Key Points
Question
Are genetic variants associated with response to anti–vascular endothelial growth factor treatment in neovascular age-related macular degeneration (nAMD)?
Findings
In this multicenter genome-wide association study including 2058 patients with nAMD, rare protein-altering variants in the C10ORF88 and UNC93B1 genes were associated with worse visual acuity response to anti–vascular endothelial growth factor therapy. The effect of these rare variants was remarkably large, as patients carrying variants in the C10orf88 and UNC93B1 genes lost a mean 6 and 5 lines, respectively, on the Early Treatment of Diabetic Retinopathy Study letter chart after treatment.
Meaning
The results of this study may be used to adapt treatment strategies to individual needs in nAMD.
This multicenter genome-wide association study identifies genetic factors associated with variability in the response to anti–vascular endothelial growth factor therapy for patients with neovascular age-related macular degeneration.
Abstract
Importance
Visual acuity (VA) outcomes differ considerably among patients with neovascular age-related macular degeneration (nAMD) treated with anti–vascular endothelial growth factor (VEGF) drugs. Identification of pharmacogenetic associations may help clinicians understand the mechanisms underlying this variability as well as pave the way for personalized treatment in nAMD.
Objective
To identify genetic factors associated with variability in the response to anti-VEGF therapy for patients with nAMD.
Design, Setting, and Participants
In this multicenter genome-wide association study, 678 patients with nAMD with genome-wide genotyping data were included in the discovery phase; 1380 additional patients with nAMD were genotyped for selected common variants in the replication phase. All participants received 3 monthly injections of bevacizumab or ranibizumab. Clinical data were evaluated for inclusion/exclusion criteria from October 2014 to October 2015, followed by data analysis from October 2015 to February 2016. For replication cohort genotyping, clinical data collection and analysis (including meta-analysis) was performed from March 2016 to April 2017.
Main Outcomes and Measures
Change in VA after the loading dose of 3 monthly anti-VEGF injections compared with baseline.
Results
Of the 2058 included patients, 1210 (58.8%) were women, and the mean (SD) age across all cohorts was 78 (7.4) years. Patients included in the discovery cohort and most of the patients in the replication cohorts were of European descent. The mean (SD) baseline VA was 51.3 (20.3) Early Treatment Diabetic Retinopathy Study (ETDRS) score letters, and the mean (SD) change in VA after the loading dose of 3 monthly injections was a gain of 5.1 (13.9) ETDRS score letters (ie, 1-line gain). Genome-wide single-variant analyses of common variants revealed 5 independent loci that reached a P value less than 10 × 10−5. After replication and meta-analysis of the lead variants, rs12138564 located in the CCT3 gene remained nominally associated with a better treatment outcome (ETDRS letter gain, 1.7; β, 0.034; SE, 0.008; P = 1.38 × 10−5). Genome-wide gene-based optimal unified sequence kernel association test of rare variants showed genome-wide significant associations for the C10orf88 (P = 4.22 × 10−7) and UNC93B1 (P = 6.09 × 10−7) genes, in both cases leading to a worse treatment outcome. Patients carrying rare variants in the C10orf88 and UNC93B1 genes lost a mean (SD) VA of 30.6 (17.4) ETDRS score letters (ie, loss of 6.09 lines) and 26.5 (13.8) ETDRS score letters (ie, loss of 5.29 lines), respectively, after 3 months of anti-VEGF treatment.
Conclusions and Relevance
We propose that there is a limited contribution of common genetic variants to variability in nAMD treatment response. Our results suggest that rare protein-altering variants in the C10orf88 and UNC93B1 genes are associated with a worse response to anti-VEGF therapy in patients with nAMD, but these results require further validation in other cohorts.
Introduction
Advanced age-related macular degeneration (AMD) is a leading cause of blindness in elderly individuals.1,2 The most vision-impairing type of advanced AMD is neovascular AMD (nAMD), which is responsible for the majority of visual acuity (VA) loss caused by this disease.3,4 Currently, the most effective treatment for nAMD is intravitreal injections of anti−vascular endothelial growth factor (VEGF) antibodies.5,6 Although this treatment has resulted in dramatic improvements in VA for many patients with nAMD, a high degree of variability in treatment response has been observed; approximately 10% of patients with nAMD show a decline in VA of at least 15 Early Treatment Diabetic Retinopathy Study (ETDRS) score letters (ie, 3 lines) on the letter chart despite treatment.5,6,7
Early identification of patients with poor treatment response is a critical step in optimizing AMD treatment. Patients classified as nonresponders based on an absence of VA improvement after anti-VEGF injections might have better outcomes with higher frequency of dosing along with regular monitoring, although, to our knowledge, there is no definitive proof of this at this time.8 Also, alternative therapies with the potential for longer action are currently being developed for nAMD,9 and it is possible that other therapeutic options will become available. Therefore, establishing which factors are involved in treatment response variability could aid in the stratification of patients for the best treatment regime or therapeutic option.10 Genetic factors have been indicated to affect treatment outcome in patients with nAMD, although contradictory results have been reported.11,12,13,14,15,16,17
Using the resources of the International AMD Genomics Consortium (IAMDGC) and additional nAMD cohorts treated with anti-VEGF therapy, we performed a multicenter genome-wide association study to (1) evaluate in a hypothesis-free approach the association of common genetic variants with VA treatment response to anti-VEGF therapy in patients with nAMD and (2) evaluate the cumulative association of rare protein-altering variants with VA treatment response to anti-VEGF therapy in patients with nAMD. Identification of pharmacogenetic associations can help to identify underlying causal genes and mechanisms and suggest potential new drug targets and might be used as robust biomarkers for precision medicine.
Methods
Study Cohorts
Retrospective data collection for patients included in the discovery and replication cohorts was carried out in multiple clinics (Table 1).18,19,20,21 The discovery cohort included patients with nAMD who were evaluated as part of the International AMD Genomics Consortium (IAMDGC) project.22 All groups collected data according to Declaration of Helsinki principles. Study participants provided informed written consent, and protocols were reviewed and approved by local ethics committees. The inclusion criteria for all study center patients can be found in the eMethods in the Supplement. Information about sex, age at first injection, and baseline VA before anti-VEGF treatment and after 3 monthly injections (within 2 weeks) was collected. Visual acuity was collected in ETDRS letters or Snellen eye chart and was transformed into logMAR for analysis.
