Key Points
Question
What is the disease burden and functional effect of chemotherapy-induced peripheral neuropathy in childhood cancer survivors?
Findings
In this cross-sectional study including 121 childhood cancer survivors, long-term deficits in clinical, electrophysiological, and functional measures of peripheral neuropathy were common, and concurrent deficits in patient-reported outcome measures suggest a significant effect. Cisplatin has a greater neurotoxicity profile than vinca alkaloids.
Meaning
Both the type of neurotoxic agent used and a targeted clinical neurological assessment are important considerations when screening childhood cancer survivors for long-term neuropathy.
This cross-sectional study comprehensively assesses chemotherapy-induced peripheral neuropathy in childhood cancer survivors to define disease burden and functional effect and aims to inform screening recommendations.
Abstract
Importance
In light of the excellent long-term survival of childhood cancer patients, it is imperative to screen for factors affecting health, function, and quality of life in long-term survivors.
Objective
To comprehensively assess chemotherapy-induced peripheral neuropathy in childhood cancer survivors to define disease burden and functional effect and to inform screening recommendations.
Design, Setting, and Participants
In this cross-sectional observational study, cancer survivors who were treated with chemotherapy for extracranial malignancy before age 17 years were recruited consecutively between April 2015 and December 2016 from a single tertiary hospital-based comprehensive cancer survivorship clinic and compared with healthy age-matched controls. Investigators were blinded to the type of chemotherapy. A total of 169 patients met inclusion criteria, of whom 48 (28.4%) were unable to be contacted or declined participation.
Exposures
Chemotherapy agents known to be toxic to peripheral nerves.
Main Outcomes and Measures
The clinical peripheral neurological assessment using the Total Neuropathy Score was compared between recipients of different neurotoxic chemotherapy agents and control participants and was correlated with neurophysiological, functional, and patient-reported outcome measures.
Results
Of the 121 childhood cancer survivors included in this study, 65 (53.7%) were male, and the cohort underwent neurotoxicity assessments at a median (range) age of 16 (7-47) years, a median (range) 8.5 (1.5-29) years after treatment completion. Vinca alkaloids and platinum compounds were the main neurotoxic agents. Clinical abnormalities consistent with peripheral neuropathy were common, seen in 53 of 100 participants (53.0%) treated with neurotoxic chemotherapy (mean Total Neuropathy Score increase, 2.1; 95% CI, 1.4-2.9; P < .001), and were associated with lower limb predominant sensory axonal neuropathy (mean amplitude reduction, 5.8 μV; 95% CI, 2.8-8.8; P < .001). Functional deficits were seen in manual dexterity, distal sensation, and balance. Patient-reported outcomes demonstrating reduction in global quality of life and physical functioning were associated with the Total Neuropathy Score. Cisplatin produced long-term neurotoxicity more frequently than vinca alkaloids.
Conclusions and Relevance
Clinical abnormalities attributable to peripheral neuropathy were common in childhood cancer survivors and persisted long term, with concurrent deficits in patient-reported outcomes. Both the type of neurotoxic agent and a targeted clinical neurological assessment are important considerations when screening survivors for long-term neuropathy. Further development of peripheral neuropathy–specific pediatric assessment tools will aid research into neuroprotective and rehabilitative strategies.
Introduction
The changing landscape of childhood cancer treatment over the last 6 decades has seen the improvement of childhood and adolescent cancer survival rates to 80% or greater for many cancer types.1,2 A child who survives 5 or more years after cancer diagnosis has a life span comparable with other persons of their age.3 In view of this excellent long-term survival, it is important to characterize the long-term morbidity from cancer treatment in the context of a significant burden of long-term health conditions in childhood cancer survivors (CCS).4,5,6
Chemotherapy-induced peripheral neuropathy (CIPN) is a potentially long-lasting adverse effect of commonly used chemotherapy agents in pediatric practice, principally vincristine and other vinca alkaloids, cisplatin, and carboplatin.7 To our knowledge, long-term outcomes of peripheral neuropathy from these agents in children remain to be fully delineated. Neuromuscular impairment and restricted participation in daily activities are observed in CCS,8,9,10 and persistent deficits in fundamental motor skills are demonstrable in children following completion of cancer treatment.11 Multiple factors, such as obesity, poor bone health, and psychosocial health, may be part of a complex interaction that limits physical activity, which in turn perpetuates and maintains long-term health conditions.4,5,12,13 Chemotherapy-induced peripheral neuropathy may be a key factor contributing to reduced physical performance, and defining its effect is an important consideration in the design of any interventional programs. While other studies have demonstrated physical limitations in CCS,8,9,14,15 this study aims to extend these findings by providing a comprehensive multimodality evaluation of CIPN in long-term survivors of extracranial childhood cancer across multiple cancer types to define the disease burden and its functional effect as well as to inform recommendations for screening in this population.
Methods
Childhood cancer survivors treated with chemotherapy for extracranial malignancy before the age of 17 years at the Kids Cancer Centre, Sydney Children’s Hospital, Sydney, Australia, were recruited consecutively between April 2015 and December 2016 from the long-term follow-up clinic. Patients with other causes of peripheral neuropathy, such as diabetes, history of critical illness neuropathy, known inherited neuropathic conditions, or other neurodevelopmental disorders, were excluded. The study was approved by the Sydney Children’s Hospitals Network Human Research Ethics Committee. Written informed consent was obtained from each participant or their parent/guardian in accordance with the Declaration of Helsinki.
