Abstract
Orthosiphon stamineus Benth. (Lamiaceae) is a valued medicinal plant in traditional folk medicine. Many pharmacological studies have demonstrated the ability of this plant to exhibit antimicrobial, antioxidant, hepatoprotection, antigenotoxic, antiplasmodial, cytotoxic, cardioactive, antidiabetic, anti-inflammatory activies. This review is a comprehensive summary of the presently available chemical, pharmacological investigations as well as the traditional and therapeutic uses of this plant. Important and different experimental data have been addressed along with a review of all phytochemicals identified in this plant, including flavonoids, terpenoids, and essential oils. O. stamineus has wide traditional and pharmacological uses in various pathophysiological conditions. Therefore, it is an attractive subject for further experimental and clinical investigations.
KEYWORDS: Misai kucing, Orthosiphon stamineus, rosmarinic acid, sinensetin
INTRODUCTION
Orthosiphon stamineus is commonly known as misai kucing and kumis kucing. O. stamineus is widely grown in Southeast Asia and the tropical countries. Leaves of this plant are known as “Java tea” and are mainly used for the purpose of making herbal tea commonly in Southeast Asia and European countries.[1] Other names for O. stamineus include Orthosiphon aristatus, Orthosiphon spicatus, Orthosiphon blaetter, kumis kucing, Indischer Nierentee, Feuilles de Barbiflore, and de Java. Orthosiphon species is categorized into two varieties: one with the white flowers (white variety) and the other with the light purple flowers (purple variety). Purple variety contains more bioactive compounds than the white one. Normally, the leaves and stem tips have medicinal values. Due to this property, this plant has extensively been subjugated traditionally to treat several human ailments and conditions such as diuretic, rheumatism, abdominal pain, kidney and bladder inflammation, edema, gout, and hypertension.[2,3,4] The leaves of O. stamineus exhibit excellent pharmacological activities such as antioxidant, antibacterial, hepatoprotective, anti-inflammatory, cytotoxic, antihypertensive, and vasodilatation.[5,6,7,8] Many pharmacopoeias such as French, Indonesian, Dutch, and Swiss have listed this plant for the treatment related to renal cleansing and function, and related disorders that include nephritis, cystitis, and urethritis. In Europe, people use the leaves of O. stamineus extract as a tonic for kidney and bladder stones, liver and gallbladder problems, and urinary tract infections. This can be used to reduce cholesterol and blood pressure. Earlier report showed that this plant contains high amount of flavones, polyphenols, bioactive proteins, glycosides, a volatile oil, and vast quantities of potassium.[8]
Owing to rich phenol content, researcher isolated more than 20 phenolic compounds from this plant including lipophilic flavones, flavonol glycosides, and caffeic acid derivatives such as rosmarinic acid (RA), 2,3-dicaffeoyltartaric acid,[8,9,10,11,12,13] and many others compounds such as sinensetin (SIN),[14,15] 30-hydroxy-5,6,7,40-tetramethoxyflavone (TMF),[16,17,18] salvigenin, scutellarein tetramethyl ether (5,6,7,40-TMF), 5-hydroxyl-6,7,30,40-TMF,[11] 5,6-dihydroxy-7,40-dimethoxyflavone,[14] eupatorin (EUP),[19,20] tetramethyl scutellarein,[21] camphor, menthone, d-terpineol, isomenthone, borneol, citronellol, carvone, geranyl acetone, damascenone, translinalool oxide, linalool, bornyl acetate, limonene, 1,8-cineol, p-cymene, b-pinene camphene, a-pinene,[22] 2-o-deacetylorthosiphol J, 3-o-deacetylorthosiphol I, 6-hydroxyorthosiphol B, 7-o-deacetylorthosiphol B,[23] norstaminolactone A,[24] neoorthosiphols A and B,[25] neoorthosiphonone A,[26] norstaminol A,[27] orthosiphol A, orthosiphol B,[28,29] orthosiphol F, orthosiphol G,[27] orthosiphols O–Q,[24] orthosiphols R–T,[30] secoorthosiphol A–C,[31] siphonols A–E,[23] staminol A,[32] a-amyrin, p-amyrin, and betulinic acid.[33] There is a significant interest in O. stamineus, as evidenced by the volume of research keen to it. Therefore, it is interesting to perform an up-to-date comprehensive review that correlates the phytochemical content of this plant with its traditional and folk medical and pharmacological uses. Therefore, this review presents an overview presentation of the work reported about the phytochemicals and the pharmacological applications of O. stamineus and its main compounds [Figure 1].