Table 1. Demographics and Clinic Characteristics of the Discovery and Replication Cohorts.
| Characteristic | Discovery Phase (n = 678) | Replication Phase (n = 1380) | Total (n = 2058) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| University Hospital of Cologne | Hadassah Medical Center–Hebrew University | Radboud University Medical Center | Centre for Eye Research Australia | Centre for Vision Research | Hadassah Medical Center–Hebrew University | Centre for Eye Research Australia | St James’s University Hospital | BRAMD18 | IVAN19,20 | EUGENDA21 | ||
| No. of patients | 155 | 113 | 121 | 119 | 170 | 146 | 88 | 220 | 215 | 542 | 169 | 2058 |
| Age at first injection, mean (SD), y | 76.9 (7.2) | 77.4 (7.5) | 77.5 (7.2) | 79.1 (7.2) | 77.4 (7.9) | 79.3 (8.8) | 79.7 (6.9) | 80.2 (5.9) | 77.6 (7) | 77.6 (7.4) | 76.7 (7.5) | 78.0 (7.4) |
| Female, % | 91 (58.7) | 68 (60.2) | 60 (49.6) | 68 (57.1) | 107 (62.9) | 89 (61.0) | 41 (46.6) | 138 (62.7) | 117 (54.4) | 317 (58.5) | 114 (68.0) | 1210 (58.8) |
| Baseline VA, mean (SD) | ||||||||||||
| logMAR | 0.657 (0.367) | 0.757 (0.616) | 0.682 (0.373) | 0.715 (0.35) | 1.039 (0.407) | 0.598 (0.562) | 0.762 (0.504) | 0.659 (0.289) | 0.509 (0.265) | 0.654 (0.333) | 0.547 (0.362) | 0.674 (0.406) |
| ETDRS score lettersa | 52.2 (18.4) | 47.2 (30.8) | 50.9 (18.7) | 49.3 (17.5) | 33.1 (20.4) | 55.1 (28.1) | 46.9 (25.2) | 52.1 (14.5) | 59.6 (13.3) | 52.3 (16.7) | 57.7 (18.1) | 51.3 (20.3) |
| Snellen eye chart | 20/100 | 20/125 | 20/100 | 20/100 | 20/250 | 20/80 | 20/125 | 20/100 | 20/63 | 20/100 | 20/80 | 20/100 |
| Change in VA after 3 mo, mean (SD)b | ||||||||||||
| logMAR | 0.051 (0.274) | 0.065 (0.515) | 0.124 (0.284) | 0.15 (0.231) | 0.107 (0.310) | 0.106 (0.384) | 0.161 (0.301 | 0.116 (0.203) | 0.085 (0.176) | 0.094 (0.237) | 0.106 (0.207) | 0.101 (0.277) |
| ETDRS score letters | 2.6 (13.7) | 3.3 (25.8) | 6.2 (14.2) | 7.5 (11.6 | 5.4 (15.5) | 5.3 (19.2) | 8.1 (15.1) | 5.8 (10.2) | 4.3 (8.8) | 4.7 (11.9) | 5.3 (10.4) | 5.1 (13.9 |
| Lines gained or lost | 0.5 | 0.6 | 1.2 | 1.5 | 1.1 | 1.1 | 1.6 | 1.1 | 0.8 | 0.9 | 1.1 | 1 |
Abbreviations: BRAMD, Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration trial cohort; ETDRS, Early Treatment Diabetic Retinopathy Study; EUGENDA, European Genetic Database study cohort; IVAN, Alternative Treatments to Inhibit VEGF in Age-related Choroidal Neovascularisation trial cohort; VA, visual acuity.
ETDRS score letter equivalents were calculated in the following manner: ETDRS score letters = 85 − (logMAR/0.02).
Change in VA was calculating by subtracting VA after 3 months of treatment from baseline VA.
Exome Array Genotyping and Quality Control
DNA samples from patients included in the discovery cohort were uniformly genotyped with a custom-modified HumanCoreExome array (Illumina) by the IAMDGC at the Center for Inherited Disease Research, Baltimore, Maryland. Genotype quality control and imputation was performed by the IAMDGC, as has been previously detailed.22 Principal components analysis (PCA) was performed, and only individuals of European descent, based on the PCA, were included in the analysis. The first 2 informative PCA eigenvalues (PC1 and PC2), which account for genetic variation in data coming from shared ancestry, were additionally used as covariates to adjust for population stratification. Identity-by-descent analysis was performed using PLINK version 1.9,23 and samples with a relatedness score (PI-HAT) greater than 0.25 were excluded.
Treatment Outcome Measurement
The outcome measure was a functional response defined as the change in VA after treatment, which was defined as the initial VA before treatment subtracted from the final VA after 3 anti-VEGF injections, and was analyzed as a continuous variable. The change in VA distributions (logMAR) was evaluated for normal distribution among each study cohort, and outliers (detected using the labeling rule; mean within 3 SDs) were adapted to mean within 2 SDs.
Genome-Wide Analyses of Common Variants
Variants with imputation quality scores (R2) greater than 0.6 and minor allele frequencies (MAF) of 0.05 or greater were included in the common variant analysis. Genome-wide single-variant association analyses using the change in VA as the testing variable were performed and included the first 2 ancestry principal components (PC1 and PC2), baseline VA, and age at first injection as covariates via a quantitative linear regression model (linear Wald testing) using the q.lm package in the EPACTS (Efficient and Parallelizable Association Container Toolbox) software version 3.2.6 (http://genome.sph.umich.edu/wiki/EPACTS). The analyses were performed separately in the 5 discovery cohorts, and covariate-adjusted estimates of the genetic effects were subsequently combined in a meta-analysis. Meta-analysis was performed with METAL24 based on effect size estimates and standard errors. Genomic control was applied if λ was greater than 1. The nominal significance level (significance level of each individual test) was set to a P value less than .05. The Bonferroni procedure was applied to account for multiple testing. A threshold for genome-wide significance was set at a P value less than 5 × 10−8 and for suggestively associated variants at a P value less than 5 × 10−5. Results were visualized using the qqman package in R version 3.2.4 (https://cran.r-project.org/web/packages/qqman/index.html) and LocusZoom.25
Genotyping and Association Analyses in Replication Cohorts
The lead variants of the loci that reached the threshold for suggestive significance were selected for genotyping in independent replication cohorts. These included single-nucleotide polymorphisms rs241692 (FHIT), rs12138564 (CCT3), rs13002976 (LOC105373426), rs242939 (CRHR1), and rs2237435 (INHBA).