A comprehensive neurotoxicity assessment was carried out with each participant. For participants younger than 17 years, this included the pediatric-modified Total Neuropathy Score (TNS) for clinical measures, the Movement Assessment Battery for Children (MABC) for functional measures, nerve conduction studies for neurophysiological measures, and the Pediatric Quality of Life Inventory Generic Core Scales (PedsQL) and the Pediatric Outcomes Data Collection Instrument (PODCI) for patient-reported outcome (PRO) measures. For participants 17 years or older, this included the TNS clinical version for clinical measures, Von Frey monofilaments, grating orientation task, and grooved peg board task for functional measures, nerve conduction studies for neurophysiological measures, and the European Organisation for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire and chemotherapy-induced peripheral neuropathy questionnaire (CIPN20) for PRO measures (eMethods and eTable 1 in the Supplement). Investigators were blinded to the type of chemotherapy.
Statistical analyses were conducted in consultation with a biostatistician using SPSS Statistics version 23 (IBM) and Prism version 7 (GraphPad). Comparisons used 2-tailed t tests and Mann-Whitney U tests for nonparametric subgroup analyses, χ2 tests for nominal data, 1-way analysis of variance for multiple groups, and Pearson or Spearman (small or ordinal data sets) correlations. Results are stated as means with standard deviations, and a P value less than .05 was considered significant. Bonferroni-corrected P values were used for correlations with multiple PROs.
Results were compared with healthy age-matched controls (age range, 6-56 years) or established population reference ranges for pediatric functional and PRO measures (eMethods and eTable 2 in the Supplement). In addition to group-level comparisons, individual item variance for each participant’s performance was calculated using the following formula: (control mean time − participant time) / control standard deviation. The 1-sample t test was used to compare the group variances with 0. The participant group mean was compared with the population mean and expressed as mean standard deviations above or below the population mean. Hierarchical linear regression models of neurophysiological and PRO measures with stepwise inclusion of predictive variables that may assist in screening CCS, including demographic, malignancy, and treatment variables, as well as concurrent clinical, neurophysiological, and functional outcomes were constructed to determine effect size (R2 increase) of each variable.
Results
A total of 169 patients met the inclusion criteria, of whom 48 (28.4%) were unable to be contacted or declined participation because of competing appointments or not wanting to participate in research. Neurotoxicity assessments were conducted in 121 CCS, of whom 65 (53.7%) were male, at a median (range) age of 16 (7.0-47.0) years, including 107 (88.4%) who received neurotoxic chemotherapy agents and 14 (11.6%) who received a protocol with no neurotoxic agents. Exposure to chemotherapy occurred a median (range) age of 4 (0-17.5) years, and the participants were tested a median (range) 8.5 (1.5-29.0) years after completion of their treatment (Table 1). Demographic characteristics are described for the entire cohort of 121 participants, but comparisons for all CIPN parameters were carried out between individual subgroups (ie, CCS who received neurotoxic chemotherapy vs controls or CCS who did not receive neurotoxic chemotherapy vs controls).
Table 1. Demographic Characteristics of Childhood Cancer Survivors.
| Characteristic | No. (%) (n = 121) |
|---|---|
| Current age, median (range), y | 16 (7.0-47.0) |
| Age at diagnosis, median (range), y | 4 (0-17.5) |
| Time since completion of treatment, median (range), y | 8.5 (1.5-29.0) |
| Body mass index, median (range)a | 20.6 (14.2-38.4) |
| Sex | |
| Male | 65 (53.7) |
| Female | 56 (46.3) |
| Type of malignancy | |
| Leukemia | 64 (52.9) |
| Lymphoma | 17 (14) |
| Solid tumor | 36 (29.8) |
| Other | 4 (3.3) |
| Type of chemotherapy | |
| Vinca alkaloid | 86 (71.1) |
| Platinum agent | 7 (5.8) |
| Both | 13 (10.7) |
| Thalidomide | 1 (0.8) |
| None | 14 (11.6) |
| No. of peripheral neurotoxic agents | |
| 1 | 88 (72.7) |
| ≥2 | 19 (15.7) |
| None | 14 (11.6) |
| Radiotherapyb | |
| Yes | 53 (43.8) |
| No | 68 (56.2) |
| Stem cell transplantation | |
| Yes | 31 (25.7) |
| No | 90 (74.4) |
| Dose reduction during treatment attributed to neuropathy | |
| Yes | 7 (5.8) |
| No | 113 (93.4) |
| Unknown | 1 (0.8) |
Body mass index calculated as weight in kilograms divided by height in meters squared.
Radiotherapy included cranial or craniospinal, extracranial involved-field, and total body irradiation.
Radiotherapy exposure was seen in 50 of 107 CCS (46.7%) who received neurotoxic chemotherapy; 12 (11.2%) had cranial or craniospinal irradiation, 25 (23.4%) had extracranial involved-field irradiation, 12 (11.2%) had total body irradiation, and 1 (0.9%) had both cranial and total body irradiation. Additionally, 21 CCS (19.6%) who received neurotoxic chemotherapy also received a bone marrow transplant. Twenty CCS (18.7%) had a body mass index (calculated as weight in kilograms divided by height in meters squared) in the overweight category (>25), and 4 (3.7%) were in the obese category (>30).
Vinca alkaloids were the most commonly used neurotoxic chemotherapy agent and were used in 86 of 121 CCS (71.1%), of whom 81 (66.9%) received vincristine as the sole neurotoxic agent and 5 (4.1%) received multiple vinca alkaloids. Platinum agents were prescribed to 20 CCS (16.5%), including 7 (5.8%) who received cisplatin, 7 (5.8%) who received carboplatin, and 6 (5.0%) who received cisplatin and carboplatin; 7 (5.8%) were prescribed platinum agents as the only neurotoxic therapy and 13 (10.7%) were prescribed platinum agents in combination with vinca alkaloids. Thalidomide was the sole neurotoxic agent in 1 participant (0.8%). Numbers of patients, age, and sex distributions for the CCS and controls in the adult and pediatric age groups for each of the CIPN parameters are presented in eTable 2 in the Supplement.
