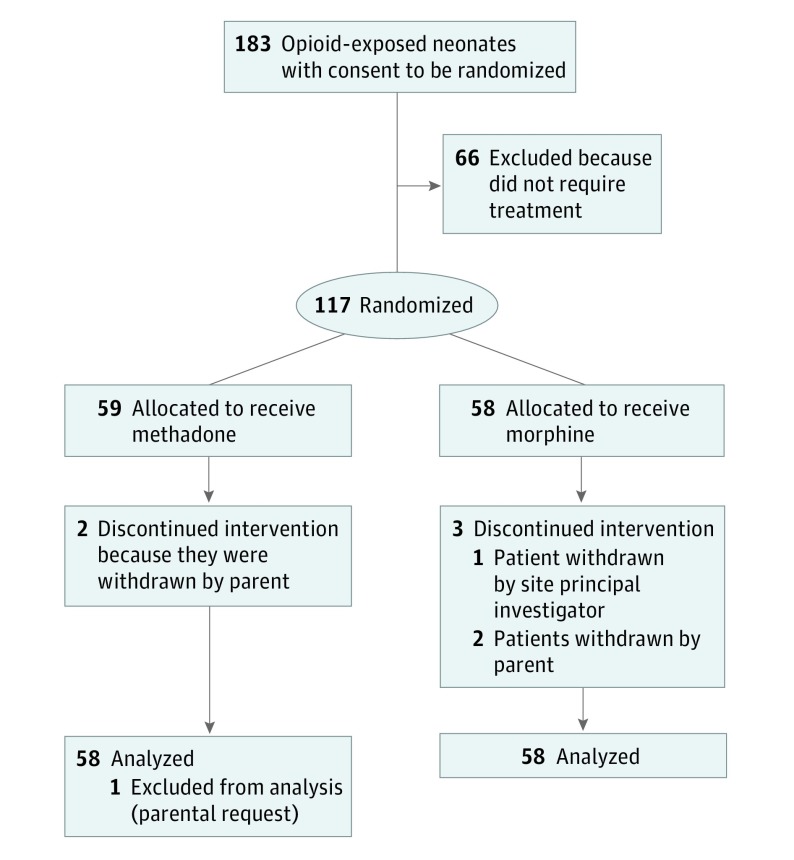
This randomized clinical trial compares the safety and efficacy of methadone vs morphine for treatment of infants with neonatal abstinence syndrome using a weight-and sign-based treatment protocol.
Key Points
Question
Does methadone or morphine have better safety and efficacy for the treatment of neonatal abstinence syndrome?
Findings
In this randomized clinical trial, 117 infants were randomized to treatment with methadone or morphine using a weight- and sign-based treatment protocol. Methadone was associated with reductions in length of hospital stay, length of hospital stay attributable to neonatal abstinence syndrome, and length of treatment after adjusting for study site and type of maternal opioid.
Meaning
Methadone is more effective than morphine only in infants needing pharmacologic treatment for neonatal abstinence syndrome.
Abstract
Importance
Although opioids are used to treat neonatal abstinence syndrome (NAS), the best pharmacologic treatment has not been established.
Objective
To compare the safety and efficacy of methadone and morphine in NAS.
Design, Setting, and Participants
In this randomized, double-blind, intention-to-treat trial, term infants from 8 US newborn units whose mothers received buprenorphine, methadone, or opioids for pain control during pregnancy were eligible. A total of 117 infants were randomized to receive methadone or morphine from February 9, 2014, to March 6, 2017. Mothers who declined randomization could consent to data collection and standard institutional treatment.
Interventions
Infants were assessed with the Finnegan Neonatal Abstinence Scoring System every 4 hours and treated with methadone or placebo every 4 hours or morphine every 4 hours. Infants with persistently elevated Finnegan scores received dose increases. Infants who exceeded a predetermined opioid dose received phenobarbital. Dose reductions occurred every 12 to 48 hours when signs of NAS were controlled with therapy, stopping at 20% of the original dose.
Main Outcomes and Measures
The primary end point was length of hospital stay (LOS). The secondary end points were LOS attributable to NAS and length of drug treatment (LOT).