Genotyping in the replication phase was performed using either KASP genotyping assay (LGC Group) or MassARRAY System with iPlex chemistry (Agena Bioscience), depending on the center performing the genotyping. All variants fell within Hardy-Weinberg equilibrium measures.
The association analysis in the replication cohort was performed in the same manner as the discovery cohort, except for the inclusion of PCA as covariates. The results of all cohorts were combined in a meta-analysis. Subsequently, an overall meta-analysis of the discovery and replication phase was performed.
Gene-Based Analysis of Rare Variants
We performed gene-based analyses using the optimal unified sequence kernel association test26 as implemented in EPACTS. Rare and low-frequency (MAF < 0.05) protein-altering variants (ie, missense, nonsense, or affecting canonical splice sites) were included in the analysis. Imputed variants were only included if the imputation quality (R2) was 0.8 or greater. Association tests were adjusted for age, baseline VA, and the first 2 ancestry principal components, and a sensitivity analysis adjusting for 10 ancestry principal components was additionally performed. To account for multiple testing, the Bonferroni procedure was applied. The summary statistics for each single variant were extracted from a comparable single-variant analysis using EPACTS.
Results
Characteristics of the Study Cohorts
We collected demographic and VA treatment response information for 2058 patients with nAMD who received anti-VEGF therapy. In the discovery phase, 678 patients from 5 different cohorts were genotyped with exome arrays by the IAMDGC22 and used for genome-wide association analyses on common and rare variants. In the replication phase, 1380 individuals from 6 different cohorts were genotyped for common variants identified in the discovery study.
Demographic information and clinical parameters of the study cohorts are described in Table 1. The mean change in VA after the loading dose of 3 monthly injections for all patients included in the study was 0.101 logMAR, which corresponded to a gain of 5.1 ETDRS score letters (ie, 1 line gain), and the mean change varied by cohort (Table 1). Age and VA at baseline have been the factors most consistently described as influencing change in VA after anti-VEGF treatment.27,28,29,30 This was also true for the total patient population and in most of the individual cohorts (eTable 1 in the Supplement). Therefore, these variables were included as covariates in all our subsequent analyses.
Association of rs12138564 in the CCT3 Gene With Response to Anti-VEGF Therapy
In the discovery phase, we performed genome-wide single-variant association analyses on the change in VA after 3 monthly anti-VEGF injections. Linear regression models were conducted on 6 089 769 quality-controlled common variants (MAF ≥ 0.05).
We identified a total of 111 variants with suggestive significance level (P < 10 × 10−5) (Figure 1A; eFigure in the Supplement). Consecutive conditional analysis revealed that these variants were distributed across 5 different loci, for which the lead variants were rs12138564, rs13002976, rs241692, rs2237435, and rs242939 (Table 2). The details of the associations per cohort are presented in eTable 2 in the Supplement.
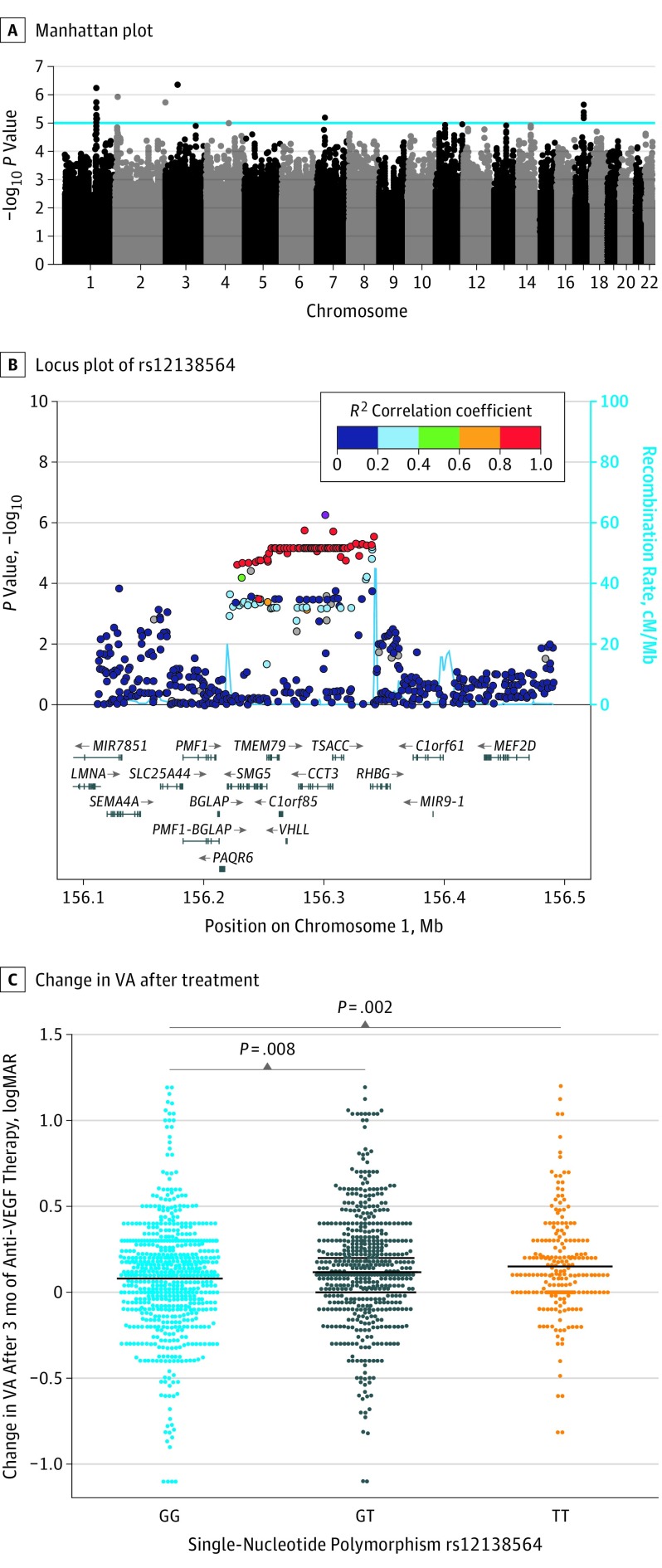
Figure 1. Single-Variant Association Analyses of Common Variants on Response to Anti–Vascular Endothelial Growth Factor (VEGF) Therapy in Neovascular Age-Related Macular Degeneration.
