Ocean Acidification and Warming induce trade-offs in development and growth of Atlantic herring embryos by altering mitochondrial function and increasing the animal’s energy demand.
Keywords: Atlantic herring, embryonic development, mitochondrial capacity, Ocean Acidification, Ocean Warming, respiration
Abstract
Atlantic herring (Clupea harengus) is a benthic spawner, therefore its eggs are prone to encounter different water conditions during embryonic development, with bottom waters often depleted of oxygen and enriched in CO2. Some Atlantic herring spawning grounds are predicted to be highly affected by ongoing Ocean Acidification and Warming with water temperature increasing by up to +3°C and CO2 levels reaching ca. 1000 μatm (RCP 8.5). Although many studies investigated the effects of high levels of CO2 on the embryonic development of Atlantic herring, little is known about the combination of temperature and ecologically relevant levels of CO2. In this study, we investigated the effects of Ocean Acidification and Warming on embryonic metabolic and developmental performance such as mitochondrial function, respiration, hatching success (HS) and growth in Atlantic herring from the Oslo Fjord, one of the spawning grounds predicted to be greatly affected by climate change. Fertilized eggs were incubated under combinations of two PCO2 conditions (400 μatm and 1100 μatm) and three temperatures (6, 10 and 14°C), which correspond to current and end-of-the-century conditions. We analysed HS, oxygen consumption (MO2) and mitochondrial function of embryos as well as larval length at hatch. The capacity of the electron transport system (ETS) increased with temperature, reaching a plateau at 14°C, where the contribution of Complex I to the ETS declined in favour of Complex II. This relative shift was coupled with a dramatic increase in MO2 at 14°C. HS was high under ambient spawning conditions (6–10°C), but decreased at 14°C and hatched larvae at this temperature were smaller. Elevated PCO2 increased larval malformations, indicating sub-lethal effects. These results indicate that energetic limitations due to thermally affected mitochondria and higher energy demand for maintenance occur at the expense of embryonic development and growth.
Introduction
The atmospheric CO2 concentration has increased dramatically since the preindustrial era, from ca. 280 μatm to ca. 410 μatm nowadays causing an increase in average ocean surface temperatures of about 0.83°C and a decrease in surface water pH of 0.1 units (Bopp et al., 2013). If the rate of emissions does not change, the level of atmospheric CO2 is expected to rise to ca. 851–1370 μatm by the year 2100 causing an average warming of 3.15°C and a decrease of 0.41 pH units in the ocean surface waters (Henson et al., 2017).
While juvenile and adult fish appear to tolerate CO2 levels far beyond average climate change predictions (>2000 μatm, Ishimatsu et al., 2008), early life stages, such as developing embryos and lecithotrophic larvae, appear to be more vulnerable to both, Ocean Acidification and Warming (Baumann et al., 2011; Chambers et al., 2014; Pimentel et al., 2014; Frommel et al., 2016; Stiasny et al., 2016; Dahlke et al., 2017; Sswat et al., 2018). This is probably due to simple modes of respiration (dermal vs. gills) during development and insufficient acid–base regulation before the formation of gills, a situation possibly exacerbated by the higher surface to volume ratio of the early stages compared to adults (Kikkawa et al., 2003; Ishimatsu et al., 2008). Moreover, developing embryos and lecithotrophic larvae are entirely dependent on parental provisioning of resources (yolk) and molecular defence mechanisms (Kamler, 2008) which may become limiting in a changing environment. Exposure to elevated PCO2 (CO2 partial pressure) has been found to adversely affect embryonic development (Tseng et al., 2013; Dahlke et al., 2017), larval growth and survival (Baumann et al., 2011) and tissue/organ health (Frommel et al., 2016) in some fish species. However, in other species, studies have not detected any effect on embryogenesis (Franke and Clemmesen, 2011; Maneja et al., 2015), hatching (Frommel et al., 2012) or growth and development (Munday et al., 2011; Frommel et al., 2012; Hurst et al., 2012, 2013; Bignami et al., 2014). Thus, it is important to understand which are the mechanisms underlying the sensitivity towards Ocean Acidification and Warming in the early life stages to assess which fish species will be affected more by the ongoing climatic changes.
Thermal acclimation and acid–base regulation may increase the metabolic costs of development, causing the reallocation of the yolk-limited resources from development and growth to maintenance (Rombough, 2011; Dahlke et al., 2017). Studies of energy metabolism in developing fish eggs have been concerned mainly with measuring levels of potential energy reserves, metabolites and relevant metabolic enzyme systems (Tocher et al., 1985; Finn et al., 1996; Finn and Fyhn, 2010), and only recently, studies have begun to address how Ocean Acidification and Warming affect the metabolism of fish embryos (Flynn et al., 2015; Pimentel et al., 2016; Dahlke et al., 2017).
In an early study, Boulekbache (1981) described the energetic charge of ATP in the developing embryos of rainbow trout (Oncorhynchus mykiss). He found a decrease of ATP/ADP during cleavage (morula–blastula), then a slight increase during gastrulation followed by a plateau. This profile could represent a heavy utilization of pre-existing ATP during the early stage followed by a neosynthesis in parallel to increased embryo cell movement and diversification (Boulekbache, 1981; Tocher et al., 1985). Since the ATP consumption profile seems to be correlated with specific moments of embryonic development and since at these stages ATP production may be limited by the endogenous resources (yolk), ATP production pathways, mitochondrial metabolism in particular, could play a key role in the embryonic sensitivity to ocean acidification and warming.
Atlantic herring (Clupea harengus) is a benthic spawner and schooling pelagic fish. It is widely distributed throughout the North Atlantic shelf regions from the East coast of North America to the West coast of Europe and the Baltic Sea Herring populations represent a major resource not only for other fish species, birds and whales (Lynam et al. 2015), but also for commercial fisheries, with annual catches of more than 2 million tones (FAO, 2018). Herring population dynamics are known to be sensitive to changes in water temperature: in the last centuries cool periods promoted the increase of herring biomass and the southward expansion of the distribution areas, while warming events exerted the opposite effect (Alheit and Hagen, 1997). Moreover, herring spawn over extended periods with a wide range of spawning locations specific to seasons and populations (Geffen, 2009). Therefore, herring populations spawning in areas predicted to be severely affected by climate changes may be more vulnerable to Ocean Acidification and Warming, especially those spawning during the summer/autumn season. For example, PCO2 values above 4000 μatm could be reached in the future at important herring spawning grounds in the Baltic Sea such as the Kiel Fjord (Melzner et al., 2013; Frommel et al., 2014) and at higher latitudes including the Skagerrak and North Sea. PCO2 is predicted to double with an increase in temperature of more than 3°C (Henson et al., 2017; van Vuuren et al., 2011: RPC 8.5). Studies of developing herring embryos from the Baltic Sea found no significant effect of high PCO2 (4600 μatm) in hatch rate, development, or otolith size (Franke and Clemmesen, 2011). However, no studies have so far addressed the combined effects of increasing temperature and PCO2 on the embryonic development of this fish.
