Key Points
Question
Is B-cell–depleting therapy more efficacious than calcineurin inhibition in maintaining relapse-free survival in children with corticosteroid-dependent nephrotic syndrome?
Findings
In this randomized clinical trial that included 120 children with corticosteroid-dependent nephrotic syndrome, a single course of rituximab therapy was associated with a significantly higher 12-month relapse-free survival rate than daily tacrolimus therapy (90.0% vs 63.3%) during 12 months of follow-up. The mean cumulative corticosteroid dose during the 12-month study period was lower with rituximab compared with tacrolimus (25.8 vs 86.3 mg/kg).
Meaning
In children with corticosteroid-dependent nephrotic syndrome, rituximab is more effective than tacrolimus in maintaining disease remission and may be considered as first-line corticosteroid-sparing therapy.
Abstract
Importance
Calcineurin inhibitors are an established first-line corticosteroid-sparing therapy for patients with corticosteroid-dependent nephrotic syndrome (CDNS), whereas B-lymphocyte–depleting therapy is mostly used as a rescue for calcineurin inhibitor–resistant cases. The positive efficacy and safety profile of rituximab raises the question of whether it could be used as a first-line alternative to calcineurin inhibitor therapy.
Objective
To compare the efficacy of rituximab and tacrolimus in maintaining relapse-free survival among children with CDNS.
Design, Setting, and Participants
A parallel-arm, open-label, randomized clinical trial was performed from May 8, 2015, to September 20, 2016, with 1-year follow-up in a single-center, tertiary care unit. A total of 176 consecutive children aged 3 to 16 years with CDNS not previously treated with corticosteroid-sparing agents were screened for eligibility.
Interventions
The children received either tacrolimus (along with tapering alternate-day prednisolone) for 12 months or a single course of rituximab (2 infusions of 375 mg/m2).
Main Outcomes and Measures
Twelve-month relapse-free survival in the intention-to-treat population.
Results
Of the 176 children screened for eligibility, 120 were randomized and all but 3 patients completed 1 year of follow-up. The groups were comparable, with mean (SD) age of 7.2 (2.8) years, 32 boys (53.3%) in each group, mean (SD) disease duration of 2.5 (1.5) years and 2.3 (1.7) in the tacrolimus and rituximab groups, respectively, disease duration less than 1 year among 15 children (25.0%) in each group, median (interquartile range) of 4 (3-5) relapses in each group, and mean (SD) cumulative prednisolone dose of 246 (48) mg/kg and 239 (52) mg/kg in the prestudy year in the tacrolimus and rituximab groups, respectively. Rituximab therapy was associated with a higher 12-month relapse-free survival rate than tacrolimus (54 [90.0%] vs 38 [63.3%] children; P < .001; odds ratio, 5.21; 95% CI, 1.93-14.07). Among the patients who experienced relapse, median time to first relapse was 40 weeks in the rituximab group and 29 weeks in the tacrolimus group. Only 2 patients in the rituximab group had more than 1 relapse during the study period compared with 10 patients in the tacrolimus group. The cumulative corticosteroid dose during the 12-month study period was lower with rituximab compared with tacrolimus (mean [SD], 25.8 [27.8] vs 86.3 [58.0] mg/kg). Although both treatments were well tolerated, mild to moderate infections were twice as common in the tacrolimus group (26 [43.3%] vs 13 [21.7%] events).
Conclusions and Relevance
In children with CDNS, rituximab appears to be more effective than tacrolimus in maintaining disease remission and minimizing corticosteroid exposure and, given its good tolerability and lack of nephrotoxic effects, may be considered as first-line corticosteroid-sparing therapy.
Trial Registration
ClinicalTrials.gov Identifier: NCT02438982; Clinical Trial Registry of India: CTRI/2014/01/004355
This randomized clinical trial compares the effect of rituximab vs tacrolimus therapy on the use of corticosteroids in children with corticosteroid-dependent nephrotic syndrome.
Introduction
Idiopathic nephrotic syndrome is the most common disorder of glomerular function in children. Most children respond well to glucocorticoid therapy; however, as many as 40% develop a complicated course resulting in frequent relapses or corticosteroid-dependent nephrotic syndrome (CDNS).1 The adverse effects of chronic glucocorticoid treatment have prompted the use of corticosteroid-sparing immunosuppressive therapies in patients with CDNS. Calcineurin inhibitors (CNIs) are presently the preferred corticosteroid-sparing drug class. However, the efficacy of CNIs varies depending on patient characteristics.2 Moreover, CNIs are potentially nephrotoxic, neurotoxic, and diabetogenic and require regular therapeutic drug monitoring.2,3,4 In patients with CDNS who experience tacrolimus intolerance or insufficient responsiveness, rituximab, a B-lymphocyte–depleting monoclonal antibody, has been demonstrated to be a valid therapeutic alternative.5,6,7 A single course of rituximab reliably retains disease remission for 6 to 12 months and the adverse effect profile observed to date is benign.5,6,7,8,9,10,11 The successful use of rituximab as second-line corticosteroid-sparing therapy has raised the question whether and when anti-B–cell therapy should be considered for first-line use to minimize corticosteroid exposure and avoid CNI toxic effects. To provide an evidence base to this discussion, we performed a randomized clinical trial comparing a single course of rituximab with standard tacrolimus maintenance therapy during a 1-year period in children with CDNS. The high incidence of childhood idiopathic nephrotic syndrome in India12 and the large catchment area of Nilratan Sircar Medical College and Hospital, with more than 1000 new children with nephrosis attending each year, permitted us to conduct the trial efficiently in a single-center effort.