Clinical Findings
Participants treated with neurotoxic chemotherapy demonstrated a significant increase in signs and symptoms of neuropathy as assessed via the TNS (mean [SD; range] score: CCS, 2.9 [3.2; 0-14]; controls, 0.8 [1.0; 0-4]; P < .001) (Table 2). Total Neuropathy Score abnormalities were present in 53 of 100 CCS (53.0%) treated with neurotoxic chemotherapy and 5 of 36 controls (14%). More prominent abnormalities of the peripheral clinical neurological assessment (TNS > 3) were more prevalent in CCS treated with a cisplatin-containing protocol (10 of 12 [83%]) compared with CCS treated with vinca alkaloids (23 of 80 [29%]), CCS treated with carboplatin without cisplatin (2 of 7 [29%]), CCS who did not receive any neurotoxic agents (2 of 13 [15%]), or controls (1 of 36 [3%]) (P = .001).
Table 2. Results of Multimodal Testing for Childhood Cancer Survivors (CCS) Exposed to Neurotoxic Chemotherapy and Controls Across Clinical, Neurophysiological, and Functional Domains.
| Test Parameter | Mean (SD) | P Value | |
|---|---|---|---|
| CCS Treated With Neurotoxic Chemotherapy (n = 107) | Controlsa | ||
| Clinical | |||
| Total Neuropathy Score | 2.9 (3.2) | 0.8 (1.0) | <.001 |
| Neurophysiological, μV | |||
| Sural sensory amplitude | 17.6 (7.9) | 23.3 (7.8) | <.001 |
| Median sensory amplitude | 60.2 (28.0) | 61.6 (21.9) | .80 |
| Functional | |||
| Participants <17 y | |||
| MABC | |||
| Combined manual dexterity | −0.1 (1.5)b | NAc | .55 |
| Manual dexterity task 1 | −0.4 (1.0) | NAc | .003 |
| Manual dexterity task 2 | −0.3 (1.0) | NAc | .03 |
| Combined aiming and catching | 0.2 (1.1) | NAc | .24 |
| Combined balance | −0.1 (1.1) | NAc | .35 |
| Balance task 1 | −0.3 (1.1) | NAc | .03 |
| Participants ≥17 y | |||
| Von Frey monofilaments, mN | 0.3 (0.24) | 0.2 (0.11) | .01 |
| Grating orientation task, mm | 2.6 (1.48) | 1.9 (0.62) | .02 |
| Grooved peg board, s | 62.6 (10.3) | 56.7 (7.7) | .008 |
Abbreviations: MABC, Movement Assessment Battery for Children; NA, not applicable.
See eTable 2 in the Supplement for numbers of controls for each parameter.
Standard deviations from the population mean.
Compared with population reference ranges.
Neurophysiological Findings
The lower limb sural sensory amplitudes were smaller in CCS exposed to neurotoxic chemotherapy compared with age-matched controls (mean reduction, 5.8 μV; 95% CI, 2.8 to 8.8; P < .001) suggesting a reduced number of functioning axons (Table 2). In comparison, no significant reduction was seen in the sural amplitude in CCS not exposed to neurotoxic chemotherapy (mean reduction, 0.4 μV; 95% CI, −6.7 to 5.9; P = .90).
A sural amplitude below the mean (SD) control recording of 23.3 (7.8) μV was observed in 65 of 85 CCS (76%) treated with neurotoxic agents (Figure 1). Compared with population reference values, a sural amplitude below the age-appropriate reference ranges was noted in 20 CCS (24%) treated with neurotoxic chemotherapy (12 of 66 [18%] with vinca alkaloids and 7 of 18 [39%] with platinum agents).16,17,18 A total of 14 of 85 (16%) had an abnormal sural amplitude as well as an abnormal TNS, fulfilling criteria for peripheral neuropathy.19 No significant difference was observed in the sural sensory conduction velocity (0.9 m/s; 95% CI, −1.3 to 3.1; P = .40).
Figure 1. Shift in Distribution of Population Sensory Amplitudes.
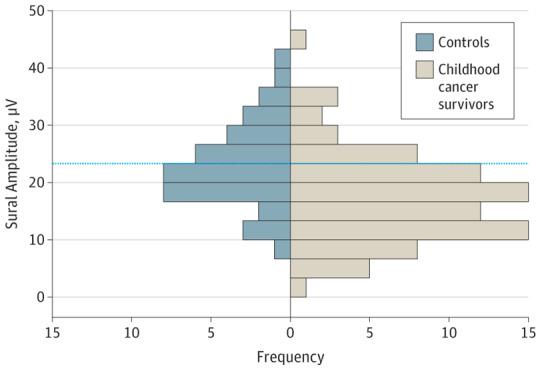
Shift in the population distribution of sural sensory amplitudes in childhood cancer survivors (n = 85) compared with controls (n = 40). The mean (SD) frequency was 23.3 (1.2) μV for controls and 17.6 (0.9) μV for survivors. A total of 65 of 85 childhood cancer survivors (76%) exposed to neurotoxic chemotherapy have sensory amplitudes below the control mean. The dotted line indicates the control mean.
In contrast, there were no significant differences in motor amplitudes between CCS treated with neurotoxic agents and controls, including amplitudes from the tibial nerve to abductor hallucis (−0.4 mV; 95% CI, −2.0 to 1.2; P = .60) and the peroneal nerve to extensor digitorum brevis (−1.0 mV; 95% CI, −2.1 to 0.2; P = .09). Comparable values were also obtained for the upper limb median (SD) sensory amplitudes for CCS (60.2 [28.0] μV) and controls (61.6 [21.9] μV) (P = .80). Individually, compared with population reference ranges, abnormal values were seen in 1 of 85 CCS (1%) for tibial motor amplitude, 3 of 44 (7%) for peroneal motor amplitude, and 4 of 103 (3.9%) for median sensory amplitude. All but 1 CCS with abnormalities in other nerves also had an abnormal sural amplitude.
