Key Points
Question
Does optical coherence tomography angiography measurement of inner macular vessel density outperform optical coherence tomography measurement of inner macular thickness in the diagnostic evaluation of glaucoma?
Findings
In a cross-sectional study including 115 patients with glaucoma and 35 healthy individuals, at 90% specificity, the sensitivity to discriminate glaucomatous eyes from healthy eyes was 60.2% for inner macular vessel density and 81.4% for inner macular thickness. The strength of the structure-function association was stronger for inner macular thickness for both linear and nonlinear regression analyses than inner macular vessel density.
Meaning
These data suggest that optical coherence tomography angiography of the macula is limited in the diagnostic evaluation of glaucoma.
Abstract
Importance
Whether optical coherence tomography angiography (OCT-A) outperforms OCT to detect glaucoma remains inconclusive.
Objective
To compare (1) the diagnostic performance for detection of glaucoma and (2) the structure-function association between inner macular vessel density and inner macular thickness.
Design, Setting, and Participants
This cross-sectional study included 115 patients with glaucoma and 35 healthy individuals for measurements of retinal thickness and retinal vessel density, segmented between the anterior boundary of internal limiting membrane and the posterior boundary of the inner plexiform layer, over the 3 × 3-mm2 macula using swept-source OCT. All participants were Chinese. Visual sensitivity corresponding to the 3 × 3-mm2 macular region was expressed in unlogged 1/lambert for investigation of the structure-function associations. Diagnostic performance was evaluated with area under the receiver operating characteristic curves (AUCs). The study was conducted between January 12, 2016, and December 12, 2016.
Main Outcomes and Measures
Area under the receiver operating characteristic curve and R2 analysis.
Results
Of the 115 patients with glaucoma, 42 (36.5%) were women (mean [SD] age, 53.5 [13.4] years); of the 35 individuals with healthy eyes, 25 (71.4%) were women (age, 60.6 [5.9] years). Inner macular vessel density and thickness were 4.3% (95% CI, 2.4%-6.1%) and 21.1 μm (95% CI, 17.4-24.9 μm) smaller, respectively, in eyes with glaucoma compared with healthy eyes. The AUC of mean inner macular thickness for glaucoma detection was greater than that of mean inner macular vessel density (difference, 0.17; 95% CI, 0.01-0.31; P = .03). At 90% specificity, the sensitivity of mean inner macular thicknesses for detection of glaucoma was greater than that of mean inner macular vessel densities (difference, 29.2%; 95% CI, 11.5%-64.6%; P = .02). The strength of the structure-function association was stronger for mean inner macular thickness than mean inner macular vessel density in the linear (difference in R2 = 0.38; 95% CI, 0.22-0.54; P < .001) and nonlinear (difference in R2 = 0.36; 95% CI, 0.21-0.51; P < .001) regression models.
Conclusions and Relevance
In this study, OCT measurement of inner macular thickness shows a higher diagnostic performance to detect glaucoma and a stronger structure-function association than the currently used OCT-A measurement of inner macular vessel density. These findings may suggest that OCT-A of the macula has a limited role in the diagnostic evaluation of glaucoma.
This cross-sectional study compares the use of optical coherence tomography angiography with optical coherence tomography in the diagnosis and evaluation of inner macular thickness and inner macular vessel density in patients with glaucoma.
Introduction
Optical coherence tomography angiography (OCT-A) is a noninvasive imaging technique that has facilitated visualization of the capillary networks in different layers of the retina. The fact that the macula has the highest density of retinal ganglion cells underscores its strategic position for detection of early vascular and structural changes in glaucoma.1 Although many studies have demonstrated that OCT-A measurement of vessel density at the macula is reduced in eyes with glaucoma, the diagnostic value of OCT-A in comparison with conventional OCT measurement of inner macular thickness for detection of glaucoma remains inconclusive.2,3,4,5,6,7 In a study examining 58 glaucomatous eyes with visual field loss confined to a single hemifield, Yarmohammadi and colleagues2 found that the visual sensitivity in the perimetrically intact hemiretina had a stronger correlation with the inner macular vessel density compared with the inner macular thickness. Monitoring 32 patients with glaucoma over a mean of 13 months, Shoji and colleagues3 showed a progressive reduction of the inner macular vessel density, whereas the inner macular thickness remained unchanged.