Results
A total of 183 mothers consented to have their infants in the study; 117 infants required treatment. Because 1 parent withdrew consent, data were analyzed on 116 infants (mean [SD] gestational age, 39.1 [1.1] weeks; mean [SD] birth weight, 3157 [486] g; 58 [50%] male). Demographic variables and risk factors were similar except for more prenatal cigarette exposure in infants who received methadone. Adjusting for study site and maternal opioid type, methadone was associated with decreased mean number of days for LOS by 14% (relative number of days, 0.86; 95% CI, 0.74-1.00; P = .046), corresponding to a difference of 2.9 days; 14% reduction in LOS attributable to NAS (relative number of days, 0.86; 95% CI, 0.77-0.96; P = .01), corresponding to a difference of 2.7 days; and 16% reduction in LOT (relative number of days, 0.84; 95% CI, 0.73-0.97; P = .02), corresponding to a difference of 2.3 days. Methadone was also associated with reduced median LOS (16 vs 20 days, P = .005), LOS attributable to NAS (16 vs 19 days, P = .005), and LOT (11.5 vs 15 days, P = .009). Study infants had better short-term outcomes than 170 nonrandomized infants treated with morphine per standard institutional protocols.
Conclusions and Relevance
With use of weight- and sign-based treatment for NAS, short-term outcomes were better in infants receiving methadone compared with morphine. Assessment of longer-term outcomes is ongoing.
Trial Registration
ClinicalTrials.gov Identifier: NCT01958476
Introduction
Misuse of opioids during pregnancy affects more than 5% of all pregnant women in the United States.1 Opioids may also be prescribed during pregnancy to control chronic pain. As the number of pregnant women exposed to opioids and other psychotropic medications has increased, so has the incidence of neonatal abstinence syndrome (NAS).2 Neonatal abstinence syndrome is a constellation of signs that involve central and autonomic nervous system dysfunction that affects more than half of infants exposed to opioids in utero, although its expression is widely variable.3,4
Although several different approaches are used to treat NAS, no universal standard exists. The use of opioids has been recommended for treatment of infants with significant NAS, with neonatal morphine solution or methadone being the most commonly used medications.5 If there is inadequate response to an opioid, a second medication may be added. However, there is significant heterogeneity in treatment approaches, and the safety and efficacy of many of these drugs have not been adequately established. In a single-center trial of NAS, Brown et al6 found significant reductions in median treatment times among 13 infants treated with methadone (15 days) compared with 18 infants treated with morphine (21 days). The few studies7,8 that compared single-drug regimens were largely retrospective and demonstrated no differences in short-term outcomes or potential advantages with the use of morphine.
Although some clinicians have recommended treating with an opioid based on the infant’s weight, others have based the dose on the severity of NAS as assessed by the Finnegan Neonatal Abstinence Scoring System (Finnegan score [FS]).9 The current trial was designed to evaluate the safety and efficacy of a novel weight- and sign-based approach to control withdrawal. To our knowledge, this was the first multisite randomized clinical trial to compare the 2 most common medications used to treat NAS, methadone and morphine.
Methods
Study Design
Reporting and analyses for this trial followed CONSORT 2010 guidelines (trial protocol in Supplement 1). Investigators, participants, staff, and statisticians were masked to treatment assignment. Only research pharmacists had access to treatment assignments. The study was approved by the institutional review boards at Tufts Medical Center, Boston, Massachusetts; Baystate Children’s Hospital, Springfield, Massachusetts; Boston Medical Center, Boston, Massachusetts; Maine Medical Center, Portland; University of Florida Health, Jacksonville; University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania; Vanderbilt University Medical Center, Nashville, Tennessee; and Women & Infants Hospital of Rhode Island, Providence. Written informed consent was obtained by study investigators at each of the 8 US sites.
The study was a randomized, double-blind, intention-to-treat trial that compared the safety and efficacy of methadone vs morphine to treat NAS. A total of 183 mothers consented to have their infants enrolled from February 9, 2014, to March 6, 2017 (when funding ended); 117 required treatment and were randomized to receive methadone or morphine (data available on 58 per group) (Figure). Randomization was 1:1 according to computer-generated randomization sequences and was stratified by site and type of maternal opioid used (buprenorphine or methadone for treatment of an opioid use disorder, opioids prescribed for chronic pain) to balance study arms. Details are provided in the eAppendix in Supplement 2. Multiple factors limited recruitment. First, study initiation was delayed by 14 months to develop the compounding protocol for the methadone, contract with Boston Laboratories, perform the testing, and receive the Investigational New Drug approval. Second, many mothers declined to enroll their infants in the randomized treatment trial because they were taking methadone and did not wish their infants to receive it. Other mothers were concerned about social or legal ramifications of enrolling their infants in the trial. Third, sites began to focus on nonpharmacologic care, which reduced the numbers of infants who required pharmacologic treatment.