Methods
Study Design
Rituximab for Relapse Prevention in Nephrotic Syndrome (RITURNS) was a prospective, single-center, open-label, 2-parallel-arm, phase 3 randomized clinical trial to test the efficacy of single-course rituximab compared with maintenance tacrolimus to maintain relapse-free survival at the end of 1 year among children with CDNS.
The study protocol (available in Supplement 1) was approved by the institutional review board of Nilratan Sircar Medical College and Hospital in December 2013, and the drug regulatory authority of India in April 2015. The institutional review board was granted continuous access to the trial data and oversaw the safety of the study patients. Informed written and audiovisual consent were obtained from the parents (and assent of patients older than 7 years) after provision of detailed oral and written information concerning the context of the study, potential benefit to the child, and comprehensive safety aspects. There was no financial compensation. Study progress was reported regularly both to the institutional review board and the National Drug Regulatory of India. An independent data safety monitoring board reviewed the study regularly.
Study Patients
All children between ages 3 and 16 years with CDNS attending the study center were consecutively screened for eligibility and, if considered eligible, invited to participate in the study. Standard definitions were used for nephrotic syndrome, remission, and relapses (eTable 1 in Supplement 2). Inclusion requirements comprised, among others, an estimated glomerular filtration rate13 (eGFR) greater than 80 mL/min/1.73 m2, current proteinuria remission, no previous exposure to a corticosteroid-sparing agent, and exclusion of a secondary form of nephrotic syndrome and active infection. All patients had undergone kidney biopsy with light and immunofluorescence microscopy within 3 months prior to enrollment. There were no changes in protocol after trial commencement. Detailed definitions and inclusion and exclusion criteria are provided in the trial protocol (Supplement 1).
Randomization and Masking
The children were randomly allocated in concealed fashion to receive either rituximab or tacrolimus along with alternate-day prednisolone over a 12-month period. Randomization was performed 1:1 using a web-based tool including stratified block randomization with varying block sizes and sex, age (≤7 vs >7 years), and renal histologic characteristics (minimal change disease vs focal segmental glomerulosclerosis) as stratification factors.14 The trial was open-label, with no masking of patients or study staff to treatment allocation.
Study Intervention
Following randomization, children received either oral tacrolimus for 12 months or a single course of rituximab infusions. In the tacrolimus arm, children received tacrolimus, 0.2 mg/kg/d, targeting trough levels of 5 to 7 ng/mL along with tapering doses of alternate-day prednisolone. The duration of tapering varied depending on the prednisolone dose at study entry; however, prednisolone was discontinued in all patients within 6 months of relapse-free survival. In the rituximab arm, children were scheduled to receive 2 to 4 rituximab infusions at weekly intervals (375 mg/m2, maximum dose, 500 mg) depending on the circulating B-cell count along with alternate-day prednisolone for 4 weeks (the protocol [Supplement 1] includes details). Owing to adequate B-cell depletion observed in all patients after the second dose, all patients received 2 infusions of rituximab. Patients who experienced 2 or more or 4 or more relapses within 6 or 12 months, respectively, were considered as treatment failures and shifted to the other experimental therapy as per center practice.
Study Procedures
Within 1 week after randomization, children received the first dose of their assigned drug. Data regarding the number of relapses, adverse effects, cumulative corticosteroid dose, circulating B-cell count (number per cubic millimeter) measured via flow cytometry, tacrolimus trough serum level, and hematologic and biochemical test results were noted during regular study visits and, if necessary, relapse.
Study medication was distributed at every visit and families were asked to return the used vials for pill counting. Medication intake and proteinuria dipstick results were recorded daily in a patient diary. Home proteinuria monitoring was performed using urine dipstick analysis. Urine protein-creatinine ratio was performed during each scheduled visit and at relapse.