These studies suggest the inner macular vessel density to be a more sensitive structural marker for detection of glaucoma compared with inner macular thickness. By contrast, Rao and colleagues4,5 showed a higher diagnostic performance of the inner macular thickness compared with the inner macular vessel density for detection of glaucoma. Some other studies reported no significant differences between inner macular thickness and inner macular vessel density measurements to discriminate eyes with glaucoma from healthy eyes.6,7
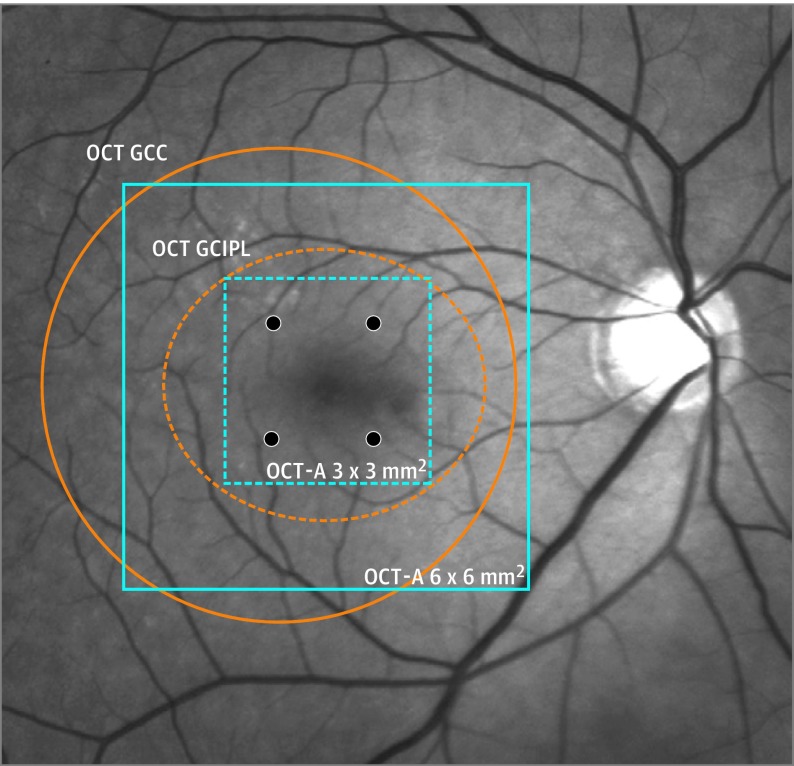
A possible explanation for the inconsistencies can be related to the fact that the region of interest selected for measurements of inner vessel density and inner retinal thickness was not in direct correspondence. Whereas the inner macular vessel density has been commonly measured in an area between 3 × 3 mm2 and 6 × 6 mm2 segmented from the anterior boundary of internal limiting membrane to the posterior boundary of the inner plexiform layer, inner macular thickness has been typically measured between the anterior boundary of the ganglion cell layer and the posterior boundary of the inner plexiform layer over an elliptical annulus centered at the fovea with outer vertical and horizontal axes of 4.0 and 4.8 mm, respectively (ie, the ganglion cell inner plexiform layer thickness) or measured between the anterior boundary of the internal limiting membrane and the posterior boundary of the inner plexiform layer over a 7-mm-diameter circle decentered from the fovea 0.75 mm temporally (ie, the ganglion cell complex thickness) (Figure 1).
Figure 1. Comparison of the Regions of Interest for Optical Coherence Tomography Angiography (OCT-A) and OCT Measurements Adopted in Clinical Studies.
