This prospective observational study investigates the incidence of fellow eye involvement in patients with unilateral typical age-related macular degeneration or polypoidal choroidal vasculopathy.
Key Points
Question
What is the incidence of fellow eye involvement in patients with unilateral exudative age-related macular degeneration with and without nonexudative neovascularization as detected by optical coherence tomography angiography and/or indocyanine green angiography?
Findings
In this prospective observational study of 95 patients with unilateral age-related macular degeneration, the estimated annual incidence of fellow eye involvement was 18.1% and 2.0% in those with and without nonexudative neovascularization, respectively, with a statistically significant difference.
Meaning
The presence of nonexudative neovascularization predisposes to the development of exudative changes; however, treatment should only commence when exudation develops.
Abstract
Importance
Since the advent of optical coherence tomography angiography (OCT-A), nonexudative neovascularization has been described in the fellow eyes of unilateral exudative age-related macular degeneration (AMD). However, there is limited literature describing the natural course and optimal management of these lesions.
Objective
To determine the incidence of fellow eye involvement in patients presenting with unilateral typical AMD or polypoidal choroidal vasculopathy and to evaluate the patterns of OCT-A changes within 6 months before the onset of exudative changes, especially focusing on nonexudative neovascularization.
Design, Setting, and Participants
Data for this study were taken from a prospective, observational cohort study involving Asian patients with exudative AMD in the Asian AMD Phenotyping Study between October 2015 and March 2016. Analyses began in June 2017. Only patients who had gradable OCT-A and indocyanine green angiography (ICGA) scans of the fellow eye at baseline and follow-up at least 6 months apart were included for the analysis. The contralateral eye was evaluated for presence of nonexudative neovascularization based on multimodal imaging, which included ICGA, spectral domain optical coherence tomography, and OCT-A.
Main Outcomes and Measures
The difference between the incidence of those with nonexudative choroidal neovascularization and those without as analyzed using log-rank test and qualitative analysis of OCT-A images.
Results
We included 95 fellow eyes of 95 patients who presented with unilateral exudative AMD with a mean (SD) age of 68.6 (8.6) years. Nonexudative neovascularization was present in 18 eyes (19%) (8 [22.9%] and 10 [19.0%] fellow eyes with typical AMD and polypoidal choroidal vasculopathy, respectively; 8 [44.4%] on OCT-A; 5 [27.8%] on ICGA; and 5 [27.8%] on both OCT-A and ICGA). Development of exudative changes was noted in 6 fellow eyes (6.3%). Four eyes developed exudation from previously noted nonexudative neovascularization, and 2 eyes arose exudative changes from de novo. The probability of developing exudation within 6 months was significantly higher in eyes with baseline nonexudative neovascularization (0.087; 95% CI, 0.0033-0.210) compared with eyes without (0.010; 95% CI, 0.0026-0.041) (P = .008). In all eyes whose OCT-A images were available immediately before the onset of exudative changes, there was an increase in the size of network vessels compared with baseline.
Conclusions and Relevance
The presence of nonexudative neovascularization may predispose to the development of exudative changes.
Introduction
Age-related macular degeneration (AMD) is a major public health concern globally and is projected to affect nearly 300 million people by 2040.1 Age-related macular degeneration is classified as early and intermediate AMD, with features of drusen and retinal pigment epithelium (RPE) changes, and late AMD, which includes exudative AMD, characterized by the presence of choroidal neovascularization (CNV).2 Clinical fundus examination showing the presence of exudation and hemorrhage is the sine qua non of CNV. However, more recently, histological studies have demonstrated the presence of nonexudative neovascularization in some eyes with early or intermediate AMD.3,4 Moreover, clinical studies have been able to detect nonexudative neovascularization as a hyperfluorescent plaque using indocyanine green angiography (ICGA).5,6 On fluorescein angiography (FA), such lesions may show window defects without signs of neovascularization or late speckled hyperfluorescent lesions lacking well-demarcated borders but without signs of typical occult CNV, such as late-phase leakage of undetermined source or pooling of dye in the subretinal space.6,7 With the advent of optical coherence tomography angiography (OCT-A), the detection of nonexudative CNV became much easier.5,6,8,9,10,11
We, and others,6,10,11,12 have reported that nonexudative CNV is observed in the fellow eyes of exudative AMD at a prevalence between 11% and 27%. However, to our knowledge, there are limited studies regarding the risk of developing exudative changes in such eyes, and therefore the clinical significance of these lesions was unclear. Some hypothesized that this nonexudative neovascularization is compensatory vessels against ischemia and protects against RPE atrophy.13 In the current longitudinal study, we investigate the incidence of fellow eye involvement in patients with unilateral exudative AMD especially focusing on nonexudative neovascularization. We also assessed the OCT-A findings of the fellow eyes before and at the onset of the exudative changes.