A, Manhattan plot of genome-wide association study for common variants in a discovery cohort (n = 678). The blue line indicates the suggestive significance threshold (P < 5 × 10−5). B, Locus plot of single-nucleotide polymorphism rs12138564 in the CCT3 gene. The light blue line and right y-axis show the observed recombination rate. C, Visual acuity (VA) change after 3 months of treatment stratified by rs12138564 genotypes in discovery and replication cohorts. The black bars indicate the mean change in VA of the beehive cluster.
Table 2. Single-Variant Association Analyses of 5 Lead Variants in the Discovery and Replication Phases.
| Chromosome | Positiona | Lead Variant | MA | Geneb | Discovery Phase (n = 678) | Replication Phase (n = 1380) | Total (n = 2058) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | |||||
| 1 | 156 291 600 | rs12138564 | T | CCT3 | 0.079 (0.016) | 5.74 × 10−7 | 0.019 (0.009) | 0.029 | 0.034 (0.008) | 1.38 × 10−5 |
| 2 | 10 678 538 | rs13002976 | G | NOL10 | −0.080 (0.016) | 1.21 × 10−6 | 0.009 (0.008) | 0.265 | −0.009 (0.008) | 0.219 |
| 3 | 60 410 187 | rs241692 | G | FHIT | 0.213 (0.042) | 4.46 × 10−7 | −0.023 (0.017) | 0.187 | 0.011 (0.016) | 0.493 |
| 7 | 41 731 053 | rs2237435 | A | INHBA | −0.081 (0.018) | 6.58 × 10−6 | 0.015 (0.009) | 0.109 | −0.005 (0.008) | 0.526 |
| 17 | 43 895 579 | rs242939 | C | CRHR1 | 0.128 (0.027) | 2.25 × 10−6 | −0.021 (0.017) | 0.214 | 0.020 (0.014) | 0.162 |
Abbreviation: MA, minor allele.
Chromosomal position according to the NCBI RefSeq hg19 human genome reference assembly.
Closest gene to the lead variant.
In the replication phase, the lead variants of the 5 associated loci were analyzed in 6 independent cohorts of patients with nAMD treated with anti-VEGF therapy, which comprised a total of 1380 patients. The results of the discovery and replication phase were combined in an overall meta-analysis of 11 cohorts, including 2058 patients with nAMD (Table 2; eTable 2 in the Supplement). The association of single-nucleotide polymorphism rs12138564 with functional treatment response remained nominally significant, showing a positive association of the minor allele with treatment outcome (β, 0.034; SE, 0.008; P = 1.38 × 10−5) (Table 2; eTable 2 in the Supplement). Single-nucleotide polymorphism rs12138564 is located in intron 8 of the CCT3 gene (Figure 1B). The other 4 lead variants did not replicate, and their association was lost after the meta-analysis of the discovery and replication cohorts (Table 2; eTable 2 in the Supplement).
The heterozygous rs12138564 GT genotype group showed an increased improvement in VA after anti-VEGF treatment compared with the reference GG genotype group (P = .008), and the homozygous TT group showed the largest improvement (P = .002). The GG genotype group showed a mean improvement in VA of 0.079 logMAR or approximately 4 ETDRS score letters (ie, 0.79 lines gained), the GT group, 0.118 logMAR or approximately 6 ETDRS score letters (ie, 1.18 lines gained), and the TT group, 0.150 logMAR or approximately 7.5 ETDRS score letters (ie, 1.5 lines gained) (Figure 1C).
Additionally, variants shown to be associated with treatment response in previous studies12,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 were not associated with VA treatment outcome at genome-wide or suggestive significance levels (P < .003 [ie, .05 / 18]) in this study (eTable 3 in the Supplement). We also analyzed 52 AMD-associated variants reported by the largest AMD case-control genome-wide association study (GWAS) performed so far.22 None of the 47 variants present in 1 or more of our study cohorts were found to be associated with VA response at either a suggestive significance level (P < .001 [ie, .05 / 47]) nor at the genome-wide significance level (eTable 4 in the Supplement).
Association of Rare Variants in C10orf88 and UNC93B1 With Response to Anti-VEGF Therapy
Using the rare variation content of the IAMDGC exome array, we analyzed the cumulative association of rare protein-altering variants with nAMD functional treatment response. We performed gene-based optimal unified sequence kernel association tests26 of quality-controlled variants with an MAF < 0.05. A total of 58 414 protein-altering variants classified as missense, nonsense, or affecting canonical splice sites distributed in a total of 14 788 genes were included in the analysis.