It is thus important to understand the effects of ocean acidification and warming on the development of herring embryos under the conditions predicted to occur at the spawning grounds by the end of the century. To do so, we incubated developing embryos of Atlantic herring from the Scandinavian coast within a cross-factorial combination of three temperatures (6–10–14°C) and two PCO2 levels (400–1100 μatm), to mirror present conditions and the conditions projected for the end of the century (RCP. 8.5; van Vuuren et al., 2011; Henson et al., 2017) for the entire developmental period, from fertilization to hatch. We analysed mitochondrial respiration and the oxygen demand of late-stage embryos (50% eye pigmentation) to investigate mitochondrial function in relation to embryonic energy demand, respectively. Furthermore, we observed hatching success (HS), larval deformities and larval length at hatch to identify constraints on performance at the whole-organism level.
Methodology
This study was conducted at the Sven Lovén Centre for Marine Science, Kristineberg Biological Station (University of Gothenburg, Sweden) between April and May 2013 in accordance with the legislation of the Swedish Board of Agriculture (Permit: 332–2012).
Experimental animals
Ripe Atlantic herring, C. harengus, were caught with gill nets during the spawning season in April 2013 in the inner Oslo Fjord (Norway). Selected fish were caught and killed with a blow on the head and were stored on ice and transported to the Sven Lovén Centre. Gametes of males (n = 3) and females (n = 3) used for in vitro fertilizations were obtained by strip spawning approximately four hours after the fish had been caught.
Experimental design
A full-factorial design with three temperatures (6, 10 and 14°C) and two PCO2 (400 μatm and 1100 μatm) was used for fertilization and incubation of herring eggs. Treatment conditions were selected to encompass ambient spawning temperatures (6–10°C and the PCO2 recorded in the year 2013) as well as water warming and acidification projected for the end of this century according to IPCC’s business-as-usual scenario (RCP 8.5, van Vuuren et al., 2011). Eggs produced by different females were incubated separately and ‘females’ were treated as biological replicates (n = 3). Each female was represented by two incubators at each treatment combination (2 × 3 females × 6 treatments = 36 incubators in total). In order to avoid biased survival estimates, only one of both incubators was used to collect samples for measurements of oxygen consumption rates and mitochondrial capacities. The second incubator was used to evaluate HS and larval morphology at hatch. Incubator classification and arrangement within experimental units was done randomly.
Fertilization protocol
The eggs of each female were stripped onto 12 plates of Polyethylene mesh (500 μm mesh size, 10 cm diameter). To optimize fertilization success and oxygenation during development, care was taken to arrange the eggs in single layer. Two out of 12 egg-plates were fertilized at each of six different temperature × PCO2 treatment combinations (Table 1) following a wet-fertilization protocol (Geffen, 1999). Individual egg-plates were placed in Petri dishes and incubated for 10 min with a milt-seawater dilution of 1:500 (10 ml, produced with milt aliquots from n = 3 males). After being carefully rinsed, the egg-plates were transferred into hatching jars filled with 1 l of filtered (0.2 μm) and UV sterilized seawater (adjusted to the respective treatment combination). The percentage of fertilized eggs on each plate (i.e. fertilization success) was determined by visual inspection under a stereomicroscope after 12 h of incubation. Mean values are shown in Table 1.
Table 1:
Mean ± SEM fertilization success of Atlantic herring (Clupea harengus) eggs fertilized at different levels of temperature and PCO2. Differences between temperature and PCO2 treatments were statistically not significant (F = 1.9, P = 0.192 and F = 0.97, P = 0.344, respectively)
| Temperature (°C) | Fertilization success (%) | |
|---|---|---|
| Control PCO2 | High PCO2 | |
| 6 | 88.8 ± 2.8 | 79.4 ± 4.3 |
| 10 | 76.0 ± 9.0 | 72.2 ± 8.6 |
| 14 | 88.9 ± 8.5 | 85.3 ± 8.0 |
Incubation
The incubation set-up is shown in Supplementary Figure S1. Herring eggs adhered to mesh-plates were incubated within transparent, bottom tapered hatching jars (Imhoff sedimentation cones, 1000 ml volume, Supplementary Fig. S1A), which were submerged into 400 l seawater baths thermostatted to different temperatures (6, 10 and 14°C, Supplementary Fig. S1B). Each incubator was sealed with a Styrofoam lid to prevent outgassing of CO2. Eggs received dim light with a daily rhythm of 12 h/12 h light/darkness. Every 24 h, 80% of the water volume of each incubator was replaced by filtered (0.2 μm) and UV-sterilized seawater (33 PSU) to avoid oxygen depletion and bacterial or fungal infestation. Herring eggs were not exposed to air during water exchange. Each water bath contained two 60-l reservoir tanks, which were used to pre-adjust exchange-seawater to the corresponding temperature and PCO2. Water temperatures of the different water baths were recorded automatically every 15 min (±0.1°C, Table 2) by a multi-channel aquarium computer (IKS-Aquastar, IKS Systems, Germany).
Table 2:
Summary table of the water parameters measured during the incubation of Atlantic herring (Clupea harengus) eggs until hatch. Data are presented as mean ± SD
| Duration (days) | Nominal T (°C) | Measured T(°C) | Oxygen (%) | PCO2 (μatm) | pHF | |||
|---|---|---|---|---|---|---|---|---|
| Control PCO2 | High PCO2 | Control PCO2 | High PCO2 | Control PCO2 | High PCO2 | |||
| 27 | 6 | 6.15 ± 0.06 | 94.40 ± 0.71 | 94.40 ± 0.61 | 415 ± 10 | 1101 ± 47 | 8.15 ± 0.02 | 7.77 ± 0.02 |
| 16 | 10 | 10.04 ± 0.06 | 94.40 ± 0.63 | 94.40 ± 0.49 | 408 ± 10 | 1050 ± 46 | 8.17 ± 0.02 | 7.79 ± 0.03 |
| 11 | 14 | 14.07 ± 0.20 | 95.00 ± 0.00 | 95.00 ± 0.00 | 403 ± 12 | 1050 ± 29 | 8.18 ± 0.02 | 7.78 ± 0.02 |
Elevated PCO2 conditions were administered by injection of pure CO2 gas into the submerged 60 l reservoir tanks by bubbling through large aeration stones (20 cm length). A multi-channel feedback system (IKS-Aquastar), connected to individual pH-probes (IKS-Aquastar) and solenoid valves were used to adjust PCO2 values. Pure CO2 was infused via perforated silicone tubes until the desired pH/PCO2 was reached. The PCO2 of the reservoir tanks was measured in situ prior to every second water exchange with an infrared PCO2 probe (Vaisala GM70, manual temperature compensation, ±10 μatm accuracy; Vaisala, Finland). The probe was equipped with an aspiration pump and sealed with a gas-permeable membrane to measure PCO2 in air equilibrated with dissolved CO2 in the water, as described by Munday et al. (2013) and Jutfelt and Hedgarde (2013). Factory calibration was confirmed by measurements of seawater previously bubbled with a technical gas mixture (1010 μatm CO2 in air; AGA Sweden). Prior to the daily water exchange, pH-values of the reservoir tanks were measured with a lab-grade pH-electrode to three decimal places (Mettler Toledo InLab Routine Pt 1000 with temperature compensation, Mettler Toledo, Switzerland), which was connected to a WTW 3310 pH-meter. A two-point calibration with NBS-buffers was performed on a daily basis. To convert NBS to the free proton concentration scale for seawater pH (Waters and Millero, 2013), the electrode was recalibrated with Tris-HCl seawater buffers (Dickson et al., 2007), which were acclimated to the corresponding incubation temperature prior to each measurement. Seawater pH-values refer to the free proton concentration scale throughout this manuscript (for summary see Table 2), Individual values for each measured parameter are available in the Open Access library PANGAEA (see ‘Data availability’ section).