Functional Assessment Findings
In child and adolescent participants, there was no significant difference between CCS treated with neurotoxic agents and population references in the mean (SD) combined score percentiles of manual dexterity (43.2 [28.6]), aiming and catching (56.4 [30.1]), or balance (46.2 [31.8]) using the MABC. However, 2 of 3 individual manual dexterity tasks and 1 of 3 balance tasks demonstrated impairment in CCS (Table 2; Figure 2).
Figure 2. Functional Deficits in Childhood Cancer Survivors (CCS).

A, Functional assessments using the Movement Assessment Battery for Children (MABC) in child and adolescent CCS exposed to neurotoxic chemotherapy compared with population reference ranges. Functional deficit is seen in 2 of 3 manual dexterity tasks and 1 of 3 balance tasks and is represented as the number of standard deviations below the population mean. The dotted line indicates the population mean and the error bars, SEM. B-D, Functional assessments in adult CCS exposed to neurotoxic chemotherapy compared with laboratory controls. B, Fingertip sensory threshold for light touch using Von Frey monofilaments (increased threshold in CCS, 0.1 mN; 95% CI, 0.03-0.2; P = .01). C, Fingertip cutaneous resolution threshold using the grating orientation task (increased threshold in CCS, 0.7 mm; 95% CI, 0.1-1.2; P = .003). D, Manual dexterity using the grooved peg board task (increased time to completion in CCS, 5.9 s; 95% CI, 1.6-10.1; P = .008). The black bar indicates the median and the error bars, interquartile ranges.
In adult participants, impaired performance was seen in the neurotoxic group compared with controls in distal sensory and motor tasks using Von Frey monofilaments testing light touch sensation (increased threshold, 0.1 mN; 95% CI, 0.03 to 0.2; P = .01), the grating orientation task testing cutaneous spatial resolution (increased threshold, 0.7 mm; 95% CI, 0.1 to 1.2; P = .003), and the grooved peg board testing manual dexterity (increased time to completion, 5.9 seconds; 95% CI, 1.6 to 10.1; P = .008) (Table 2; Figure 2). This translates to a difference of 0.4, 0.5, and 0.6 SDs, respectively, compared with controls. Additional matching for sex for the grooved peg board, given known sex differences in performance (eMethods in the Supplement) demonstrated a similar difference in the mean, which did not reach statistical significance in the context of a smaller sample size (increased time to completion, 4.7 seconds; 95% CI, 0.1 to 9.5; P = .06).
Comparison of CCS who did not receive any neurotoxic chemotherapy with controls did not demonstrate any functional differences except in the grating orientation task in the adult group (increased threshold, 0.5 mm; 95% CI, 0.003 to 1.0; P = .049). However, there were only small sample sizes in these subgroups (8 child and adolescent participants and 6 adult participants).
Patient-Reported Outcomes
Parent proxy reports for child and adolescent participants in the neurotoxic group demonstrated significant impairment and participation restriction across multiple domains, including the PedsQL physical domain (−0.4 SD; 95% CI, −0.6 to −0.1; P = .01) and psychosocial domain (−0.8 SD; 95% CI, −1.1 to −0.5; P < .001) and the PODCI sports and physical functioning domain (−0.4 SD; 95% CI, −0.8 to −0.1; P = .008). In comparison, child/adolescent self-report demonstrated impairment in the PedsQL psychosocial domain only (−0.3 SD; 95% CI, −0.5 to −0.05; P = .02) (eTable 3 in the Supplement).
In adult participants in the neurotoxic group, greater symptom report and poorer functioning and quality of life (QOL) were evident in all domains of the EORTC quality-of-life questionnaire (reduced physical functioning, 5.0 points; 95% CI, 0.9 to 9.1; P < .001) and CIPN20 questionnaire, except the autonomic symptom scale (increased neuropathy symptoms, 4.7 points; 95% CI, 1.2 to 8.2; P < .001) (eTable 3 in the Supplement). The 14 participants not exposed to chemotherapy (8 child and adolescent participants and 6 adult participants) were not different from controls in any of the domains.
Correlations
The clinical findings (TNS) were associated with the neurophysiological findings as well as the PRO measures for child and adolescent CCS (lower limb sural amplitude: Pearson correlation coefficient [r] = −0.33; P = .002; parent-reported PedsQL physical domain: Spearman correlation coefficient [rs] = −0.44; P < .001; parent-reported PedsQL psychosocial domain: rs = −0.43; P < .001; parent-reported PedsQL overall function domain: rs = −0.45; P < .001; parent-reported PODCI sport and physical function domain: rs = −0.31; P = .02; self-reported PedsQL physical domain: rs = −0.29; P = .03; self-reported PedsQL psychosocial domain: rs = −0.33; P = .01; self-reported PedsQL overall function domain: rs = −0.34; P = .01; and self-reported PODCI sport and physical function domain: rs = −0.38; P = .008). Notably, there was also a positive correlation between the parent-reported and self-reported overall function PedsQL (r = 0.66; P < .001) and PODCI sport and physical function (r = 0.66; P < .001) domains.
Adult and pediatric functional outcomes did not correlate with TNS, neurophysiological, or PRO measures. Importantly, no correlation was seen between vincristine dose and TNS or neurophysiological measures.
Risk Factors and Predictors
The TNS was the most significant predictive variable for PRO as well as neurophysiological outcomes, making it a valuable screening tool for CIPN-related deficits (Table 3). Following univariate analysis, radiotherapy (any location), sural amplitude, and TNS were the most probable significant predictors of PROs. Multivariate hierarchical linear regression demonstrated that the TNS was able to predict 25.6% of variability in parent-reported PedsQL scores (R2 = 0.38; β coefficient = −0.54; P < .001) and 22.6% in self-reported PedsQL scores (R2 = 0.24; β coefficient = −0.52; P = .001), 28.6% in parent-reported PODCI sport and physical function domain (R2 = 0.40; β coefficient = −0.57; P < .001), and 46.5% in the EORTC CIPN20 questionnaire (R2 = 0.47; β coefficient = 0.77; P < .001). The TNS was also the only significant predictor of neurophysiological (sural amplitude) outcomes, predicting 10% of the variability (R2 = 0.09; β coefficient = −0.32; P = .004) (Table 3). Importantly, age at diagnosis, time since completion of treatment, body mass index, type of malignancy, type of chemotherapy, and bone marrow transplantation were not predictive of neurophysiological or patient-reported deficits.