Relapse was identified through urine dipstick and confirmed by urine protein-creatinine ratio. Relapse was defined by 3+ or 4+ results on albuminuria dipstick testing for 3 consecutive early-morning specimens, and remission was defined by urine albumin nil or trace results for 3 consecutive early-morning specimens (eTable 1 in Supplement 2). Circulating B-cell count was measured at the time of enrollment; at the end of weeks 2, 4, 12, 26, 39, and 52; and at the time of any relapse. Retrospective data from the previous year regarding relapses and cumulative corticosteroid dose were also collected.
Study Outcomes
The primary end point was the 12-month, relapse-free survival rate. Prespecified key secondary end points were the frequency of relapses, time to first relapse, cumulative prednisolone dosage (milligrams per kilogram per year), changes in serum biochemistry, peripheral blood B-cell count, the number of children not receiving corticosteroids, and the rates of adverse events. Adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 3.15
Statistical Analysis
Based on previous study findings, 50% of patients in the tacrolimus arm and 80% of those in the rituximab arm were assumed to remain relapse free during the 12-month observation period.16,17 Sixty patients per arm were required to demonstrate superiority at 90% power and 5% 2-sided, type I error rate, including an expected 20% dropout rate.
The primary end point was analyzed using the χ2 test based on the intention-to-treat set. For 1 patient with missing primary end point information, the most common value in the respective group was imputed.18 As a sensitivity analysis, the primary end point was also analyzed based on the per-protocol set.
A logistic regression model was used to evaluate the influence of treatment on the odds of having at least 1 relapse within the 12-month observation period, taking into account the stratification variables (sex, age, renal histopathologic status) and adjusting for disease duration, which is known to influence the risk of relapse. Kaplan-Meier curves were used to visualize time to first relapse and graphically compare the treatment groups. In addition, time to first relapse was analyzed using a Cox proportional hazards regression model including the same variables. All secondary end points were analyzed based on the intention-to-treat set without imputation. The B-cell counts over time were visualized for patients who experienced at least 1 relapse vs patients without a relapse. For analysis of safety end points, all patients who were treated for at least 1 day were included.
Data are expressed as absolute number (percentage), median (25th quantile; 75th quantile), mean (SD), mean difference, odds ratios (ORs) and hazard ratio with 95% CIs as appropriate. Except for the primary end point, all further analyses are of descriptive values. Significance level was set at P = .05 with 2-tailed, unpaired testing. Analyses were performed with the use of SAS software, version 9.4 (SAS Institute).
Results
Patient Population
A total of 176 patients were screened between May 8 and August 10, 2015, of whom 120 were randomized; the study end date was September 20, 2016. Many patients in our region do not have access to steroid-sparing drugs such as mycophenolate mofetil (MMF), tacrolimus, or rituximab because of financial constraints and therefore face long-term steroid maintenance therapy. Following ethical approval of this study in December 2013, it took nearly 1.5 years to begin patient enrollment in May 2015, because of delay for acquiring approval from drug regulatory authority of India. Most of the patients during this period were waiting to be recruited in this trial to receive the expensive steroid-sparing agents free of cost, and, after opening the window of patient recruitment, most of the eligible patients were recruited quickly.
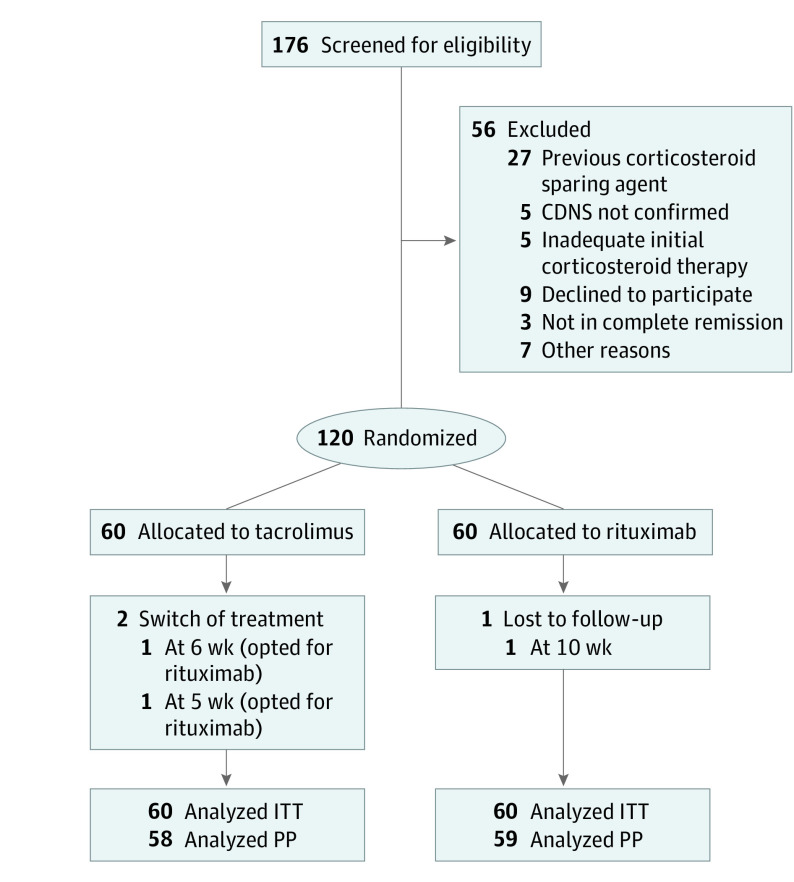
The baseline demographic and clinical characteristics were well balanced between the treatment groups (Table 1), and 91.7% (55 of a total of 60 in each group) of patients in each group reported adverse effects of corticosteroid treatment at the time of enrollment. The predominant histologic type in both groups was minimal-change nephrotic syndrome. Diary reviews and returned pill counts suggested good drug adherence in the tacrolimus group. Three patients were excluded from the per-protocol set owing to loss of follow-up or treatment switching (Figure 1).