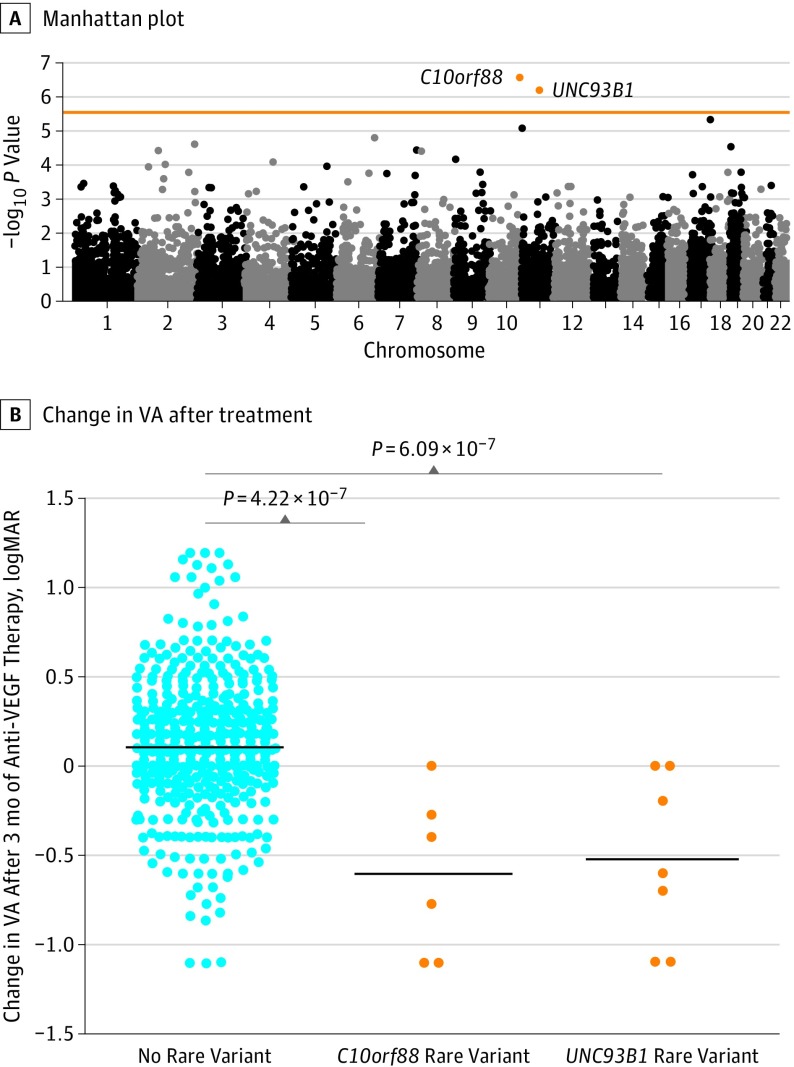
We identified 2 genes associated with VA treatment response at a gene-based genome-wide significance level (P < 3.38 × 10−6 [ie, .05 / 14 788]): chromosome 10 open reading frame 88 (C10orf88; P = 4.22 × 10−7) and unc-93 homologue B1 (UNC93B1; P = 6.09 × 10−7); carriers of rare variants in these genes showed worse VA response compared with noncarriers (Table 3; Figure 2A). Sensitivity analysis adjusting for 10 ancestry principal components showed comparable results (C10orf88: P = 2.24 × 10−7; UNC93B1: P = 1.65 × 10−7). Patients who did not carry a rare variant in either C10orf88 or UNC93B1 gained on average 0.109 logMAR or 5.5 ETDRS score letters (ie, 1.0 line gained) of VA after treatment. In contrast, the mean change in VA after treatment for carriers of rare variants in C10orf88 was a loss of 0.609 logMAR or 30.6 ETDRS score letters (ie, 6.09 lines). The carriers of rare variants in UNC93B1 showed a substantial decrease in VA after treatment as well, losing on average 0.529 logMAR or 26.5 ETDRS score letters (ie, 5.29 lines) (Figure 2B).
Table 3. Gene-Based Analysis of Rare Variants on Response to Anti–Vascular Endothelial Growth Factor Therapy in Neovascular Age-Related Macular Degeneration.
| Gene | Chromosome | Chromosomal Positiona | No. of Rare Variants | RAC | P Value |
|---|---|---|---|---|---|
| C10orf88 | 10 | 124 692 082 to 124 712 511 | 3 | 7 | 4.22 × 10−7 |
| UNC93B1 | 11 | 67 765 163 to 67 770 499 | 2 | 14 | 6.09 × 10−7 |
Abbreviation: RAC, rare allele count.
Chromosome and chromosomal position according to the NCBI RefSeq hg19 human genome reference assembly.
Figure 2. Gene-Based Analysis of Rare Variants on Response to Anti–Vascular Endothelial Growth Factor (VEGF) Therapy in Neovascular Age-Related Macular Degeneration.
A, Manhattan plot of genes analyzed in gene-based rare variant test. The horizontal line indicates the genome-wide significance level (P < 3.38 × 10−6 [ie, .05 / 14 788]). B, Visual acuity (VA) change after 3 months of treatment stratified by noncarriers as well as carriers of rare variants in C10orf88 and UNC93B1. Comparisons are conducted using the optimal unified sequence kernel association test. The black bars indicate the mean change in VA.
All variants included in the burden tests for these 2 genes had a high imputation quality score (R2) of 1 and showed the same direction of the association. For C10orf88, 3 rare protein-altering variants were included; 2 led to an amino acid change (c.412G>A; p.Glu138Lys and c.827T>C; p.Ile276Thr) and 1 variant introduced a stop codon (c.1258C>T; p.Gln420*). The p.Glu138Lys (rare allele count = 3; P = 4.96 × 10−4) and p.Ile276Thr (rare allele count = 3; P = 2.33 × 10−5) variants individually showed a nominal association with worse response to treatment. The variant p.Gln420* was present in only 1 individual and did not show an association at the single-variant level (P = .12). The variants p.Glu138Lys and p.Gln420* had a combined annotation-dependent depletion score greater than 20, which indicates that they are among the 1% most deleterious substitutions in the human genome (eTable 5 in the Supplement).
Two variants contributed to the burden of the UNC93B1 gene, both leading to an amino acid change (c.385C>A; p.Leu129Ile and c.626C>T; p.Pro209Leu). Both variants individually showed a nominal association with worse response to treatment (p.Leu129Ile: P = 3.33 × 10−7; p.Pro209Leu: P = 4.21 × 10−7) and had an assigned combined annotation dependent depletion score greater than 20 (eTable 5 in the Supplement). These 2 variants are in high linkage disequilibrium (R2 approximately 1) and were simultaneously present in 7 individuals, all belonging to the Hadassah Medical Center–Hebrew University cohort from Jerusalem, Israel.