Table 3. Hierarchical Multiple Linear Regression Models for Neurophysiological and Patient-Reported Outcomes.
| Outcome | Neuropsychological | Patient-Reported Outcomes in CCS <17 y | Patient-Reported Outcomes in CCS ≥17 y | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sural Amplitude | Parent-Reported PedsQL | Self-reported PedsQL | Parent-Reported PODCI Sports and Physical | EORTC CIPN20 Symptom Report | |||||||||||
| R 2 | β Coefficient | P Value | R 2 | β Coefficient | P Value | R 2 | β Coefficient | P Value | R 2 | β Coefficient | P Value | R 2 | β Coefficient | P Value | |
| Predictor 1: radiotherapy | −0.003 | −0.02 | .86 | 0.08 | −0.13 | .28 | −0.01 | 0.06 | .66 | 0.05 | −0.08 | .49 | 0.02 | 0.03 | .84 |
| Predictor 2: sural amplitude | NA | NA | NA | 0.13 | 0.15 | .22 | 0.02 | 0.09 | .51 | 0.11 | 0.16 | .18 | −0.01 | 0.43 | .007 |
| Predictor 3: Total Neuropathy Score | 0.09 | −0.32 | .004 | 0.38 | −0.54 | <.001 | 0.24 | −0.52 | .001 | 0.40 | −0.57 | <.001 | 0.47 | 0.77 | <.001 |
| Overall model ANOVA, P value | .01 | <.001 | .002 | <.001 | <.001 | ||||||||||
Abbreviations: ANOVA, analysis of variance; CCS, childhood cancer survivors; CIPN20, chemotherapy-induced peripheral neuropathy questionnaire; EORTC, European Organisation for Research and Treatment of Cancer; PedsQL, Pediatric Quality of Life Inventory Generic Core Scales; PODCI, Pediatric Outcomes Data Collection Instrument.
Discussion
Comprehensive, multimodal assessment of long-term survivors of extracranial childhood malignancy identified coherent abnormalities attributable to CIPN across objective and subjective measures. This study included participants who received multiple forms of treatment for a wide range of cancer types, increasing the generalizability of the findings. Clinical abnormalities are common and evident early in CCS and are accompanied by an overall reduction in the number of axons, manifesting as a length-dependent, predominantly sensory axonal neuropathy. Abnormalities are more prevalent following exposure to cisplatin, suggesting a greater neurotoxicity profile. Clinical abnormalities were correlated with deficits in PROs and QOL, suggesting that these changes are clinically relevant and important to monitor in CCS. There may be multiple risk factors that predispose CCS to the development of CIPN, including the type of neurotoxic agent and a genetic predisposition, and further delineation of these risk factors is critical to neuroprotective strategies.7,20 Recent evidence of benefit from targeted exercise intervention in pediatric inherited neuropathy provides the rationale for further similar therapeutic research in patients with CIPN.21
The widespread reduction in sural amplitude following exposure to neurotoxic chemotherapy, with 23.5% of CCS below population reference ranges, suggests an irreversible reduction in the number of functioning axons. This reduction is not evident in the CCS subgroup not exposed to neurotoxic chemotherapy. In contrast to the predominantly motor acute vincristine neuropathy reported in children,22,23 our study identified a predominantly sensory long-term neuropathy with both vinca alkaloids and platinum agents, similar to findings in adults.24 This may be due to lack of protection from the blood-nerve barrier in the sensory dorsal root ganglia25 as well as an underappreciation of acute sensory manifestations during chemotherapy treatment in the pediatric population. Additionally, long-term negative sensory symptoms, such as numbness, may not be recognized if present from an early age. Electrophysiological assessments by Jain et al26 within 3 years and by Ramchandren et al15 within 7 years after completion of treatment report a predominantly motor neuropathy. Both studies lacked a control group and used exceptionally broad reference ranges for the sural amplitude,27 which may have precluded the recognition of a sensory neuropathy. A larger study by Tay et al28 reported a mixed motor and sensory neuropathy 4 years after completion of vincristine treatment in 68.3% of patients. The predominantly sensory neuropathy that we observed in a smaller proportion of vincristine-treated participants at a longer median follow-up of 8.5 years suggests that neural recovery from acute vincristine injury occurs over several years and that motor nerves may recover better than sensory nerves. The concurrent presence of sensory neuropathy in all but 1 patient with abnormal motor studies in our cohort reinforces the long-term susceptibility of sensory nerves, with additional motor nerve involvement in some cases.
A key finding in this study is the greater long-term neurotoxicity of cisplatin compared with vinca alkaloids, previously underrecognized in the pediatric population.7 Young adults systematically assessed 13 years after treatment with cisplatin demonstrate long-term clinical sensory neuropathy in up to 72%,29,30,31 with little or no electrophysiological recovery.32,33 In comparison, 30% to 40% of adults treated with vincristine had symptoms or signs of neuropathy up to 3 years after completion of treatment, with continued long-term improvement and at least partial electrophysiological recovery.34,35 These differences in neurotoxicity profiles may be caused by differential underlying mechanisms. Platinum agents have been shown to be toxic to the cell bodies in the dorsal root ganglia, causing anterograde neuronal loss rather than the retrograde axonopathy described with vinca alkaloids, which may be more amenable to recovery.36,37 While carboplatin is generally considered less neurotoxic than cisplatin,38 some of the CCS receiving carboplatin had comparable neurotoxicity with those receiving cisplatin. However, its neurotoxicity in isolation is difficult to ascertain because of the small number of patients in this cohort receiving carboplatin alone.