Figure. CONSORT Flow Diagram.
Inclusion and Exclusion Criteria
Mothers treated with methadone or buprenorphine for an opioid use disorder or an opioid for treatment of chronic pain and receiving prenatal care (at least 1-2 obstetric visits in the third trimester) were eligible for study inclusion. Mothers with a known psychiatric diagnosis could receive additional psychotropic drugs. Mothers were still included if they used illicit drugs during the pregnancy. Mothers were excluded if they consumed more than 3 oz of alcohol per week during the pregnancy (confounding effects of fetal alcohol exposure). Urine toxicology testing was performed in mothers at the time of delivery. All infants were born at 37 weeks’ gestation or later (by solid obstetric dating criteria or early ultrasonography; premature infants were excluded because of a more variable response to prenatal and postnatal opioid exposure) and without evidence of sepsis, major congenital anomalies, or genetic disorders. Infants had urine and meconium toxicologic tests performed at birth.
Observational Comparison Cohort
Mothers who declined randomization could consent to standardized institutional weight-based morphine treatment and data collection. Inclusion and exclusion criteria were the same as those used in the randomized clinical trial.
Preparations
The commercial preservative-free neonatal morphine was diluted to 0.2 mg/mL. The commercial methadone contained 15% alcohol as a preservative. Because alcohol could affect short- and longer-term outcomes, the US Food and Drug Administration (FDA) required that a preservative-free methadone solution be prepared using methadone powder (Mallinckrodt Inc). However, the FDA first required development of compounding procedures using Good Clinical Practice guidelines. Boston Analytical tested the compounded solution (0.4 mg/mL) for sterility, concentration, stability, and purity during a 2-week period because it would be difficult to compound the solution daily. After these studies were completed, the Investigational New Drug and Certificate of Confidentiality were issued. Extensive training of each site’s pharmacy was conducted to standardize compounding procedures and ensure the safety of the formulation. The pharmacist at each site randomized the infant and assigned the therapy.
Finnegan Scoring
Infants were assessed by nursing staff using standardized FSs every 4 hours as previously described.1,10 All nursing staff completed interobserver reliability training, which was achieved when the same item and total score were obtained by 2 independent raters using both training videos and infants enrolled in the study.11
Treatment Protocol
Pharmacologic treatment was initiated when the FS was 8 or higher on 2 consecutive occasions or 12 or higher on 1 assessment. If this occurred when research pharmacy staff was unavailable, the infants could receive 1 dose of morphine before randomization. The dose approach using weight and FS is outlined in Table 1. Infants received methadone alternating with placebo every 4 hours (each agent administered every 8 hours to maintain blinding) or morphine every 4 hours. The methadone, placebo, and morphine looked identical, ensuring the blinding for all staff. If the infant continued to have an FS of 8 or higher for 2 consecutive scores or had 1 score of 12 or higher, the dose was increased to the next level. If the FS remained elevated despite increasing to a predetermined maximal opioid dose, phenobarbital (20-mg/kg loading dose followed by 4-5 mg/kg daily) was administered. Doses were increased until control of withdrawal was achieved. Although phenobarbital was not considered to be a study drug, a protocol was provided to each site (eTable 1 in Supplement 2). The study drug was then decreased by 10% every 12 to 48 hours (with FSs generally <8) and treatment stopped at 20% of the initial dose. Infants were then observed for 48 hours before discharge.
Table 1. Treatment Schedule of the Neonatal Abstinence Syndrome Study Druga.
| Level | Finnegan Score | Starting Daily Dose, mg/kgb |
|---|---|---|
| Morphine, 0.2 mg/mL | ||
| 1 | 8-10 | 0.3 |
| 2 | 11-13 | 0.5 |
| 3 | 14-16 | 0.7 |
| 4 | ≥17 | 0.9 |
| Methadone, 0.4 mg/mL | ||
| 1 | 8-10 | 0.3 |
| 2 | 11-13 | 0.5 |
| 3 | 14-16 | 0.7 |
| 4 | ≥17 | 0.9 |
Information on the treatment protocol is shown in the Treatment Protocol subsection of the Methods section.
Morphine was given every 4 hours; methadone was given every 8 hours, with placebo given every 8 hours to maintain the blinding.