Table 1. Baseline Demographic, Clinical, and Biologic Characteristics of the Patients According to Randomization Group in the Intention-to-Treat Populationa.
| Characteristic | Tacrolimus (n = 60) | Rituximab (n = 60) |
|---|---|---|
| Demographics | ||
| Male, No. (%) | 32 (53.3) | 32 (53.3) |
| Age, y | 7.2 (2.8) | 7.1 (2.8) |
| Anthropometry | ||
| Weight, kg | 27.8 (8.8) | 27.5 (8.6) |
| Height, cm | 115 (16) | 114 (15) |
| Height z score | −1.2 (0.6) | −1.4 (0.7) |
| BMI z score | 2.2 (0.9) | 2.2 (1.0) |
| Disease history | ||
| Duration of disease, y | 2.5 (1.5) | 2.3 (1.7) |
| Duration of disease <1 y, No. (%) | 15 (25.0) | 15 (25.0) |
| No. of relapse episodes per patient in prestudy year, median (IQR)b | 4 (3-5) | 4 (3-5) |
| Renal histologic status | ||
| Minimal change glomerulopathy, No. (%) | 42 (70.0) | 43 (71.7) |
| Focal segmental glomerulosclerosis, No. (%) | 18 (30.0) | 17 (28.3) |
| Prednisolone therapy | ||
| Cumulative prednisolone dose in prestudy year, mg/kg/y | 246 (48) | 239 (52) |
| Current prednisolone dose, mg/kg/d | 1.3 (0.2) | 1.3 (0.2) |
| Serum biochemistry | ||
| Albumin, g/dL | 4.34 (0.81) | 4.18 (0.73) |
| Cholesterol, mg/dL | 115 (24) | 109 (23) |
| eGFR, mL/min/1.73 m2 | 103.0 (10.8) | 100.2 (8.6) |
| Patients with hypertension, No. (%) | 24 (40.0) | 20 (33.3) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; IQR, interquartile range.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; cholesterol to millimoles per liter, multiply by 0.0259.
Unless otherwise indicated, values are given as mean (SD).
Median (25th quantile-75th quantile).
Figure 1. Trial Flowchart.
CDNS indicates corticosteroid-dependent nephrotic syndrome; ITT, intention-to-treat; and PP, per protocol.
Primary Outcome
The 12-month relapse-free survival rate in the intention-to-treat set was significantly higher with rituximab compared with tacrolimus (54 [90.0%] vs 38 [63.3%]; P < .001; OR, 5.21; 95% CI, 1.93-14.07). The sensitivity analyses confirmed the results of the primary analysis. Logistic regression analysis showed an adjusted 88% relative risk reduction in the odds of relapse associated with the use of rituximab vs tacrolimus (eTable 2 in Supplement 2).
Secondary Outcomes
Time to First Relapse
Among the relapsing patients, the median time to first relapse was 40 weeks in the rituximab group and 29 weeks in the tacrolimus group. Figure 2 illustrates the differences in time to first relapse between the groups.
Figure 2. Probability of Relapse-Free Survival According to Treatment Group.
The 12-month relapse-free survival rate was significantly higher with rituximab compared with tacrolimus (log rank P < .001).
The relative risk of developing a relapse was 5 times higher in the tacrolimus group compared with the rituximab group in the Cox proportional hazards regression model (eTable 2 in Supplement 2). The additive effect of treatment and histopathologic diagnosis are illustrated in the eFigure in Supplement 2.
Number of Relapses
The overall relapse rate declined in both treatment groups relative to the prestudy year by a median of 3 relapses in the tacrolimus group and 4 relapses in the rituximab group. Only 2 patients in the rituximab group had more than 1 relapse during the study period compared with 10 patients in the tacrolimus group. Treatment failure was reported in 2 patients (3.3%) in the rituximab group compared with 6 patients (10.0%) in the tacrolimus group. The detailed trial results regarding relapses during the study period are given in Table 2.