Figure 1.
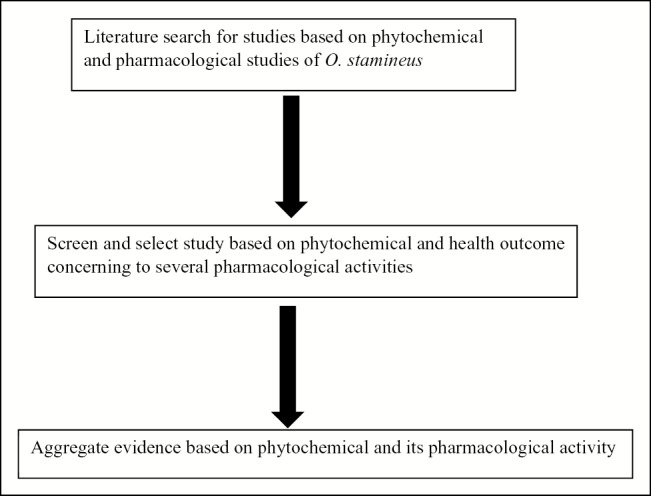
Overview chart of the proposed approach to the literature review
PHYTOCHEMICAL STUDY OF O. STAMINEUS
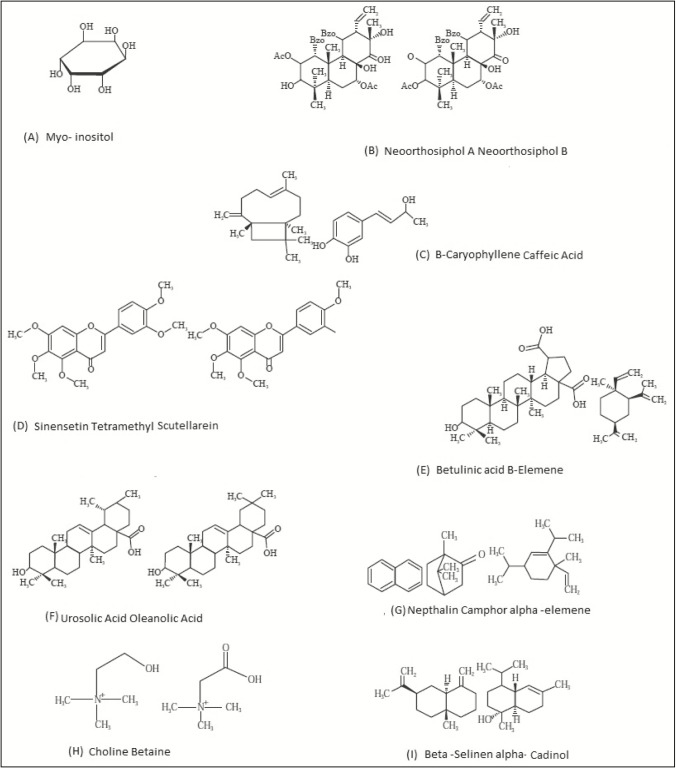
The phytochemical study of kumis kucing grown in Asia has been conducted extensively since the 1930s. The scientists have identified more than hundreds of chemical compounds and classified them as monoterpenes, diterpenes, triterpenes, saponins, flavonoids, organic acids, and so on. Moreover, earlier study has recognized 69 chemical compounds in the essential oil extracted from the leaves of O. stamineus. They were 1-octen-3-ol, β-bourbene, β-caryophyllene, α-humulene, β-elemene, phenylacetaldehyde, caryophyllene oxide, β-pinene, camphene, 3-octanol, limonene, cis-2-octenal, 2-heptenal, trans, cis-octa-3-5-dien-2-one, 1-methylnaphthalene, α-muniolene, trans, trans-octa-3-5-dien-2-one, 2-amylfuran, menthone, carvone, methyl chavicol, α-pinene, tridecane, ρ-cymene, pentenylfuran, hexanal, naphthalene, benzaldehyde, eugenol, linalool, trans-linalool oxide, δ-cadinene, trans-2-(cis)-6-nonadienale, methyl eugenol, trans-2-hexanal, camphor, citronellol, α-copaene, borneol, dodecane, α-cubebene, geranylacetane, δ-terpineol, acetophenone, trans-anethole, germacrene D, decanal, δ-elemene, 1,8-cineol, 4-heptenal, isomenthone, β-cyclocitral, damascenone, dehydroionone, cis-linalool oxide, undecane, bornyl acetate, 2-methyl naphthalene, β-ionone, perillen, safranal, hexahydrofamesylacetone, hexan-1-ol, 2,6,6-trimethyl-2-cyclohexene-1,4-dione, isobornyl acetate, trans, trans-deca-2,4-dienal, cis-caryophyllene, germacrene, and cis-3-hexen-1-ol.[22] Few years ago, seven triterpenes—namely, ursolic acid, oleanolic acid, betulinic acid, hydroxyl betulinic acid, maslinic acid, a-amyrin, and b-amyrin—have been isolated from the leaves of O. stamineus. Recently, one compound a-amyrin was isolated for the first time from this plant. Some other compounds detected were b-caryophyllene, a-humulene, b-elemene, 1-octen-3-ol, b-bourbonene, b-pinene, caryophyllene oxide, camphene, and limonene. These are all major compounds obtained from the hydro distilled essential oils of the leaves and stems of O. stamineus. Alternatively, a-pinene, 1,8-cineol, borneol, linalool, camphor, eugenol, p-cymene, carvone, bornyl acetate, and d-cadinene were reported as minor components of O. stamineus leaf and stem oils.[33] Some of the structures of chemical constituents of O. stamineus are mentioned in Figure 2. In recent days, several analytical methods were used for the analysis of bioactive constituents found in O. stamineus. Saidan et al.[34] developed and validated a novel reverse-phase high-performance liquid chromatography (RP-HPLC) method for the quantification of four marker compounds—RA, 3ʹ-hydroxy-5,6,7,4ʹ-TMF, SIN, and EUP—in numerous O. stamineus leaf extracts using RP-HPLC-diode-array detection at 320nm using a gradient mobile phase of 0.1% formic acid:acetonitrile at a flow rate of 1mL/min on reverse-phase acclaim polar advantage II C18 column (3 µm, 3×150mm) with 18min separation time. Recently, Hashim et al.[35] developed and validated high-performance thin-layer chromatography method and simultaneously quantified four compounds—RA, 3ʹ-hydroxy-5,6,7,4ʹ-TMF, SIN, and EUP—found in ethanol, 50% ethanol, and water extract of O. stamineus leaves. The linearity of RA, TMF, SIN, and EUP were obtained between 10 and 100ng/spot with high correlation coefficient value (R2) of more than 0.986. The limit of detection was found to be 122.47±3.95 (RA), 43.38±0.79 (SIN), 17.26±1.16 (TMF), and 46.80±1.33ng/spot (EUP).
Figure 2.
