array (Figure 1). Size-exclusion chromatography was performed using a Superdex 200 10/300 GL column (GE Healthcare Bio-Sciences, Pittsburgh, PA) on an ÄKTA chromatography system (GE Healthcare) compared to the elution pattern of a commercial mixture of proteins (MWGF1000, MilliporeSigma). SDS-PAGE was performed using NuPAGE 4–12% Bis-Tris precast polyacrylamide gels with SeeBlue Plus2 molecular weight markers (Thermo Fisher). Immunoblotting was performed after transfer to PVDF membranes using alkaline phosphate-conjugated anti-6x-His monoclonal antibody (Thermo Fisher) detected using Vector Red (Vector Laboratories). Sialoglycan-binding specificity was tested by incubating Siglec-8-COMP on a 135-glycan slide-printed array containing 125 sialoglycans and 10 neutral glycans (Li et al. 2017) (Supplementary data, Table 1). Binding was detected by washing and incubating with Alexa Fluor 488-labeled anti-His antibody (Qiagen 35310).
Lectin blotting and immunoblotting
Siglec-8 ligands were resolved by SDS gel electrophoresis on composite agarose–acrylamide gels (2% agarose and 1.5% acrylamide or as indicated) for 2.5 h at 80 V, conditions suitable for resolution of large glycoprotein molecules (Jia et al. 2015; Yu et al. 2017). Samples in GuHCl buffers (see below) were dialyzed against urea buffer (1 M urea, 20 mM sodium phosphate, pH 7.4) prior to electrophoresis. Resolved proteins were electroblotted onto polyvinylidene fluoride (PVDF) membranes (iBlot, Thermo Fisher). Membranes were blocked with 5% nonfat milk dissolved in PBS supplemented with 0.1% Tween-20 (PBST) for 30 min. Siglec-8-Fc (1 μg) and horseradish peroxidase (HRP)-conjugated anti-human Fc (MilliporeSigma, 0.7 μg) were incubated in a total of 50 μL of PBST for 30 min on ice, then diluted to 2 mL with PBST. Blots were overlaid with the precomplexed mixture for 16 h at 4°C. Alternatively, blots were incubated with one of the two anti-aggrecan antibodies at 1 μg/mL in PBST: (i) mAb 7D4 against the G1-IGD-G2 domains (Bio-Rad, Hercules, CA) or (ii) polyclonal PA1-1745 against the G3 domain (Thermo Fisher). Blots were washed and then incubated with appropriate HRP-conjugated secondary antibodies in PBST for 1 h at ambient temperature. HRP (Siglec-8-Fc or antibody binding) was detected using enhanced chemiluminescence (GE Healthcare). Molecular weight markers included (as indicated) SeeBlue Plus2 or HiMark (Thermo Fisher).
Human airways
Tracheae were obtained from four human organ donors. Donors were 47–57 years old at the time of death, included three women and one man, two smokers and two nonsmokers, with one reporting allergic rhinitis but no other reports of airway disease. Causes of death included anoxia secondary to cardiac arrest (two donors), head trauma and stroke. Organs were flushed and stored in HTK solution or UW solution and kept on ice for up to 24 h prior to dissection (Latchana et al. 2014). The trachea was dissected free of surrounding tissue, transferred to ice-cold RPMI-1640 containing antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin), and further processed for histology and/or protein extraction.
Human airway protein extraction and size-exclusion chromatography
Tracheae were cut into small pieces, frozen in liquid nitrogen and pulverized using a liquid nitrogen-chilled mortar and pestle. Based on the weight of pulverized tissue, extraction buffer was added (6 M GuHCl (OmniPur, MilliporeSigma), 5 mM EDTA, 10 mM sodium phosphate (pH 6.5), plus protease inhibitor cocktail (MilliporeSigma P8340)) at a ratio of 10 mL/g of pulverized tissue. The suspension was incubated 16 h at 4°C with end-over-end mixing. After centrifugation at 22,000 × g for 30 min, the pellet was discarded and the supernatant was used immediately or stored at −20°C.