Methods
Patients
Data for this study was taken from a prospective, observational cohort study involving patients with exudative AMD in the Asian AMD Phenotyping Study.14 Detailed methodology has been published elsewhere.15,16,17 The study was approved by the Singhealth Centralized institutional review board and was conducted according to the tenets of the Declaration of Helsinki.18 Written informed consent was obtained from each patient before participation in the study. Patients suspected of new presentation of exudative AMD, polypoidal choroidal vasculopathy (PCV), or retinal angiomatous proliferation (RAP) were invited to participate, and eligibility was confirmed after clinical examination and review of angiography results by retinal specialists. The current study is an extension of our previous study,12 and the data for this study was taken from patients with unilateral AMD who were recruited between October 2015 and March 2016 at the Singapore National Eye Centre and had gradable OCT-A and ICGA of the fellow eye at baseline and follow-up at least 6 months apart. Only patients with a minimum of 6 months of follow-up were considered for analysis, which started in June 2017.
Clinical Examinations
All patients had a standardized history and clinical examination, including measurement of best-corrected visual acuity, slitlamp biomicroscopy indirect fundus examination, and underwent FA and ICGA performed with the Heidelberg Spectralis HRA (Heidelberg Engineering) or flash camera (TRC-50DX, Topcon) and swept-source OCT-A (SS-OCT-A) (DRI OCT Triton, Topcon), together with spectral domain OCT with enhanced depth imaging mode (Spectralis, Heidelberg Engineering). Thirty degrees of retina were scanned along the horizontal planes through the foveal center. The averaging systems of OCT were used and 30 images were averaged for each scan.
Diagnosis of Typical AMD and PCV
Exudative AMD was diagnosed when there was evidence of CNV associated with nondrusenoid retinal pigment epithelium detachment, serous or hemorrhagic retinal detachment, subretinal hemorrhage, or subretinal exudation.14 According to FA, ICGA, and OCT findings, patients with exudative AMD were divided into 2 subgroups consisting of those with typical CNV and those with PCV. Polypoidal choroidal vasculopathy was diagnosed based on the criteria of the Japanese Ophthalmological Society,19 which define PCV as the presence of a polypoidal lesion with a branching vascular network on ICGA results, with concomitant exudation or hemorrhage.
SS-OCT-A Image Acquisition
All eyes underwent SS-OCT-A imaging at baseline or during their early follow-up period. Enface images were used to evaluate presence of flow signals.12,20,21 The SS OCT Angio is based on Topcon OCT Angiography Ratio Analysis algorithm.22 The instrument uses a light source with a center wavelength of 1050 nm and scans at speed of 100 000 A-scans per second, which yields axial resolution of 8 μm and depth of 2.4 dB/mm. Each OCT-A volume scan contains 320 × 320 pixels and covers an area of 3 × 3 mm. The OCT Angiography Ratio Analysis algorithm is based on detecting the degree of motion between consecutive OCT images. Briefly, OCT B-scan images are collected at the same transverse location 4 times. These 4 images are registered with each other using registration algorithm. The degree of motion is then calculated to allow extraction of blood flow. This procedure is then repeated for different Y-position in the retina to achieve the 3-dimensional data set and to reconstruct en face OCT-A images.