End Points
The primary end point was length of hospital stay (LOS). Secondary end points were LOS related to treatment for NAS (birth to last day of study drug treatment plus 2 days for observation after treatment was stopped), length of treatment with the study drug (LOT), need for supplemental phenobarbital, weight gain during the hospitalization, and the need for dose escalation of the study drug. When the trial was designed, LOS was the standard outcome measure for smaller NAS studies. However, this variable can be influenced by multiple medical and social factors; thus, the secondary outcomes were analyzed.
Statistical Analysis
A total sample size of 184 was projected to attain 80% power to detect a difference in mean LOS of 2.3 days, assuming that LOS demonstrated a Poisson distribution and mean LOS in the shorter LOS group was 30 days or less. Baseline variables were described by treatment group. Analyses of treatment effect were performed according to the intention-to-treat principle. All statistical tests were 2-sided and at a significance level of α = .05. Secondary end points were not accounted for in the power calculations and were therefore considered to be exploratory. Count data were analyzed using negative binomial regression, binary data with logistic regression, and weight gain with linear regression. Analyses were adjusted for the stratifying variables used in randomization (site, type of opioid that the mother received). In addition, by inserting an interaction term, we tested whether treatment effect was modified by the type of opioid that the mother was taking. Because all infants whose mothers received opioids for pain were randomized to a single group by chance, we performed a sensitivity analysis that excluded those infants. In unadjusted analyses of count data, medians were also compared between treatment groups using the Wilcoxon rank sum test. Adjusted medians could not be calculated because of small cell sizes. The infants treated with morphine in the observational cohort were compared with all randomized infants and infants in the randomized morphine arm by using similar statistical methods. This comparison was not planned at the time of study initiation because it was not anticipated that so many mothers would refuse randomization but agree to data collection. Additional explanation of statistical methods is provided in the eAppendix in Supplement 2. Analyses were performed using SAS software, version 9.4_M3 (SAS Institute Inc).
Results
A total of 183 mothers consented to have their infants in the study; 117 infants required treatment. Because 1 parent withdrew consent, data were analyzed on 116 infants (mean [SD] gestational age, 39.1 [1.1] weeks; mean [SD] birth weight, 3157 [486] g; 58 [50%] male). Demographic variables and risk factors were similar between groups (Table 2 and Table 3). The number of mothers smoking 5 or more cigarettes per day was greater in the methadone group, and the number of infants initially cared for in the newborn unit was greater in the methadone group.
Table 2. Characteristics of Mothers and Infants at Baselinea.
| Characteristic | Randomized | Nonrandomized | |
|---|---|---|---|
| Methadone (n = 58) | Morphine (n = 58) | Morphine (n = 170)b | |
| Mothers | |||
| Opioid (stratification factor) | |||
| Buprenorphine | 20 (34.5) | 19 (32.8) | 75 (44.1) |
| Methadone | 38 (65.5) | 35 (60.3) | 89 (52.4) |
| Prescription opioids for pain | 0 | 4 (6.9) | 6 (3.5) |
| Smoking status | |||
| Smoked during pregnancy | 49 (84.5) | 44 (75.9) | 128 (75.3) |
| Smoked >5 cigarettes/d | 33 (56.9) | 15 (25.9) | 81 (47.7) |
| Missing | 5 (8.6) | 6 (10.3) | 20 (11.8) |
| Urine toxicology test result | |||
| Positive for at least 1 illicit substance | 13 (22.4) | 16 (27.6) | 33 (19.4) |
| Missing | 4 (6.9) | 4 (6.9) | 7 (4.1) |
| Psychiatric diagnoses | 42 (72.4) | 37 (63.8) | 116 (68.2) |
| Psychiatric medication during pregnancy | 20 (35.7) | 16 (27.6) | 49 (29.2) |
| Infants | |||
| Gestational age, mean (SD), wk | 39.2 (1.2) | 39.1 (1.1) | 39.2 (1.3) |
| Birth weight, mean (SD), g | 3186 (488) | 3128 (487) | 3125 (483) |
| Male | 29 (50) | 29 (50) | 82 (48.2) |
| Apgar score ≥7 | |||
| 1 min | 54 (93.1) | 53 (91.4) | 162 (95.3) |
| 5 min | 57 (98.3) | 57 (98.3) | 170 (100) |
| Race/ethnicity | |||
| White | 46 (79.3) | 42 (72.4) | 129 (75.9) |
| Hispanic | 6 (10.3) | 6 (10.3) | 7 (4.1) |
| Other | 6 (10.3) | 10 (17.2) | 16 (9.4) |
| Head circumference mean (SD), cm | 33.9 (1.7) | 33.7 (1.8) | 33.7 (1.5) |
| Age at treatment start, mean (SD), d | 3.2 (1.3) | 3.5 (1.6) | 3.4 (1.9) |
| Urine toxicology test result | |||
| Positive | 41 (74.6) | 42 (75.0) | 127 (84.1) |
| Missing | 3 (5.2) | 2 (3.4) | 19 (11.2) |
| Site of initial care (before treatment) | |||
| NICU | 3 (5.2) | 10 (17.2) | 34 (20.0) |
| Newborn unit | 55 (94.8) | 48 (82.8) | 130 (76.5) |
| Other | 0 | 0 | 6 (3.5) |
| Single dose of morphine before randomization | 23 (39.7) | 17 (29.3) | NA |
Abbreviations: NA, not applicable; NICU, neonatal intensive care unit.