For size-exclusion chromatography, an aliquot of tissue extract was dialyzed against running buffer (4 M GuHCl, 10 mM sodium phosphate, pH 7.0) using Float-A-Lyzer G2 100 kDa MWCO (Spectrum Labs, Rancho Dominguez, CA). Dialyzed sample (4.5 mL) was loaded onto a HiPrep 26/60 Sephacryl S-500 HR column (GE Healthcare) on an ÄKTA chromatography system (GE Healthcare) at a flow rate of 0.8 mL/min. After injection, 48 mL (~15% of the total column volume) of eluate was discarded, then 1.8-mL fractions were collected until a full column volume (320 mL) was eluted. Aliquots from each fraction were dotted onto PVDF membranes using a Bio-Dot Microfiltration Apparatus (Bio-Rad) and probed for Siglec-8-Fc binding as above. Positive fractions were individually dialyzed in urea buffer before resolution by composite agarose–acrylamide gel electrophoresis and Siglec-8-Fc blotting as described above. Fractions were pooled based on electrophoretic migration for further purification by Siglec-8-COMP affinity chromatography.
Siglec-8-COMP affinity chromatography
Medium from HEK293F cells expressing Siglec-8-COMP was filtered (0.22-μm Stericup, MilliporeSigma) and cooled to 4°C. A 1-mL packed volume of nickel Sepharose resin (GE Healthcare 17-5268-01) was prewashed with 30 mL of binding buffer (500 mM NaCl, 20 mM sodium phosphate, 20 mM imidazole, pH 7.4). The medium containing Siglec-8-COMP (200 mL, >2 μg/mL) was cycled through the prewashed nickel beads for 24 h at 4°C using a peristaltic pump. The column was then washed with 30 mL of High-Salt Elution Buffer (1 M urea, 1 M NaCl, 20 mM sodium phosphate, 20 mM imidazole, pH 7.4) and then equilibrated with 30 mL of urea buffer. Combined fractions from size-exclusion chromatography were dialyzed against urea buffer and precleared by cycling three times through 1 mL of nickel Sepharose resin (without Siglec-COMP) that had been pre-equilibrated with 30 mL of urea buffer. The precleared sample was then loaded onto the Siglec-8-COMP column and cycled through three times. The column was then washed with 10 mL of a low salt wash (1 M urea, 150 mM NaCl, 20 mM sodium phosphate, 20 mM imidazole, pH 7.4), collecting 2-mL fractions. Siglec-8 ligand was eluted using high-salt elution buffer collecting one 0.5-mL fraction and subsequent 1-mL fractions. Salt elution released Siglec-8 ligands without eluting Siglec-8-COMP. Equal volumes of fractions were loaded on composite gels and analyzed as described above.
As indicated, purified Sigelc-8 ligands were treated at 37°C with enzymes prior to resolving and Siglec-8-Fc blotting. Ligands were dialyzed against PBS prior to incubation with sialidase, ChABC, keratanase I, keratanase II or aggrecanase (ADAMTS-4) as indicated in the figure legends. Control ligands were treated similarly without enzymes.
To test whether Siglec-8 ligand bound to immobilized Siglec-8-COMP via its sialoglycan-binding site, elution with synthetic glycans was tested (Figure 4E). Purified Siglec-8-COMP (44 μg) was immobilized on 140 μL of magnetic nickel Sepharose beads (GE Healthcare 28967388). Size-separated S8-1M in urea buffer (200 μL) was incubated with the Siglec-8-COMP beads overnight mixing end-over-end at 4°C. The beads were washed three times with 500 μL of 100 mM NaCl, 20 mM imidazole, 10 mM sodium phosphate pH 7.4. Siglec-8-COMP beads with bound S8-1M were suspended in urea buffer and distributed equally into microcentrifuge tubes. The urea buffer was removed and replaced with 30 μL of urea buffer alone or the same buffer containing 15 mM of synthetic β-azidoethylglycosides of LacNAc, 3-sialyl LacNAc, 6-sulfo-3-sialyl LacNAc or 6′-sulfo-3-sialyl LacNAc. After 24 h at 4°C, the supernatant was collected and any remaining ligand eluted in 30 μL of 500 mM imidazole in urea buffer. Equivalent aliquots from the glycan and imidazole elutions were resolved by composite gel electrophoresis and blots probed for Siglec-8-Fc binding.