Nonexudative CNV
The definition of nonexudative CNV has been as described in our previous article.12 En face SS-OCT-A was used to access the presence of flow signals or neovascular tissue.20,21 Automated layer segmentation for outer retina (70.2 μm below the junction between inner plexiform layer and inner nuclear layer to Bruch membrane) and choriocapillaris (Bruch membrane to 10.4 μm below Bruch membrane) were used for the analysis. We evaluated the accuracy of automated segmentation and performed manual adjustment if necessary in all cases.21 The eyes were diagnosed as having neovascularization when the scans of the outer retinal layer, where flow signals are normally absent, showed a vascular network. In cases in which outer retinal slabs showed faint flow signal of neovascularization, the choriocapillaris slab was also used as a reference. Where the neovascular membrane had a distinct border, quantitative analysis of the neovascular membrane was performed using the in-built software system (IMAGEnet6, Topcon) by manually outlining the contour. Size measurement was challenging in 1 case because the neovascular membrane had indistinct borders. In ICGA results, the eyes were diagnosed as having neovascularization when they exhibited focal choroidal hyperfluorescence (plaque) in late frame of ICGA results with or without clear evidence of neovascular networks. These fellow eyes were regarded as unaffected before they demonstrated the clinical symptoms or active polyps or exudation.
Patient Follow-up
To be enrolled in the current study, patients were required to have OCT-A imaging at baseline (when the first eye presented) and at least 6 months later. Patients were followed up at 1- to 3-month intervals, depending on the activity of the disease. At each visit, patients underwent visual acuity test, OCT, and a slitlamp biomicroscopy. Patients were diagnosed as having the fellow eye involved when OCT results detected exudative changes.
Statistical Analysis
Statistical analysis was carried out using JMP software, version 11.0 (SAS Institute). Log-rank test was used for the analysis of the difference between the incidence of those with nonexudative CNV and those without.
Results
We included 95 eyes of 95 patients with unilateral exudative AMD. Thirty-five patients (36.8%) had typical AMD, and 60 (63.2%) had PCV in the first eyes. Overall, the mean (SD) age at presentation was 68.6 (8.6) years, and 55 patients (57%) were men. Most patients were Chinese (86 [89.6%]), and there were 3 Indian (3.1%), 6 Malay (6.3%), and 1 Filipino (1.1%) patients. The mean (SD) visual acuity at presentation was 0.19 (0.16) logMAR units. Of the 95 patients, 18 (18.9%) had nonexudative neovascularization (8 [22.9%] and 10 [19.0%] fellow eyes with typical AMD and PCV, respectively; 8 [44.4%] on OCT-A; 5 [27.8%] on ICGA; and 5 [27.8%] on both OCT-A and ICGA) (eTable in the Supplement). Fluorescein angiography showed window defect in 13 cases (72.2%), minimal or no leakage in 4 cases (22.2%), and oozing in 1 case (5.6%). All 18 cases had RPE elevation on structural OCT results. The mean age was similar between patients with nonexudative neovascularization and those without (mean [SD], 68.8 [8.05] vs 68.4 [10.27] years; 95% CI, −3.9 to 5.25; P = .70). Most patients were men (45 [58%] and 9 [50%] in those with and without nonexudative neovascularization, respectively; odds ratio, 1.40; 95% CI, 0.44-4.49; P = .43). The median follow-up period was 343 days.