Finally, we extracted the results for the genes in which a burden of rare variation has been shown in AMD (CFH, CFI, TIMP3, and SLC16A8).22 A suggestive association (P < .013 [ie, .05 / 4]) was found for SLC16A8 (P = .007) (eTable 6 in the Supplement); none of the genes reached the genome-wide significance level.
Discussion
We have undertaken a multicenter pharmacogenetic study of patients with nAMD and performed both GWAS of common variants and a gene-based analysis for the cumulative effect of rare variants. We evaluated the role of genetic variation on the change in VA after the loading dose of 3 monthly injections. Visual acuity is the most relevant outcome measure for patients, as it directly influences their quality of life.49
Our GWAS for common variants had 80% power to detect variants of moderate effects, explaining at least 6.2% of the variance in treatment response.50 However, no single-variant associations were found at the genome-wide significance level, suggesting a limited association of individual common variants with treatment outcome. We identified the variant rs12138564, located in intron 8 of the CCT3 gene, to be suggestively associated with treatment response after replication analysis, and therefore, this variant may merit further investigation in alternative cohorts.
We did not find any of the previously reported variants12,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,51 to be associated with functional response. This may be because of differences between the studies in response definition and patient population in terms of disease stage and other environmental factors or because of spurious findings. However, our results support the notion that none of the previously identified genetic markers are individually strong determinants of overall functional treatment response. Furthermore, we also did not find an association for any of the 52 AMD-associated variants reported in the largest AMD GWAS performed so far.22
The rare variant burden test revealed 2 genes associated with primary functional response at a genome-wide level: C10orf88 and UNC93B1. Therefore, these genes merit further evaluation in other large replication cohorts. The function of C10orf88 is still uncharacterized, and therefore the biological link to treatment response in nAMD is unclear. It has been suggested that common variants in C10orf88 are associated with vitamin D levels, although it cannot be excluded that the effect might be driven by the neighboring gene, ACADSB.52 Additionally, C10orf88 is expressed at low levels in the retina and retinal pigment epithelium/choroid.53 UNC93B1 is involved in the innate and adaptive immune response by regulating toll-like receptor signaling.54,55,56,57,58 Therefore, our results may point toward an immune component in treatment response in AMD.
Carriers of rare variants (MAF ≤ 1%) in the C10orf88 and UNC93B genes lost on average 6.09 and 5.29 lines of vision, respectively, on the ETDRS letter chart. The association of rare variants with VA outcome after treatment was very large, making these findings potentially relevant for the clinical practice.59 Large changes in VA after treatment are rare; however, they are seen in clinical practice and clinical trials.19,20,60,61 For example, in the Alternative Treatments to Inhibit VEGF in Age-related Choroidal Neovascularisation clinical trial data set included in this study,19,20 5.7% of patients lost 15 or more EDTRS score letters (ie, 3.0 lines) after 3 monthly injections and 1.5% of patients lost 25 or more EDTRS score letters (ie, 5.0 lines) at this time point.
Limitations
This study had limitations. Whether our findings on the functional response can be related to an anatomical response remains to be further investigated.62 We chose this time interval because 3 loading injections are administered widely in contrast to the follow-up treatment, which is variable per clinic, and its effect on VA outcomes.63 Most patients show the most improvement in their VA after the first 3 monthly injections5; thus, this time interval can be predictive of a longer-term response.32 Moreover, long-term treatment response is likely to be affected by nonadherence to treatment protocols. However, patients may improve after the 3-month time point, and therefore the effect of the identified genetic variants on secondary or long-term response would need to be evaluated. We investigated the treatment response to bevacizumab or ranibizumab therapy in a combined analysis. Future studies will require an evaluation of the association of these genetic variants and treatment outcome after aflibercept treatment, as this drug is now routinely used in the clinical setting for nAMD treatment. The anatomical changes, such as retinal or sub-retinal hemorrhage, retinal pigment epitheliumtears, and increase in macular thickness, may account for severe VA loss (≥15 ETDRS letters). Information on such factors was not available in this study; therefore, follow-up studies are needed to investigate if these variants are associated with anatomical variables after anti-VEGF treatment in nAMD. Another limitation is that 4 of 6 carriers of rare variants in the C10orf88 gene and all carriers of rare variants in the UNC93B1 gene were from the Jerusalem cohort. Therefore, this finding may be related to the genetic nature of that specific population.
Conclusions
Our multicenter GWAS suggests that the variability in primary functional treatment outcome in nAMD is probably not explained by large effects of common variants. However, our results suggest that rare genetic variants may have large effects on treatment outcome after 3 monthly anti-VEGF injections; these results require further validation in other cohorts.
eMethods. Inclusion criteria for study participants.
eTable 1. Association of baseline variables with response to anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration.
eTable 2. Single-variant association analyses of lead variants in the discovery and replication phases by cohort.
eTable 3. Single-variant association analysis of variants previously associated with response to anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration.
eTable 4. Single-variant association analysis of variants previously associated with age-related macular degeneration.
eTable 5. Rare protein-altering variants in C10orf88 and UNC93B1 included in the gene-based analysis.
eTable 6. Gene-based analysis of rare variants in genes previously associated with age-related macular degeneration.
eFigure. Q-Q plot of the single-variant association analysis of response to anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration.