The OCT-A measurements of vessel density at the macula (3 × 3-mm2 [dashed blue lines]; 6 × 6 mm2 [solid blue lines]) and OCT measurements of ganglion cell complex (GCC) (Optovue Inc) (orange circle) and ganglion cell inner plexiform layer (GCIPL) thicknesses (orange ellipse) (Carl Zeiss Meditec). The 4 visual field locations (24-2) corresponding to the 3 x 3-mm2 central macular region are highlighted in black.
The discrepancies in the segmentation of retinal layers and the selection of retinal regions for vessel density and tissue thickness measurements likely account for the divergent findings in the literature. The present study standardized the retinal layers for segmentation and the region of interest for analysis to enable a fair comparison between OCT-A and OCT measurements for detection of glaucoma. By extracting vessel density and retinal thickness data contained between the anterior boundary of the internal limiting membrane and the posterior boundary of the inner plexiform layer over the central 3 × 3-mm2 macular region, we compared (1) the diagnostic performance for detection of glaucoma and (2) the structure-function associations between inner macular thickness and inner macular vessel density measurements.
Methods
Participants
Two hundred seventy-four individuals of Chinese descent, including 228 patients with primary open-angle glaucoma and 46 people with healthy eyes, were consecutively recruited at the University Eye Center, the Chinese University of Hong Kong, between January 12, 2016, and December 12, 2016. Patients with glaucoma were recruited from the glaucoma clinic, whereas healthy individuals were recruited from the general eye clinic. The study was conducted in accordance with the ethical standards stated in the Declaration of Helsinki8 and was approved by the Kowloon Central/Kowloon East ethics committee with written informed consent obtained. There was no financial compensation for the participants.
The inclusion criteria were visual acuity of at least 20/40 Snellen without history of macular disease, neurologic disease, and refractive or retinal surgery. The participants underwent a comprehensive ophthalmic examination that included measurements of axial length (partial coherence laser interferometry; Carl Zeiss Meditec), central corneal thickness (ultrasonographic pachymetry), intraocular pressure (Goldmann applanation tonometry), biomicroscopy examination of the optic disc, and gonioscopy. All patients had retinal nerve fiber layer (RNFL) thickness, inner macular thickness, inner macular vessel density, and visual field examination performed on the same day (details described below). Glaucomatous eyes had a narrowed neuroretinal rim and optic disc excavation on slitlamp biomicroscopy with corresponding RNFL abnormalities in the OCT RNFL thickness deviation map (ie, ≥20 superpixels of RNFL thickness below the lower 99th centile of the reference ranges). Participants with healthy eyes had intraocular pressure less than 21 mm Hg and no evidence of optic disc, RNFL, macular, or visual field abnormalities in both eyes. After excluding 102 participants with poor-quality OCT-A images (described below), 19 individuals with poor-quality OCT images (image quality index <40), and 3 participants with unreliable visual field results, 115 patients with glaucoma and 35 individuals with healthy eyes were included in the analysis. If both eyes were eligible for inclusion, only the eye with a higher image quality index in the OCT-A image was included. To determine the test-retest variability of inner macular vessel density measurement, 17 participants with healthy eyes and 25 patients with glaucoma were randomly selected and invited to have an additional set of OCT-A measurements performed in the same visit.