Structures of some compounds found in Orthosiphon stamineus (A–I)
In recent times, See Tiam et al.[36] showed the impacts of various methods such as mechanical grinding, ultrasonic-assisted extraction (UAE), microwave-assisted extraction, and also sample pretreatments using acid and alkali on the microstructure of plant sample for the extraction of bioactive compounds from O. stamineus leaf. The results exposed good diffusion of bioactive compound of approximately 86%–95% of the total extraction yield quantified by conventional Soxhlet extraction method. Chemical pretreatments normally reported weaker microstructure disruption; thus, a slight enhancement on the extraction yields was observed. In this case, acid reagent is more appropriate for the pretreatment, as the presence of alkali decomposes the bioactive compounds. In another study, Sree et al.[37] developed an HPLC method in which chromatographic separation was carried out using a mobile phase methanol–acetic acid–water (10:2:88, v/v) as solvent A and methanol–acetic acid–water (90:2:8, v/v) as solvent B and programed in gradient. In another experiment, an RP-HPLC method was developed using an isocratic system with a flow rate of 1mL/min, a column temperature of 25°C with a mobile phase of acetonitrile:isopropyl alcohol:20mM phosphate buffer (NaH2PO4) (30:15:55, v/v). The UV detection was set at 340nm. The injection volume was 20 µL of solution with a run time less than 20min for each injection. The peaks were detected at 340nm and identified using reference standards.[38]
PHARMACOLOGICAL ACTIVITIES
Antiproliferative and cytotoxic activities
O. stamineus contains a number of phenolic flavonoid compounds that play a significant role in the treatment of various types of ailments. A study showed that norstaminolactone A, orthosiphols A, B, D, E, K, L, M, N, O, P, and Q, nororthosiphonolide A, orthosiphonone A, norstaminone A, and neoorthosiphol A compounds showed weak-to-mild antiproliferative activity when tested for their cytotoxic activity against highly liver metastatic colon 26-L5 carcinoma and human HT-1080 cell lines. Only norstaminolactone A showed potent antiproliferative activity with inhibitory concentration (IC50) value of 2.16mg/mL against highly liver metastatic colon 26-L5 carcinoma cell line.[13,24,27,39] Numerous recent studies showed the cellular and molecular mechanism of the antiproliferative activity of O. stamineus extracts.[38,40,41] Previous reported works provides better understanding of mechanism of action of anticancer effect of the plant. In another study, Salleh et al.[41] showed that ethyl acetate fraction (hot water extract) of O. stamineus could prevent the growth of human hepatocellular carcinoma cell line (HepG2) by inducing apoptosis. In this process, nuclear condensation and fragmentation as well as mitochondrial membrane dysfunction (sign of apoptosis) were noticed in the HepG2 cells. The study also suggested that ethyl acetate fraction of O. stamineus is a possible candidate for further development of chemopreventive agent for human liver cancer. Other major detected constituents of O. stamineus in the ethyl acetate fraction were RA and caffeic acid, and these were suspected to have synergistically backed cancer cell apoptosis. In an in vivo experiment, 50% ethanolic extract of Orthosiphon act as anticancer against colorectal tumor in nude mice. In this experiment, orally administered two doses of 100 and 200mg/kg body weight (BW) of the extract of O. stamineus over 4 weeks suppressed tumor growth by 47% and 83%, respectively.[40] Stampoulis et al.[27] found that the methanol extract of O. stamineus leaves showed a cytotoxic activity against liver metastatic colon 26-L5 carcinoma cells. Remarkably, its chloroform fraction showed the strongest activity. In another study, compounds of O. stamineus from Myanmar showed mild-to-weak antiproliferative activity toward highly malignant liver metastatic colon 26-L5 carcinoma and human HT-1080 fibrosarcoma cell lines.[42] Awale et al.[43] further studied the possible cytotoxic activity of compounds isolated from Japanese O. stamineus toward highly malignant liver metastatic murine colon 26-L5 carcinoma and human HT-1080 fibrosarcoma cell lines. Now day’s attention is given in using natural anti-angiogenic compounds instead of synthetic drugs. Sahib et al.[44] investigated the effect of methanolic extract of O. stamineus and found that this extract can enhance the anticancer efficacy of tamoxifen, an estrogen receptor antagonist. The extract by itself does not exert any appreciable effect. It was also seen that the antiproliferative activity of tamoxifen toward MCF-7 hormone-sensitive breast cancer cells was raised up by fivefold, when it was coadministered with the extract. In general, O. stamineus synergistically enhanced the activity of tamoxifen against hormone-responsive breast cancer cells in vitro. Therefore, it is suggested to be useful as an adjuvant for treating metastatic breast cancer. Another research conducted by Abdelwahab et al.[45] correlated the antiapoptotic property with antioxidant and phenolic compound content. The antiapoptotic and antioxidant activities of O. stamineus aqueous–methanolic extract and its fractions were examined. The results revealed that ethyl acetate fraction contains highest total phenolic content and antioxidant activity, whereas the chloroform fraction was found to have the highest flavonoid content. Cell death induced by H2O2 was dose-dependently inhibited by the pretreatment with the ethyl acetate fraction. O. stamineus not only increased the expression of Bcl-2, but also decreased the expression of Bax, and ultimately reduced H2O2-induced apoptosis. Outcome of the search indicated that the antiapoptotic effect of O. stamineus might be ascribable to its antioxidant and phenolic compound contents.
Anti-inflammatory and analgesic activities
The cell and animal model studies have provided strong evidence supporting the traditional use of O. aristatus as a therapy for inflammatory disorders. Active constituents such as EUP, SIN, and ursolic acid were found to be the key anti-inflammatory agents.[20,46] Yam et al.[20] studied and investigated the anti-inflammatory activity on chemical constituents of the fractionated chloroform extract. Different techniques were applied to investigate the anti-inflammatory effect: anti-peritoneal capillary permeability, in vitro nitric oxide (NO) scavenging activity, and carrageenan-induced hind paw edema in rats. The results showed that oral administration of the flavonoid-rich chloroform fraction at 500 and 1000mg/kg reduced edema and caused NO and dye leakage to the peritoneal cavity. Phytochemical screening of the fraction confirms the presence of SIN and EUP. In another research, Akowuah and Zhari[47] used rat and mouse models to carry out both anti-inflammatory and analgesic activities of standardized 50% methanol extract of O. stamineus. The result showed that oral administration of up to 1000mg/kg of the extract produced an anti-inflammatory effect as established by a reduction in the hind paw edema in rats pretreated with carrageenan. The analgesic activity was confirmed using the acetic acid–induced writhing test and formalin-induced licking test (late phase) in mice and rats; although oral administration of higher dose of the extract up to 1000mg/kg did not show any effect on the tail flick and hot plate tests in mice. In another study, it was found that natural compounds isolated from O. stamineus inhibit NO production in rats.[48] Although NO is an important signaling molecule, its excessive production triggers tissue damage and release of pro-inflammatory cytokines such as tumor necrosis factor, interferon, and interleukin.[48]