Proteomic mass spectrometry
Affinity-purified salt-eluted S8-1M and S8-600K were desalted by centrifugal ultrafiltration (Amicon Ultra 0.5 mL 100 K, MilliporeSigma). Following reduction with 10 mM dithiothreitol for 1 h at 55°C and carbamidomethylation with 20 mM iodoacetamide in the dark for 45 min, proteins were digested with sequence-grade recombinant Lys-C and trypsin (Promega, Madison, WI) at 37°C for 16 h. The resulting peptides were purified by Pierce C18 Tips (Thermo Fisher). Peptides were eluted with 60% acetonitrile in 0.1% TFA.
Purified salt-eluted S8-250K was further resolved by preparative SDS-PAGE on a 3–8% NuPAGE precast gel with HiMark-prestained protein standards (Thermo Fisher). Based on the migration of standards, the section of gel containing resolved S8-250K was excised, cut into small pieces, washed with 40 mM ammonium bicarbonate and acetonitrile and then reduced and alkylated as above (using 10 mM dithiothreitol then 55 mM iodoacetamide). The pieces were rewashed with bicarbonate and acetonitrile and rehydrated in 50 mM ammonium bicarbonate containing Lys-C and trypsin and incubated at 37°C for 16 h. The resulting peptides were extracted by sequential incubations with 20, 50 and 80% acetonitrile in 5% aqueous formic acid. The washes were combined, dried and purified using Pierce C18 Tips.
Purified peptides were reconstituted in 39 μL of mobile phase A (0.1% formic acid in water) and 1 μL of mobile phase B (80% acetonitrile and 0.1% formic acid in water), passed through a Nanosep MF centrifugal device (0.2 μm, Pall, Port Washington, NY) and transferred to an autosampler vial at 4°C. Samples were analyzed using an Orbitrap Fusion Lumos tribrid mass spectrometer (Thermo Fisher) equipped with UltiMate3000 RSLCnano liquid chromatograph using a C18 analytical column (Acclaim PepMap 300, 150 × 0.075 mm, Thermo Fisher). After injection (6 μL) peptides were eluted using a multistep gradient from mobile phase A to B at a flow rate of 300 μL/min over 90 min. Peptides were fragmented using higher energy collisional dissociation, electron transfer dissociation, and collision-induced dissociation. The electrospray ionization voltage was 2.2 kV and the capillary temperature 280°C. Full scan mass spectra were acquired in the positive ion mode over the range m/z = 400 to 1600 using the Orbitrap mass analyzer in profile format with a mass resolution setting of 30,000. MS2 scans were collected in the quadrupole or ion trap for the most intense ions in the Top-Speed mode within a 3-s cycle, in centroid format, using Fusion instrument software (version 2.0, Thermo Fisher) with the following parameters: isolation width 4 m/z units, normalized collision energy 30%, charge state 2+ ~ 5+, activation Q 0.25 and activation time 30 ms. Real-time dynamic exclusion was enabled to preclude reselection of previously analyzed precursor ions, with the following parameters: repeat count 1, exclusion duration 35 s and mass tolerance within 10 ppm.
Data were processed using Proteome Discoverer (version 1.4, Thermo Fisher) and searched against the human-specific SwissProt-reviewed protein database (downloaded 18-Oct-2017). Indexed databases for Lys-C/tryptic digests were created allowing for up to three missed internal cleavage sites, one fixed modification (carboxyamidomethylcysteine, +57.021 Da) and variable modifications (methionine oxidation, +15.995 Da). Precursor ion mass tolerances for spectra acquired using the Orbitrap and linear ion trap (LTQ) were set to 10 ppm. The fragment ion mass tolerance was set to 0.8 Da. High-probability assignments were inspected for validity.