Data are presented as number (%) or mothers or infants unless otherwise indicated.
Nineteen infants in the nonrandomized cohort received clonidine plus morphine for first-line treatment at 1 site.
Table 3. Treatment Characteristics.
| Characteristic | No. (%) of Infants | P Value | |
|---|---|---|---|
| Methadone (n = 58) | Morphine (n = 58) | ||
| Initial site of NAS care | |||
| NICU | 17 (29.3) | 17 (29.3) | .90 |
| Special care unit | 9 (15.5) | 7 (12.1) | |
| General pediatric unit | 16 (27.6) | 18 (31.0) | |
| Newborn unit | 16 (27.6) | 16 (27.6) | |
| Starting daily dose of study drug, mg/kg | |||
| 0.3 (Level 1) | 26 (44.8) | 32 (55.2) | .52 |
| 0.5 (Level 2) | 25 (43.1) | 21 (36.2) | |
| 0.7 (Level 3) | 7 (12.1) | 5 (8.6) | |
| Maximum Finnegan score before starting treatment, mean (SD) | 12.9 (2.9) | 12.6 (2.8) | .60 |
| Primary feeding during hospitalization | |||
| Formula only | 22 (37.9) | 32 (55.2) | .06 |
| Breast milk (exclusive or with formula supplementation) | 36 (62.1) | 26 (44.8) | |
Abbreviations: NAS, neonatal abstinence syndrome; NICU, neonatal intensive care unit.
Overall interobserver reliability for FS for the study was 81%, with 7 of 8 centers performing at more than 90% and 1 center that enrolled a small number of infants not performing as expected. A total of 13 adverse events were recorded (equally distributed between groups), including shallow breathing, bradycardia, oxygen desaturation, lethargy, poor feeding, hypothermia, and emesis. One infant who received methadone had a serious adverse event that involved apnea, lethargy, and hypothermia, which prompted readmission to the neonatal intensive care unit. The dose of study drug was decreased in response to the adverse events, and all infants responded well and continued in the study. In response to the adverse events, the protocol was amended to permit more rapid weaning of study drug (12-48 hours), and no additional adverse events were recorded.
Unadjusted analyses found no statistically significant differences in mean primary or secondary outcomes between the study groups. Adjusting for treatment site and maternal opioid type demonstrated that compared with morphine, methadone was associated with a 14% reduction in mean relative LOS (relative number of days, 0.86; 95% CI, 0.74-1.00; P = .046), corresponding to a difference of 2.9 days; 14% reduction in LOS attributable to NAS (relative number of days, 0.86; 95% CI, 0.77-0.96; P = .01), corresponding to a difference of 2.7 days; and 16% reduction in LOT (relative number of days, 0.84; 95% CI, 0.73-0.97; P = .02), corresponding to a difference of 2.3 days. Methadone was also associated with statistically significant reductions in the median total LOS (16 vs 20 days, P = .005), median LOS related to NAS (16 vs 19 days, P = .005), and median LOT (11.5 vs 15 days, P = .009) compared with morphine (Table 4). The statistically significant decreases remained after sensitivity analyses omitted the 4 infants exposed to maternal prescription opioids for chronic pain (eTable 2 in Supplement 2). Although use of phenobarbital was less common in infants treated with methadone, the difference was nonsignificant (odds ratio, 0.44; 95% CI, 0.18-1.09; P = .07). Results of the per protocol analysis (eTable 3 in Supplement 2) agreed with the intention-to-treat analysis.