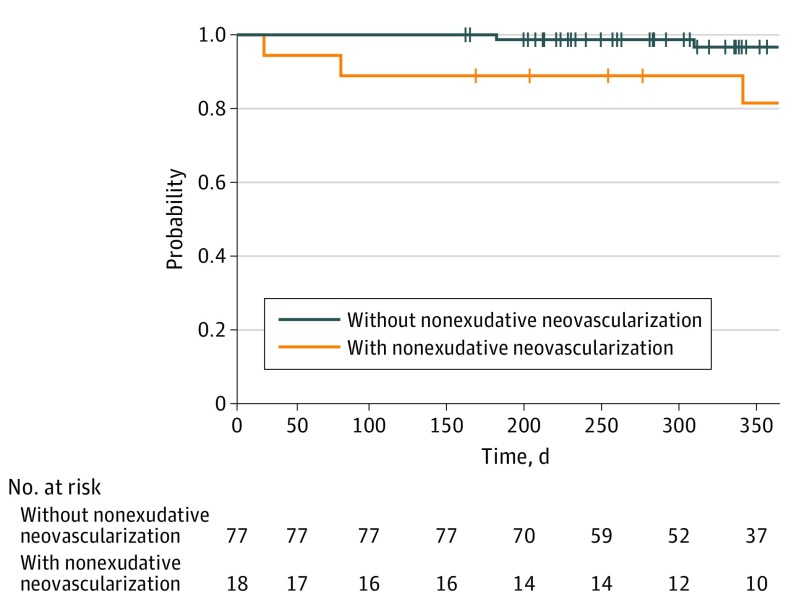
During the follow-up period, development of exudative changes was noted in 6 fellow eyes: 4 of 18 eyes (22.2%) with nonexudative neovascularization and 2 of 77 eyes (2.6%) without nonexudative neovascularization (odds ratio, 10.3; 95% CI, 1.34-124.0; P = .01, Fisher exact test). Mean (SD) visual acuity at baseline and at the time of exudative changes was 0.11 (0.10) and 0.08 (0.07), respectively. When classified according to modality of detection at baseline, exudation developed in 1 of 8 eyes (13%) detected with OCT-A alone, 1 of 5 eyes (20%) detected with ICGA alone, and 1 of 5 eyes (20%) detected with both modalities. The period between diagnosis and exudative changes was within 365 days in 5 eyes. The other eye developed exudative changes at 1275 days. The period between diagnosis and fellow eye involvement was shorter in eyes with nonexudative neovascularization than those without (Figure 1; P = .01, log-rank test). The risk ratio for those with nonexudative neovascularization relative to those without was 7.24 (95% CI, 1.37-53.1; P = .20). Estimated annual incidence of fellow eye involvement was 18.1% (95% CI, 8.5%-40.4%) and 2.0% (95% CI, 0.44%-8.40%) in those with and without nonexudative neovascularization, respectively. Exudative change developed in 2 of 35 fellow eyes (5.71%) in typical AMD and 4 of 60 fellow eyes (6.32%) in PCV, respectively, with no statistical differences between the 2 groups (P > .99).
Figure 1. Time to Developing Exudative Changes.
Exudative change was used as an end point.
All OCT-A images recorded before and immediately after the onset of exudative changes were scrutinized. From the last OCT-A examination, exudative changes were noted at days 183, 101, 70, and 18 from nonexudative neovascularization and 299 and 170 days from de novo. In all 3 eyes whose OCT-A images were available within 6 months before the onset of exudative changes, there was an increase in the size of network vessels (Figure 2 and Figure 3). In another case, OCT-A imaging was available only at the time of exudation, which also showed increased flow compared with baseline (eFigure 1 in the Supplement). In contrast, there was no change in the size and shape of nonexudative neovascularization in the fellow eyes that did not develop exudative changes (Figure 4). There was a slight increase in leakage on FA before the onset of exudative changes in 1 of the eyes without nonexudative neovascularization at baseline (eFigure 2 in the Supplement), and neovascularization developed pigment epithelial detachment from preexisting RPE undulation in the other. The first sign of exudative changes on OCT was subretinal fluid in 4 fellow eyes and pigment epithelial detachment in 2 fellow eyes.
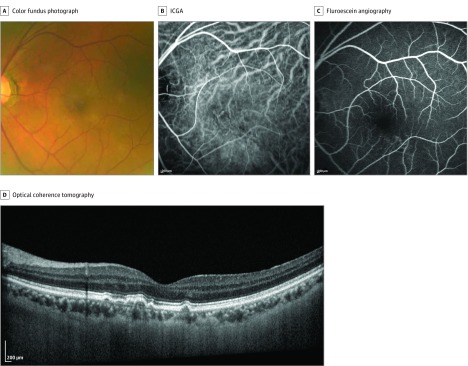
Figure 2. Left Eye of a Man With Nonexudative Neovascularization.