OCT-A Measurement of Inner Macular Vessel Density
The instrument and scan protocol for OCT-A were based on a commercially available platform (Triton OCT; Topcon Corp), whereas the analysis and measurement of vessel density were performed in customized software programmed in MATLAB (MathWorks Inc). The OCT-A was performed with a volume scan (320 × 320 pixels) over a 3 × 3-mm2 macular region centered at the fovea. The distance between the B-scans and the A-scans was 9.375 and 5.0 μm, respectively. Each B-scan was consecutively scanned 4 times to measure the decorrelation signals of blood flow. The scan duration was approximately 4 seconds. The instrument software then automatically segmented the individual retinal layers in the individual B-scans and applied OCT-A ratio analysis to generate OCT-A images.9 All OCT-A images were manually reviewed to ensure correct segmentation of the B-scans. A total of 102 participants (132 images from 93 patients with glaucoma and 15 images from 9 control participants) were excluded because of poor-quality OCT-A images. The excluded images had 1 or more of the following characteristics: an image quality index less than 40, motion artifacts, segmental loss in image signal, and indistinct vessel boundaries (eFigure 1 in the Supplement). The reasons for exclusion were distributed similarly between the control and glaucoma groups (eTable 1 in the Supplement). No significant differences in demographics and biometric variables were found between the included and the excluded individuals in the control (eTable 2 in the Supplement) the glaucoma (eTable 3 in the Supplement) groups.
Retinal vessels between the anterior boundary of the internal limiting membrane and the posterior boundary of the inner plexiform layer revealed in the OCT-A images were exported to a computer for measurement of inner macular vessel density through a threshold strategy similar to that of other studies,10,11,12 using a custom program developed in MATLAB (MathWorks Inc) (eFigure 2A in the Supplement). Large retinal vessels (retinal arterioles and venules) were first detected by global thresholding on contrast-stretched gray-scale OCT-A images (eFigure 2B in the Supplement). The OCT-A images were then de-noised by applying a nonlocal means filter (eFigure 2C in the Supplement) and binarized by local adaptive thresholding for detection of presumably retinal capillaries after exclusion of the large retinal vessels (eFigure 2D in the Supplement).13,14 Binarization helps to delineate the vessel boundaries that may not be well demarcated under high magnification in the original gray-scale OCT-A images. The central elliptical region (major axis, 1.2 mm; minor axis, 1.0 mm) containing the foveal avascular zone was excluded from the measurement (eFigure 2E in the Supplement). The mean inner macular vessel density was calculated as the proportion of retinal capillaries, as determined from the binarized OCT-A images, over the 3 × 3-mm2 macular region after exclusion of the central elliptical region and large retinal vessels. Superior, superotemporal, inferotemporal, inferior, inferonasal, and superonasal inner macular vessel densities were measured from six 60° sectors in the 3 × 3-mm2 macular region (eFigure 2E in the Supplement).
OCT Measurement of Inner Macular Thickness
Inner macular thickness over the central 3 × 3-mm2 macular region was measured from the OCT wide-field scan (12 × 9 mm2, 512 × 256 pixels) using proprietary software provided by the manufacturer (OCT Data Collector; Topcon Corp). The duration of the wide-field scan was approximately 1.3 seconds. The same inner retinal layers were segmented and the same 3 × 3-mm2 macular region for measurements of mean and regional inner macular vessel densities was selected for measurements of mean and regional inner macular thicknesses after exclusion of the central elliptical region (eFigure 2F in the Supplement).
Visual Field Measurement
Perimetry was performed using the standard automatic white-on-white perimetry (Swedish Interactive Threshold Algorithm Standard, 24-2, Humphrey field analyzer II-I; Carl Zeiss Meditec). A visual field was considered reliable when the fixation losses were 20% or less and the false-positive errors were 15% or less. Only reliable visual field results were included in the analysis. The mean visual sensitivity corresponding to the same 3 × 3-mm2 macular region selected for the OCT and OCT-A measurements was calculated from taking the mean of visual sensitivity threshold values (expressed in unlogged 1/lambert) at the central 4 visual field locations.