Table 4. Primary and Secondary Outcomes.
| Outcome | Methadone (n = 58) | Morphine (n = 58) | Methadone-Morphine Comparison | |||
|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | |||||
| Statisticb (95% CI) | P Value | Statisticb (95% CI) | P Value | |||
| LOS | ||||||
| Mean (SD) | 21.8 (15.0) | 23.2 (8.8) | 0.94 (0.80 to 1.11) | .48 | 0.86 (0.74 to 1.00) | .046 |
| Median (IQR) | 16 (14 to 22) | 20 (16 to 27)c | ||||
| LOS attributable to NAS | ||||||
| Mean (SD) | 18.9 (7.9) | 21.1 (6.9) | 0.89 (0.79 to 1.02) | .09 | 0.86 (0.77 to 0.97) | .01 |
| Median (IQR) | 16 (14 to 22) | 19 (16 to 25)c | ||||
| LOT | ||||||
| Mean (SD) | 14.7 (8.0) | 16.6 (6.9) | 0.89 (0.76 to 1.04) | .14 | 0.84 (0.73 to 0.97) | .02 |
| Median (IQR)c | 11.5 (10 to 17) | 15 (12 to 19)c | ||||
| Phenobarbital, No. (%)d | 10 (17.2) | 17 (29.3) | 0.45 (0.19 to 1.10) | .08 | 0.44 (0.18 to 1.09) | .07 |
| Infants needing a dose increase, No. (%)d | 22 (37.9) | 28 (48.3) | 0.57 (0.27 to 1.20) | .14 | 0.51 (0.23 to 1.14) | .10 |
| Weight gain, mean (SD), g/d | 8.4 (13.9) | 11.2 (14.2) | −2.7 (−7.8 to 2.3) | .30 | −3.2 (−8.0 to 1.7) | .20 |
Abbreviations: IQR, interquartile range; LOS, length of hospital stay; LOT, length of drug treatment; NAS, neonatal abstinence syndrome.
Adjusted for site and type of maternal opioid (methadone, buprenorphine, or opioids for pain).
Statistics are relative numbers of days for LOS, LOS attributable to NAS, and LOT; odds ratios for phenobarbital and infants needing a dose increase; and difference for weight gain.
Difference in medians statistically significant (P = .009).
Does not include 4 infants whose mothers were treated with opioids for pain because of sparse strata, leading to problems with model estimation.
To determine the effect of the study treatment alone, the combined randomized cohort was compared with the nonrandomized cohort. Randomized infants had statistically significantly shorter adjusted total LOS, LOS attributable to NAS, and LOT and received phenobarbital less frequently than did nonrandomized infants (eTable 4 in Supplement 2). However, no statistically significant differences were found in any outcomes in infants randomized to receive morphine compared with the observational cohort, demonstrating that any beneficial effects were primarily attributable to methadone.
Discussion
Despite recommendations that opioids should be used for treatment of NAS, no universal evidence-based pharmacologic treatment strategy exists. In addition, no drug is approved for use in infants by the FDA.5,12 A few studies8,13 that evaluated the efficacy of opioids in NAS in a small number of infants identified a shorter LOS and LOT in infants treated with methadone. We also found that treatment of NAS with methadone was modestly better than treatment with morphine in improving short-term outcomes. Our study is the only multicenter comparison trial to date and suggests that the choice of opioid in infants with NAS can affect the duration of treatment independent of the opioid exposure during pregnancy. Stratification by maternal opioid produced comparable cohorts except for more mothers of methadone-treated infants smoking 5 or more cigarettes per day. Cigarette smoking is known to be associated with the need for higher doses of opioids and longer LOS and LOT in infants with NAS, possibly because of the ability of nicotine to induce the cytochrome P450 and glucuronidase genes.14,15 Despite this potential confounder, methadone was still associated with better outcomes.
The differential response to the type of opioid used to treat NAS resides in the unique characteristics of each drug. The active R enantiomer of methadone possesses greater μ-opioid receptor agonist activity than morphine but has lower receptor affinity. Metabolism of methadone by cytochrome P450 isoenzymes can vary by as much as 30-fold in liver and 11-fold in gut.16 The higher fat solubility, protein binding, and volume of distribution of methadone prolongs the half-life and allows a longer dose interval.17 Morphine is a μ- and κ-opioid receptor agonist metabolized via gestationally regulated hepatic glucuronidation and eliminated by the kidney.17 Although morphine is typically administered every 3 to 4 hours in newborns, the dose regimen of methadone is more variable, and the drug can be given every 8 to 24 hours. The longer dose interval for methadone compared with morphine is another beneficial effect. Recently, Hall et al18 reported that lengthening the methadone dose interval (supported by pharmacokinetic data) reduced LOS and LOT by 3 days. Conceivably, a pharmacokinetic-based methadone dose regimen might have further improved outcome in our study.19
The treatment approach permitted us to control the signs of NAS more quickly and wean the dose more rapidly. However, adverse events were reported that were equally distributed between the treatment groups. These adverse effects are recognized as potential complications of opioid administration. Because our treatment approach might have contributed to these events, we changed the treatment protocol during the trial to allow more rapid weaning of the study drug (from a minimum of 24 hours to 12 hours). This change minimized subsequent events.