The reduction in the number of residual axons in this cohort suggests a smaller axonal reserve following exposure to neurotoxic chemotherapy. The natural history of aging involves a reduction in axonal responses over the life span,17,39 which, in the context of a reduced axonal reserve, may confer a greater susceptibility to reduced function at an earlier age. Childhood cancer survivors are also at an increased risk of metabolic syndrome and diabetes.13,40,41 Diabetes is one of the leading causes of peripheral neuropathy worldwide, and it is being increasingly recognized that impaired glucose tolerance, prediabetes, metabolic syndrome, and obesity can also manifest peripheral neuropathy.42,43,44 Consequently, CCS may be at a greater risk of diabetic and prediabetic neuropathy, and this underscores the need for vigilance in this population as well as the importance of screening for early signs of peripheral neuropathy. Chemotherapy-induced peripheral neuropathy–related impediments in physical function may also result in a reduced inclination for regular exercise, adding a further metabolic and cardiovascular risk factor.
From a functional perspective, reduced manual dexterity and distal sensation were seen in children and adults on objective testing. Behavioral sciences literature suggests the use of 0.2, 0.5, and 0.8 SDs to demonstrate meaningful clinical differences of small, moderate, and large effect size, respectively. This would suggest that the 0.4 to 0.8 SD deficits observed in the generic health-related QOL and specific neuropathy parent-reported and patient-reported outcomes may have clinical relevance.45,46,47,48 However, the clinical significance of the 0.3 to 0.4 SD differences seen in the pediatric functional tasks and 0.4 to 0.6 SD differences in the adult functional tasks are more difficult to interpret. These relatively modest differences might reflect the large inbuilt reserve in pediatric nerves, and the implications of a reduction in this axonal reserve may only become evident with a longer period of observation as the current cohort gets older and potentially accumulates more neurological deficits, as observed in other CCS.49,50
The stepwise acquisition of new skills during development in children might mean that more significant deficits would present with motor or sensory disability, whereas milder deficits may only present with a failure to achieve their premorbid potential rather than loss of skills. This change in developmental trajectory can be difficult to appreciate. As such, the PRO measures demonstrate global reduction in QOL and more specific impairment in physical function on the PODCI and EORTC questionnaires, suggesting that these deficits on formal testing may be relevant. Other studies have also reported deficits in strength and mobility,8,9,14 balance,51 and postural control52 as well as poorer QOL outcomes53 in CCS. However, the present study links CIPN-specific measures with long-term QOL, providing an important and comprehensive assessment of the association of long-term neuropathy with patient function. Importantly, we have demonstrated the TNS as a significant predictor of adult and pediatric PROs. While deficits evident in functional measures and PROs may be multifactorial, the TNS was specifically designed to capture peripheral neurotoxicity, has been validated against multiple neuropathy measures,54,55,56 and is correlated with objective neurophysiological measures in CCS, enabling greater confidence in attributing abnormalities to peripheral neuropathy. The TNS is based solely on clinical assessment and has less interindividual variability than neurophysiological testing, making it a valuable screening tool to identify patients at higher risk of disability from peripheral neuropathy.
Limitations
This study had limitations. Although tested several years after completion of their chemotherapy, it is unlikely that participants developed peripheral nerve injury from other causes during this time, given the young age of the cohort. Furthermore, comorbidities and preexisting conditions known to cause nerve injury, such as diabetes, renal failure, and critical illness neuropathy, were specific exclusion criteria. While there is a wide range of time elapsed since completion of treatment among the survivors, the median time since completion of treatment is 8.5 years. Late onset of neurological deficits with an increase in cumulative incidence up to 30 years after completion of therapy has been observed in large databases of CCS49,50 and may be compounded by additional comorbidities that occur as late effects. The administration of different functional and PRO measures for adults and children limited the numbers of participants in some subgroups, including CCS who did not receive any neurotoxic chemotherapy. Further studies are required to develop a greater understanding of CIPN in high-risk CCS, particularly those treated with platinum agents. The functional and PRO measures used in the pediatric participants were not specifically designed for use in CIPN, and only a subset of MABC measures identified functional impairments. As such, the development of responsive, pediatric CIPN–specific outcomes measures would be important for further clinical research into neuroprotective strategies and exercise interventions.
Conclusions
Long-term deficits in clinical, electrophysiological, and functional measures of peripheral neuropathy were common in our young cohort of CCS with concurrent deficits in PRO measures. Multimodal testing with inclusion of objective neurophysiological measures and subjective PRO measures is important to be able to attribute abnormalities to CIPN and determine their clinical relevance. Both the type of neurotoxic agent used and a targeted clinical neurological assessment are important considerations when screening CCS for long-term neuropathy. The effect of CIPN may be greater in the presence of other comorbidities, such as diabetes, and also as CCS get older. Development of standardized, CIPN-specific pediatric assessment tools, further research into the acute development and progression of neuropathy, and delineation of genetic and nongenetic predisposing factors are essential for the development of neuroprotective interventional, and rehabilitative strategies to optimize the long-term QOL for CCS.
eMethods. Description of clinical, neurophysiological, functional, and patient-reported outcome measures.
eTable 1. Multimodal assessment techniques.
eTable 2. Number of participants <17 years and ≥17 years who had each component of the CIPN assessment with age and gender distributions in each of the groups analysed.
eTable 3. General and neuropathy-specific parent-reported and self-reported outcomes for childhood cancer survivors exposed to neurotoxic chemotherapy and controls.