Antioxidant property
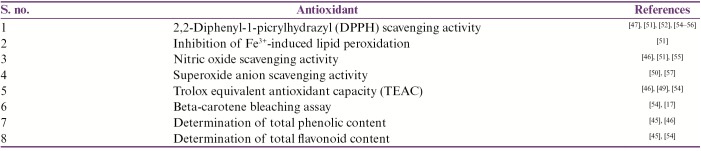
A number of studies showed that O. stamineus has excellent antioxidant property. A research conducted by Awale et al.[30] showed that different kinds of extracts of O. stamineus (distilled water, 50% aqueous methanol, methanol, 70% aqueous acetone, and chloroform extracts) produced different radical scavenging activities, using a 1,1-diphenyl-2-picrylhydrazyl in vitro model system. The acetone extract showed the highest activity. Another study showed variations in the total phenolic compounds, ranging from 6.7 to 10.1mg caffeic acid/g dry weight of the methanol extract. They also proved using different in vitro methods (superoxide scavenging and xanthine oxidase) that O. stamineus extract showed potential antioxidant activity.[17,49] Akowuah et al.[50] in another experiment investigated the antioxidative potency of various fractions of O. stamineus extract using an in vitro model of 1,1-diphenyl-2-picrylhydrazyl scavenging. The results showed antioxidant potency comparable to that of some standard antioxidants, including quercetin and butylated hydroxyanisole. The highest antioxidant activity showed by acetone extract was more than that of the aqueous methanol, methanol, and chloroform extracts. In another study, Yam et al.[51] demonstrated the antioxidant potency of the methanol extract of O. stamineus using 1,1-diphenyl-2-picrylhydrazyl radical scavenging, Fe3+-induced lipid peroxidation inhibiting activities, and trolox equivalent antioxidant capacity in in vitro models. In a recent study, ethanol and aqueous extracts of O. stamineus were evaluated in vitro for their antioxidant, antimicrobial as well as their immunomodulatory properties on human peripheral blood mononuclear cells.[52] The antioxidant activity was determined by 2,2-diphenyl-1-picrylhydrazyl radical scavenging method, whereas the antibacterial effectiveness was carried out by both disc diffusion method and minimum inhibitory concentration (MIC) against four bacterial strains (gram-positive and gram-negative). In addition, (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used for the investigation of immunomodulatory effect of the extracts. The result showed that aqueous extract of O. stamineus exhibited significant free radical scavenging activity with IC50 of 9.6 µg/mL, whereas the IC50 for the ethanol extract was 21.4 µg/mL. These results showed that O. stamineus showed high antioxidant activity and could be considered as an immunomodulatory agent also.[52] In another study, an ultrasound-assisted extraction (UAE) with ethanol was used to extract the compounds responsible for the antioxidant activities of misai kucing (O. stamineus).[53] For the optimization of four independent variables such as ethanol concentration (%), amplitude (%), duty cycle (W/s), and extraction time (min), response surface methodology (RSM) was used. Based on the optimal conditions, the experimental values were reported to be close to the predicted value by RSM modeling (P > 0.05), which showed the suitability of UAE for extracting the antioxidants of misai kucing. RA, kaempferol-rutinoside, and SIN were identified by HPLC–mass spectrometry.[53] The different antioxidant activities are mentioned in Table 1.
Table 1.
Antioxidant activities of Orthosiphon stamineus
Antimicrobial activity
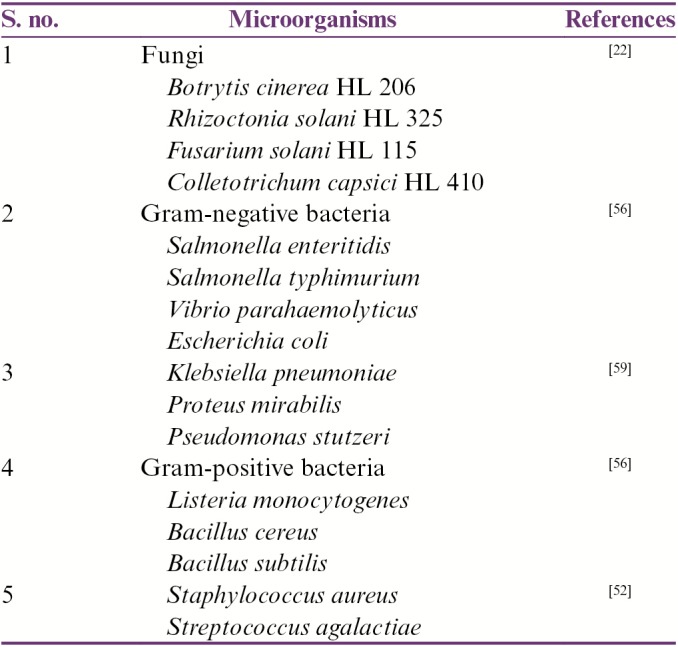
O. stamineus is rich in phenolic secondary metabolites, and some of them also have antimicrobial activity. An in vitro study using a disc diffusion assay showed that the extracts of this plant have antimicrobial activity against the selected foodborne bacteria.[58] The variable antibacterial activity was shown when 50% methanol extract of O. stamineus was tested against Bacillus subtilis, B. cereus, Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, Vibrio parahaemolyticus, Salmonella enteritidis, S. typhimurium, and Klebsiella pneumoniae, with the highest growth inhibitory action seen against V. parahaemolyticus, a bacterium that causes mild gastroenteritis in humans on consumption of contaminated seafood. O. stamineus has excellent antimicrobial properties. The extract showed antibacterial activity on serotypes c and d of Streptococcus mutans (MIC = 7.8–23.4mg/mL). However, later on, it was found that when treated in the presence of 5% sucrose, the potency gets decreased to approximately one-half, but no change was found in other type. The 50% methanol extract of O. stamineus inhibited B. subtilis, B. cereus, S. aureus, L. monocytogenes, E. coli, V. parahaemolyticus, S. enteritidis, S. typhimurium, and K. pneumoniae.[20] The antimicrobial activities are given in Table 2.
Table 2.
Reported antimicrobial activities of Orthosiphon stamineus extracts against selected microorganisms
HEPATOPROTECTIVE ACTIVITY
O. stamineus extract was found to reduce the necrotic changes in rat liver, and it inhibited the elevation of serum alanine transaminase and aspartate transaminase levels when treated with different doses (125, 250, 500, and 1000mg/kg). The hepatoprotective effect was caused by its antioxidant and free radical scavenging properties.[51] In another study, methanol extract of leaves at a dose of 200mg/kg showed hepatoprotective activity on paracetamol-induced rats. It is indicated that these properties were due to the ability to prevent the depletion of the tissue glutathione level.[60] As far as its aqueous extract is concerned, it lowered bilirubin level in jaundiced rat. This may be due to the increasing activity of glucuronyl transferase that facilitated hepatic conjugation of bilirubin or increased bilirubin binding by albumin.[61] Yam et al.[51] carried out an experiment on the methanolic extract of O. stamineus and reported its hepatoprotective effect. Alshawsh et al.[52] and Maheswari et al.[60] showed the hepatoprotective effect of ethanolic and methanolic extracts by using rat models for CCl4, thioacetamide-, and paracetamol-induced hepatotoxicity, respectively. The results of liver function tests and histology studies monitored the progression of hepatotoxicity. These studies supported the hepatoprotective effect of this extract.