Siglec overlay histochemistry
Tissues were fixed in neutral 4% paraformaldehyde in PBS at 4°C for 16 h, embedded in paraffin, sectioned to 5 μm and captured on glass slides. Following deparaffinization, the slides were heated briefly in 10 mM sodium citrate (pH 6.0) for antigen retrieval and cooled in PBST. As indicated, slides were overlaid with 100 mM sodium acetate pH 6.0 with or without keratanase I or keratanase II, or with 50 mM Tris-HCl, 50 mM sodium acetate, pH 8.0 with or without ChABC at enzyme concentrations noted in the figure legends. In some experiments, sequential treatments with ChABC and keratanase were performed. Enzyme treatments were performed at 37°C in a humidified chamber. After enzyme treatments or control incubations, the slides were washed with PBST, blocked with 10 mg/mL BSA in PBST for 30 min, enzyme-blocking reagent (Dako North America, Carpinteria, CA) for 5 min and then with Fc Receptor Blocker (Innovex Biosciences, Richmond, CA) for 30 min, all at ambient temperature. Siglec-8-Fc (20 μg/mL) was preincubated with AP-conjugated goat anti-human secondary antibody (2 μg/mL, product 109-055-008, Jackson Immunoresearch, West Grove, PA) then overlaid on blocked slides and incubated 16 h at 4°C. In some experiments, slides were overlaid with Siglec-8-Fc for 16 h at 4°C, washed, and then with secondary antibody for 4 h at ambient temperature. Slides were washed with 0.1% Tween-20 in 100 mM Tris-HCl pH 8.3 for 10 min and developed using Vector Red AP substrate (Vector Laboratories, Burlingame, CA). Slides were washed with water, counterstained with hematoxylin QS (Vector Laboratories), dehydrated, mounted in Krystalon (MilliporeSigma) and imaged using a Nikon Eclipse 90i microscope.
Eosinophil apoptosis
Siglec-8 ligand induction of apoptosis of human eosinophils was tested by incubating freshly isolated peripheral blood eosinophils with affinity-purified salt-eluted S8-250K ligand. To generate a matched positive and negative control, a portion of S8-250K was treated with cold periodate to selectively cleave sialic acid (Neu5Ac) glycerol side chains (Reuter et al. 1989) and abolish Siglec-8 binding (Figure 10A) and then with sodium borohydride to selectively reduce the resulting aldehyde to an unreactive hydroxyl group. Equal aliquots of purified S8-250K were treated with or without 6 mM sodium periodate for 30 min on ice in the dark. Glycerol (0.3 M) was then added followed by 10 mM sodium borohydride for 30 min on ice. The oxidized (negative control) and intact S8-250K (test sample) were dialyzed extensively against HEPES-buffered RPMI (Thermo Fisher 72400120) containing penicillin (100 U/mL) and streptomycin (100 μg/mL). Dialyzed samples were sterilized (0.22-μm filter) and stored frozen prior to use.
Written informed consent for blood donation (up to 180 mL) was obtained using an institutional review board-approved protocol at the Northwestern University Feinberg School of Medicine. Eosinophils were purified from peripheral blood using density gradient centrifugation, erythrocyte hypotonic lysis and CD16 immunomagnetic negative selection (Miltenyi Biotech, San Diego, CA), as previously described (Hudson et al. 2009). Purity and initial viability were consistently >95%, as determined by flow cytometry and 4′-6-diamidino-2-phenylindole (DAPI) exclusion (Thermo Fisher). Cells were cultured in RPMI 1640 medium containing 10% FCS and antibiotics with 30 ng/mL recombinant human IL-5 (R&D Systems) for 24 h. IL-5-primed eosinophils (2 × 105) were incubated for 18–24 h at 37°C with equal aliquots of control treated (test sample) and periodate oxidized (negative control) S8-250K, then apoptosis was assessed by means of flow cytometry using fluorescein isothiocyanate–Annexin V (BD Biosciences, San Jose, CA) and DAPI labeling (Carroll et al. 2017).
Supplementary Material
Acknowledgements
The authors thank Dr Phillip Rendle, Victoria University of Wellington, and Dr Andrew Muscroft-Taylor, Callaghan Innovation, for providing the expression plasmid for truncated keratanase II.
Abbreviations
- BAP
biotin acceptor peptide
- ChABC
chondroitinase ABC
- COMP
cartilage oligomeric matrix protein
- CS
chondroitin sulfate
- DAPI
4′-6-diamidino-2-phenylindole
- GuHCl
guanidinium hydrochloride
- HRP
horseradish peroxidase;
- ITIM
immunoreceptor tyrosine-based inhibitory motif
- KS
keratan sulfate
- LacNAc
N-acetyllactosamine (Galβ1-4GlcNAc)
- PBS
Dulbecco’s phosphate-buffered saline
- PCR
polymerase chain reaction
- PVDF
polyvinylidene fluoride
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Funding
This work was performed under the auspices of the Lung Inflammatory Disease Program of Excellence in Glycoscience (LIDPEG) supported by the National Heart, Lung, and Blood Institute (Grant HL107151). A.G.G. and R.N.P. were supported in part by The Chemistry-Biology Interface Program at Johns Hopkins University (T32 GM080189). B.S.B. was also supported by the National Institute of Allergy and Infectious Diseases (Grant AI072265) and both B.S.B and R.L.S. received support from National Institute of Allergy and Infectious Diseases AI136443. K.A. and M.T. were supported in part by an NIH P41 grant from the National Institute of General Medical Sciences (Grant GM103490).