Data collection
Whole-embryo oxygen consumption
Oxygen consumption rates (MO2) of late-stage embryos (at 50% eye pigmentation) were measured in closed, temperature-controlled respiration chambers (OXY0 41 A, Collotec Meßtechnik GmbH, Germany, Supplementary Fig. S2) following methodologies described by Schiffer et al. (2014). All measurements were performed in duplicates (with two respiration chambers) at the same developmental stage (~50% eye pigmentation) and treatment as during incubation. Staging was done by visual inspection during the daily water exchange. Development times until 50% eye pigmentation (and hatching) did not differ between PCO2 treatments (Supplementary Fig. S3 and Table S1), as was demonstrated for Baltic herring under more extreme PCO2 conditions (4 600 μatm, Franke and Clemmesen, 2011). The stage at 50% eye pigmentation was selected because it represents a clearly discernible developmental landmark (Hill and Johnston, 1997) at which the embryonic cardiocirculatory system, and thus metabolic capacity, is already well-developed (Hill and Johnston, 1997). For each run, ~20 (±3) eggs were loaded into each of the two respiration chambers. The chambers were previously filled with a volume of ~2 ml sterilized seawater, whereby each chamber was alternately used for different PCO2 treatments. The eggs were placed on a polyethylene mesh (500 μm mesh size) with a magnetic micro-stirrer (3 mm) underneath to avoid oxygen stratification within the respiration chamber (see Supplementary Fig. S2). The change in oxygen saturation was detected by micro-optodes (fiber-optic microsensor, flat broken tip, diameter: 140 μm, PreSens GmbH, Germany) connected to a Microx TX3 (PreSens GmbH, Germany). Recordings were stopped after 60 min (at 6°C) or as soon as the oxygen saturation declined below 80% air saturation (20–40 min at 10 and 14°C). After each run, the wet mass per egg and the exact water volume of the respiration chamber was determined by weighing on a precision balance (±0.01 mg). Bacterial oxygen consumption (always below 5%) and optode drift (always below 1%) was determined by blank measurements before and after three successive runs with eggs. Given that egg masses did not differ between temperature and PCO2 treatments (Supplementary Fig. S3), MO2 was expressed as (nmol O2 egg−1 h−1) according to the following formula: MO2 = DO2 * Vol/NEggs, where DO2 is the decline in oxygen saturation (nmol l−1 h−1), Vol is the water volume of the respiration (ml) chamber and NEggs is the number of eggs.
Mitochondrial function
Mitochondrial function was measured in a cellular suspension of late-stage eggs (at 50% eye pigmentation) as described in Dahlke et al. (2017). Briefly, one hundred eggs from n = 3 females were gently ground on ice in a glass potter filled with 2-ml ice-cold modified mitochondrial respiration medium MiR05 (0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 160 mM sucrose, 1 g l−1 bovine serum albumin, pH 7.4, 380 mOsmol l−1) (Iftikar and Hickey, 2013; Gnaiger et al., 2015). The resulting suspension was collected avoiding the collection of the eggshells and mitochondrial respiration was analysed using Oroboros Oxygraph-2k™ respirometers (Oroboros Instruments, Innsbruck, Austria). The oxygen flux (nmol O2 (egg* h)−1) was recorded and calculated in real-time using Oroboros DatLab 5.2.1.51 (Oroboros Instruments, Innsbruck, Austria). Measurements were conducted in MiR05 buffer equilibrated to atmospheric PCO2 and acclimation temperature of the eggs. The cO2 ranged from atmospheric saturation (ca. 370 nmol ml−1) to 150 nmol ml−1. A substrate–uncoupler–inhibitor titration (SUIT) protocol was used to investigate the capacities of the single components of the electron transport system (ETS) measured as oxygen consumption attributable to each component (nmol O2 (egg* h)−1). In detail: ETS capacity was measured by step-wise (1 μM each) titration of carbonyl cyanide p-(trifluoromethoxy)phenyl-hydrazone (FCCP) in the presence of Complex I (CI) and Complex II (CII) substrates (10 mM glutamate, 2 mM malate, 10 mM pyruvate and 10 mM succinate). CI, CII and Complex III (CIII) were inhibited by the addition of 0.5 μM rotenone, 5 mM malonate and 2.5 μM antimycin a, respectively. All chemicals were obtained from Sigma-Aldrich (Germany).
Hatching success
Once hatching started, free-swimming larvae were collected in the morning, euthanized with an overdose of tricaine methane sulphonate (MS-222) and counted after visual examination for morphological deformities under a stereomicroscope. The incidence of larval deformities was quantified as the percentage of hatchlings that exhibited severe deformations of the yolk sac, cranium or vertebral column. HS, defined as the percentage of non-malformed larvae that hatched from fertilized eggs, was calculated as:
where Li represents the number of hatched larvae, Ld is the number of deformed larvae and Ef is the number of fertilized eggs (shown in Table 1).
Larval size at hatch
Subsamples of 10–30 non-malformed larvae of three females at each treatment combination were photographed for subsequent measurements of larval standard length (SL) using Olympus image analysis software (Stream Essentials©, ± 1 μm). Only samples obtained from the same daily cohort (during peak-hatch at each temperature treatment) were used for statistical comparison between PCO2 treatments.
Data analysis
All data are presented as mean ± SEM.
Statistical analyses were conducted using R 3.2.0 (R Core Team, 2015) and the level of statistical significance was set at P < 0.05 for all the statistical tests.
Normal distribution and homoscedasticy of the data were assessed by Shapiro–Wilk and Bartlett’s tests, respectively.