A, Color fundus photograph. B, Indocyanine green angiography (ICGA) showing nonexudative neovascularization located at subfovea. C, Fluorescein angiography showing window defect. D, Cross-sectional optical coherence tomography showing undulation of retinal pigment epithelium line.
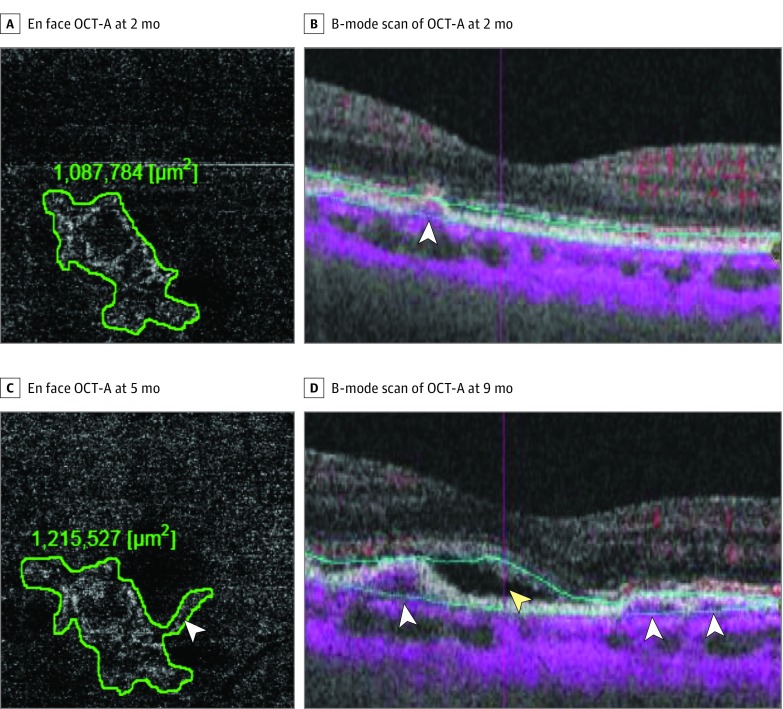
Figure 3. Left Eye of a Man With Nonexudative Neovascularization That Developed Exudative Changes.
Follow-up examinations at 2 (A and B), 5 (C), and 9 (D) months. Optical coherence tomography angiography (OCT-A) scans through the fovea showing flow signal between retinal pigment epithelium and Bruch membrane (B) and vascular flow within the outer retina corresponding to the irregular retinal pigment epithelium elevation (A). Neovascularization increased in size on en face OCT-A from 1 087 000 μm2 to 1 212 527 μm2 (A vs C) before the onset of exudative changes (D). Double layer sign (white arrowheads) can be seen to increase from 2 months (B) to 9 months (D). Exudation developed at 9 months as evidenced by presence of subretinal fluid (yellow arrowhead).
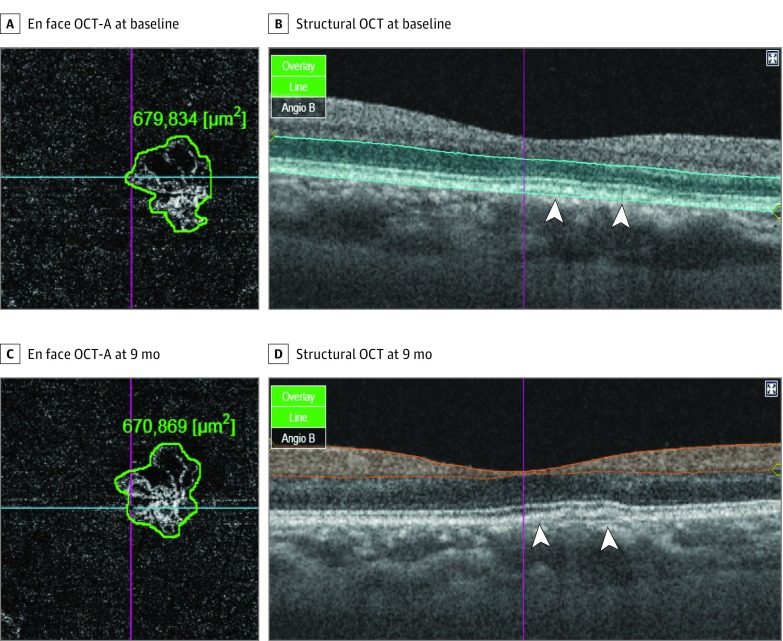
Figure 4. Indolent Nonexudative Neovascularizations Detected by Indocyanine Green Angiography (ICGA) and Optical Coherence Tomography Angiography (OCT-A).