References
- 1.Friedman DS, O’Colmain BJ, Muñoz B, et al. ; Eye Diseases Prevalence Research Group . Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, et al. . Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844-851. [PMC free article] [PubMed] [Google Scholar]
- 3.Colijn JM, Buitendijk GHS, Prokofyeva E, et al. ; EYE-RISK consortium; European Eye Epidemiology (E3) consortium . Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017;124(12):1753-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferris FL III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640-1642. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. ; MARINA Study Group . Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T; ANCHOR Study Group . Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57-65.e5. [DOI] [PubMed] [Google Scholar]
- 7.Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye (Lond). 2016;30(2):270-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holz FG, Tadayoni R, Beatty S, et al. . Identifying predictors of anti-VEGF treatment response in patients with neovascular age-related macular degeneration through discriminant and principal component analysis. Ophthalmic Res. 2017;58(1):49-55. [DOI] [PubMed] [Google Scholar]
- 9.Villegas VM, Aranguren LA, Kovach JL, Schwartz SG, Flynn HW Jr. Current advances in the treatment of neovascular age-related macular degeneration. Expert Opin Drug Deliv. 2017;14(2):273-282. [DOI] [PubMed] [Google Scholar]
- 10.van Asten F, Rovers MM, Lechanteur YT, et al. . Predicting non-response to ranibizumab in patients with neovascular age-related macular degeneration. Ophthalmic Epidemiol. 2014;21(6):347-355. [DOI] [PubMed] [Google Scholar]
- 11.Tsilimbaris MK, López-Gálvez MI, Gallego-Pinazo R, Margaron P, Lambrou GN. Epidemiological and clinical baseline characteristics as predictive biomarkers of response to anti-VEGF treatment in patients with neovascular AMD. J Ophthalmol. 2016;2016:4367631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59(1):1-18. [DOI] [PubMed] [Google Scholar]
- 13.Lotery AJ, Gibson J, Cree AJ, et al. ; Alternative Treatments to Inhibit VEGF in Patients with Age-Related Choroidal Neovascularisation (IVAN) Study Group . Pharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age-related macular degeneration in the IVAN Study. Ophthalmology. 2013;120(12):2637-2643. [DOI] [PubMed] [Google Scholar]
- 14.Hagstrom SA, Ying GS, Maguire MG, et al. ; CATT Research Group; IVAN Study Investigators . VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy in age-related macular degeneration. Ophthalmology. 2015;122(8):1563-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagstrom SA, Ying GS, Pauer GJ, Huang J, Maguire MG, Martin DF; CATT Research Group . Endothelial PAS domain-containing protein 1 (EPAS1) gene polymorphisms and response to anti-VEGF therapy in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2014;121(8):1663-1664.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagstrom SA, Ying GS, Pauer GJ, et al. ; Comparison of AMD Treatments Trials Research Group . Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2013;120(3):593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagstrom SA, Ying GS, Pauer GJ, et al. ; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group . VEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy: Comparison of Age-Related Macular Degeneration Treatments Trials (CATT). JAMA Ophthalmol. 2014;132(5):521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schauwvlieghe AME, Dijkman G, Hooymans JM, et al. . Comparing the Effectiveness of Bevacizumab to Ranibizumab in Patients with Exudative Age-Related Macular Degeneration: the BRAMD Study. PLoS One. 2016;11(5):e0153052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarthy U, Harding SP, Rogers CA, et al. ; IVAN Study Investigators . Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399-1411. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarthy U, Harding SP, Rogers CA, et al. ; IVAN study investigators . Alternative Treatments to Inhibit VEGF in Age-related Choroidal Neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258-1267. [DOI] [PubMed] [Google Scholar]
- 21.Saksens NTM, Geerlings MJ, Bakker B, et al. . Rare genetic variants associated with development of age-related macular degeneration. JAMA Ophthalmol. 2016;134(3):287-293. [DOI] [PubMed] [Google Scholar]
- 22.Fritsche LG, Igl W, Bailey JN, et al. . A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48(2):134-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purcell S, Neale B, Todd-Brown K, et al. . PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruim RJ, Welch RP, Sanna S, et al. . LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S, Emond MJ, Bamshad MJ, et al. ; NHLBI GO Exome Sequencing Project—ESP Lung Project Team . Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am J Hum Genet. 2012;91(2):224-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR; MARINA Study Group . Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246-252. [DOI] [PubMed] [Google Scholar]
- 28.Kaiser PK, Brown DM, Zhang K, et al. . Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144(6):850-857. [DOI] [PubMed] [Google Scholar]
- 29.Ying GS, Huang J, Maguire MG, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials Research Group . Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smailhodzic D, Muether PS, Chen J, et al. . Cumulative effect of risk alleles in CFH, ARMS2, and VEGFA on the response to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2012;119(11):2304-2311. [DOI] [PubMed] [Google Scholar]
- 31.Dedania VS, Grob S, Zhang K, Bakri SJ. Pharmacogenomics of response to anti-VEGF therapy in exudative age-related macular degeneration. Retina. 2015;35(3):381-391. [DOI] [PubMed] [Google Scholar]
- 32.Menghini M, Kurz-Levin MM, Amstutz C, et al. . Response to ranibizumab therapy in neovascular AMD: an evaluation of good and bad responders. Klin Monbl Augenheilkd. 2010;227(4):244-248. [DOI] [PubMed] [Google Scholar]
- 33.Wang VM, Rosen RB, Meyerle CB, et al. . Suggestive association between PLA2G12A single nucleotide polymorphism rs2285714 and response to anti-vascular endothelial growth factor therapy in patients with exudative age-related macular degeneration. Mol Vis. 2012;18:2578-2585. [PMC free article] [PubMed] [Google Scholar]
- 34.Chang W, Noh DH, Sagong M, Kim IT. Pharmacogenetic association with early response to intravitreal ranibizumab for age-related macular degeneration in a Korean population. Mol Vis. 2013;19:702-709. [PMC free article] [PubMed] [Google Scholar]
- 35.Lazzeri S, Figus M, Orlandi P, et al. . VEGF-A polymorphisms predict short-term functional response to intravitreal ranibizumab in exudative age-related macular degeneration. Pharmacogenomics. 2013;14(6):623-630. [DOI] [PubMed] [Google Scholar]