Table 2. Primary and Secondary Study End Points According to Treatment Group in the Intention-to-Treat Populationa.
| End Point | Tacrolimus | Rituximab | Between-Group Difference (95% CI)b |
|---|---|---|---|
| Primary, No. | 60 | 60 | |
| Patients with sustained remission (0-12 mo), No. (%) | 38/60 (63.3) | 54/60 (90.0) | 5.21 (1.93 to 14.07) |
| Secondary, No. | 58 | 59 | |
| Frequency of patients with relapse (0-12 mo), No. (%) | |||
| 0 Relapse | 37 (63.8) | 53 (89.8) | |
| 1 Relapse | 11 (19.0) | 4 (6.8) | |
| 2 Relapses | 6 (10.3) | 2 (3.4) | |
| 3 Relapses | 4 (7.0) | 0 | |
| Change in relapse rate from prestudy year, median (IQR)c | −3 (−4 to −2) | −4 (−4 to −3) | |
| Patients with sustained remission (0-6 mo), No. (%) | 48/58 (82.8) | 59/59 (100) | |
| Patients with treatment failure, No. (%) | 6/60 (10.0) | 2/60 (3.3) | 0.31 (0.06 to 1.60) |
| Serum albumin at month 12, g/dL | 4.87 (0.78) | 5.63 (0.99) | 0.76 (0.43 to 1.09) |
| 12-mo Change in serum albumin, g/dL | 0.54 (0.96) | 1.47 (0.49) | 0.93 (0.65 to 1.21) |
| Serum cholesterol at month 12, mg/dL | 98.4 (16.1) | 79.6 (22.1) | −18.8 (−25.8 to −11.7) |
| 12-mo Change in serum cholesterol, mg/dL | −16.8 (19.8) | −29.6 (17.8) | −12.8 (−19.7 to −5.9) |
| eGFR at month 12, mL/min/1.73 m2 | 111.8 (11) | 118.4 (11) | 6.6 (2.5 to 10.7) |
| 12-mo eGFR change, mL/min/1.73 m2 | 8.4 (6.9) | 18.2 (8.3) | 9.8 (7.0 to 12.6) |
| Cumulative prednisolone dose in study year, mg/kg | 86.3 (58.0) | 25.8 (27.8) | −60.5 (−77.1 to −43.9) |
| Change in cumulative prednisolone dose from prestudy year, mg/kg | −161 (68) | −213 (49) | −52.5 (−74.2 to −30.8) |
| Prednisolone dose at month 12, mg/kg/d | 0.62 (1.33) | 0.19 (0.76) | −0.43 (−0.82 to 0.03) |
| 12-mo Change in prednisolone dose, mg/kg/d | −0.70 (1.32) | −1.12 (0.74) | −0.42 (−0.81 to 0.03) |
| Children not receiving corticosteroids at month 12, No. (%) | 46/58 (79.3) | 55/59 (93.2) | 0.28 (0.08 to 0.92)d |
| Height z score at month 12 | −1.20 (0.48) | −1.00 (0.63) | 0.19 (−0.01 to 0.40) |
| 12-mo Absolute change in height z score | 0.07 (0.08) | 0.42 (0.13) | 0.35 (0.31 to 0.39) |
| BMI z score at month 12 | 1.66 (0.76) | 1.63 (0.79) | −0.03 (−0.31 to 0.26) |
| 12-mo Absolute change in BMI z score | −0.51 (0.18) | −0.61 (0.30) | −0.10 (−0.19 to 0.01) |
| Patients with hypertension at month 12, No. (%) | 3/58 (5.2) | 2/59 (3.4) | 0.64 (0.10 to 4.00)d |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); eGFR, estimated glomerular filtration rate; IQR, interquartile range.
SI conversion factors: To convert albumin to grams per liter, multiply by 10; cholesterol to millimoles per liter, multiply by 0.0259.
Unless otherwise indicated, values are given as mean (SD).
The between-group difference is given as mean difference or odds ratio and corresponding 95% CI with the tacrolimus group being the reference.
Median (25th quantile-75th quantile).
Odds ratio.
Biochemical Measures
The children receiving rituximab exhibited higher serum albumin and lower serum cholesterol levels than the tacrolimus-treated patients at 12 months (mean difference, 0.76; 95% CI, 0.43-1.09, and −18.8; 95% CI, −25.8 to −11.7). The eGFR increased from baseline to the 12-month visit in both treatment arms; however, the final eGFR was higher in the rituximab group (mean difference, 6.6; 95% CI, 2.5-10.7).