References
- 1.Australian Institute of Health and Welfare . Cancer in Adolescents and Young Adults in Australia. No. 62; Cat. no. CAN 59. Canberra, ACT: Australian Institute of Health and Welfare; 2011. [Google Scholar]
- 2.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36(4):277-285. [DOI] [PubMed] [Google Scholar]
- 3.Youlden DR, Baade PD, Hallahan AR, Valery PC, Green AC, Aitken JF. Conditional survival estimates for childhood cancer in Australia, 2002-2011: a population-based study. Cancer Epidemiol. 2015;39(3):394-400. [DOI] [PubMed] [Google Scholar]
- 4.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanday S. Increasing morbidity in childhood-onset cancer survivors. Lancet Oncol. 2015;16(5):e202. [DOI] [PubMed] [Google Scholar]
- 6.Yeh JM, Hanmer J, Ward ZJ, et al. Chronic conditions and utility-based health-related quality of life in adult childhood cancer survivors. J Natl Cancer Inst. 2016;108(9):djw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kandula T, Park SB, Cohn RJ, Krishnan AV, Farrar MA. Pediatric chemotherapy induced peripheral neuropathy: a systematic review of current knowledge. Cancer Treat Rev. 2016;50:118-128. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman MC, Mulrooney DA, Steinberger J, Lee J, Baker KS, Ness KK. Deficits in physical function among young childhood cancer survivors. J Clin Oncol. 2013;31(22):2799-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118(3):828-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143(9):639-647. [DOI] [PubMed] [Google Scholar]
- 11.Naumann FL, Hunt M, Ali D, Wakefield CE, Moultrie K, Cohn RJ. Assessment of fundamental movement skills in childhood cancer patients [published correction appears in Pediatr Blood Cancer. 2016;63(3):574]. Pediatr Blood Cancer. 2015;62(12):2211-2215. [DOI] [PubMed] [Google Scholar]
- 12.Neville KA, Cohn RJ. Bone health in survivors of childhood cancer. Lancet Diabetes Endocrinol. 2015;3(7):496-497. [DOI] [PubMed] [Google Scholar]
- 13.Touyz LM, Cohen J, Neville KA, et al. Changes in body mass index in long-term survivors of childhood acute lymphoblastic leukemia treated without cranial radiation and with reduced glucocorticoid therapy. Pediatr Blood Cancer. 2017;64(4). [DOI] [PubMed] [Google Scholar]
- 14.Ness KK, Jones KE, Smith WA, et al. Chemotherapy-related neuropathic symptoms and functional impairment in adult survivors of extracranial solid tumors of childhood: results from the St Jude Lifetime Cohort Study. Arch Phys Med Rehabil. 2013;94(8):1451-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramchandren S, Leonard M, Mody RJ, et al. Peripheral neuropathy in survivors of childhood acute lymphoblastic leukemia. J Peripher Nerv Syst. 2009;14(3):184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burke D, Skuse NF, Lethlean AK. Sensory conduction of the sural nerve in polyneuropathy. J Neurol Neurosurg Psychiatry. 1974;37(6):647-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esper GJ, Nardin RA, Benatar M, Sax TW, Acosta JA, Raynor EM. Sural and radial sensory responses in healthy adults: diagnostic implications for polyneuropathy. Muscle Nerve. 2005;31(5):628-632. [DOI] [PubMed] [Google Scholar]
- 18.Preston DC, Shapiro BE. Electromyography and Neuromuscular Disorders. 3rd ed. Philadelphia, PA: Elsevier Saunders; 2013. [Google Scholar]
- 19.England JD, Gronseth GS, Franklin G, et al. ; American Academy of Neurology; American Association of Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation . Distal symmetric polyneuropathy: a definition for clinical research. Neurology. 2005;64(2):199-207. [DOI] [PubMed] [Google Scholar]
- 20.Beutler AS, Kulkarni AA, Kanwar R, et al. Sequencing of Charcot-Marie-Tooth disease genes in a toxic polyneuropathy. Ann Neurol. 2014;76(5):727-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burns J, Sman AD, Cornett KM, et al. ; FAST Study Group . Safety and efficacy of progressive resistance exercise for Charcot-Marie-Tooth disease in children: a randomised, double-blind, sham-controlled trial. Lancet Child Adolesc Health. 2017;1(2):106-113. doi: 10.1016/S2352-4642(17)30013-5 [DOI] [PubMed] [Google Scholar]
- 22.Toopchizadeh V, Barzegar M, Rezamand A, Feiz AH. Electrophysiological consequences of vincristine contained chemotherapy in children: a cohort study. J Pediatr Neurol. 2009;7(4):351-356. doi: 10.3233/JPN-2009-0333 [DOI] [Google Scholar]
- 23.Courtemanche H, Magot A, Ollivier Y, et al. Vincristine-induced neuropathy: atypical electrophysiological patterns in children. Muscle Nerve. 2015;52(6):981-985. [DOI] [PubMed] [Google Scholar]
- 24.Haim N, Epelbaum R, Ben-Shahar M, Yarnitsky D, Simri W, Robinson E. Full dose vincristine (without 2-mg dose limit) in the treatment of lymphomas. Cancer. 1994;73(10):2515-2519. [DOI] [PubMed] [Google Scholar]
- 25.Allen DT, Kiernan JA. Permeation of proteins from the blood into peripheral nerves and ganglia. Neuroscience. 1994;59(3):755-764. [DOI] [PubMed] [Google Scholar]
- 26.Jain P, Gulati S, Seth R, Bakhshi S, Toteja GS, Pandey RM. Vincristine-induced neuropathy in childhood ALL (acute lymphoblastic leukemia) survivors: prevalence and electrophysiological characteristics. J Child Neurol. 2014;29(7):932-937. [DOI] [PubMed] [Google Scholar]
- 27.Hyllienmark L, Ludvigsson J, Brismar T. Normal values of nerve conduction in children and adolescents. Electroencephalogr Clin Neurophysiol. 1995;97(5):208-214. [DOI] [PubMed] [Google Scholar]
- 28.Tay CG, Lee VWM, Ong LC, Goh KJ, Ariffin H, Fong CY. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. 2017;64(8). [DOI] [PubMed] [Google Scholar]