Establishment of a universal treatment protocol is complicated by significant practice variation and the use of additional medications, such as phenobarbital and clonidine.13 Phenobarbital functions to increase the breakdown of drugs metabolized by cytochrome P450, whereas clonidine (an α2-adrenergic agonist) reduces central sympathetic outflow. A previous report20 that compared phenobarbital with clonidine coadministered with morphine found little difference in LOS and LOT. Our weight- and sign-based treatment strategy permitted use of phenobarbital when a predetermined maximum opioid dose did not adequately control withdrawal. Although methadone reduced the use of phenobarbital, adherence to the protocol alone decreased the need for supplemental medication as shown by comparisons with the nonrandomized cohort.
Our study has implications for the treatment of NAS. A commercially available methadone solution that is preservative free and safe for newborns is needed. Recently, Kraft et al21 demonstrated that sublingual buprenorphine was more effective than morphine in the treatment of NAS. However, the buprenorphine formulation contained significant amounts of alcohol, which may ultimately limit the widespread use of this drug. Most drugs used to treat newborns are adult formulations that contain preservatives that have not been proven to be safe and could affect neurodevelopmental outcome. Extensive prestudy work was undertaken to guarantee the stability, purity, and sterility of our methadone preparation. Such expensive and time-consuming processes highlight the need to develop safe, commercially available formulations designed specifically for newborns.22
Although it is possible that methadone is better than morphine for treatment of NAS, a small retrospective study7 found lower Bayley III mean composite cognitive and motor scores in methadone-treated infants compared with those receiving morphine. Our ongoing neurodevelopmental follow-up of infants through 18 to 24 months should provide additional information on the longer-term safety of opioid treatment in NAS.
Limitations
The complexity of the present trial introduced some limitations. As stated previously, we did not meet our recruitment goal for reasons detailed in the Study Design section. This inability to meet the recruitment goal highlights the difficulty of conducting large trials of pregnant women taking opioids and infants with NAS. However, we recruited 170 additional infants as a standard of care control arm to compare randomized and nonrandomized infants being treated for NAS.
The infant’s need for medical treatment was based on the FS, a widely used assessment tool that is subjective and has significant interobserver variability. We used a well-structured training program that had been used in other studies (eg, the Maternal Opioid Treatment: Human Experimental Research study, using the same training personnel and approaches) to minimize variability.1 Lastly, our results were stratified by site to minimize the variation of supplementary approaches (eg, nonpharmacologic care) adopted by participating institutions in response to NAS. Nonetheless, it is possible that individual institutions implemented changes in treatment protocols during the 4-year recruitment period that might have affected outcome, although randomization at each site should account for this possibility.
Conclusions
Methadone was better than morphine in improving short-term NAS outcomes. Even modest reductions in LOS could significantly decrease the economic effect of NAS, considering the thousands of infants treated each year.23 A more complete understanding of the factors that determine the severity of NAS and the long-term safety of different treatment approaches is needed. Such an understanding will help refine best practices and reduce the societal and financial burden of NAS while improving short- and longer-term outcomes in this highly vulnerable population.