Multi factorial analyses of variance (two-way ANOVA) including the female parent ID as covariance were used to evaluate whether temperature and PCO2 and the combination of both factors had an effect on the parameters object of this study. The two-way ANOVA was followed by Tukey’s HSD test for temperature and Student’s t-test for CO2.
In addition, MO2 data were analyzed with the female-specific egg mass included in the model as covariate.
The temperature coefficient Q10 was calculated according to the equation:
R: respiration rate
T: temperature at which the respiration rate was measured.
Results
Mitochondrial function and whole-embryo respiration
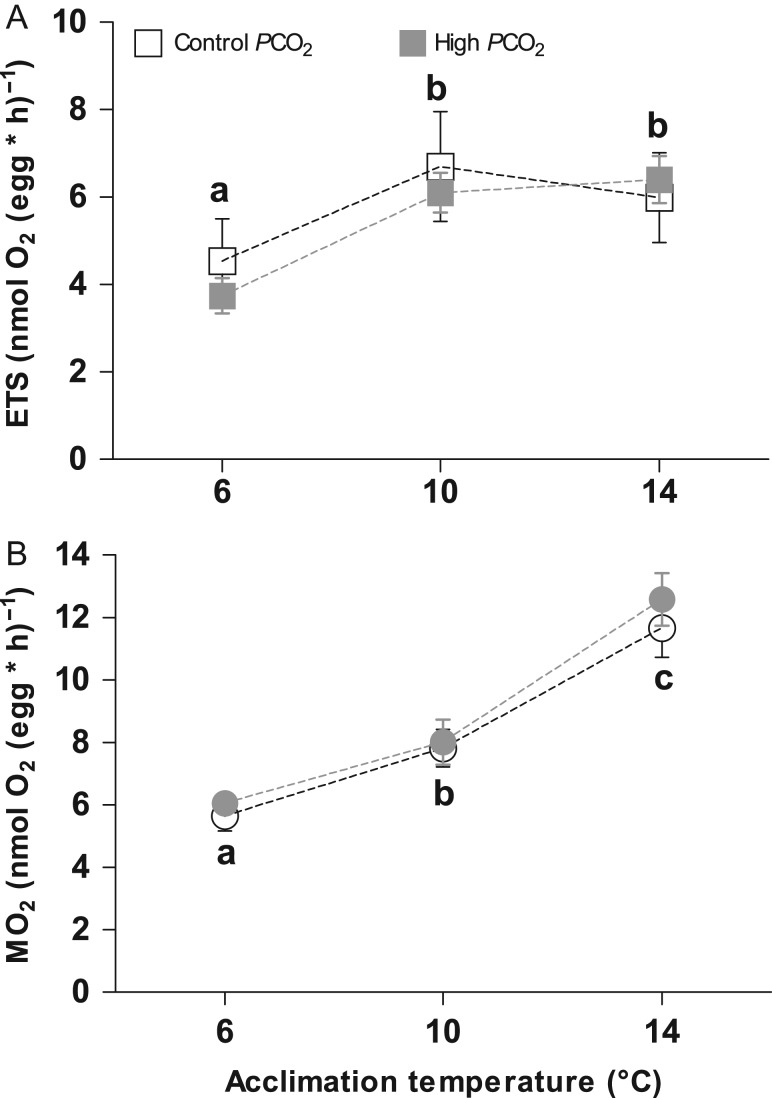
The in vivo oxygen consumption rates (MO2) of Atlantic herring embryos were affected only by temperature (F= 173.87, P < 0.001, Fig. 1b). In general, MO2 increased with temperature in a non-linear fashion: expressed as Q10, the increase in MO2 between 6 and 10°C (Control PCO2: 2.30 ± 0.23; High PCO2: 2.00 ± 0.20) was lower than between 10 and 14°C (Control PCO2: 2.27 ± 0.14; High PCO2: 3.17 ± 0.27).
Figure 1:
Respiration performance and mitochondrial capacity of Atlantic herring (Clupea harengus) embryos at 50% eye pigmentation stage. Values are reported as mean ± SEM. Panel A: Electron Transport System (ETS) capacity. Open squares: control PCO2 (400 μatm), solid squares: high PCO2 (1100 μatm). Panel B: Whole-embryo respiration. Open circles: control PCO2 (400 μatm), solid circles: high PCO2 (1100 μatm). Different letters within panels indicate significant differences (P < 0.05) between temperature treatments independent of the CO2 treatment.
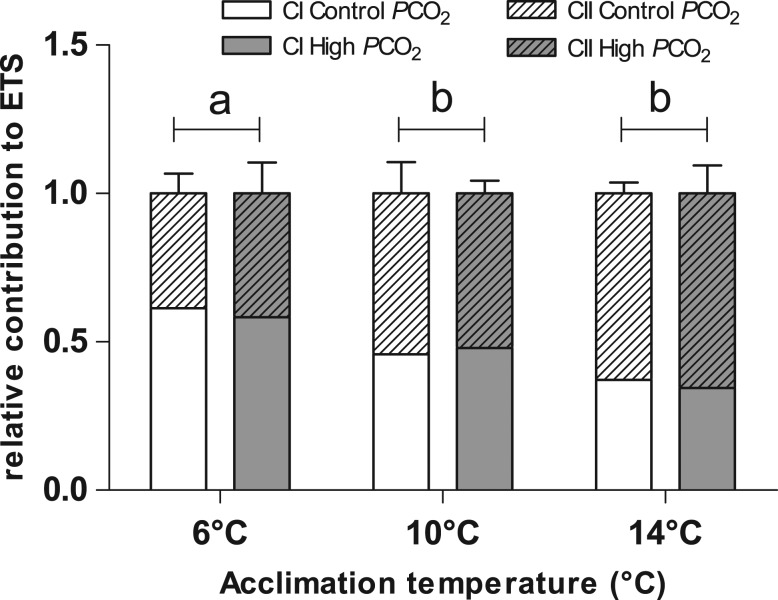
In vitro, the mitochondrial oxygen flux corresponding to the maximum capacity of the ETS was affected only by temperature (F= 5.61, P = 0.019, Fig. 1a) and increased between 6 and 10°C (P = 0.04), but, unlike whole-embryo MO2 (Fig. 1), reached a plateau between 10 and 14°C (P > 0.05). CI and CII contributed differently to the ETS according to temperature (F = 17.28, P < 0.001, Fig. 2). CI contribution declined with increasing temperature while CII contribution increased (Fig. 2). At 14°C, only 37% of the ETS capacity was contributed from CI, compared with 62% at 6°C (Fig. 2).
Figure 2:
Contribution (%) of Complex I and Complex II to the electron transport system (ETS) in the embryos of Atlantic herring (Clupea harengus). Embryonic stage: 50% eye pigmentation. Values are reported as mean ± SEM. Open bars: Complex I, dashed bars: Complex II. Open bars: control PCO2 (400 μatm), solid bars: high PCO2 (1100 μatm). Different letters indicate statistical differences (P < 0.05) between temperature treatments independent of the CO2 treatment.