A and B, Right eye of a woman. Cross-sectional OCT-A scans through the fovea showing slightly undulated retinal pigment epithelium line (B, arrowheads) and clearly detects vascular flow within the outer retinal slab (A). The size of nonexudative neovascular membrane (A) was 679 834 μm2. There was no change in the size (670 869 μm2) and vascularity of the neovascularization at 9-month follow-up (C and D).
Discussion
Development of exudative lesion in the second eye in patients with AMD has major impact on the quality of life of these patients. The annual incidence of the fellow eye involvement in unilateral exudative AMD is reported to be between 4% to 19%, with no difference between white and Asian patients. Additionally, a study from Japan reported that the cumulative incidence of involvement in fellow eyes with exudative AMD was 3.4% in 1 year, 9.3% in 3 years, and 11.3% in 5 years, with no difference between typical AMD and PCV.23 Similarly, a study from Korea reported that among 47 fellow eyes, PCV developed in 9 fellow eyes (19.1%) during the mean (SD) follow-up period of 30.3 (12.2) months.24 The annual incidence of fellow eye involvement in the current study was 20.1%, which was comparable with that in previous studies.
We, and others,6,10,11,12 have reported that roughly 1 in 5 fellow eyes of patients with unilateral exudative AMD has nonexudative neovascularization. In the current cohort, nonexudative neovascularization was found in 18 (19%) fellow eyes at baseline. As reported in previous studies using ICGA,6,7,10,11 nonexudative CNV did not show late-phase leakage of undetermined source or pooling of dye in the subretinal space, which defines occult CNVs. Management of these lesions remains unclear. This is owing to a lack of knowledge of the risk of development of exudation in such lesions. In the current study, we have made several important new observations. First, nonexudative neovascularization carries significant risk of developing exudation (estimated annual incidence, 18.1%). Second, few eyes without prior nonexudative neovascularization developed exudative neovascularization de novo (estimated annual incidence, 2.0%). Thus, eyes with nonexudative neovascularization are at almost 10 times higher risk of developing exudative changes than eyes without these lesions. Our finding is similar to a recent study in a predominantly Caucasian population that demonstrated the risk of exudation was 15.2 times lower for eyes without subclinical macular neovascularization compared with eyes with detectable neovascularization.25 Therefore it is important to detect such nonexudative neovascularization so that these patients can be counseled and monitored appropriately.
To our knowledge, there is currently no clinical data to support the treatment for nonexudative neovascularization before exudation occurs. In the current study, we were able to detect increase in size of vascular network noninvasively using serial OCT-A, corroborating a previous study.25 However, we observed that such enlargement may occur for a certain time before exudation eventually occurs (Figure 3). Therefore, initiation of treatment is also not clearly indicated even in the presence of increase in lesion size if there is no exudation. Based on our current understanding, we recommend OCT-A at baseline examination and frequent follow-up and detailed counseling regarding self-monitoring in these patients.
Several factors have been reported to be associated with fellow eye involvement by exudative AMD or PCV. Previous studies have reported that presence of both drusen and pigmentary abnormalities are risk factors for developing exudative AMD.26,27 Elevation or disturbance of RPE have been observed on OCT before the diagnosis of the fellow eye conversion.28 Development of PCV in fellow eyes has also been associated with RPE atrophy23 and presence of late geographic hyperfluorescence on ICGA.24 We recently reported that presence of pachychoroid epitheliopathy is associated with nonexudative neovascularization.12 With the advent of OCT-A, we can now noninvasively confirm that many of these areas of RPE disturbance actually harbors neovascularization and monitor these lesions longitudinally.