Statistical Analysis
The test-retest variabilities of inner macular vessel density measurement were expressed in intraclass correlation coefficient and repeatability coefficient. Normality assumption was tested by using the Shapiro-Wilk test and inspecting histograms. Independent t test and Mann-Whitney test were used to compare normally distributed and nonnormally distributed data, respectively. χ2 Analysis was used to compare categorical data. A general linear model was used for pairwise comparison of inner macular vessel density and inner macular thickness between the control and the glaucoma groups after adjusting for covariates. Receiver operating characteristic (ROC) regression analysis was used to compare the diagnostic performance of inner macular vessel density and inner macular thickness measurements for discrimination between healthy and glaucomatous eyes after adjusting for covariates.15,16,17 The structure-function association was evaluated using linear and nonlinear (second-order polynomial) regression models. To compare the strength of structure-function association between inner macular vessel density and inner macular thickness, bootstrap resampling (n = 1000) was performed to generate the 95% bias-corrected CIs of coefficients of determination. All P values were 2-tailed. Statistical analyses were performed with Stata, version 14.0 (StataCorp) and R, version 3.3.2 (R Foundation for Statistical Computing).
Results
eTable 4 in the Supplement summarizes the demographics of 150 participants, including 115 patients with glaucoma (visual field mean deviation [MD] [SD], −9.4 [8.6] dB) and 35 healthy individuals (visual field MD, −0.5 [1.2] dB). The patients with glaucoma were younger (mean [SD] age, 53.5 [13.4] vs 60.6 [5.9] years) and more myopic (spherical equivalent, −4.4 [3.8] vs −1.3 [2.4] diopters [D]) than the healthy individuals (P < .001). In addition, there were more women in the healthy group (25 [71.4%] vs 42 [36.5%]). There were no significant differences between the 2 groups in the proportions of participants with diabetes or hypertension. Fifty-five patients (47.8%) had mild glaucoma (MD,≥−6 dB) (among whom 24 had preperimetric glaucoma), 27 (23.5%) had moderate glaucoma (−6>MD>12 dB), and 33 (28.7%) had advanced glaucoma (MD 12 dB or less). The test-retest variabilities of mean inner macular vessel density measurement were low. In the healthy group, the intraclass correlation coefficient was 0.877 (95% CI, 0.694-0.954) and the repeatability coefficient was 3.67% (95% CI, 2.75%-5.50%). In the glaucoma group, the coefficients were 0.924 (95% CI, 0.836-0.966) and 3.85% (95% CI, 3.02%-5.31%), respectively.
Diagnostic Performance of OCT-A and OCT Measurements for Glaucoma Detection
Inner macular vessel density was smaller in the glaucoma group (42.1 [4.9%]) than the healthy group (46.3 [4.4%]), before and after adjusting for age, axial length, sex, and image quality index (difference, 4.3%; 95% CI, 2.4%-6.1%; P < .001). Likewise, inner macular thickness was smaller in the former (77.0 [16.6] μm) than the latter (98.2 [6.4] μm) group, before and after adjusting for the covariates (difference, 21.1 μm; 95% CI, 17.4-24.9 μm; P < .001). The AUCs for discrimination between glaucomatous and healthy eyes were greater for the mean and regional inner macular thicknesses (range, 0.81-0.93) than the mean and regional inner macular vessel densities (range, 0.54-0.83) (range, P < .001 to P ≤ .04) after adjusting for age, axial length, sex, and image quality index (Table). At 90% specificity, the sensitivity was 77.0% (95% CI, 59.6%-91.7%) for the mean inner macular thickness and 47.8% (95% CI, 16.2%-64.0%) for the mean inner macular vessel density (difference, 29.2%; 95% CI, 11.5%-64.6%; P = .02). Among the regional measurements, the inferotemporal inner macular vessel density and the inferotemporal inner macular thickness showed the highest diagnostic performance for detection of glaucoma and the AUCs were 0.83 (95% CI, 0.73-0.90) and 0.93 (95% CI, 0.86-0.97), respectively (difference, 0.11, 95% CI, 0.03 to 0.23; P = .01). At 90% specificity, the inferotemporal inner macular thickness had a higher sensitivity (81.4%; 95% CI, 67.2-88.1) for detection of glaucoma than the inferotemporal inner macular vessel density (60.2%; 95% CI, 39.5-73.1) (difference, 21.2%; 95% CI, 14.1%-54.0%; P = .008). Examples of OCT-A inner macular vessel density maps and OCT inner macular thickness maps from a healthy eye (Figure 2), an eye with mild glaucoma (Figure 3), and an eye with advanced glaucoma (Figure 4) are shown.