Cumulative Prednisolone Dose
Whereas both tacrolimus and rituximab treatment resulted in a significant decrease in the cumulative dose of prednisolone from the prestudy year, the cumulative corticosteroid dose administered during the 12-month study period was lower in the rituximab group compared with the tacrolimus group (25.8 [27.8] vs 86.3 [58.0] mg/kg) (Table 2). At 12 months, 55 of 59 children (93.2%) of the rituximab group compared with 46 of 58 (79.3%) in the tacrolimus group were not receiving corticosteroids.
Anthropometry
While the height-for-age z score increased and body mass index BMI z score decreased over 1 year in both treatment arms compared with baseline, the absolute changes were greater with rituximab (mean difference, 0.35; 95% CI, 0.31-0.39 for height z score and −0.10; 95% CI, −0.19 to 0.01 for body mass index z score). Two patients in the rituximab group and 3 in the tacrolimus group had hypertension.
Therapeutic Monitoring
In the tacrolimus group, mean (SD) trough tacrolimus serum levels at day 14 were 6.0 (0.5) μg/L. Among the patients who developed relapses, the mean (SD) level at the time of the first relapse was 5.9 (0.4) μg/L.
In the rituximab group, the peripheral blood B-cell count decreased to less than 5/mm3 in all patients following 2 doses of rituximab and no patient required further doses (Figure 3). B-cell counts had recovered to the reference range for age in 11.9% (7 of 59), 39.0% (23 of 59), and 93.2% (55 of 59) of patients at 6, 9, and 12 months, respectively. Starting at 4 weeks and persisting for 9 months after treatment, the 6 patients who subsequently developed relapses displayed higher B-cell counts than the 54 who did not relapse. All relapses occurred after full B-cell recovery.
Figure 3. Course of Circulating B-Cell Counts in the Rituximab Group .
Data are expressed on a logarithmic scale as median and interquartile range.
Adverse Events
A total of 268 adverse events were recorded: 145 in the tacrolimus arm and 123 in the rituximab arm (eTable 3 in Supplement 2). Grade 2 events were observed more often in the tacrolimus arm (51 vs 24 events). The difference was mainly accounted for by infections, which occurred twice as often in the tacrolimus than rituximab group (26 [43.3%] vs 13 [21.7%] grade 2/3 infectious events). There were a total of 23 transfusion reactions with rituximab, most of which were mild and transient (grade 1 events), and none required hospitalization. No fatality or serious adverse events occurred in either study arm.
Discussion
To our knowledge, this study is one of the largest randomized clinical trials of corticosteroid-sparing agents performed to date in children with CDNS and represents the first prospective comparison of tacrolimus and rituximab therapy in this condition. We demonstrate a significant and clinically relevant reduction of relapse rates by primary use of rituximab compared with standard CNI therapy. The cumulative relapse risk at 12 months was reduced by 80% in the rituximab group (adjusted for age, sex, renal histopathologic status, and previous disease duration). The difference in the primary end point went along with reduced corticosteroid exposure, better catch-up growth, and higher eGFR in the rituximab-treated children.
In concordance with previous observations, both treatment protocols effectively reduced relapse rates and allowed efficient corticosteroid sparing. The 63% 12-month relapse-free survival rate observed with tacrolimus was comparable to that of previous studies.16,19 In the rituximab arm, no relapse episodes occurred within the first 6 months and relapse-free survival was 90% at 12 months. Somewhat lower 12-month remission rates (40%-80%) were observed in previous trials, possibly owing to the fact that rituximab was usually administered as a last therapeutic option in patients with high disease activity who had not responded to other steroid-sparing therapies.6,7,11,20,21 Also, whereas most previous rituximab studies adopted a single-dose protocol, 2 infusions were administered in this trial, potentially leading to more-persistent B-cell depletion.6,7,9,22,23
In addition to the direct medical and psychological benefit of the reduced relapse incidence, several clinical benefits attributable to more efficient corticosteroid sparing were observed in the rituximab group. These benefits included significant catch-up growth, a slightly more marked reduction of overweight, and 20% lower serum cholesterol levels at the end of the study period, confirming findings of a previous uncontrolled trial in a limited number of adults and children with corticosteroid-dependent or frequently relapsing nephrotic syndrome.9 Furthermore, eGFR was significantly higher in the rituximab group, most likely because of the absence of CNI-associated renal vasoconstriction and/or chronic nephrotoxicity.24,25
We confirmed several important relapse risk factors, which were independent of the pharmacologic therapy applied. Young children and those with longer previous disease duration were more prone to relapse during corticosteroid-sparing treatment, likely reflecting their greater underlying disease activity.9 Likewise, although both drugs were confirmed to be effective in reducing relapse frequency across histopathologic entities, patients with focal segmental glomerulosclerosis retained a higher relative relapse risk than those with minimal change in disease in both treatment arms.9,26 Inferior disease control by corticosteroid-sparing therapies in focal segmental glomerulosclerosis, including rituximab, has previously been reported.27
Therapeutic drug monitoring revealed that, although there was no evidence for an association of relapse risk and tacrolimus trough blood levels, the patients who relapsed 6 to 12 months after rituximab administration displayed significantly earlier B-cell recovery, with cell count differences emerging as early as 4 weeks post dosing. Future research should explore whether and how the duration of disease remission can be optimized by individualized, B-cell count–guided rituximab administration.