- 29.Strumberg D, Brügge S, Korn MW, et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13(2):229-236. [DOI] [PubMed] [Google Scholar]
- 30.Earl HM, Connolly S, Latoufis C, et al. Long-term neurotoxicity of chemotherapy in adolescents and young adults treated for bone and soft tissue sarcomas. Sarcoma. 1998;2(2):97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krarup-Hansen A, Fugleholm K, Helweg-Larsen S, et al. Examination of distal involvement in cisplatin-induced neuropathy in man: an electrophysiological and histological study with particular reference to touch receptor function. Brain. 1993;116(pt 5):1017-1041. [DOI] [PubMed] [Google Scholar]
- 32.LoMonaco M, Milone M, Batocchi AP, Padua L, Restuccia D, Tonali P. Cisplatin neuropathy: clinical course and neurophysiological findings. J Neurol. 1992;239(4):199-204. [DOI] [PubMed] [Google Scholar]
- 33.Sghirlanzoni A, Silvani A, Scaioli V, Pareyson D, Marchesan R, Boiardi A. Cisplatin neuropathy in brain tumor chemotherapy. Ital J Neurol Sci. 1992;13(4):311-315. [DOI] [PubMed] [Google Scholar]
- 34.Postma TJ, Benard BA, Huijgens PC, Ossenkoppele GJ, Heimans JJ. Long-term effects of vincristine on the peripheral nervous system. J Neurooncol. 1993;15(1):23-27. [DOI] [PubMed] [Google Scholar]
- 35.Casey EB, Jellife AM, Le Quesne PM, Millett YL. Vincristine neuropathy: clinical and electrophysiological observations. Brain. 1973;96(1):69-86. [DOI] [PubMed] [Google Scholar]
- 36.Park SB, Krishnan AV, Lin CS, Goldstein D, Friedlander M, Kiernan MC. Mechanisms underlying chemotherapy-induced neurotoxicity and the potential for neuroprotective strategies. Curr Med Chem. 2008;15(29):3081-3094. [DOI] [PubMed] [Google Scholar]
- 37.Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: what do we know about mechanisms? Neurosci Lett. 2015;596:90-107. [DOI] [PubMed] [Google Scholar]
- 38.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17(1):409-422. [DOI] [PubMed] [Google Scholar]
- 39.Tavee JO, Polston D, Zhou L, Shields RW, Butler RS, Levin KH. Sural sensory nerve action potential, epidermal nerve fiber density, and quantitative sudomotor axon reflex in the healthy elderly. Muscle Nerve. 2014;49(4):564-569. [DOI] [PubMed] [Google Scholar]
- 40.Meacham LR, Sklar CA, Li S, et al. Diabetes mellitus in long-term survivors of childhood cancer: increased risk associated with radiation therapy: a report for the Childhood Cancer Survivor Study. Arch Intern Med. 2009;169(15):1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neville KA, Cohn RJ, Steinbeck KS, Johnston K, Walker JL. Hyperinsulinemia, impaired glucose tolerance, and diabetes mellitus in survivors of childhood cancer: prevalence and risk factors. J Clin Endocrinol Metab. 2006;91(11):4401-4407. [DOI] [PubMed] [Google Scholar]
- 42.Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist. 2008;14(1):23-29. [DOI] [PubMed] [Google Scholar]
- 43.Stino AM, Smith AG. Peripheral neuropathy in prediabetes and the metabolic syndrome. J Diabetes Investig. 2017;8(5):646-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Brien PD, Hinder LM, Callaghan BC, Feldman EL. Neurological consequences of obesity. Lancet Neurol. 2017;16(6):465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 46.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395-407. [DOI] [PubMed] [Google Scholar]
- 47.Maringwa J, Quinten C, King M, et al. ; EORTC PROBE Project and Brain Cancer Group . Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol. 2011;22(9):2107-2112. [DOI] [PubMed] [Google Scholar]
- 48.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. Pharmacoeconomics. 1999;15(2):141-155. [DOI] [PubMed] [Google Scholar]
- 49.Goldsby RE, Liu Q, Nathan PC, et al. Late-occurring neurologic sequelae in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(2):324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells EM, Ullrich NJ, Seidel K, et al. Longitudinal assessment of late-onset neurologic conditions in survivors of childhood central nervous system tumors: a Childhood Cancer Survivor Study report. Neuro Oncol. 2018;20(1):132-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varedi M, McKenna R, Lamberg EM. Balance in children with acute lymphoblastic leukemia. Pediatr Int. 2017;59(3):293-302. [DOI] [PubMed] [Google Scholar]
- 52.Einarsson EJ, Patel M, Petersen H, et al. Decreased postural control in adult survivors of childhood cancer treated with chemotherapy. Sci Rep. 2016;6:36784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fardell JE, Vetsch J, Trahair T, et al. Health-related quality of life of children on treatment for acute lymphoblastic leukemia: a systematic review. Pediatr Blood Cancer. 2017;64(9). [DOI] [PubMed] [Google Scholar]
- 54.Cavaletti G, Cornblath DR, Merkies IS, et al. ; CI-PeriNomS Group . The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol. 2013;24(2):454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavaletti G, Frigeni B, Lanzani F, et al. ; Italian NETox Group . The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12(3):210-215. [DOI] [PubMed] [Google Scholar]
- 56.Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53(8):1660-1664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Description of clinical, neurophysiological, functional, and patient-reported outcome measures.
eTable 1. Multimodal assessment techniques.
eTable 2. Number of participants <17 years and ≥17 years who had each component of the CIPN assessment with age and gender distributions in each of the groups analysed.
eTable 3. General and neuropathy-specific parent-reported and self-reported outcomes for childhood cancer survivors exposed to neurotoxic chemotherapy and controls.