Limitations
There are limitations to our study. First, among patients who were initially diagnosed as having unilateral AMD, this study included only patients who had follow-up longer than 6 months. It is possible that the fellow eyes without nonexudative neovascularization require a longer time to develop exudative changes compared with those with nonexudative neovascularization. With further follow-up, there could be no difference in incidence of exudative changes between fellow eyes with or without nonexudative neovascularization. Additionally, some patients were lost to follow-up, and the long-term incidence of exudative changes may be underestimated or overestimated. Second, timing of follow-up interval and OCT-A scans were not uniform. Thus, some patients lacked OCT-A images immediately before the onset of exudative changes. In the current study, we used the SS-OCT system, which provides a better signal-to-noise ratio compared with spectral domain OCT system.29,30,31,32 However, relatively high background noise may still limit our ability to visualize the neovascularization and calculate vessel density. Additionally, the OCT-A devise used in the current study lacks follow-up mode ensuring that the exact same retinal area was imaged at every follow-up visit.
Conclusions
This study is the first to describe a longitudinal observation to assess the long-term conversion of nonexudative neovascularization in the Asian population, to our knowledge. The presence of nonexudative neovascularization may predispose to the development of exudative changes.
eTable. Summary of baseline characteristics of patients with and without non-exudative neovascularization
eFigure 1. Left eye of a 62-year old man with non-exudative neovascularization that developed exudative changes.
eFigure 2. Right eye of a 63-year old woman that developed exudative changes from de novo
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. [DOI] [PubMed] [Google Scholar]
- 2.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. [DOI] [PubMed] [Google Scholar]
- 3.Green WR, McDonnell PJ, Yeo JH. Pathologic features of senile macular degeneration. Ophthalmology. 1985;92(5):615-627. [PubMed] [Google Scholar]
- 4.Spraul CW, Grossniklaus HE. Characteristics of Drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115(2):267-273. [DOI] [PubMed] [Google Scholar]
- 5.Bottoni FG, Aandekerk AL, Deutman AF. Clinical application of digital indocyanine green videoangiography in senile macular degeneration. Graefes Arch Clin Exp Ophthalmol. 1994;232(8):458-468. [DOI] [PubMed] [Google Scholar]
- 6.Hanutsaha P, Guyer DR, Yannuzzi LA, et al. Indocyanine-green videoangiography of drusen as a possible predictive indicator of exudative maculopathy. Ophthalmology. 1998;105(9):1632-1636. [DOI] [PubMed] [Google Scholar]
- 7.Querques G, Srour M, Massamba N, et al. Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54(10):6886-6892. [DOI] [PubMed] [Google Scholar]
- 8.Carnevali A, Cicinelli MV, Capuano V, et al. Optical coherence tomography angiography: a useful tool for diagnosis of treatment-naïve quiescent choroidal neovascularization. Am J Ophthalmol. 2016;169:189-198. [DOI] [PubMed] [Google Scholar]
- 9.Carnevali A, Capuano V, Sacconi R, et al. OCT angiography of treatment-naïve quiescent choroidal neovascularization in pachychoroid neovasculopathy. Ophthalmol Retina. 2017;1(4):328-332. doi: 10.1016/j.oret.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 10.Palejwala NV, Jia Y, Gao SS, et al. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015;35(11):2204-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roisman L, Zhang Q, Wang RK, et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology. 2016;123(6):1309-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanagi Y, Mohla A, Lee W-K, et al. Prevalence and risk factors for nonexudative neovascularization in fellow eyes of patients with unilateral age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2017;58(9):3488-3495. [DOI] [PubMed] [Google Scholar]
- 13.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137(3):496-503. [DOI] [PubMed] [Google Scholar]