Treatment choices in CDNS are not only driven by efficacy but also by drug tolerability and safety considerations. Our head-to-head comparison showed, apart from a minimally compromised eGFR and common mild infusion reactions to rituximab, excellent tolerability of both treatment protocols. Mild intercurrent infections appeared to occur more frequently in the tacrolimus group. None of the serious adverse events anecdotally reported after rituximab exposure, such as progressive multifocal leukoencephalopathy,28 pulmonary fibrosis, Pneumocystis jiroveci pneumonia, fulminant myocarditis, and ulcerative colitis, were observed in the present study; however, sample size and duration of therapy were underpowered to estimate the true incidence of such rare complications.
A further relevant aspect to consider regarding the choice of corticosteroid-sparing therapy in childhood CDNS is treatment adherence, which is often suboptimal with chronic CNI and glucocorticoid therapy. The reliable achievement of disease remission by a single course of intravenous therapy without the need for maintenance oral drug intake is a major practical argument in favor of rituximab to many families. Furthermore, the cumulative cost of treatment is an issue, particularly in developing countries. Within the 12-month time frame considered in this study, the cost of 2 doses of rituximab was up to 20% less than the cumulative expense for even the generic tacrolimus preparation used in India.
Limitations
We recognize several limitations to our study. The intrinsic difference in efficacy of tacrolimus and rituximab may have been somewhat underestimated by study design owing to the longer corticosteroid coadministration in the tacrolimus arm. However, we cannot exclude temporary study drug nonadherence in the tacrolimus arm, although patient diaries, returned pill counts, and tacrolimus blood levels at time of relapse suggested good treatment adherence. In addition, the 12 months’ exposure to the study drugs did not permit a valid assessment of the long-term safety of both drugs in children with nephrotic syndrome. Furthermore, the efficacy and safety of the drugs may vary depending on patient ethnicity and geographic location; thus, the findings of this single-center trial in India may not be readily generalizable to other ethnic groups and regions. Furthermore, changes in quality of life with the 2 treatment protocols were not assessed systematically. Finally, our trial was designed to compare the corticosteroid-sparing potential of monotherapies. It is possible that better long-term remission at acceptable adverse effect rates would have been achieved and the difference in efficacy would have been attenuated by combining different corticosteroid-sparing agents. Future research will need to explore the usefulness of such combined therapies, which may include the sequential use of rituximab and low-toxicity immunomodulatory agents, such as mycophenolate mofetil,29,30 to avoid the long-term risks of repetitive B-cell depletion, such as opportunistic infections, autoimmune pathologic effects, and formation of neutralizing antibodies.31
Conclusions
The findings of the study indicate that rituximab is more effective than tacrolimus over a 12-month period in maintaining disease remission and minimizing corticosteroid exposure. Given its good tolerability and lack of nephrotoxic effects, rituximab may be considered as first-line corticosteroid-sparing therapy in children with CDNS.
RITURNS Protocol version 1.2 (Final Version)
eTable 1. Definitions of Categories of NS, Relapse, Remission
eTable 2. Multivariable Logistic Regression Analysis of Having at Least One Relapse Within 12 Months and Cox Regression Analysis of Time to First Relapse
eTable 3. Adverse Events by Treatment Group
eFigure. Probability of Relapse-Free Survival According to Treatment and Histopathological Diagnosis
References
- 1.Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children. Cochrane Database Syst Rev. 2013;10(10):CD002290. [DOI] [PubMed] [Google Scholar]
- 2.Lombel RM, Gipson DS, Hodson EM; Kidney Disease: Improving Global Outcomes . Treatment of steroid-sensitive nephrotic syndrome: new guidelines from KDIGO. Pediatr Nephrol. 2013;28(3):415-426. [DOI] [PubMed] [Google Scholar]
- 3.Morgan C, Sis B, Pinsk M, Yiu V. Renal interstitial fibrosis in children treated with FK506 for nephrotic syndrome. Nephrol Dial Transplant. 2011;26(9):2860-2865. [DOI] [PubMed] [Google Scholar]
- 4.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4(4):583-595. [DOI] [PubMed] [Google Scholar]
- 5.Iijima K, Sako M, Nozu K. Rituximab for nephrotic syndrome in children. Clin Exp Nephrol. 2017;21(2):193-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamei K, Ito S, Nozu K, et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol. 2009;24(7):1321-1328. [DOI] [PubMed] [Google Scholar]
- 7.Kemper MJ, Gellermann J, Habbig S, et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol Dial Transplant. 2012;27(5):1910-1915. [DOI] [PubMed] [Google Scholar]
- 8.Kamei K, Ogura M, Sato M, Sako M, Iijima K, Ito S. Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol. 2016;31(1):89-95. [DOI] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Ruggiero B, Cravedi P, et al. ; Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease or Focal Segmental Glomerulosclerosis (NEMO) Study Group . Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol. 2014;25(4):850-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iijima K, Sako M, Nozu K, et al. ; Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group . Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273-1281. [DOI] [PubMed] [Google Scholar]
- 11.Ravani P, Ponticelli A, Siciliano C, et al. Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int. 2013;84(5):1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banh TH, Hussain-Shamsy N, Patel V, et al. Ethnic differences in incidence and outcome of childhood nephrotic syndrome. Clin J Am Soc Nephrol. 2016;11(10):1760-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Muñoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randomisation and Online Databases for Clinical Trials. http://www.sealedenvelope.com. Accessed May 2, 2015.