Antihypertensive, hypoglycemic, hypolipidemic, and anti-obesity activities
Many works have been reported of antihypertensive activity of O. stamineus. A study showed that methylripariochromene A (100mg/kg) isolated from the leaves of O. stamineus decreased systolic blood pressure and heart rate, when it was injected subcutaneously into conscious male spontaneously hypertensive rats.[61] It also showed a concentration-dependent clamp down of retrenchments induced by high potassium, phenylephrine, or prostaglandin F2α in endothelium-denuded rat thoracic aorta. Furthermore, it exhibited a noticeable overpowering of contractile force without a noteworthy reduction in the heart rate in isolated bilateral guinea pig atria (negative inotropic effect). Lastly, it increased urine flow rate and absolute excretion of sodium, potassium, and chloride for 3h after oral administration to saline-preloaded fasted rats. All these outcomes specify that methylripariochromene A of O. stamineus possesses an antihypertensive property considered to cause vasodilation, decreased cardiac output, and diuresis. Mariam et al.[62] evaluated the acute effects of aqueous O. stamineus extract on blood glucose levels in both normal and diabetic rats. The results showed that administration of O. stamineus aqueous extract (OSAE) at 1000mg/kg produced hypoglycemic and antihyperglycemic effects in normal and streptozotocin (STZ)-induced diabetic rats, respectively. In this experiment, 14-day oral treatment was carried out with an aqueous extract of O. stamineus on plasma glucose and lipid profiles in normal and STZ-induced diabetic male Wistar rats. They reported reduction of plasma glucose levels in both euglycemic and hyperglycemic animals on oral administration of the extract at 200–1000mg/kg. Overall results showed that OSAE is effective as an antihyperglycemic and an antihyperlipidemic agent in diabetic rats. Matsubara et al.[61] remarked the excellency of this plant in the treatment of hypertension. In another experiment, a combined nutraceutical study containing alcoholic extract of O. stamineus was carried out. Leaves reduced the systolic and diastolic blood pressure as well as pulse pressure of the patients with hypertensive dyslipidemia after 8 weeks of treatment. The nutraceutical tested contained berberine, monacolin, and policosanols, in addition to O. stamineus extract.[63] In another study, 50% methanolic extract was orally administered to spontaneous hypertensive rats at doses of 250, 500, and 1000mg/kg BW. Systolic blood pressure of the rats decreased from 150 to 114 mmHg after 2 weeks’ treatment of dosing. Potency of the extract was comparable to irbesartan that was administered at 20mg/kg BW in the positive control group. Antihypertensive effects of the extract were planned to be facilitated by diuresis and natriuresis.[64] Recently Azam et al.[65] reported that aqueous, ethanolic, 50% aqueous ethanolic and methanolic extract, when given orally at a dose of 500 mg/kg body weight (bw) for 14 days to diabetic rats induced via intraperitoneal injection of 60 mg/kg bw STZ showed OS aqueous extract (OSAE) caused a reversal of diabetes mellitus comparable to that of 10 mg/kg bw glibenclamide. Nuclear magnetic resonance metabolomics approach using pattern recognition combined with multivariate statistical analysis was applied in the rat urine to study the effect. A total of 15 urinary metabolites, whose levels changed significantly on treatment, were identified as the biomarkers of OSAE in diabetes. Another recent study conducted by Seyedan et al.[66] reported antiglycemic effect of O. stamineus. In this experiment, ethanolic extract of leaves of O. stamineus (200 and 400mg/kg) and its major compound (RA, 10mg/kg) in obese mice (C57BL/6) induced by a high-fat diet with continuous supplementation with O. stamineus extract (200 and 400mg/kg) for 8 weeks considerably reduced BW gain. However, supplementation with RA, a constituent in the extract, produced only a slight reduction in BW gain compared to the high-fat diet control group. Result showed that the ethanolic extract of leaves of O. stamineus can significantly reduce a gain in BW and possess hypolipidemic and anti-obesity effects, thus guarding against the adverse effects of high-fat diet-induced obesity. Recently, Shafaei et al.[67] reported in vitro angiotensin-converting enzyme (ACE) inhibition activity of different extracts of O. stamineus leaves and their main flavonoids, namely RA, SIN, EUP, and 3ʹ-hydroxy-5,6,7,4ʹ-TMF. The in vitro ACE inhibition activity relied on determining the hippuric acid formation from ACE-specific substrate (hippuryl–histidyl–leucine) by the action of ACE enzyme.
Gastroprotective activity
The Malay traditional uses of O. stamineus in the treatment of gastric ailments are compelling reasons for investigating its possible gastroprotective effect.[68] In an experiment, the anti-ulcerogenic activity of 50% methanol extract of O. stamineus leaves was evaluated against ethanol-induced ulcers in male Sprague Dawley rats. The results exposed a significant dose-dependent (125, 250, 500, and 1000mg/kg) decrease in the ulcer index (UI) and gastric mucosal damage and lipid peroxidation along with an increase in mucus secretion. It was established that the extract possesses a gastroprotective property that is endorsed by its ability to inhibit lipid peroxidation and stimulate gastric mucus secretion to the high concentration of RA. In another in vivo model experiment, the anti-ulcer activity of Orthosiphon leaves extract was evaluated through several parameters that involve gastric acidity, number of ulcers, diameters of ulcers, UI, and healing ratio. The dose levels of Orthosiphon leaves extract that was used in this study were 250 and 500mg/kg, respectively. The results showed that Orthosiphon leaves extract have significantly different gastric ulcer healing properties when compared to control group. It was also supported by histopathological exmination.[69]
CONCLUSION
O. stamineus Benth. is a valued medicinal plant, growing well in many countries, especially Southeast Asian countries. This plant has a great potential value for cultivation because it contains secondary metabolites with interesting biological activities. Numerous compounds have been isolated from this plant, and this species has been used in the treatment and prevention of several illnesses such as diarrhea, inflammation, and intestinal disorders. Many experiments have been conducted to validate its pharmacological uses. This review has presented a comprehensive overview on the phytochemistry, and phytochemical and pharmacological applications of O. stamineus and its main compounds. In addition, clinical evaluation of the possible toxicity related to these isolated compounds needs to be assessed for finding their application as a biotherapeutical medicine.