Viable hatch and length at hatch
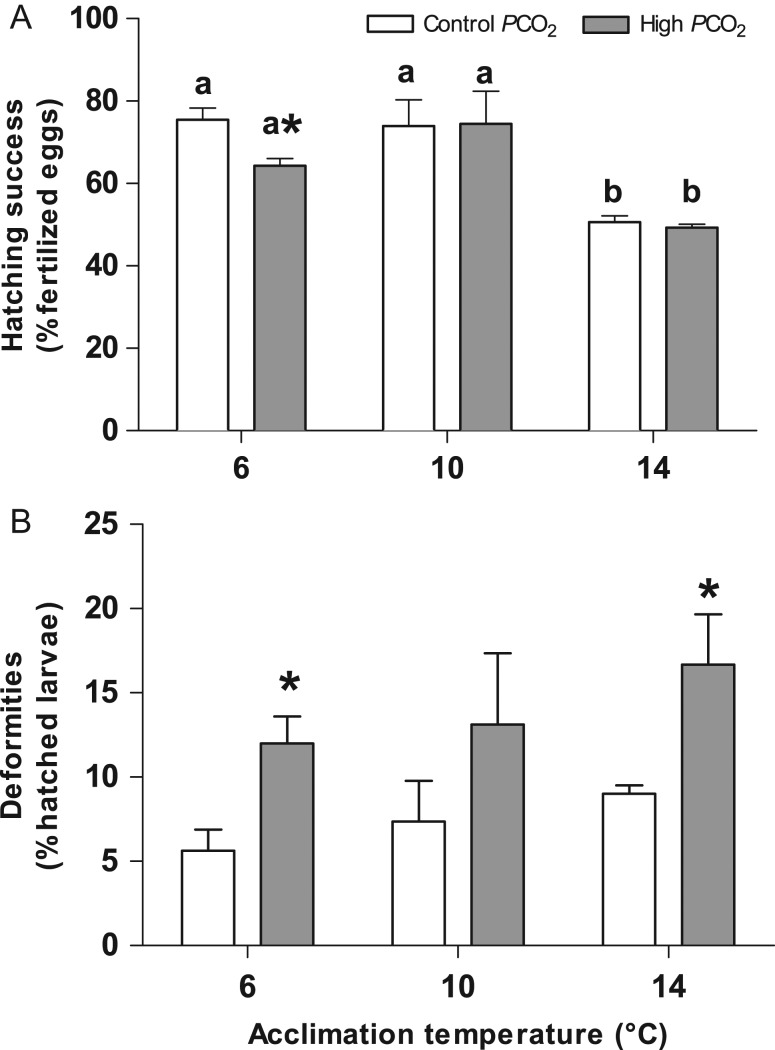
HS (Fig. 3) was significantly affected by temperature (F = 14.07, P = 0.001) with a reduction of hatched larvae at 14°C compared with the other acclimation groups (6–14°C: P = 0.005; 10–14°C: P = 0.001; Fig. 3a). Elevated PCO2 had no significant effects on HS but caused a significant reduction (P = 0.02681) of the HS in the group incubated at 6°C (64.26 ± 1.72%) compared to the group incubated at the same temperature but under control PCO2 (75.43 ± 2.85%).
Figure 3:
Viable hatch of Atlantic herring (Clupea harengus). Values are reported as mean ± SEM. Panel A: hatching success as percentage of fertilized eggs that hatch. Open bars: control PCO2 (400 μatm), solid bars: high PCO2 (1100 μatm). Panel B: Larval malformations as percentage of hatched larvae. Open bars: control PCO2 (400 μatm), solid bars: high PCO2 (1100 μatm). Different letters indicate statistical differences (P < 0.05) between temperature treatments, * indicates significant differences (P < 0.05) between CO2 groups at the same temperature.
The proportion of larvae hatching with severe morphological malformations was higher in the groups incubated under high PCO2 (F = 13.03, P = 0.004, Fig. 3b) with percentages almost doubled compared with the groups incubated under control PCO2 (tab. 1). Larval malformations were not significantly correlated with increasing temperatures (F = 1.67, P > 0.05) and there was no interactive effect between temperature and CO2.
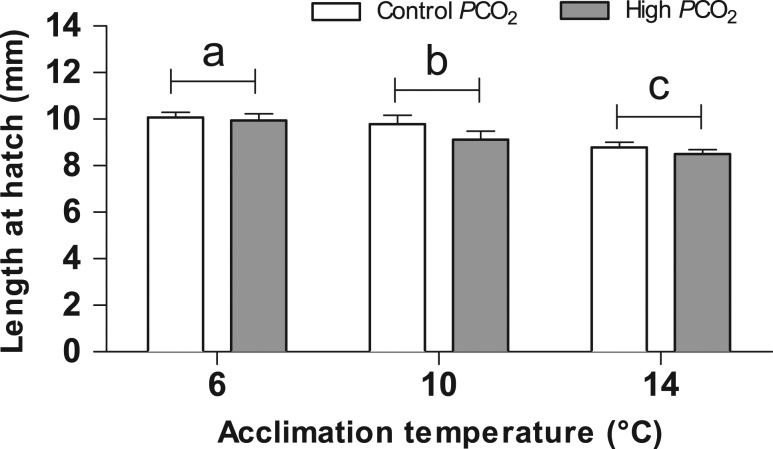
SL at hatch (Fig. 4) was significantly affected by temperature (F= 43.12, P < 0.001) with a trend toward reduction with warming. SL was not affected by elevated PCO2 (F = 9.11, P > 0.05).
Figure 4:
Length at hatch (mm) of Atlantic herring larvae (Clupea harengus). Values are expressed as mean ± SEM. Open bars: control PCO2 (400 μatm), solid bars: high PCO2 (1100 μatm). Different letters indicate significant differences (P < 0.05) between temperature treatments independent of the CO2 treatment.
Discussion
In this study, we analysed the development and mitochondrial function of Atlantic herring (C. harengus) embryos that were incubated to either current water conditions, or to conditions projected for the end of this century in waters surrounding the Scandinavian coast; one of the main spawning grounds of this species in the North Atlantic.
In general, we found that elevated temperature reduced HS and high PCO2 caused larval malformation. Mitochondrial function was not affected by elevated PCO2; however, temperature played a major role in shaping mitochondrial respiration, with subsequent effects on embryonic respiration and body length at hatching; which is in line with other studies on Atlantic herring (Geffen, 2002; Peck et al., 2012).
The capacity of the ETS increased with temperature between 6°C and 10°C without a further increase at 14°C; however, the relative contribution of CI and CII to the ETS changed with temperature (for the entire range 6–14°C), with the contribution of CI being negatively correlated to temperature. In developing teleost fish, embryos mainly rely on carbohydrates during the initial phase of development, until blastula (Kamler, 2008), then catabolize amino acids from protein (benthophils) or free amino acids (FAA, pelagophils), together with lipids (Finn and Fyhn, 2010). In a study on Atlantic cod (Gadus morhua) eggs, Fyhn and Serigstad (1987) showed that the FAA content of the yolk was depleted by ~90% during spawning to hatching, but without a corresponding increase in the protein content of the developing embryo. Moreover, they found that alanine, serine, leucine, isoleucine, lysine, and valine (in that order) quantitatively dominated the amino acids pool, and accounted for ~75% of the decrease. Alanine, serine, leucine, isoleucine and lysine enter the TCA cycle at the citrate synthase step, via pyruvate (alanine and serine) or via acetyl-coA and acetoacetyl-coA (leucine, isoleucine and lysine); both of which are fed into CI and CII. Only valine and isoleucine enter the cycle via succinyl-coA and feed directly into CII. Taking this into account, the reduction of CI contribution to the ETS, in favour of CII, could indicate a shift in metabolic pathways from the preferred CI feeding amino acids (alanine, serine, leucine, isoleucine and lysine) to CII feeding amino acids (valine and isoleucine), as a result of increasing temperature. However, several studies have reported a reduced contribution of CI to the ETS with decreasing temperature in adult fish and embryos, with suggested causes being either a lack of substrates or a change in membrane fluidity (Hilton et al., 2010; Iftikar et al., 2015; Dahlke et al., 2017). These two hypotheses are not contradictory, but complement (and even cause) each other.
A shift in ETS contribution from CI to CII results in a less efficient ATP production pathway, since each cycle of the TCA cycle theoretically produces ~7.5 ATP from CI, but only ~1.5 ATP from CII. The decreased ATP provision at higher temperatures would need compensation by an increase in embryonic respiration (MO2) as seen at 14°C in this study. In addition to the shift in the contribution of the individual complexes to ETS, increasing temperature may also cause a rise in mitochondrial uncoupling, increasing oxygen demand to compensate for the increased proton leak (Weinstein and Somero, 1998; Hardewig et al., 1999). Therefore, an animal’s respiratory rate (MO2) may increase in order to partially compensate for these constraints. However, a limit may be reached where the animal is no longer able to provide oxygen to mitochondria or aerobically produce enough ATP; which may lead to constraints on performance, the onset of anaerobic metabolism and eventually death (Hardewig et al., 1999; Pörtner, 2002).
In this study, we identified several negative effects of decreased ATP production efficiency at a higher incubation temperature (14°C). There was a decreased HS at this elevated temperature and the larvae that hatched at 14°C were smaller than the larvae from other incubation temperatures, indicating that less energy was available for development. Therefore, high temperature (14°) may have limited mitochondrial function, which is mirrored at the whole-organism level, by the decreased length and HS. This provides a link between thermal sensitivity of energy metabolism and the effects of warming at the whole-organism level.
Elevated PCO2 caused a significant increase in larval deformities. This is similar to the findings of Frommel et al. (2014), which showed that elevated PCO2 caused significant organ damage and reduced growth in the larvae of Atlantic herring. However, in another study on Atlantic herring embryos, (Franke and Clemmesen, 2011) found no significant effect of elevated PCO2 (levels up to 4635 μatm) on egg mortality or the occurrence of embryonic malformations. These contrasting findings could be partially explained by the different origins of the herring populations. The herring used in this study and the study by Frommel et al. (2014) came from the Scandinavian coast, while the herring used in the study by Franke and Clemmesen came from the Kiel Fjord in the Baltic Sea, where PCO2 levels are above 2300 μatm due to upwelling events (Thomsen et al., 2010). Atlantic herring display high plasticity in physiological tolerance (Geffen, 2009; Peck et al., 2012), allowing different populations to spawn in different seasons and live in a broad range of temperatures and salinities (Geffen, 2009). Herring lay adhesive benthic eggs (Nash et al., 2009; Schmidt et al., 2009) and therefore encounter potentially challenging hydrographic conditions during egg development, since bottom waters are often depleted of oxygen and enriched in CO2, relative to surface waters. These results contribute to the growing evidence of differences in the sensitivity towards Ocean Acidification and Warming between herring populations (Franke and Clemmesen, 2011; Sswat et al., 2018) and compared to pelagic spawners such as Atlantic cod, flounder and many tropical reef species (Chambers et al., 2014; Munday et al., 2016; Dahlke et al., 2017).
Conclusions
Our study assessed the effects of combined Ocean Acidification and Warming on developing eggs of Atlantic herring. By studying such effects at both the cellular level (e.g. mitochondrial functioning) and the organism level (e.g. body size at hatching), this study provides a link between the thermal sensitivity of an individual’s energetic metabolism with the fitness of the individual as a whole.
Elevated temperature significantly affected mitochondrial function by shifting the relative ETS contribution from CI to CII. This may decrease ATP production, which could lead to a mismatch between the energy produced by the mitochondria and the energy requested by the organism for maintaining metabolism; which in turn could reduce the energy allocation to development indicated by reduced length at hatch.
Elevated PCO2 did not affect HS; however, it did increase the occurrence of malformed larvae. This suggests that exposure to near future acidification levels may cause sub-lethal cellular damage that may not be reflected in vitality and survival rates. These sub-lethal effects of ocean acidification may present the largest risk to individuals and populations (Briffa et al., 2012). For example, smaller larval size at hatch may increase the risk of predation and reduce foraging ability (Miller et al., 1988).
Furthermore, herring populations experience high fishing mortality in addition to other environmental stressors such as pollution and hypoxia. Therefore, potential effects of ocean acidification and warming must be added to the list of anthropogenic perturbations leading to increased mortality in fish early life stages.
Supplementary Material
Acknowledgements
The authors are grateful to Bengt Lundve and the all Kristineberg Biological Station team for the logistical support. We thank Dana Graulich for technical assistance and Catriona Clemmesen and Michael Sswat for providing the herrings.
Data availability
The datasets containing the physiological and morphological parameters measured in this study and the data regarding the incubation physico-chemical parameters are available from the Open Access library PANGAEA (www.pangaea.de; https://doi.pangaea.de/10.1594/PANGAEA.884123 and https://doi.pangaea.de/10.1594/PANGAEA.884124).
Funding
This study was funded by the German research program BIOACID phase II by the Bundesministerium für Bildung und Forschung (FKZ 03F0655B), by the PACES research program of the Alfred Wegener Institute Helmholtz Centre for Polar and Marine Research and by the Association of European Marine Biological Laboratories (ASSEMBLE, grant agreement no. 227799).
Author contributions
E.L., F.T.D., F.C.M., D.S. and H.-O.P. designed the experiment; E.L., F.T.D. and F.C.M. collected, analysed and interpreted the data; E.L., F.T.D., F.C.M., D.S. and H.-O.P wrote the article.