- 15.Common Terminology Criteria for Adverse Events; v3.0 (CTCAE). https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Published August 9, 2006. Accessed November 9, 2013.
- 16.Wang W, Xia Y, Mao J, et al. Treatment of tacrolimus or cyclosporine A in children with idiopathic nephrotic syndrome. Pediatr Nephrol. 2012;27(11):2073-2079. [DOI] [PubMed] [Google Scholar]
- 17.Gulati A, Sinha A, Jordan SC, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol. 2010;5(12):2207-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clin Trials. 2008;5(3):225-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang EM, Lee ST, Choi HJ, et al. Tacrolimus for children with refractory nephrotic syndrome: a one-year prospective, multicenter, and open-label study of Tacrobell, a generic formula. World J Pediatr. 2016;12(1):60-65. [DOI] [PubMed] [Google Scholar]
- 20.Kamei K, Okada M, Sato M, et al. Rituximab treatment combined with methylprednisolone pulse therapy and immunosuppressants for childhood steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2014;29(7):1181-1187. [DOI] [PubMed] [Google Scholar]
- 21.Guigonis V, Dallocchio A, Baudouin V, et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23(8):1269-1279. [DOI] [PubMed] [Google Scholar]
- 22.Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1308-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravani P, Rossi R, Bonanni A, et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. 2015;26(9):2259-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4(2):481-508. [DOI] [PubMed] [Google Scholar]
- 25.Gellermann J, Weber L, Pape L, Tönshoff B, Hoyer P, Querfeld U; Gesellschaft für Pädiatrische Nephrologie (GPN) . Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome. J Am Soc Nephrol. 2013;24(10):1689-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha A, Bagga A. Rituximab therapy in nephrotic syndrome: implications for patients’ management. Nat Rev Nephrol. 2013;9(3):154-169. [DOI] [PubMed] [Google Scholar]
- 27.Sinha A, Bhatia D, Gulati A, et al. Efficacy and safety of rituximab in children with difficult-to-treat nephrotic syndrome. Nephrol Dial Transplant. 2015;30(1):96-106. [DOI] [PubMed] [Google Scholar]
- 28.Boren EJ, Cheema GS, Naguwa SM, Ansari AA, Gershwin ME. The emergence of progressive multifocal leukoencephalopathy (PML) in rheumatic diseases. J Autoimmun. 2008;30(1-2):90-98. [DOI] [PubMed] [Google Scholar]
- 29.Basu B, Mahapatra TK, Mondal N. Mycophenolate mofetil following rituximab in children with steroid-resistant nephrotic syndrome. Pediatrics. 2015;136(1):e132-e139. [DOI] [PubMed] [Google Scholar]
- 30.Filler G, Huang SH, Sharma AP. Should we consider MMF therapy after rituximab for nephrotic syndrome? Pediatr Nephrol. 2011;26(10):1759-1762. [DOI] [PubMed] [Google Scholar]
- 31.Ahn YH, Kang HG, Lee JM, Choi HJ, Ha IS, Cheong HI. Development of antirituximab antibodies in children with nephrotic syndrome. Pediatr Nephrol. 2014;29(8):1461-1464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RITURNS Protocol version 1.2 (Final Version)
eTable 1. Definitions of Categories of NS, Relapse, Remission
eTable 2. Multivariable Logistic Regression Analysis of Having at Least One Relapse Within 12 Months and Cox Regression Analysis of Time to First Relapse
eTable 3. Adverse Events by Treatment Group
eFigure. Probability of Relapse-Free Survival According to Treatment and Histopathological Diagnosis