Conflict of interest statement
B.S.B. has current or recent consulting or scientific advisory board arrangements with, or has received honoraria from, Sanofi-Aventis, GlaxoSmithKline, TEVA, AstraZeneca and Allakos Inc. and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and UpToDate™ and is a co-inventor on existing Siglec-8-related patents that have been licensed to Allakos and thus may be entitled to a share of royalties received from Allakos by Johns Hopkins University on the potential sales of such products. B.S.B. is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by the Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
References
- Akhtar S, Kerr BC, Hayes AJ, Hughes CE, Meek KM, Caterson B. 2008. Immunochemical localization of keratan sulfate proteoglycans in cornea, sclera, and limbus using a keratanase-generated neoepitope monoclonal antibody. Invest Ophthalmol Vis Sci. 49:2424–2431. [DOI] [PubMed] [Google Scholar]
- Angata T, Margulies EH, Green ED, Varki A. 2004. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 101:13251–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnig C, Frossard N, Levy BD. 2018. Towards targeting resolution pathways of airway inflammation in asthma. Pharmacol Ther. 186:98–113. [DOI] [PubMed] [Google Scholar]
- Bochner BS. 2016. "Siglec"ting the allergic response for therapeutic targeting. Glycobiology. 26:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner BS, Alvarez RA, Mehta P, Bovin NV, Blixt O, White JR, Schnaar RL. 2005. Glycan array screening reveals a candidate ligand for siglec-8. J Biol Chem. 280:4307–4312. [DOI] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Bayliss MT, Nieduszynski IA. 1998. Human aggrecan keratan sulfate undergoes structural changes during adolescent development. J Biol Chem. 273:26408–26414. [DOI] [PubMed] [Google Scholar]
- Brown GM, Huckerby TN, Morris HG, Abram BL, Nieduszynski IA. 1994. Oligosaccharides derived from bovine articular cartilage keratan sulfates after keratanase II digestion: Implications for keratan sulfate structural fingerprinting. Biochemistry. 33:4836–4846. [DOI] [PubMed] [Google Scholar]
- Campanero-Rhodes MA, Childs RA, Kiso M, Komba S, Le NC, Warren J, Otto D, Crocker PR, Feizi T. 2006. Carbohydrate microarrays reveal sulphation as a modulator of siglec binding. Biochem Biophys Res Commun. 344:1141–1146. [DOI] [PubMed] [Google Scholar]
- Carroll DJ, O'Sullivan JA, Nix DB, Cao Y, Tiemeyer M, Bochner BS. 2017. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol. 141:2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR. 1983. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 258:8848–8854. [PubMed] [Google Scholar]
- Caterson B, Melrose J. 2018. Keratan sulphate, a complex glycosaminoglycan with unique functional capability. Glycobiology. 28:182–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Chou CC, Hsieh HW, Tu Z, Lin CH, Nycholat C, Fukuda M, Khoo KH. 2015. Efficient Mapping of sulfated glycotopes by negative ion mode nanoLC-MS/MS-based sulfoglycomic analysis of permethylated glycans. Anal Chem. 87:6380–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak AY, Sharma GVM, Kononov LO, Krishna PR, Levinsky AB, Kochetkov NK, Rao AVR. 1992. 2-Azidoethyl glycosides: Glycosides potentially useful for the preparation of neoglycoconjugates. Carbohydr Res. 223:303–309. [Google Scholar]
- Cho JY, Song DJ, Pham A, Rosenthal P, Miller M, Dayan S, Doherty TA, Varki A, Broide DH. 2010. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 11:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosnier C, Bustamante LY, Bartholdson SJ, Bei AK, Theron M, Uchikawa M, Mboup S, Ndir O, Kwiatkowski DP, Duraisingh MT et al. 2011. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature. 480:534–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton JN, Gilroy DW. 2016. Resolution of inflammation: A new therapeutic frontier. Nat Rev Drug Discov. 15:551–567. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. 2000. Keratan sulfate: Structure, biosynthesis, and function. Glycobiology. 10:951–958. [DOI] [PubMed] [Google Scholar]