References
- Alheit J, Hagen E (1997) Long-term climate forcing of European herring and sardine populations. Fish Oceanogr 2: 130–139. [Google Scholar]
- Baumann H, Talmage SC, Gobler CJ (2011) Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat Clim Change 2: 38–41. [Google Scholar]
- Bignami S, Sponaugle S, Cowen RK (2014) Effects of ocean acidification on the larvae of a high-value pelagic fisheries species, mahi-mahi coryphaena hippurus. Aquatic Biol 21: 249–260. [Google Scholar]
- Bopp L, Resplandy L, Orr JC, Doney SC, Dunne JP, Gehlen M, Halloran P, Heinze C, Ilyina T, Seferian R (2013) Multiple stressors of ocean ecosystems in the 21st century: projections with CMIP5 models. Biogeosciences 10: 6225–6245. [Google Scholar]
- Boulekbache H. (1981) Energy metabolism in fish development. Amer Zool 21: 377–389. [Google Scholar]
- Briffa M, de la Haye K, Munday PL (2012) High CO2 and marine animal behaviour: potential mechanisms and ecological consequences. Mar Pollut Bull 64: 1519–1528. [DOI] [PubMed] [Google Scholar]
- Chambers RC, Candelmo AC, Habeck EA, Poach ME, Wieczorek D, Cooper KR, Greenfield CE, Phelan BA (2014) Effects of elevated CO2 in the early life stages of summer flounder, Paralichthys dentatus, and potential consequences of ocean acidification. Biogeosciences 11: 1613–1626. [Google Scholar]
- Dahlke FT, Leo E, Mark FC, Portner HO, Bickmeyer U, Frickenhaus S, Storch D (2017) Effects of ocean acidification increase embryonic sensitivity to thermal extremes in Atlantic cod, Gadus morhua. Glob Chang Biol 23: 1499–1510. [DOI] [PubMed] [Google Scholar]
- Dickson AG, Sabine CL, Christian JR (2007) Guide to best practices for ocean CO2 measurements. North Pacific Marine Science Organization.
- FAO (2018) The State of World Fisheries and Aquaculture—Meeting the sustainable development goals. Rome. Licence: CC BY-NC-SA 3.0 IGO.
- Finn RN, Fyhn HJ (2010) Requirement for amino acids in ontogeny of fish. Aquac Res 41: 684–716. [Google Scholar]
- Finn RN, Fyhn HJ, Henderson RJ, Evjen MS (1996) The sequence of catabolic substrate oxidation and enthalpy balance of developing embryos and yolksac larvae of turbot (Scophthalmus maximus L.). Comp Biochem Physiol 115A: 133–151. [Google Scholar]
- Flynn EE, Bjelde BE, Miller NA, Todgham AE (2015) Ocean acidification exerts negative effects during warming conditions in a developing Antarctic fish. Conserv Physiol 3: cov033. doi:10.1093/conphys/cov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke A, Clemmesen C (2011) Effect of ocean acidification on early life stages of Atlantic herring (Clupea harengus L.). Biogeosciences 8: 3697–3707. [Google Scholar]
- Frommel AY, Maneja RH, Lowe D, Pascoe CK, Geffen AJ, Folkvord A, Piatkowski U, Clemmesen C (2014) Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol Appl 24: 1131–1143. [DOI] [PubMed] [Google Scholar]
- Frommel AY, Margulies D, Wexler JB, Stein MS, Scholey VP, Williamson JE, Bromhead D, Nicol S, Havenhand J (2016) Ocean acidification has lethal and sub-lethal effects on larval development of yellowfin tuna, Thunnus albacares. J Exp Mar Bio Ecol 482: 18–24. [Google Scholar]
- Frommel AY, Schubert A, Piatkowski U, Clemmesen C (2012) Egg and early larval stages of Baltic cod, Gadus morhua, are robust to high levels of ocean acidification. Mar Biol 160: 1825–1834. [Google Scholar]
- Fyhn HJ, Serigstad B (1987) Free amino acids as energy substrate in developing eggs and larvae of the cod Gadus morhua. Mar Biol 96: 335–341. [Google Scholar]
- Geffen AJ. (1999) Variations in sperm motility of the Atlantic herring Clupea harengus. Mar Biol 134: 637–643. [Google Scholar]
- Geffen AJ. (2002) Length of herring larvae in relation to age and hatching order. J Fish Biol 60: 479–485. [Google Scholar]
- Geffen AJ. (2009) Advances in herring biology: from simple to complex, coping with plasticity and adaptability. ICES J Mar Sci 66: 1688–1695. [Google Scholar]
- Gnaiger E, Boushel R, Søndergaard H, Munch‐Andersen T, Damsgaard R, Hagen C, Díez‐Sánchez C, Ara I, Wright‐Paradis C, Schrauwen P (2015) Mitochondrial coupling and capacity of oxidative phosphorylation in skeletal muscle of Inuit and Caucasians in the Aarctic winter. Scand J Med Sci Sports 25: 126–134. [DOI] [PubMed] [Google Scholar]
- Hardewig I, Peck LS, Pörtner HO (1999) Thermal sensitivity of mitochondrial function in the Antarctic notothenioid Lepidonotothen nudifrons. J Comp Physiol B 169: 597–604. [Google Scholar]
- Henson SA, Beaulieu C, Ilyina T, John JG, Long M, Seferian R, Tjiputra J, Sarmiento JL (2017) Rapid emergence of climate change in environmental drivers of marine ecosystems. Nat Commun 8: 14682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Johnston IA (1997) Photomicrographic atlas of Atlantic herring embryonic development. J Fish Biol 51(5): 960–977. [Google Scholar]
- Hilton Z, Clements KD, Hickey AJ (2010) Temperature sensitivity of cardiac mitochondria in intertidal and subtidal triplefin fishes. J Comp Physiol B 180: 979–990. [DOI] [PubMed] [Google Scholar]
- Hurst TP, Fernandez ER, Mathis JT (2013) Effects of ocean acidification on hatch size and larval growth of walleye pollock (Theragra chalcogramma). ICES J Mar Sci 70: 812–822. [Google Scholar]
- Hurst TP, Fernandez ER, Mathis JT, Miller JA, Stinson CM, Ahgeak EF (2012) Resiliency of juvenile walleye pollock to projected levels of ocean acidification. Aquat Biol 17: 247–259. [Google Scholar]
- Iftikar FI, Hickey AJ (2013) Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS One 8: e64120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iftikar F, Morash A, Cook D, Herbert N, Hickey AJ (2015) Temperature acclimation of mitochondria function from the hearts of a temperate wrasse (Notolabrus celidotus). Comp Biochem Physiol A Mol Integr Physiol 184: 46–55. [DOI] [PubMed] [Google Scholar]
- Ishimatsu A, Hayashi M, Kikkawa T (2008) Fishes in high-CO2, acidified oceans. Mar Ecol Prog Ser 373(1): 295–302. [Google Scholar]