Financial support and sponsorship
Kamran Ashraf would like to acknowledge Universiti Teknologi MARA for the financial support under the reference number 600-IRMI/MyRA 5/3/LESTARI (079/2017).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Indubala J, Ng LT. Herbs: the green pharmacy of Malaysia. Kuala Lumpur, Malaysia: Vinpress; 2000. pp. 76–7. [Google Scholar]
- 2.Hegnauer R. Chemotaxonomic der Planzen. Vol. IV. Birkhäuser Verlag: Stuggart; 1966. pp. 314–6. [Google Scholar]
- 3.Wangner H. Parmacietic biology: drugs and their inhalants. 2nd ed. Stuttgart, Germany: Gustav Fischer; 1982. pp. 45–90. [Google Scholar]
- 4.Eisai PT. Indonesia Medicinal Herb Index in Indonesia. 2nd ed. Godjah, Mada: University Press; 1995. pp. 239–63. [Google Scholar]
- 5.Chung WG, Roh HK, Kim HM, Cha YN. Monooxygenase in N-demethylation of caffeine; identified by using inducer treated rat liver microsomes that are characterized with testosterone metabolic patterns. Chem Biol Interact. 1998;113:1–14. doi: 10.1016/s0009-2797(97)00109-9. [DOI] [PubMed] [Google Scholar]
- 6.Masuda T, Masuda K, Shiragami S, Jitoe A, Nakatani N. Orthosiphol A and B, novel diterpenoid inhibitors of TPA (12-O-tetradecanoylphorbol–13–acetate)–induced inflammation, from Orthosiphon stamineus. Tetrahedron. 1992;48:6787–92. [Google Scholar]
- 7.Beaux D, Fleurentin J, Mortier F. Effect of extracts of Orthosiphon stamineus Benth., Hieracium pilosella L., Sambucus nigra L., and Arctostaphylos uva-ursi (L.) Spreng. in rats. Phytother Res. 1999;13:222–5. doi: 10.1002/(SICI)1099-1573(199905)13:3<222::AID-PTR447>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 8.Tezuka Y, Stampoulis P, Banskota AH, Awale S, Tran KQ, Saiki I, et al. Constituents of the Vietnamese medicinal plant Orthosiphon stamineus. Chem Pharm Bull. 2000;48:1711–9. doi: 10.1248/cpb.48.1711. [DOI] [PubMed] [Google Scholar]
- 9.Sumaryono W, Proksch P, Wray V, Witte L, Hartmann T. Qualitative and quantitative analysis of the phenolic constituents from Orthosiphon aristatus. Planta Med. 1991;57:176–80. doi: 10.1055/s-2006-960060. [DOI] [PubMed] [Google Scholar]
- 10.Olah NK, Radu L, Mogoşan C, Hanganu D, Gocan S. Phytochemical and pharmacological studies on Orthosiphon stamineus Benth.(Lamiaceae) hydroalcoholic extracts. J Pharm Biomed Anal. 2003;33:117–23. doi: 10.1016/s0731-7085(03)00227-9. [DOI] [PubMed] [Google Scholar]
- 11.Takeda Y, Matsumoto T, Terao H, Shingu T, Futatsuishi Y, Nohara T, et al. Orthosiphol D and E, minor diterpenes from Orthosiphon stamineus. Phytochemistry. 1993;33:411–5. [Google Scholar]
- 12.Yam MF, Asmawi MZ, Basir R. An investigation of the anti-inflammatory and analgesic effects of Orthosiphon stamineus leaf extract. J Med Food. 2008;11:362–8. doi: 10.1089/jmf.2006.065. [DOI] [PubMed] [Google Scholar]
- 13.Yam MF, Ang LF, Basir R, Salman IM, Ameer OZ, Asmawi MZ. Evaluation of the anti-pyretic potential of Orthosiphon stamineus Benth. standardized extract. Inflammopharmacology. 2009;17:50–4. doi: 10.1007/s10787-008-8038-3. [DOI] [PubMed] [Google Scholar]
- 14.Yuliana ND, Khatib A, Link-Struensee AMR, Ijzerman AP, Rungkat-Zakaria F, Young HC, et al. Adenosine Al receptor binding activity of methoxy flavonoids from Orthosiphon stamineus. Planta Medica. 2009;75:132–6. doi: 10.1055/s-0028-1088379. [DOI] [PubMed] [Google Scholar]
- 15.Mohamed EA, Mohamed AJ, Asmawi MZ, Sadikun A, Ebrika OS, Yam MF. Antihyperglycemic effect of Orthosiphon stamineus Benth leaves extract and its bioassay-guided fractions. Molecules. 2011;16:3787–801. doi: 10.3390/molecules16053787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pietta PG, Mauri PL, Gardana C, Bruno A. High-performance liquid chromatography with diode-array ultraviolet detection of methoxylated flavones in Orthosiphon leaves. J Chromatogr A. 1991;547:439–42. [Google Scholar]
- 17.Akowuah GA, Zhari I, Norhayati I, Sadikun A, Khamsah SM. Sinensetin, eupatorin, 3’-hydroxy-5,6,7,4’-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004;87:559–66. [Google Scholar]
- 18.Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical scavenging activity. Food Chem. 2005;93:311–7. [Google Scholar]
- 19.Hossain MA, Mizanur Rahman SM. Isolation and characterisation of flavonoids from the leaves of medicinal plant Orthosiphon stamineus. Arab J Chem. 2015;8:218–21. [Google Scholar]
- 20.Yam MF, Lim V, Salman IM, Ameer OZ, Ang LF, Rosidah N, et al. HPLC and anti-inflammatory studies of the flavonoid rich chloroform extract fraction of Orthosiphon stamineus leaves. Molecules. 2010;15:4452–66. doi: 10.3390/molecules15064452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banskota AH, Tezuka Y, Le Iran Q, Kadota S. Chemical constituents and biological activities of Vietnamese medicinal plants. Curr Topics Med Chem. 2003;3:227–48. doi: 10.2174/1568026033392516. [DOI] [PubMed] [Google Scholar]
- 22.Hossain MA, Ismail Z, Rahman A, Kang SC. Chemical composition and anti-fungal properties of the essential oils and crude extracts of Orthosiphon stamineus Benth. Ind Crops Prod. 2008;27:328–34. [Google Scholar]
- 23.Awale S, Tezuka Y, Banskota AH, Ketut Adnyana I, Kadota S. Highly-oxygenated isopimarane-type diterpenes from Orthosiphon stamineus of Indonesia and their nitric oxide inhibitory activity. Chem Pharma Bull. 2003;51:268–75. doi: 10.1248/cpb.51.268. [DOI] [PubMed] [Google Scholar]