- Funderburgh JL. 2002. Keratan sulfate biosynthesis. IUBMB Life. 54:187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PS, Shimizu K, Grant AV, Rafaels N, Zhou LF, Hudson SA, Konno S, Zimmermann N, Araujo MI, Ponte EV et al. 2010. Polymorphisms in the sialic acid-binding immunoglobulin-like lectin-8 (Siglec-8) gene are associated with susceptibility to asthma. Eur J Hum Genet. 18:713–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden KG, Yim NC, Griggs LJ, Weisbach JA. 1971. Gel electrophoresis of mucous glycoproteins. I. Effect of gel porosity. Biochemistry. 10:3105–3109. [DOI] [PubMed] [Google Scholar]
- Hudson SA, Bovin NV, Schnaar RL, Crocker PR, Bochner BS. 2009. Eosinophil-selective binding and proapoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6'-sulfated sialyl Lewis x. J Pharmacol Exp Ther. 330:608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson SA, Herrmann H, Du J, Cox P, Haddad el-B, Butler B, Crocker PR, Ackerman SJ, Valent P, Bochner BS. 2011. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil siglec-8 expression. J Clin Immunol. 31:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yu H, Fernandes SM, Wei Y, Gonzalez-Gil A, Motari MG, Vajn K, Stevens WW, Peters AT, Bochner BS et al. 2015. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 135:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano G, Almanan M, Bochner BS, Zimmermann N. 2013. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: Role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 132:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikly KK, Bochner BS, Freeman SD, Tan KB, Gallagher KT, D'alessio KJ, Holmes SD, Abrahamson JA, Erickson-Miller CL, Murdock PR et al. 2000. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 105:1093–1100. [DOI] [PubMed] [Google Scholar]
- Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, Zhu Z, Tiemeyer M, Bochner BS. 2015. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 135:1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. 2012. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacol Ther. 135:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchana N, Peck JR, Whitson B, Black SM. 2014. Preservation solutions for cardiac and pulmonary donor grafts: A review of the current literature. J Thorac Dis. 6:1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauder RM, Huckerby TN, Nieduszynski IA. 1995. The structure of the keratan sulphate chains attached to fibromodulin isolated from bovine tracheal cartilage: Oligosaccharides generated by keratanase II digestion. Glycoconj J. 12:651–659. [DOI] [PubMed] [Google Scholar]
- Lauder RM, Huckerby TN, Nieduszynski IA. 1997. The structure of the keratan sulphate chains attached to fibromodulin from human articular cartilage. Glycoconj J. 14:651–660. [DOI] [PubMed] [Google Scholar]
- Li W, Hulswit RJG, Widjaja I, Raj VS, McBride R, Peng W, Widagdo W, Tortorici MA, van Dieren B, Lang Y et al. 2017. Identification of sialic acid-binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci USA. 114:E8508–E8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Crocker PR, Paulson JC. 2014. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 14:653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao H, Kano G, Hudson SA, Brummet M, Zimmermann N, Zhu Z, Bochner BS. 2013. Mechanisms of Siglec-F-induced eosinophil apoptosis: A role for caspases but not for SHP-1, Src kinases, NADPH oxidase or reactive oxygen. PLoS One. 8:e68143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL. 2004. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 101:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan SJ, Sharma RS, McKenzie EJ, Richards HE, Zhang J, Prescott A, Crocker PR. 2013. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling. Blood. 121:2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. 2010. Sialidase enhances recovery from spinal cord contusion injury. Proc Natl Acad Sci USA. 107:11561–11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa I, Connaris H, Taylor M, Zaitsev V, Wilson JC, Kiefel MJ, von Itzstein M, Taylor G. 2004. Sialic acid recognition by Vibrio cholerae neuraminidase. J Biol Chem. 279:40819–40826. [DOI] [PubMed] [Google Scholar]
- Nutku E, Aizawa H, Hudson SA, Bochner BS. 2003. Ligation of Siglec-8: A selective mechanism for induction of human eosinophil apoptosis. Blood. 101:5014–5020. [DOI] [PubMed] [Google Scholar]