- 14.Cheung CM, Bhargava M, Laude A, et al. Asian age-related macular degeneration phenotyping study: rationale, design and protocol of a prospective cohort study. Clin Exp Ophthalmol. 2012;40(7):727-735. [DOI] [PubMed] [Google Scholar]
- 15.Cheung CM, Li X, Mathur R, et al. A prospective study of treatment patterns and 1-year outcome of Asian age-related macular degeneration and polypoidal choroidal vasculopathy. PLoS One. 2014;9(6):e101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ting DS, Ng WY, Ng SR, et al. Choroidal thickness changes in age-related macular degeneration and polypoidal choroidal vasculopathy: a 12-month prospective study. Am J Ophthalmol. 2016;164:128-36.e1. [DOI] [PubMed] [Google Scholar]
- 17.Lim FP, Wong CW, Loh BK, et al. Prevalence and clinical correlates of focal choroidal excavation in eyes with age-related macular degeneration, polypoidal choroidal vasculopathy and central serous chorioretinopathy. Br J Ophthalmol. 2016;100(7):918-923. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.Japanese Study Group of Polypoidal Choroidal V. [Criteria for diagnosis of polypoidal choroidal vasculopathy]. Nippon Ganka Gakkai Zasshi. 2005;109(7):417-427. [PubMed] [Google Scholar]
- 20.Cheung CM, Yanagi Y, Mohla A, et al. Characterization and differentiation of polypoidal choroidal vasculopathy using swept source optical coherence tomography angiography. Retina. 2017;37(8):1464-1474. [DOI] [PubMed] [Google Scholar]
- 21.Teo KYC, Yanagi Y, Lee SY, et al. Comparison of optical coherence tomography angiographic changes after anti-vascular endothelial growth factor therapy alone or in combination with photodynamic therapy in polypoidal choroidal vasculopathy [published online August 1, 2017]. Retina. [DOI] [PubMed] [Google Scholar]
- 22.Stanga PE, Tsamis E, Papayannis A, Stringa F, Cole T, Jalil A. Swept-Source Optical Coherence Tomography Angio™ (Topcon Corp, Japan): technology review. Dev Ophthalmol. 2016;56:13-17. [DOI] [PubMed] [Google Scholar]
- 23.Ueta T, Iriyama A, Francis J, et al. Development of typical age-related macular degeneration and polypoidal choroidal vasculopathy in fellow eyes of Japanese patients with exudative age-related macular degeneration. Am J Ophthalmol. 2008;146(1):96-101. [DOI] [PubMed] [Google Scholar]
- 24.Kim YT, Kang SW, Chung SE, Kong MG, Kim JH. Development of polypoidal choroidal vasculopathy in unaffected fellow eyes. Br J Ophthalmol. 2012;96(9):1217-1221. [DOI] [PubMed] [Google Scholar]
- 25.de Oliveira Dias JRR, Zhang Q, Garcia JMBMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki M, Kawasaki R, Uchida A, et al. Early signs of exudative age-related macular degeneration in Asians. Optom Vis Sci. 2014;91(8):849-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferris FL III, Wilkinson CP, Bird A, et al. ; Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amissah-Arthur KN, Panneerselvam S, Narendran N, Yang YC. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye (Lond). 2012;26(3):394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Told R, Ginner L, Hecht A, et al. Comparative study between a spectral domain and a high-speed single-beam swept source OCTA system for identifying choroidal neovascularization in AMD. Sci Rep. 2016;6:38132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novais EA, Adhi M, Moult EM, et al. Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence tomography angiography compared to spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;164:80-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Chen C-L, Chu Z, et al. Automated quantitation of choroidal neovascularization: a comparison study between spectral-domain and swept-source OCT angiograms. Invest Ophthalmol Vis Sci. 2017;58(3):1506-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller AR, Roisman L, Zhang Q, et al. Comparison between spectral-domain and swept-source optical coherence tomography angiographic imaging of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2017;58(3):1499-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Summary of baseline characteristics of patients with and without non-exudative neovascularization
eFigure 1. Left eye of a 62-year old man with non-exudative neovascularization that developed exudative changes.
eFigure 2. Right eye of a 63-year old woman that developed exudative changes from de novo