Trial Protocol
eAppendix. Statistical Analysis
eTable 1. Phenobarbital Dosing
eTable 2. Sensitivity analyses omitting the four infants exposed to maternal prescription opioids for chronic pain
eTable 3. Per Protocol Analysis: Primary and Secondary Outcomes
eTable 4. Comparison to Non-randomized Infants: Primary and Secondary Outcomes
References
- 1.Jones HE, Kaltenbach K, Heil SH, et al. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patrick SW, Davis MM, Lehmann CU, Cooper WO. Increasing incidence and geographic distribution of neonatal abstinence syndrome: United States 2009 to 2012. J Perinatol. 2015;35(8):650-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraft WK, Stover MW, Davis JM. Neonatal abstinence syndrome: Pharmacologic strategies for the mother and infant. Semin Perinatol. 2016;40(3):203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5(1):47-55. [PMC free article] [PubMed] [Google Scholar]
- 5.Hudak ML, Tan RC; Committee on Drugs; Committee on Fetus and Newborn; American Academy of Pediatrics . Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540-e560. [DOI] [PubMed] [Google Scholar]
- 6.Brown MS, Hayes MJ, Thornton LM. Methadone versus morphine for treatment of neonatal abstinence syndrome: a prospective randomized clinical trial. J Perinatol. 2015;35(4):278-283. [DOI] [PubMed] [Google Scholar]
- 7.Burke S, Beckwith AM. Morphine versus methadone treatment for neonatal withdrawal and impact on early infant development [published online July 25, 2017]. Glob Pediatr Health. doi: 10.1523/JNEUROSCI.0531-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young ME, Hager SJ, Spurlock D Jr. Retrospective chart review comparing morphine and methadone in neonates treated for neonatal abstinence syndrome. Am J Health Syst Pharm. 2015;72(23)(suppl 3):S162-S167. [DOI] [PubMed] [Google Scholar]
- 9.Stover MW, Davis JM. Opioids in pregnancy and neonatal abstinence syndrome. Semin Perinatol. 2015;39(7):561-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DʼApolito KC. Assessing neonates for neonatal abstinence: are you reliable? J Perinat Neonatal Nurs. 2014;28(3):220-231. [DOI] [PubMed] [Google Scholar]
- 11.D’Apolito K, Finnegan L Assessing signs and symptoms of neonatal abstinence using the Finnegan Scoring Tool: an inter-observer reliability program, NeoAdvances.com. 2010. https://www.neoadvances.com/. Accessed May 1, 2018.
- 12.Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010;10(10):CD002059. [DOI] [PubMed] [Google Scholar]
- 13.Patrick SW, Kaplan HC, Passarella M, Davis MM, Lorch SA. Variation in treatment of neonatal abstinence syndrome in US children’s hospitals, 2004-2011. J Perinatol. 2014;34(11):867-872. [DOI] [PubMed] [Google Scholar]
- 14.Jones HE, Heil SH, Tuten M, et al. Cigarette smoking in opioid-dependent pregnant women: neonatal and maternal outcomes. Drug Alcohol Depend. 2013;131(3):271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaltenbach K, Holbrook AM, Coyle MG, et al. Predicting treatment for neonatal abstinence syndrome in infants born to women maintained on opioid agonist medication. Addiction. 2012;107(suppl 1):45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrari A, Coccia CP, Bertolini A, Sternieri E. Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res. 2004;50(6):551-559. [DOI] [PubMed] [Google Scholar]
- 17.Ward RM, Drover DR, Hammer GB, et al. The pharmacokinetics of methadone and its metabolites in neonates, infants, and children. Paediatr Anaesth. 2014;24(6):591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall ES, Meinzen-Derr J, Wexelblatt SL. Cohort analysis of a pharmacokinetic-modeled methadone weaning optimization for neonatal abstinence syndrome. J Pediatr. 2015;167(6):1221-5.e1. [DOI] [PubMed] [Google Scholar]
- 19.Wiles JR, Isemann B, Mizuno T, et al. Pharmacokinetics of oral methadone in the treatment of neonatal abstinence syndrome: a pilot study. J Pediatr . 2015;167(6):1214-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surran B, Visintainer P, Chamberlain S, Kopcza K, Shah B, Singh R. Efficacy of clonidine versus phenobarbital in reducing neonatal morphine sulfate therapy days for neonatal abstinence syndrome: a prospective randomized clinical trial. J Perinatol. 2013;33(12):954-959. [DOI] [PubMed] [Google Scholar]
- 21.Kraft WK, Adeniyi-Jones SC, Chervoneva I, et al. Buprenorphine for the treatment of the neonatal abstinence syndrome. N Engl J Med. 2017;376(24):2341-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckley LA, Salunke S, Thompson K, Baer G, Fegley D, Turner MA. Challenges and strategies to facilitate formulation development of pediatric drug products: Safety qualification of excipients. Int J Pharm. 2018;536(2):563-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corr TE, Hollenbeak CS. The economic burden of neonatal abstinence syndrome in the United States. Addiction. 2017;112(9):1590-1599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Statistical Analysis
eTable 1. Phenobarbital Dosing
eTable 2. Sensitivity analyses omitting the four infants exposed to maternal prescription opioids for chronic pain
eTable 3. Per Protocol Analysis: Primary and Secondary Outcomes
eTable 4. Comparison to Non-randomized Infants: Primary and Secondary Outcomes