- 24.Awale S, Tezuka Y, Banskota AH, Kouda K, Tun KM, Kadota S. Four highly oxygenated isopimarane-type diterpenes of Orthosiphon stamineus. Planta Medica. 2002;68:286–8. doi: 10.1055/s-2002-23137. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya H, Bohgaki T, Ohashi K. Two novel migrated pimarane-type diterpenes, neoorthosiphols A and B, from the leaves of Orthosiphon aristatus (Lamiaceae) Chem Pharma Bull. 1991;47:911–2. doi: 10.1248/cpb.48.433. [DOI] [PubMed] [Google Scholar]
- 26.Awale S, Tezuka Y, Kobayashi M, Ueda JY, Kadota S. Neoorthosiphonone A; a nitric oxide (NO) inhibitory diterpene with new carbon skeleton from Orthosiphon stamineus. Tetrahedron Lett. 2004;45:1359–62. [Google Scholar]
- 27.Stampoulis P, Tezuka Y, Banskota AH, Tran KQ, Saiki I, Kadota S. Staminolactones A and B and nor staminol A: three highly oxygenated staminane-type diterpenes from Orthosiphon stamineus. Organic Lett. 1999;1:1367–70. doi: 10.1021/ol990216+. [DOI] [PubMed] [Google Scholar]
- 28.Masuda T, Masuda K, Nakatani N. Orthosiphol A; a highly oxygenated diterpene from the leaves of Orthosiphon stamineus. Tetrahedron Lett. 1992;33:945–6. [Google Scholar]
- 29.Nguyen MTT, Awale S, Tezuka Y, CMen-Hsiung C, Kadota S. Staminane- and isopimarane-type diterpenes from Orthosiphon stamineus of Taiwan and their nitric oxide inhibitory activity. J Natural Products. 2004;67:654–8. doi: 10.1021/np030471+. [DOI] [PubMed] [Google Scholar]
- 30.Awale S, Tezuka Y, Banskota AH, Kadota S. Inhibition of NO production by highly-oxygenated diterpenes of Orthosiphon stamineus and their structure-activity relationship. Biol Pharma Bull. 2003;26:468–73. doi: 10.1248/bpb.26.468. [DOI] [PubMed] [Google Scholar]
- 31.Awale S, Tezuka Y, Banskota AH, Kadota S. Siphonols A-E: novel nitric oxide inhibitors from Orthosiphon stamineus of Indonesia. Bioorganic Med Chem Lett. 2003;13:31–5. doi: 10.1016/s0960-894x(02)00854-5. [DOI] [PubMed] [Google Scholar]
- 32.Stampoulis P, Tezuka Y, Banskota AH, Kim Qui T, Saiki I, Kadola S, et al. A novel diterpene from Orthosiphon stamineus. Tetrahedron Lett. 1999;40:4239–42. [Google Scholar]
- 33.Hossain MA, Ismail Z. Isolation and characterization of triterpenes from the leaves of Orthosiphon stamineus. Arab J Chem. 2013;6:295–8. [Google Scholar]
- 34.Saidan NH, Abdalrahim AFA, Hamil MSR, Abdul Majid AMS, Ismail Z. A novel reverse phase high-performance liquid chromatography method for standardization of Orthosiphon stamineus leaf extracts. Pharmacog Res. 2015;7:23–31. doi: 10.4103/0974-8490.147195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashim S, Beh HK, Hamil MS, Ismail Z, Majid AM. High-performance thin-layer chromatography method development, validation, and simultaneous quantification of four compounds identified in standardized extracts of Orthosiphon stamineus. Pharmacognosy Res. 2016;8:238–43. doi: 10.4103/0974-8490.188872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.See Tiam Y, Tee Siau N, Ang Teck CH, Chan R, Yusoff C, Ngoh Ge C. Assessment of various pretreatment and extraction methods for the extraction of bioactive compounds from Orthosiphon stamineus leaf via. microstructures analysis. Int J Food Eng. 2016;12:711–7. [Google Scholar]
- 37.Sree NV, Sri PU, Ramarao N. Neuro-protective properties of Orthosiphon stamineus (Benth) leaf methanolic fraction through antioxidant mechanisms on SH-SY5Y cells: an in-vitro evaluation. Int J Pharm Sci Res. 2015;6:1115–25. [Google Scholar]
- 38.Dolečková I, Rárová L, Grúz J, Vondrusová M, Strnad M, Kryštof V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves. Fitot. 2012;83:1000–7. doi: 10.1016/j.fitote.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Awale S, Tezuka Y, Banskota AH, Kouda K, Tun KM, Kadota S. Five novel highly oxygenated diterpenes of Orthosiphon stamineus from Myanmar. J Nat Prod. 2001;64:592–6. doi: 10.1021/np000607t. [DOI] [PubMed] [Google Scholar]
- 40.Ahamed MBK, Aisha AFA, Nassar ZD, Siddiqui JM, Ismail Z, Omari SMS, et al. Cat’s whiskers tea (Orthosiphon stamineus) extract inhibits growth of colon tumor in nude mice and angiogenesis in endothelial cells via suppressing VEGFR phosphorylation. Nutri Cancer. 2012;64:89–99. doi: 10.1080/01635581.2012.630160. [DOI] [PubMed] [Google Scholar]
- 41.Salleh SA, Rajab NF, Abdullah NR, Ismail Z, Mouatt P, Dowell A, et al. In vitro chemopreventive activity of an ethyl acetate fraction derived from hot water extract of Orthosiphon stamineus in HepG2 cells. J Med Plants Res. 2011;5:1892–9. [Google Scholar]
- 42.Sundarammal S, Thirugnanasampandan R, Selvi MT. Chemical composition analysis and antioxidant activity evaluation of essential oil from Orthosiphon thymiflorus (Roth.) Sleesen. Asian Pac J Trop Biomed. 2012:S112–5. [Google Scholar]
- 43.Awale S, Tezuka Y, Banskota AH, Shimoji S, Taira K, Kadota S. Norstaminane- and isopimarane-type diterpenes of Orthosiphon stamineus from Okinawa. Tetrahedron. 2002;58:5503–12. [Google Scholar]
- 44.Sahib HB, Ismail Z, Othman NH, Abdul Majid AMS. Orthosiphon stamineus Benth. methanolic extract enhances the anti-proliferative effects of tamoxifen on human hormone dependent breast cancer. Inter J Pharmacol. 2009;5:273–6. [Google Scholar]
- 45.Abdelwahab SI, Mohan S, Elhassan MM, Al-Mekhlafi N, Mariod AA, Abdul AB, et al. Antiapoptotic and antioxidant properties of Orthosiphon stamineus Benth (cat’s whiskers): intervention in the Bcl-2-mediated apoptotic pathway. Evid Based Complement Alternat Med. 2011;2011:156765. doi: 10.1155/2011/156765. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Hsu CL, Hong BOH, Shan YU, Yen GC. Antioxidant and anti-inflammatory effects of Orthosiphon aristatus and its bioactive compounds. J Agric Food Chem. 2010;58:2150–6. doi: 10.1021/jf903557c. [DOI] [PubMed] [Google Scholar]
- 47.Akowuah GA, Zhari I. Effect of extraction temperature on stability of major polyphenols and antioxidant activity of Orthosiphon stamineus leaf. J Herbs Spices Med Plant. 2010;16:160. [Google Scholar]
- 48.Kuo PC, Schroeder RA. The emerging multifaceted roles of nitric oxide. Ann Surg. 1995;221:220–35. doi: 10.1097/00000658-199503000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akowuah GA, Zhari I, Sadikun A, Norhayati I. HPTLC densitometric analysis of Orthosiphon stamineus leaf extracts and inhibitory effect on xanthine oxidase activity. Pharm Biol. 2006;44:65–70. [Google Scholar]
- 50.Akowuah GA, Zhari I, Norhayati I, Sadikun A. Radical scavenging activity of methanol leaf extracts of Orthosiphon stamineus. Pharm Biol. 2005;42:629–35. [Google Scholar]
- 51.Yam MF, Basir R, Asmawi MZ, Ismail Z. Antioxidant and hepatoprotective effects of Orthosiphon stamineus Benth. standardized extract. Am J Chin Med. 2007;35:115–26. doi: 10.1142/S0192415X07004679. [DOI] [PubMed] [Google Scholar]
- 52.Alshawsh MA, Abdulla MA, Ismail S, Amin ZA, Qader SW, Hadi HA, et al. Free radical scavenging, antimicrobial and immunomodulatory activities of Orthosiphon stamineus. Molecules. 2012;17:5385–95. doi: 10.3390/molecules17055385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin YT, Labbe RG, Shetty K. Inhibition of Vibrio parahaemolyticus in seafood systems using oregano and cranberry phytochemical synergies and lactic acid. Innovat Food Sci Emerging Technol. 2005;6:453–8. [Google Scholar]
- 54.Chew KK, Khoo MZ, Ng SY, Thoo YY, Aida WMW, Ho CW. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int Food Res J. 2011;18:1427–35. [Google Scholar]
- 55.Farhan M, Abdul Raza S, Pin KY, Chuah AL. Antioxidant activity and phenolic content of different parts of Orthosiphon stamineus grown under different light intensities. J Tropical Forest Sci. 2012;24:173–7. [Google Scholar]
- 56.Ho CH, Noryati I, Sulaiman SF, Rosma A. In vitro antibacterial and antioxidant activities of Orthosiphon stamineus Benth. extracts against food-borne bacteria. Food Chem. 2010;122:1168–72. [Google Scholar]
- 57.Olennikov DN, Tankhaeva LM. Physicochemical characteristics and antioxidant activity of melanoidin pigment from the fermented leaves of Orthosiphon stamineus. Brazilian J Pharmacog. 2011;22:284–90. [Google Scholar]
- 58.Ho SK, Tan CP, Thoo YY, Abas F, Ho CW. Ultrasound-assisted extraction of antioxidants in misai kucing (Orthosiphon stamineus) Molecules. 2014;19:12640–59. doi: 10.3390/molecules190812640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laikangbam R, Devi MD, Singh SR. Anti-bacterial efficacy of elite medicinal plants on urolithiasis inducing flora. J Food Agric Environ. 2009;7:40–5. [Google Scholar]
- 60.Maheswari K, Marymmal R, Venkatanarayan R. Hepatoprotective activity of Orthosiphon stamineus on liver damages caused by paracetamol in rats. Jordan J Biol Sci. 2008;1:105–8. [Google Scholar]
- 61.Matsubara T, Bohgaki T, Watarai M, Suzuki H, Ohashi K, Shibuya H. Antihypertensive actions of methylripariochromene A from Orthosiphon aristatus, an Indonesian traditional medicinal plant. Biol Pharm Bull. 1999;22:1083–8. doi: 10.1248/bpb.22.1083. [DOI] [PubMed] [Google Scholar]
- 62.Mariam A, Amawi MZ, Sadikun A. Hypoglycaemic activity of the aqueous extract of Orthosiphon stamineus. Fitoterapia. 1996;67:465–8. [Google Scholar]
- 63.Cicero AFG, De Sando V, Izzo R, Vasta A, Trimarco A, Borghi C. Effect of a combined nutraceutical containing Orthosiphon stamineus effect on blood pressure and metabolic syndrome components in hypertensive dyslipidaemic patients: a randomized clinical trial. Complement Ther Clin Pract. 2012;18:190–1. doi: 10.1016/j.ctcp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Azizan NA, Ahmad Mohamed K, Ahmad MZ, Asmawi Z. The in vivo antihypertensive effects of standardized methanol extracts of Orthosiphon stamineus on spontaneous hypertensive rats: a preliminary study. African J Pharmacol. 2012;6:376–9. [Google Scholar]
- 65.Azam AA, Pariyani R, Safinar I, Ismail A, Khatib A, Abas F, et al. Urinary metabolomics study on the protective role of Orthosiphon stamineus in streptozotocin induced diabetes mellitus in rats via 1H NMR spectroscopy. BMC Complement Altern Med. 2017;17:278. doi: 10.1186/s12906-017-1777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seyedan A, Alshawsh MA, Alshagga MA, Mohamed Z. Antiobesity and lipid lowering effects of Orthosiphon stamineus in high-fat diet-induced obese mice. Planta Med. 2017;83:684–92. doi: 10.1055/s-0042-121754. [DOI] [PubMed] [Google Scholar]
- 67.Shafaei A, Khan S, Aisha AFA, Abdul Majid AMS, Hamdan MR, Mordi MN, et al. Flavonoids-rich Orthosiphon stamineus extract as new candidate for angiotensin I-converting enzyme inhibition: a molecular docking study. Molecules. 2016;21:1500. doi: 10.3390/molecules21111500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yam MF, Ang LF, Salman IM, Ameer OZ, Lim V, Ong LM, et al. Orthosiphon stamineus leaf extract protects against ethanol-induced gastropathy in rats. J Med Food. 2009;12:1089–97. doi: 10.1089/jmf.2008.0005. [DOI] [PubMed] [Google Scholar]
- 69.Yuniarto A, Susilawati E, Ismi Khairunnisa I, Dadang Juanda D, Finna Setiawan F. Antioxidant and gastric ulcer healing effect of Orthosiphon stamineus (Benth.) leaves extract in aspirin-induced rats. Asian J Pharm Clin Res. 2017;10:397–9. [Google Scholar]