- Nutku-Bilir E, Hudson SA, Bochner BS. 2008. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 38:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propster JM, Yang F, Rabbani S, Ernst B, Allain FH, Schubert M. 2016. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci USA. 113:E4170–E4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G, Schauer R, Szeiki C, Kamerling JP, Vliegenthart JF. 1989. A detailed study of the periodate oxidation of sialic acids in glycoproteins. Glycoconj J. 6:35–44. [DOI] [PubMed] [Google Scholar]
- Schleimer RP, Schnaar RL, Bochner BS. 2016. Regulation of airway inflammation by Siglec-8 and Siglec-9 sialoglycan ligand expression. Curr Opin Allergy Clin Immunol. 16:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaar RL. 2016. Glycobiology simplified: Diverse roles of glycan recognition in inflammation. J Leukoc Biol. 99:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko A, Dohmae N, Takio K, Yamashita K. 2003. Beta 1,4-galactosyltransferase (beta 4GalT)-IV is specific for GlcNAc 6-O-sulfate. Beta 4GalT-IV acts on keratan sulfate-related glycans and a precursor glycan of 6-sulfosialyl-Lewis X. J Biol Chem. 278:9150–9158. [DOI] [PubMed] [Google Scholar]
- Steward M, Berezovskaya Y, Zhou H, Shediac R, Sun C, Miller N, Rendle PM. 2015. Recombinant, truncated B. circulans keratanase-II: Description and characterisation of a novel enzyme for use in measuring urinary keratan sulphate levels via LC–MS/MS in Morquio A syndrome. Clin Biochem. 48:796–802. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Drickamer K, Schnaar RL, Etzler ME, Varki A. 2017. Discovery and classification of glycan-binding proteins In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH et al., editors. Essentials of Glycobiology, 3rd Edition Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; p. 361–372. [Google Scholar]
- Vaillancourt VT, Bordeleau M, Laviolette M, Laprise C. 2012. From expression pattern to genetic association in asthma and asthma-related phenotypes. BMC Res Notes. 5:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti LE, De Pauli CP, Giacomelli CE. 2006. The binding of Ni(II) ions to hexahistidine as a model system of the interaction between nickel and His-tagged proteins. J Inorg Biochem. 100:192–200. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Aebi M, Packer NH, Seeberger PH, Esko JD, Stanley P, Hart G, Darvill A, Kinoshita T et al. 2015. Symbol nomenclature for graphical representations of glycans. Glycobiology. 25:1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Schnaar RL, Crocker PR. 2017. I-type lectins In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH et al., editors. Essentials of Glycobiology, 3rd Edition Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; p. 453–467. [Google Scholar]
- Virgintino D, Perissinotto D, Girolamo F, Mucignat MT, Montanini L, Errede M, Kaneiwa T, Yamada S, Sugahara K, Roncali L et al. 2009. Differential distribution of aggrecan isoforms in perineuronal nets of the human cerebral cortex. J Cell Mol Med. 13:3151–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten S, Yousefi S, Seitz M, Jakob SM, Schaffner T, Seger R, Takala J, Villiger PM, Simon HU. 2005. Siglec-9 transduces apoptotic and nonapoptotic death signals into neutrophils depending on the proinflammatory cytokine environment. Blood. 106:1423–1431. [DOI] [PubMed] [Google Scholar]
- Voulgaraki D, Mitnacht-Kraus R, Letarte M, Foster-Cuevas M, Brown MH, Barclay AN. 2005. Multivalent recombinant proteins for probing functions of leucocyte surface proteins such as the CD200 receptor. Immunology. 115:337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi H, Choi OH, Hubbard W, Lee HS, Canning BJ, Lee HH, Ryu SD, von Gunten S, Bickel CA, Hudson SA et al. 2008. Inhibition of FcepsilonRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 121:499–505. [DOI] [PubMed] [Google Scholar]
- Yu H, Gonzalez-Gil A, Wei Y, Fernandes SM, Porell RN, Vajn K, Paulson JC, Nycholat CM, Schnaar RL. 2017. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology. 27:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.