- 36.Cruz-Gonzalez F, Cabrillo-Estévez L, López-Valverde G, Cieza-Borrella C, Hernández-Galilea E, González-Sarmiento R. Predictive value of VEGF A and VEGFR2 polymorphisms in the response to intravitreal ranibizumab treatment for wet AMD. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):469-475. [DOI] [PubMed] [Google Scholar]
- 37.Hautamäki A, Kivioja J, Vavuli S, et al. . Interleukin 8 promoter polymorphism predicts the initial response to bevacizumab treatment for exudative age-related macular degeneration. Retina. 2013;33(9):1815-1827. [DOI] [PubMed] [Google Scholar]
- 38.Hautamäki A, Kivioja J, Seitsonen S, et al. . The IL-8, VEGF, and CFH polymorphisms and bevacizumab in age-related macular degeneration. Ophthalmology. 2014;121(4):973-973.e1. [DOI] [PubMed] [Google Scholar]
- 39.Hermann MM, van Asten F, Muether PS, et al. . Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2014;121(4):905-910. [DOI] [PubMed] [Google Scholar]
- 40.Matsumiya W, Honda S, Yanagisawa S, Miki A, Nagai T, Tsukahara Y. Evaluation of clinical and genetic indicators for the early response to intravitreal ranibizumab in exudative age-related macular degeneration. Pharmacogenomics. 2014;15(6):833-843. [DOI] [PubMed] [Google Scholar]
- 41.Medina FM, Alves Lopes da Motta A, Takahashi WY, et al. . Pharmacogenetic effect of complement factor H gene polymorphism in response to the initial intravitreal injection of bevacizumab for wet age-related macular degeneration. Ophthalmic Res. 2015;54(4):169-174. [DOI] [PubMed] [Google Scholar]
- 42.Piermarocchi S, Miotto S, Colavito D, Leon A, Segato T. Combined effects of genetic and non-genetic risk factors affect response to ranibizumab in exudative age-related macular degeneration. Acta Ophthalmol. 2015;93(6):e451-e457. [DOI] [PubMed] [Google Scholar]
- 43.Bakbak B, Ozturk BT, Zamani AG, et al. . Association of apolipoprotein E polymorphism with intravitreal ranibizumab treatment outcomes in age-related macular degeneration. Curr Eye Res. 2016;41(6):862-866. [DOI] [PubMed] [Google Scholar]
- 44.Lazzeri S, Orlandi P, Piaggi P, et al. . IL-8 and VEGFR-2 polymorphisms modulate long-term functional response to intravitreal ranibizumab in exudative age-related macular degeneration. Pharmacogenomics. 2016;17(1):35-39. [DOI] [PubMed] [Google Scholar]
- 45.Veloso CE, Almeida LN, Nehemy MB. CFH Y402H polymorphism and response to intravitreal ranibizumab in Brazilian patients with neovascular age-related macular degeneration. Rev Col Bras Cir. 2014;41(6):386-392. [DOI] [PubMed] [Google Scholar]
- 46.Lorés-Motta L, van Asten F, Muether PS, et al. . A genetic variant in NRP1 is associated with worse response to ranibizumab treatment in neovascular age-related macular degeneration. Pharmacogenet Genomics. 2016;26(1):20-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riaz M, Lorés-Motta L, Richardson AJ, et al. . GWAS study using DNA pooling strategy identifies association of variant rs4910623 in OR52B4 gene with anti-VEGF treatment response in age-related macular degeneration. Sci Rep. 2016;6:37924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah AR, Williams S, Baumal CR, Rosner B, Duker JS, Seddon JM. Predictors of response to intravitreal anti-vascular endothelial growth factor treatment of age-related macular degeneration. Am J Ophthalmol. 2016;163:154-166.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown GC. Vision and quality-of-life. Trans Am Ophthalmol Soc. 1999;97:473-511. [DOI] [PubMed] [Google Scholar]
- 50.Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19(1):149-150. [DOI] [PubMed] [Google Scholar]
- 51.Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Pharmacogenetics of complement factor H Y402H polymorphism and treatment of neovascular AMD with anti-VEGF agents: a meta-analysis. Sci Rep. 2015;5:14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahn J, Yu K, Stolzenberg-Solomon R, et al. . Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19(13):2739-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagner AH, Anand VN, Wang WH, et al. . Exon-level expression profiling of ocular tissues. Exp Eye Res. 2013;111:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177(2):265-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452(7184):234-238. [DOI] [PubMed] [Google Scholar]
- 56.Harris KG, Coyne CB. UNC93b induces apoptotic cell death and is cleaved by host and enteroviral proteases. PLoS One. 2015;10(10):e0141383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohno H, Chen Y, Kevany BM, et al. . Photoreceptor proteins initiate microglial activation via Toll-like receptor 4 in retinal degeneration mediated by all-trans-retinal. J Biol Chem. 2013;288(21):15326-15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart EA, Wei R, Branch MJ, Sidney LE, Amoaku WM. Expression of Toll-like receptors in human retinal and choroidal vascular endothelial cells. Exp Eye Res. 2015;138:114-123. [DOI] [PubMed] [Google Scholar]
- 59.Koch KR, Muether PS, Hermann MM, Hoerster R, Kirchhof B, Fauser S. Subjective perception versus objective outcome after intravitreal ranibizumab for exudative AMD. Graefes Arch Clin Exp Ophthalmol. 2012;250(2):201-209. [DOI] [PubMed] [Google Scholar]
- 60.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ; CATT Research Group . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin DF, Maguire MG, Fine SL, et al. ; Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group . Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keane PA, Liakopoulos S, Chang KT, et al. . Relationship between optical coherence tomography retinal parameters and visual acuity in neovascular age-related macular degeneration. Ophthalmology. 2008;115(12):2206-2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnston RL, Carius HJ, Skelly A, Ferreira A, Milnes F, Mitchell P. A retrospective study of ranibizumab treatment regimens for neovascular age-related macular degeneration (nAMD) in Australia and the United Kingdom. Adv Ther. 2017;34(3):703-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Inclusion criteria for study participants.
eTable 1. Association of baseline variables with response to anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration.
eTable 2. Single-variant association analyses of lead variants in the discovery and replication phases by cohort.
eTable 3. Single-variant association analysis of variants previously associated with response to anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration.
eTable 4. Single-variant association analysis of variants previously associated with age-related macular degeneration.
eTable 5. Rare protein-altering variants in C10orf88 and UNC93B1 included in the gene-based analysis.
eTable 6. Gene-based analysis of rare variants in genes previously associated with age-related macular degeneration.
eFigure. Q-Q plot of the single-variant association analysis of response to anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration.