Abstract
Importance
Altered neurodevelopmental trajectories are thought to reflect heterogeneity in the pathophysiologic characteristics of schizophrenia, but whether neural indicators of these trajectories are associated with future psychosis is unclear.
Objective
To investigate distinct neuroanatomical markers that can differentiate aberrant neurodevelopmental trajectories among clinically high-risk (CHR) individuals.
Design, Setting, and Participants
In this prospective longitudinal multicenter study, a neuroanatomical-based age prediction model was developed using a supervised machine learning technique with T1-weighted magnetic resonance imaging scans of 953 healthy controls 3 to 21 years of age from the Pediatric Imaging, Neurocognition, and Genetics (PING) study and then applied to scans of 275 CHR individuals (including 39 who developed psychosis) and 109 healthy controls 12 to 21 years of age from the North American Prodrome Longitudinal Study 2 (NAPLS 2) for external validation and clinical application. Scans from NAPLS 2 were collected from January 15, 2010, to April 30, 2012.
Main Outcomes and Measures
Discrepancy between neuroanatomical-based predicted age (hereafter referred to as brain age) and chronological age.
Results
The PING-derived model (460 females and 493 males; age range, 3-21 years) accurately estimated the chronological ages of the 109 healthy controls in the NAPLS 2 (43 females and 66 males; age range, 12-21 years), providing evidence of independent external validation. The 275 CHR individuals in the NAPLS 2 (111 females and 164 males; age range, 12-21 years) showed a significantly greater mean (SD) gap between model-predicted age and chronological age (0.64 [2.16] years) compared with healthy controls (P = .008). This outcome was significantly moderated by chronological age, with brain age systematically overestimating the ages of CHR individuals who developed psychosis at ages 12 to 17 years but not the brain ages of those aged 18 to 21 years. Greater brain age deviation was associated with a higher risk for developing psychosis (F = 3.70; P = .01) and a pattern of stably poor functioning over time, but only among younger CHR adolescents. Previously reported evidence of accelerated reduction in cortical thickness among CHR individuals who developed psychosis was found to apply only to those who were 18 years of age or older.
Conclusions and Relevance
These results are consistent with the view that neuroanatomical markers of schizophrenia may help to explain some of the heterogeneity of this disorder, particularly with respect to early vs later age of onset of psychosis, with younger and older individuals having differing intercepts and trajectories in structural brain parameters as a function of age. The results also suggest that baseline neuroanatomical measures are likely to be useful in estimating onset of psychosis, especially (or only) among CHR individuals with an earlier age of onset of prodromal symptoms.
This longitudinal multicenter study uses a machine learning model to investigate distinct neuroanatomical markers that can differentiate aberrant neurodevelopment trajectories among clinical high-risk individuals
Key Points
Question
Are deviations from the normal neuroanatomical maturation pattern associated with future psychosis among clinically high-risk individuals?
Findings
In this longitudinal multicenter study, clinically high-risk individuals between 12 and 17 years of age showed exaggerated deviation in neuroanatomical maturity at the time of baseline evaluation, which in turn was associated with a greater risk for developing psychosis and a pattern of stably poor functioning, while accelerated reduction in cortical thickness among clinically high-risk individuals who developed psychosis was found to apply only to those who were 18 years of age or older.
Meaning
Clinically high-risk individuals expressing prodromal symptoms of psychosis in early adolescence show contemporaneous signs of neuroanatomical vulnerability, which may be useful for estimating onset of psychosis.
Introduction
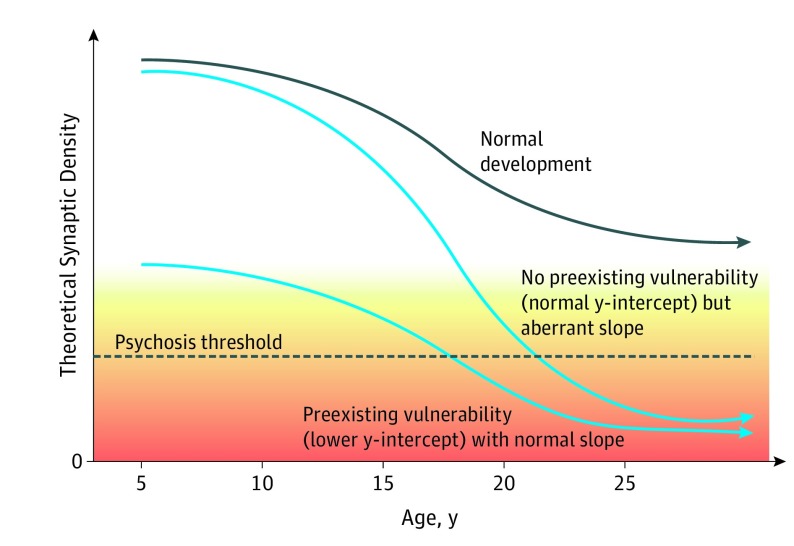
Both early (prenatal and perinatal) and late (adolescent) neurodevelopmental disturbances are hypothesized to play a role in the abnormalities of brain structure and function associated with schizophrenia.1,2 Disturbances originating earlier in life (eg, resulting from the interplay of genetic factors and obstetric complications) would be expected to affect brain integrity from birth onward and could therefore help to explain children with subtle motor, cognitive, and social-affective deficits during childhood and earlier ages at onset of full psychosis (ie, early to mid-teens).1,3,4,5,6,7,8,9,10,11,12,13 In contrast, disturbances that emerge during late adolescence and early adulthood (eg, via abnormal neuromaturational events and/or environmental factors) could help to explain individuals with normal premorbid psychological health and a more acute onset of psychotic symptoms and functional impairment in the late teens and early 20s.1,2,13,14,15,16,17 Although these 2 sets of processes likely co-occur in at least some cases, in isolation they lead to differential estimations concerning the extent to which neuroanatomical measures can aid in the detection of risk for psychosis prior to onset (Figure 1).1,2,18,19 Specifically, individuals who are relatively more affected by early neurodevelopmental disturbances would be expected to manifest greater neuroanatomical deviation relative to age-matched peers prior to onset of psychosis,5,6 while those who are relatively more affected by later neurodevelopmental disturbances would be expected to be neuroanatomically similar to age-matched peers throughout most of the premorbid period but to show a rapidly increasing deviation prior to the onset of psychosis.1,2,14,17
Figure 1. Model of Neurodevelopmental Trajectories of Cortical Synaptic Density in Association With Onset of Psychosis .
Possible paths to schizophrenia, with the gradation of colors from yellow to orange representing the increasing severity of psychotic symptoms.1,2,18,19
Consistent with the theoretical framework just outlined, prior work has observed a general pattern of greater neuroanatomical compromise among patients with schizophrenia who have a history of early-life risk exposures (eg, obstetric complications) and/or an earlier age at onset of schizophrenia.5,6,7,9,20 However, these cross-sectional case-control studies cannot comment on the timing of the appearance or course of the neuroanatomical changes or their potential relevance to estimation of onset of psychosis. To address these questions more definitively, prospective longitudinal evaluation is required.
Here, we relied on the early vs late neurodevelopmental influences model to guide our set of hypotheses, given that this model makes differential estimations related to the timing of onset of psychosis in association with different trajectories of neurodevelopmental disturbances. To quantify deviation from a normative neuromaturational trajectory on an individual basis, we sought a computationally efficient method to capture variance in a comprehensive set of neuroanatomical measures most sensitive to age-related differences using a machine learning approach. Prior studies using this method have yielded “brain age” estimates based on magnetic resonance imaging (MRI) scans that closely track with the true chronological ages of typically developing individuals.21,22 Such a metric has been shown to be highly replicable,23,24,25,26,27,28,29 heritable,23 and robust to confounders, such as scanner-related noise24,26 and head motion.24
Prior studies of adult samples of patients with schizophrenia have applied this framework to show a pattern of accelerated brain aging in the patient groups compared with controls.25,27 However, it remains unknown whether greater deviation in neuroanatomical maturity is associated with clinical outcomes in adolescents and young adults with a risk of psychosis. In this study, we generated a neuroanatomical-based age prediction model using the Pediatric Imaging, Neurocognition, and Genetics (PING) study MRI data set, which consists of scans from typically developing individuals across a wide age range from childhood to early adulthood.21,30 We then validated the PING-derived model in application to the baseline MRI scans of the healthy controls (HCs) and individuals at clinically high risk (CHR) at the baseline evaluation in the North American Prodromal Longitudinal Study 2 (NAPLS 2) sample.14,31
We hypothesized that the PING-derived model would accurately predict individuals’ chronological ages when applied to the scans from the healthy cohort in the NAPLS 2, providing evidence of independent external validation. We then used the validated model to test our primary hypotheses that CHR individuals who were younger at the time of ascertainment (as a proxy for an earlier age at onset of prodromal symptoms) will show an estimated neuroanatomical-based predicted age older than their chronological ages and that this greater deviation in neuroanatomical maturity will be associated with a greater risk for developing psychosis and a pattern of stably poor functioning during 1 year of follow-up.
Methods
The current study was approved by expedited review by the Yale University Human Subjects Committee. The study protocol and consent form were reviewed and approved by the institutional review boards at each of the 8 participating data collection sites (University of California, Los Angeles; Emory University; Harvard Medical School; Zucker Hillside Hospital; University of North Carolina; University of California, San Diego; University of Calgary; and Yale University). All participants provided written informed consent.
PING Cohort
Publicly available PING data were obtained through the PING portal (http://pingstudy.ucsd.edu) and used for building multivariate models predicting chronological age in typically developing individuals using their T1-weighted MRI scans (N = 953; 3-21 years of age).
NAPLS 2 Cohort
Clinically high-risk individuals and their matched HCs were recruited through the NAPLS 2 Consortium, which consisted of 8 sites in North America studying the prodrome period of psychosis.31 The CHR individuals were assessed based on the Structured Interview for Psychosis Risk Syndromes.32
The NAPLS 2 participants included in this study were those with T1-weighted MRI scans available who were between 12 and 21 years of age at the baseline assessment. Scans from NAPLS 2 were collected from January 15, 2010, to April 30, 2012. In total, 39 CHR individuals developed psychosis (“converted”; CHR-C), 236 CHR individuals did not convert to psychosis (CHR-NC), and 109 were HCs. The demographic characteristics of the 3 groups are shown in the Table (see eAppendix in the Supplement and previous publications for additional details about the PING21,30 and NAPLS 214,31,33 Consortia).
Table. Demographic Characteristics of Participants.
| Characteristic | HC (n = 109) | CHR-NC (n = 236) | CHR-C (n = 39) | Statistical Test for Significance (2-Tailed) | P Value | Post Hoc Tukey Testa |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 17.02 (2.44) | 17.30 (2.10) | 17.20 (2.23) | F = 0.61 | .55 | NA |
| Male sex, No. (%) | 66 (60.6) | 142 (60.2) | 23 (59.0) | χ2 = 0.038 | .98 | NA |
| Education level, mean (SD), y | 10.31 (2.44) | 10.37 (2.07) | 10.35 (2.26) | F = 0.03 | .96 | NA |
| Paternal education score, mean (SD)b | 6.43 (1.73) | 6.14 (1.66) | 6.42 (2.01) | F = 1.32 | .99 | NA |
| Maternal education score, mean (SD)b | 6.87 (1.46) | 6.26 (1.52) | 6.56 (1.74) | F = 5.94 | .003 | HC, CHR-C > CHR-NC |
| Race/ethnicity, No. (%) | ||||||
| White | 58 (53.2) | 151 (64.0) | 26 (66.7) | χ2 = 5.73 | .06 | NA |
| Hispanic or Latino | 24 (22.0) | 40 (16.9) | 7 (17.9) | χ2 = 1.43 | .49 | NA |
| Black | 24 (22.0) | 30 (12.7) | 4 (10.3) | χ2 = 5.46 | .07 | NA |
| Asian | 8 (7.3) | 17 (7.2) | 3 (7.7) | χ2 = 0.14 | .99 | NA |
| First Nations | 2 (1.8) | 5 (2.1) | 1 (2.6) | χ2 = 0.14 | .93 | NA |
| Interracial | 14 (12.8) | 24 (10.2) | 3 (7.7) | χ2 = 0.13 | .93 | NA |
| Taking antipsychotics, No. (%)c | 0 | 53 (22.5) | 12 (30.8) | χ2 = 1.25 | .26d | NA |
| Scale of Prodromal Symptoms score, mean (SD) | ||||||
| Positive | 1.14 (1.58) | 11.89 (3.59) | 13.05 (3.59) | F = 459.8 | <.001 | CHR-C > CHR-NC > HC |
| Negative | 1.92 (2.75) | 12.11 (6.20) | 12.21 (6.28) | F = 134.9 | <.001 | CHR-C, CHR-NC > HC |
| Disorganization | 0.67 (1.21) | 4.94 (3.08) | 7.67 (4.29) | F = 119.6 | <.001 | CHR-C > CHR-NC > HC |
| General | 1.51 (2.41) | 9.34 (4.24) | 9.21 (3.89) | F = 166.6 | <.001 | CHR-C, CHR-NC > HC |
| GAF scores at baseline | 82.18 (11.41) | 48.50 (10.72) | 46.28 (9.63) | F = 166.6 | <.001 | HC > CHR-C, CHR-NC |
Abbreviations: CHR-C, clinically high-risk individuals who converted to psychosis; CHR-NC, clinically high-risk individuals who did not convert to psychosis; GAF, Global Assessment of Functioning; HC, healthy controls; NA, not applicable.
The greater than symbol indicates direction of the significant results.
Parental education scored as follows: 1, no schooling; 2, some primary school; 3, completed primary school; 4, some high school; 5, completed high school; 6, some college, technical school, or undergraduate education; 7, completed college, technical school, or undergraduate education; 8, some graduate or professional school; and 9, completed graduate or professional school.
Because this was a naturalistic study, individuals were treated in their respective communities according to prevailing standards and the judgment of the treating clinicians, who were often primary care physicians rather than psychiatrists.
χ2 Test performed within CHR group.
Neuroimaging Data Acquisition and Image Processing
High-resolution, T1-weighted brain images were acquired using 3-T scanners for PING and NAPLS 2. Image postprocessing steps were performed using the FreeSurfer software suite, version 5.3 (http://surfer.nmr.mgh.harvard.edu/)34,35,36,37 (eAppendix in the Supplement).
Multivariate Modeling for Estimating Age
We preselected a limited number of neuroanatomical measures (N = 92) to minimize redundant information, yet we included volume measures from all parcels provided by the Desikan atlas38 (see eTable 1 in the Supplement for a complete list). Prior to model fitting, sex differences and scanner-specific offsets were estimated per predictor and adjusted using a linear mixed model. For model training, we used penalized regression with L2 norms (ridge regression) to avoid overfitting.39 For simplicity, the predicted age from neuroanatomical measures will be referred to as the brain age. The deviation of the predicted age from the individuals’ chronological age (brain age − chronological age) will be referred as the brain age gap. To directly compare the magnitude of the brain age gap across the full age range, the bias was estimated and accounted for in the model (see eAppendix, eFigure 1, and eFigure 2 in the Supplement for methodological details).
Model Training, Validation, and Application
All structural scans available from the PING data set were used as the discovery data set to build a model estimating individuals’ true ages. A repeated, nested cross-validation method was used to optimize the tuning parameter λ and derive unbiased estimates of model performance, which were evaluated by minimizing the mean absolute error (MAE).40 For the purpose of assessing generalizability, the PING-derived model was then applied without modification to the baseline MRI data of the HCs and CHR individuals from NAPLS 2 and adolescents with a first episode of psychosis (n = 14) as an additional external validation test (eAppendix in the Supplement).
Association With Clinical Measures
We examined associations between brain age gap and prodromal status based on the Structured Interview for Psychosis Risk Syndromes32 and the Global Assessment of Functioning Scale41 as an index of overall functioning assessed at baseline and 12-month follow-up or at conversion to psychosis.
Statistical Analysis
To compare brain age across groups, the generalized linear model was used with chronological age, group, and chronological age × group interaction as independent variables. When a chronological age × group interaction was detected, secondary analyses were performed on the brain age gap using analysis of variance when clinical samples were partitioned into younger (12-17 years of age) and older adolescents (18-21 years of age) because the deflection point underlying a significant chronological age × group interaction occurred at around 17 years of age (which is also the median age in the included sample). Age at baseline evaluation was highly correlated with age at onset of psychosis among individuals who converted (eFigure 3 in the Supplement) because most developed full-blown psychotic symptoms in less than a year from ascertainment. A receiver operating characteristic analysis was used to estimate classification accuracy between CHR-C and CHR-NC individuals using the brain age gap as the sole predictor. For post hoc t tests, a Bonferroni correction was applied for multiple comparisons as noted. Statistical tests were 2-sided with significance set at P < .05.
Results
Brain Age Model Performance and External Validation
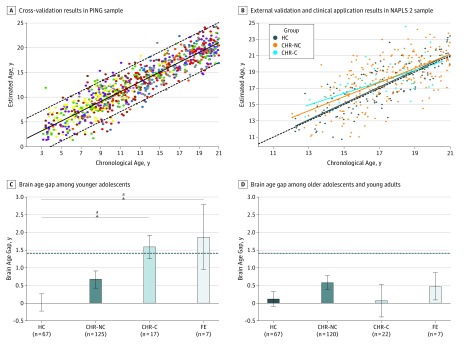
Magnetic resonance imaging scans of typically developing individuals from the PING cohort (n = 953; age range, 3-21 years; 460 females and 493 males) were used for the model training phase. Then, the validated PING-derived model was applied to MRI scans of the HCs (n = 109; age range, 12-21 years; 43 females and 66 males) and CHR individuals (n = 275; age range, 12-21 years; 111 females and 164 males) in the NAPLS 2 sample for external validation and clinical application. The models built using the cross-validation procedure in the PING cohort explained 84% of the chronological age variance (P < .001) with an MAE of 1.69 years (Figure 2A). When this PING-derived model was applied to an independent external data set (ie, the HC group in the NAPLS 2 cohort), it accounted for 51% of the variance (P < .001) in chronological age, with an MAE of 1.41 years (Figure 2B). This degree of generalizability is comparable to the cross-validation results within the PING sample when the age range was restricted to match that of the NAPLS 2 sample (age range, 12-21 years; n = 449; MAE = 1.55; R2 = 65%; P < .001). See eTable 2 in the Supplement for model parameters.
Figure 2. Brain Age Model Building, Validation, and Application.
A, Cross-validation results within the Pediatric Imaging, Neurocognition, and Genetics (PING) study sample with bias adjustment. Estimated brain age is plotted as a function of observed chronological age at baseline magnetic resonance imaging scan. The solid diagonal line indicates linear fit, and the dashed diagonal lines indicate the 95% prediction interval. Colors correspond to different scanners (R2 = 0.84; mean absolute error [MAE] = 1.69 years; mean error = –0.001 year). B, Optimized brain age model trained with the PING sample was applied without modification to the healthy control (HC), clinically high-risk with no conversion to psychosis (CHR-NC), and clinically high-risk with conversion to psychosis (CHR-C) individuals in the North American Prodrome Longitudinal Study 2 (NAPLS 2). A significant CHR group by chronological age interaction was observed (P = .047). Colors correspond to different groups, and the dashed line indicates where predicted and chronological age perfectly meet (R2 = 0.51; MAE = 1.41 years; mean error = –0.06 year). C, Bar plots of mean (SE) brain age gap by diagnostic groups among younger adolescents (<17 years of age). Both CHR-C individuals and patients with a first episode of psychosis (FE) showed an increased brain age gap compared with HCs (CHR-C, P = .02; FE = 0.02). The dashed line indicates MAE for brain age model performance in NAPLS 2 HCs. D, Bar plots of mean (SE) brain age gap by diagnostic groups among older adolescents and young adults (17-21 years of age). There were no significant differences of brain age gap by group (P > .10). The dashed line indicates MAE for brain age model performance in NAPLS 2 HCs. All error bars indicate SE.
aP < .05, corrected.
Association of Psychosis Risk With Brain Age Gap
Next, the validated brain age model was applied to CHR individuals. Overall, the CHR group showed a significantly increased mean (SD) brain age gap (0.64 [2.16] years) compared with HCs (P = .008). As shown in Figure 2B, a significant chronological age × CHR group interaction (t = –1.99; P = .047) was observed, whereby brain age was systematically overestimated among younger adolescents (≤17 years of age) in both CHR-C and CHR-NC individuals but not among those 18 years of age or older. To further characterize chronological age as a potential moderating variable, statistical tests were performed for younger and older adolescents separately, defined using a median split of 17.04 years (eTable 3 in the Supplement). Among younger adolescents, the mean brain age gap was significantly greater than zero for CHR-C individuals (1.59 years; P < .001, corrected) and CHR-NC individuals (0.67; P = .004, corrected) but not for HCs (0.06; P = .99, corrected). Furthermore, the brain age gap differed significantly across groups for younger adolescents (F = 3.70; P = .01). Post hoc tests revealed that the brain age gap of CHR-C individuals was significantly higher compared with HCs (1.58 years; P = .02, corrected). The brain age gap for CHR-NC individuals was also greater than for HCs but not significantly so (0.67 years; P = .15, corrected). There were no significant differences in brain age gap between groups among older adolescents (F = 1.09; P = .35).
In the receiver operating characteristic analysis among CHR individuals 17 years of age or younger, the brain age gap was a significant predictor of conversion to psychosis with an area under the curve of 0.63 (P = .046, permutation test with 5000 iterations; eFigure 4 in the Supplement), and these effects were not explained by exposure to antipsychotic medication (eFigure 5 in the Supplement).
Brain Age Gap in Adolescents With a First Episode of Psychosis
Adolescents with a first episode of psychosis also exhibited a significantly greater mean brain age gap compared with their matched controls (1.17 years; P = .001, permutation test). When divided into younger and older groups based on chronological age and compared with data from the NAPLS 2 cohort, younger patients with a first episode of psychosis showed a larger brain age gap compared with HCs (1.86 years; P = .02, permutation test) but did not significantly differ from CHR-NC individuals or CHR-C individuals. Generalized linear model analysis revealed a significant linear association of the severity of psychotic illness with the brain age gap only in younger adolescents (categorical variables ordered as ordinal variables: HCs < CHR-NC individuals < CHR-C individuals < individuals with a first episode of psychosis; group as ordinal variable; t = 3.31; P = .001; Figure 2C) and not in older adolescents (Figure 2D).
Association of Brain Age Gap With Global Functioning Outcome
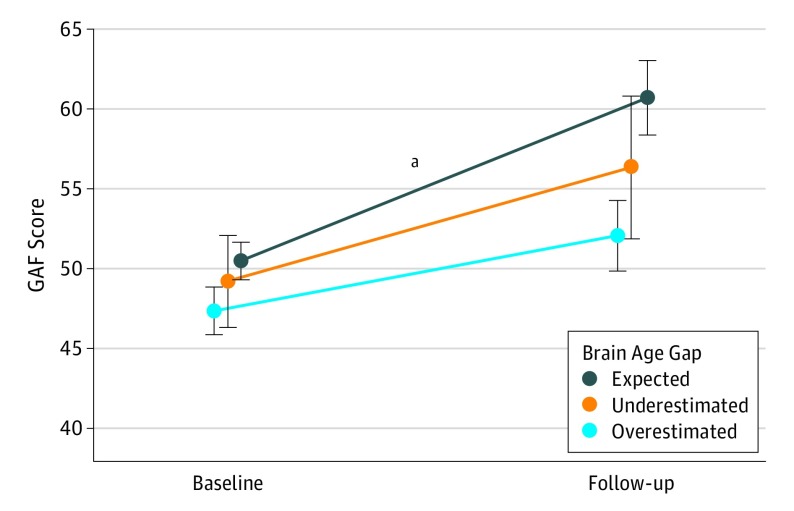
Next, we evaluated the association between brain age gap and Global Assessment of Functioning Scale scores at baseline and 12-month follow-up or at conversion to psychosis among the CHR individuals from NAPLS 2. Overall, Global Assessment of Functioning Scale scores assessed at follow-up significantly improved compared with the baseline scores (t = 6.01; P < .001). The younger adolescent group of CHR individuals was divided into 3 groups with a threshold set by ±MAE of the brain age model in external validation. As shown in Figure 3, CHR individuals falling within the expected range showed improved functioning at follow-up relative to baseline (10.21 points; t = 3.87; P < .001, corrected), whereas the Global Assessment of Functioning Scale scores of the individuals with a brain age gap beyond ±MAE showed a stable pattern of poor functioning during this interval. Similar results were observed when CHR-C individuals were excluded (eFigure 6 in the Supplement).
Figure 3. Course of Functioning From Baseline to 12-Month Follow-up by Brain Age Gap.
Global Assessment of Functioning Scale (GAF) scores at baseline and 12-month follow-up among clinically high-risk individuals according to whether their brain age gap scores were in the expected range vs underestimated or overestimated using the mean absolute error of the brain age model as a threshold (underestimated: brain age gap < −1.5; expected: −1.5 ≤ brain age gap ≤1.5 years; and overestimated: 1.5 < brain age gap). Mean GAF scores improved from baseline to follow-up when the brain age gap was within the mean absolute error range (P < .001) but did not improve for individuals with an underestimated or overestimated brain age gap. Clinically high-risk individuals who converted to psychosis were disproportionately represented among those with an overestimated brain age gap (underestimated, n = 0; expected, n = 8; overestimated, n = 9), and a similar pattern was observed even when converters were excluded (eFigure 6 in the Supplement).
aP < .001, corrected.
Prediction of Conversion to Psychosis Among CHR Individuals
In the interest of completeness, we also attempted to use baseline neuroanatomical measures derived from T1-weighted MRI data to directly predict conversion to psychosis among the NAPLS 2 CHR sample (71 converters and 436 nonconverters) using standard machine learning approaches, but the trained models derived from our data set did not significantly predict conversion to psychosis (see eAppendix in the Supplement for methodological details, results, and discussion).
Discussion
In this study, we successfully reproduced a neuroanatomical-based age prediction model using a typically developing cohort (PING sample) and successfully validated the model in an independent sample (NAPLS 2 HCs). When a validated brain age prediction model is applied to structural MRI data from adolescent and young adult individuals at CHR for psychosis, the CHR individuals show a systematic overestimation of brain age that in turn is associated with a higher risk for conversion to psychosis and a pattern of stably poor functioning. Consistent with the neurodevelopmental framework, these associations were found to be specific to individuals with earlier ages at onset of prodromal symptoms of psychosis (≤17 years of age) and also generalized to younger individuals with a first episode of psychosis, possibly representing an aberrant neurodevelopmental trajectory with a lower y-intercept (Figure 1).1,2,18,19 Because the model primarily fitted cortical gray matter measures with negative weights, older predicted age in these samples would be generally associated with reduced gray matter structures. This pattern of findings is consistent with the notion that contemporaneous neuroanatomical vulnerability is characteristic of individuals expressing prodromal symptoms in early adolescence5,6,7,9,20 and imply that a greater brain age gap may be useful in prediction of early-onset forms of psychosis.
Progressive Changes in Brain Structures in Converters to Psychosis
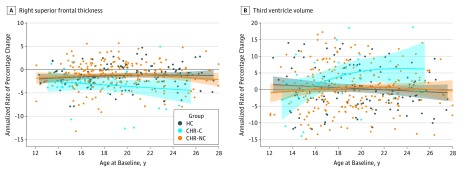
According to the neurodevelopmental framework (Figure 1),1,2,18,19 older adolescent and young adult CHR individuals with more acute-onset forms of psychosis would be expected to show normal brain maturation premorbidly and an increasing deviation around the time of onset of psychosis.1,2,14 Prior studies of longitudinal changes in brain structure in this sample (NAPLS 2) demonstrated an accelerated rate of cortical thinning in the superior and medial prefrontal regions and a greater rate of expansion of the third ventricle among CHR individuals who converted to psychosis compared with those who did not and with HCs.14,42,43 Age was assessed as a covariate in these studies; the reported outcomes were robust to age when its effects were “regressed out” in this way. However, given the results of the present investigation and the predictions of the neurodevelopmental framework (Figure 1),1,2,18,19 it may be more informative to evaluate age as a moderator in such analyses. To do so, we revisited the longitudinal data14 and plotted annualized rates of change in these neuroanatomical structures as a function of age at the time of the baseline scan. As shown in Figure 4,14,42,43 the differential rates of change in structural brain parameters among those who converted to psychosis apply only to those who were 18 years of age or older at baseline. In contrast, among younger individuals (12-17 years of age), the groups did not differ in the rate of prefrontal cortical thinning or third ventricle expansion.
Figure 4. Annualized Rate of Percentage Change.
A, Annualized rate of percentage change in the right superior frontal thickness, plotted as a function of age at baseline. B, Annualized rate of percentage change in third ventricle volume, plotted as a function of age at baseline. Locally weighted smoothing curve with 95% CI (colored area) is shown for each diagnostic group. With the use of a generalized linear model, a significant group (clinically high risk with conversion to psychosis [CHR-C] vs healthy control [HC]) by chronological age interaction was observed (right superior frontal thickness: P = .03; third ventricle: P = .001). Outliers defined by studentized deleted residuals exceeding ±3 were excluded (2 HCs, 4 individuals at clinically high risk with no conversion to psychosis [CHR-NC], and 2 individuals at CHR-C). Further details about demographics, image processing steps, and principal findings are reported in prior publications.14,42,43
Given that the neuroanatomical parameters in prefrontal regions undergo robust maturational changes during later adolescence44,45,46,47 and that the converters exhibiting an accelerated rate of cortical thinning are overrepresented in this age range, CHR individuals with an earlier age at onset of prodromal symptoms of schizophrenia with a contemporaneous neuroanatomical deficit (lower y-intercept) may still be at risk for experiencing later neuromaturational disturbances (aberrant slope) once they become older adolescents.
Limitations
Although the brain age approach can quantitatively assess deviations in brain maturation at the single individual level, such deviations are not likely to be specific to a particular disorder. By design, the fitted model parameters are data driven, characterizing the regularized maturation pattern among brain structures that tracks with variations in chronological age among typically developing individuals. In other words, this composite metric is not optimized for detecting risk of schizophrenia per se and would presumably be sensitive to any condition in which individuals deviate from the normal pattern of age-related neuroanatomical change during childhood or adolescence. However, the regional brain measures that contribute heavily to estimating chronological age in a typically developing population (ie, our top 25 hits as shown in eTable 2 in the Supplement) show considerable overlap with key regions in which prior studies have shown gray matter differences between CHR individuals who convert to psychosis and those who do not.17,48 These overlapping regions include regional gray matter volume measures, including the frontal, temporal, and parietal cortex, as well as hippocampal and amygdala volume.
Training models to predict conversion to psychosis as a categorical outcome seems like an intuitively appealing approach, but we were not successful in building such models using our data set. The performance of such models may be limited by the difficulty in adequately accounting for the backdrop of variation in normative adolescent brain development and the likelihood of heterogeneity in the pathways leading to full psychosis among individuals at CHR. However, it may be possible to increase the sensitivity to outcomes on the schizophrenia spectrum by constraining the spatial topologic parameters of brain age classifiers to brain regions previously associated with risk of psychosis and by incorporating neuroimaging parameters from other modalities. Eventually, the brain age and outcome-based machine learning approaches may be combinable with other clinical predictors49,50 to provide added traction on the problem of predicting the onset of psychosis among CHR individuals. To further validate whether our findings are in accord with the neurodevelopmental model, additional investigation is required to confirm whether neuroanatomical abnormality among younger CHR individuals is in fact affected by exposure to early neurodevelopmental risk factors (eg, obstetric complications) and/or present with an insidious onset in terms of premorbid functioning.
Conclusions
The brain age approach used in this study captured neuromaturational deviation associated with risk of psychosis only among younger CHR adolescents. This finding and the evidence that only CHR converters at the age of 18 years or older show a differential rate of progressive neuroanatomical changes over time are consistent with the view that differing intercepts and trajectories in structural brain parameters as a function of age contribute to heterogeneity in the timing of the onset and course of schizophrenia.
eAppendix. Details Regarding Subject Recruitment, Assessment, Image Acquisition, Image Processing, Image Analysis, Model Training and Validation, Methodological Considerations, and Estimation of Conversion to Psychosis among CHR Individuals
eTable 1. List of Factors for Brain Age Estimation Model
eTable 2. Top 25 Features for Brain Age Estimation Model Sorted by Absolute Weight
eTable 3. Clinical Symptoms and IQ Differences Between Younger and Older CHR Individuals
eFigure 1. Adjusting for Scanner Related Offsets
eFigure 2. Bias Adjustment and Generalization
eFigure 3. Age at Baseline and Psychosis Onset Among Converters
eFigure 4. Brain Age Gap as an Estimator of Conversion to Psychosis
eFigure 5. Antipsychotic Medication Exposure
eFigure 6. GAF Analysis with Non-Converters Only
References
- 1.Cannon TD, van Erp TGM, Bearden CE, et al. . Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29(4):653-669. [DOI] [PubMed] [Google Scholar]
- 2.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57(7):637-648. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44(7):660-669. [DOI] [PubMed] [Google Scholar]
- 4.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35(3):528-548. doi: 10.1093/schbul/sbn187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon TD, van Erp TGM, Rosso IM, et al. . Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59(1):35-41. [DOI] [PubMed] [Google Scholar]
- 6.Van Erp TGM, Saleh PA, Rosso IM, et al. . Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159(9):1514-1520. doi: 10.1176/appi.ajp.159.9.1514 [DOI] [PubMed] [Google Scholar]
- 7.Verdoux H, Geddes JR, Takei N, et al. . Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry. 1997;154(9):1220-1227. doi: 10.1176/ajp.154.9.1220 [DOI] [PubMed] [Google Scholar]
- 8.Rosso IM, Bearden CE, Hollister JM, et al. . Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26(2):367-378. [DOI] [PubMed] [Google Scholar]
- 9.Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lönnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157(5):801-807. doi: 10.1176/appi.ajp.157.5.801 [DOI] [PubMed] [Google Scholar]
- 10.Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20(3):441-451. [DOI] [PubMed] [Google Scholar]
- 11.Bearden CE, Rosso IM, Hollister JM, Sanchez LE, Hadley T, Cannon TD. A prospective cohort study of childhood behavioral deviance and language abnormalities as predictors of adult schizophrenia. Schizophr Bull. 2000;26(2):395-410. [DOI] [PubMed] [Google Scholar]
- 12.Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull. 2000;26(2):379-393. [DOI] [PubMed] [Google Scholar]
- 13.Haas GL, Sweeney JA. Premorbid and onset features of first-episode schizophrenia. Schizophr Bull. 1992;18(3):373-386. doi: 10.1093/schbul/18.3.373 [DOI] [PubMed] [Google Scholar]
- 14.Cannon TD, Chung Y, He G, et al. ; North American Prodrome Longitudinal Study Consortium . Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77(2):147-157. doi: 10.1016/j.biopsych.2014.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gur RE, Cowell P, Turetsky BI, et al. . A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55(2):145-152. [DOI] [PubMed] [Google Scholar]
- 16.Thompson PM, Vidal C, Giedd JN, et al. . Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98(20):11650-11655. doi: 10.1073/pnas.201243998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pantelis C, Velakoulis D, McGorry PD, et al. . Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281-288. [DOI] [PubMed] [Google Scholar]
- 18.Chung Y, Cannon TD. Brain imaging during the transition from psychosis prodrome to schizophrenia. J Nerv Ment Dis. 2015;203(5):336-341. doi: 10.1097/NMD.0000000000000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cannon TD. How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends Cogn Sci. 2015;19(12):744-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Callaghan E, Gibson T, Colohan HA, et al. . Risk of schizophrenia in adults born after obstetric complications and their association with early onset of illness: a controlled study. BMJ. 1992;305(6864):1256-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown TT, Kuperman JM, Chung Y, et al. . Neuroanatomical assessment of biological maturity. Curr Biol. 2012;22(18):1693-1698. doi: 10.1016/j.cub.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke K, Luders E, May A, Wilke M, Gaser C. Brain maturation: predicting individual BrainAGE in children and adolescents using structural MRI. Neuroimage. 2012;63(3):1305-1312. doi: 10.1016/j.neuroimage.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 23.Cole JH, Poudel RPK, Tsagkrasoulis D, et al. . Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. Neuroimage. 2017;163:115-124. doi: 10.1016/j.neuroimage.2017.07.059 [DOI] [PubMed] [Google Scholar]
- 24.Liem F, Varoquaux G, Kynast J, et al. . Predicting brain-age from multimodal imaging data captures cognitive impairment. Neuroimage. 2017;148:179-188. doi: 10.1016/j.neuroimage.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 25.Koutsouleris N, Davatzikos C, Borgwardt S, et al. . Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull. 2014;40(5):1140-1153. doi: 10.1093/schbul/sbt142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franke K, Ziegler G, Klöppel S, Gaser C; Alzheimer’s Disease Neuroimaging Initiative . Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010;50(3):883-892. doi: 10.1016/j.neuroimage.2010.01.005 [DOI] [PubMed] [Google Scholar]
- 27.Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS. Accelerated brain aging in schizophrenia: a longitudinal pattern recognition study. Am J Psychiatry. 2016;173(6):607-616. doi: 10.1176/appi.ajp.2015.15070922 [DOI] [PubMed] [Google Scholar]
- 28.Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H; Alzheimer’s Disease Neuroimaging Initiative . BrainAGE in mild cognitive impaired patients: predicting the conversion to Alzheimer’s disease. PLoS One. 2013;8(6):e67346. doi: 10.1371/journal.pone.0067346.s003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao B, Mwangi B, Hasan KM, et al. . Development and validation of a brain maturation index using longitudinal neuroanatomical scans. Neuroimage. 2015;117:311-318. doi: 10.1016/j.neuroimage.2015.05.071 [DOI] [PubMed] [Google Scholar]
- 30.Jernigan TL, Brown TT, Hagler DJ Jr, et al. ; Pediatric Imaging, Neurocognition and Genetics Study . The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. Neuroimage. 2016;124(pt B):1149-1154. doi: 10.1016/j.neuroimage.2015.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Addington J, Cadenhead KS, Cornblatt BA, et al. . North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142(1-3):77-82. doi: 10.1016/j.schres.2012.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGlashan T, Walsh B, Woods S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. New York, NY: Oxford University Press; 2010. [Google Scholar]
- 33.Cannon TD, Sun F, McEwen SJ, et al. . Reliability of neuroanatomical measurements in a multisite longitudinal study of youth at risk for psychosis. Hum Brain Mapp. 2014;35(5):2424-2434. doi: 10.1002/hbm.22338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 35.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis, II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207. [DOI] [PubMed] [Google Scholar]
- 36.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischl B, Salat DH, Busa E, et al. . Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355. [DOI] [PubMed] [Google Scholar]
- 38.Desikan RS, Ségonne F, Fischl B, et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- 39.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22. doi: 10.1109/TPAMI.2005.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Filzmoser P, Liebmann B, Varmuza K. Repeated double cross validation. J Chemometrics. 2009;23(4):160-171. doi: 10.1002/cem.1225 [DOI] [Google Scholar]
- 41.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 42.Chung Y, Jacobson A, He G, et al. . Prodromal symptom severity predicts accelerated gray matter reduction and third ventricle expansion among clinically high risk youth developing psychotic disorders. Mol Neuropsychiatry. 2015;1(1):13-22. doi: 10.1159/000371887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung Y, Haut KM, He G, et al. ; North American Prodrome Longitudinal Study (NAPLS) Consortium . Ventricular enlargement and progressive reduction of cortical gray matter are linked in prodromal youth who develop psychosis. Schizophr Res. 2017;189:169-174. doi: 10.1016/j.schres.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gogtay N, Giedd JN, Lusk L, et al. . Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101(21):8174-8179. doi: 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 2010;20(3):534-548. doi: 10.1093/cercor/bhp118 [DOI] [PubMed] [Google Scholar]
- 46.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24(38):8223-8231. doi: 10.1523/JNEUROSCI.1798-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tamnes CK, Herting MM, Goddings A-L, et al. . Development of the cerebral cortex across adolescence: a multisample study of inter-related longitudinal changes in cortical volume, surface area, and thickness. J Neurosci. 2017;37(12):3402-3412. doi: 10.1523/JNEUROSCI.3302-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borgwardt SJ, McGuire PK, Aston J, et al. . Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106(2-3):108-114. doi: 10.1016/j.schres.2008.08.007 [DOI] [PubMed] [Google Scholar]
- 49.Cannon TD, Cadenhead K, Cornblatt B, et al. . Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cannon TD, Yu C, Addington J, et al. . An individualized risk calculator for research in prodromal psychosis. Am J Psychiatry. 2016;173(10):980-988. doi: 10.1176/appi.ajp.2016.15070890 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Details Regarding Subject Recruitment, Assessment, Image Acquisition, Image Processing, Image Analysis, Model Training and Validation, Methodological Considerations, and Estimation of Conversion to Psychosis among CHR Individuals
eTable 1. List of Factors for Brain Age Estimation Model
eTable 2. Top 25 Features for Brain Age Estimation Model Sorted by Absolute Weight
eTable 3. Clinical Symptoms and IQ Differences Between Younger and Older CHR Individuals
eFigure 1. Adjusting for Scanner Related Offsets
eFigure 2. Bias Adjustment and Generalization
eFigure 3. Age at Baseline and Psychosis Onset Among Converters
eFigure 4. Brain Age Gap as an Estimator of Conversion to Psychosis
eFigure 5. Antipsychotic Medication Exposure
eFigure 6. GAF Analysis with Non-Converters Only