- Jutfelt F, Hedgarde M (2013) Atlantic cod actively avoid CO2 and predator odour, even after long-term CO2 exposure. Front Zool 10: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamler E. (2008) Resource allocation in yolk-feeding fish. Rev Fish Biol Fish 18: 143. [Google Scholar]
- Kikkawa T, Ishimatsu A, Kita J (2003) Acute CO2 tolerance during the early developmental stages of four marine teleosts. Environ Toxicol 18: 375–382. [DOI] [PubMed] [Google Scholar]
- Lynam CP, Llope M, Möllmann C, Helaouët P, Bayliss-Brown GA, Stenseth NC (2015) Interaction between top-down and bottom-up control in marine food webs. PNAS 114(8): 1952–1957. 10.1073/pnas.1621037114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneja RH, Frommel AY, Browman HI, Geffen AJ, Folkvord A, Piatkowski U, Durif CMF, Bjelland R, Skiftesvik AB, Clemmesen C (2015) The swimming kinematics and foraging behavior of larval Atlantic herring (Clupea harengus L.) are unaffected by elevated pCO2. J Exp Mar Bio Ecol 466: 42–48. [Google Scholar]
- Melzner F, Thomsen J, Koeve W, Oschlies A, Gutowska MA, Bange HW, Hansen HP, Körtzinger A (2013) Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar Biol 160: 1875–1888. [Google Scholar]
- Miller TJ, Crowder LB, Rice JA, Marschall EA (1988) Larval size and recruitment mechanisms in fishes: Toward a conceptual framework. Can J Fish Aquat Sci 45: 1657–1670. [Google Scholar]
- Munday PL, Gagliano M, Donelson JM, Dixson DL, Thorrold SR (2011) Ocean acidification does not affect the early life history development of a tropical marine fish. Mar Ecol Prog Ser 423: 211–221. [Google Scholar]
- Munday PL, Pratchett MS, Dixson DL, Donelson JM, Endo GGK, Reynolds AD, Knuckey R (2013) Elevated CO2 affects the behavior of an ecologically and economically important coral reef fish. Mar Biol 160: 2137–2144. 10.1007/s00227-012-2111-6. [DOI] [Google Scholar]
- Munday PL, Welch MJ, Allan BJ, Watson S-A, McMahon SJ, McCormick MI (2016) Effects of elevated CO2 on predator avoidance behaviour by reef fishes is not altered by experimental test water. PeerJ 4: e2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash RDM, Dickey-Collas M, Kell LT (2009) Stock and recruitment in North Sea herring (Clupea harengus); compensation and depensation in the population dynamics. Fish Res 95: 88–97. [Google Scholar]
- Peck MA, Kanstinger P, Holste L, Martin M (2012) Thermal windows supporting survival of the earliest life stages of Baltic herring (Clupea harengus). ICES J Mar Sci 69: 529–536. [Google Scholar]
- Pimentel MS, Faleiro F, Dionisio G, Repolho T, Pousao-Ferreira P, Machado J, Rosa R (2014) Defective skeletogenesis and oversized otoliths in fish early stages in a changing ocean. J Exp Biol 217: 2062–2070. [DOI] [PubMed] [Google Scholar]
- Pimentel MS, Faleiro F, Marques T, Bispo R, Dionísio G, Faria AM, Machado J, Peck MA, Pörtner H-O, Pousão-Ferreira P (2016) Foraging behaviour, swimming performance and malformations of early stages of commercially important fishes under ocean acidification and warming. Clim Change 137: 495–509. [Google Scholar]
- Pörtner H-O. (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A Mol Integr Physiol 132: 739–761. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing. http://www.R-project.org (last accessed 25 February 2018).
- Rombough P. (2011) The energetics of embryonic growth. Respir Physiol Neurobiol 178: 22–29. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Harms L, Pörtner H-O, Mark FC, Storch D (2014) Pre-hatching seawater pCO2 affects development and survival of zoea stages of Arctic spider crab Hyas araneus. MEPS 501: 127–139. 10.3354/meps10687. [DOI] [Google Scholar]
- Schmidt JO, Van Damme CJ, Röckmann C, Dickey-Collas M (2009) Recolonisation of spawning grounds in a recovering fish stock: recent changes in North Sea herring. Sci Mar 73: 153–157. [Google Scholar]
- Sswat M, Stiasny MH, Jutfelt F, Riebesell U, Clemmesen C (2018) Growth performance and survival of larval Atlantic herring, under the combined effects of elevated temperatures and CO2. PLoS One 13(1): e0191947 10.1371/journal.pone.0191947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiasny MH, Mittermayer FH, Sswat M, Voss R, Jutfelt F, Chierici M, Puvanendran V, Mortensen A, Reusch TB, Clemmesen C (2016) Ocean acidification effects on Atlantic cod larval survival and recruitment to the fished population. PLoS One 11(8): e0155448 10.1371/journal.pone.0155448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen J, Gutowska M, Saphörster J, Heinemann A, Trübenbach K, Fietzke J, Hiebenthal C, Eisenhauer A, Körtzinger A, Wahl M (2010) Calcifying invertebrates succeed in a naturally CO2-rich coastal habitat but are threatened by high levels of future acidification. Biogeosciences 7: 3879. [Google Scholar]
- Tocher DR, Fraser AJ, Sargent JR, Gamble JC (1985) Fatty acid composition of phospholipids and neutral lipids during embryonic and early larval development in Atlantic herring (Clupea harengus, L.). Lipids 20: 69–74. [DOI] [PubMed] [Google Scholar]
- Tseng Y-C, Hu MY, Stumpp M, Lin L-Y, Melzner F, Hwang P-P (2013) CO2-driven seawater acidification differentially affects development and molecular plasticity along life history of fish (Oryzias latipes). Comp Biochem Physiol A Mol Integr Physiol 165: 119–130. [DOI] [PubMed] [Google Scholar]
- van Vuuren DP, Edmonds J, Kainuma M, Riahi K, Thomson A, Hibbard K, Hurtt GC, Kram T, Krey V, Lamarque J-F, et al. (2011) The representative concentration pathways: an overview. Clim Change 109: 5–31. [Google Scholar]
- Waters JF, Millero FJ (2013) The free proton concentration scale for seawater pH. Mar Chem 149: 8–22. [Google Scholar]
- Weinstein RB, Somero GN (1998) Effects of temperature on mitochondrial function in the Antarctic fish Trematomus bernacchii. J Comp Physiol B 168: 190–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets containing the physiological and morphological parameters measured in this study and the data regarding the incubation physico-chemical parameters are available from the Open Access library PANGAEA (www.pangaea.de; https://doi.pangaea.de/10.1594/PANGAEA.884123 and https://doi.pangaea.de/10.1594/PANGAEA.884124).