Table. Diagnostic Performance of Inner Macular Vessel Density and Inner Macular Thickness for Discrimination Between Glaucomatous and Healthy Eyes.
| Site | Inner Macular Thickness | Inner Macular Vessel Density | P Valuea | Difference |
|---|---|---|---|---|
| Area Under the ROC Curve (95% CI) | ||||
| Mean | 0.89 (0.79-0.97) | 0.73 (0.60-0.85) | .03 | 0.17 (0.01-0.31) |
| SN | 0.81 (0.66-0.92) | 0.57 (0.46-0.73) | <.001 | 0.25 (0.12-0.40) |
| S | 0.82 (0.69-0.91) | 0.54 (0.41-0.72) | .003 | 0.28 (0.03-0.42) |
| ST | 0.85 (0.75-0.93) | 0.55 (0.37-0.73) | <.001 | 0.31 (0.10-0.50) |
| IN | 0.85 (0.72-0.94) | 0.56 (0.43-0.70) | <.001 | 0.29 (0.16-0.43) |
| I | 0.87 (0.76-0.93) | 0.75 (0.59-0.86) | .04 | 0.13 (0.02-0.31) |
| IT | 0.93 (0.86-0.97) | 0.83 (0.73-0.90) | .01 | 0.11 (0.03-0.23) |
| Sensitivity at 80% Specificity (95% CI) | ||||
| Mean | 82.3 (67.3-95.4) | 60.2 (39.8-76.5) | .04 | 22.1 (4.4-47.8) |
| SN | 67.3 (46.8-86.6) | 38.9 (24.1-56.9) | .01 | 28.4 (4.4-45.1) |
| S | 69.9 (56.4-81.6) | 31.0 (17.7-45.9) | <.001 | 38.9 (16.8-57.5) |
| ST | 70.8 (52.9-83.2) | 31.9 (14.7-47.0) | .001 | 38.9 (16.8-61.9) |
| IN | 69.9 (51.9-84.8) | 43.4 (26.9-59.8) | .001 | 26.5 (14.2-53.1) |
| I | 75.2 (56.9-86.2) | 56.6 (34.1-76.5) | .04 | 18.6 (1.8-46.9) |
| IT | 85.8 (71.2-93.1) | 74.3 (58.8-87.4) | .01 | 11.5 (5.3-39.0) |
| Sensitivity at 90% Specificity (95% CI) | ||||
| Mean | 77.0 (59.6-91.7) | 47.8 (16.2-64.0) | .02 | 29.2 (11.5-64.6) |
| SN | 59.3 (39.6-81.4) | 35.4 (21.9-56.9) | .02 | 23.9 (0.9-43.4) |
| S | 66.4 (51.2-80.0) | 26.5 (13.3-48.6) | <.001 | 39.9 (11.5-55.7) |
| ST | 67.3 (51.8-79.8) | 29.2 (15.6-50.4) | <.001 | 38.1 (18.5-61.1) |
| IN | 66.4 (48.5-83.3) | 33.6 (16.5-50.0) | <.001 | 32.8 (23.9-63.7) |
| I | 73.5 (58.7-86.8) | 51.3 (30.8-75.9) | .03 | 22.2 (2.2-50.9) |
| IT | 81.4 (67.2-88.1) | 60.2 (39.5-73.1) | .008 | 21.2 (14.1-54.0) |
Abbreviations: I, inferior; IN, inferonasal; IT, inferotemporal; ROC, receiver operating characteristic; S, superior; SN, superonasal; ST, superotemporal.
Comparisons of area under the ROC curves were performed with ROC regression analysis with adjustment for age, sex, axial length, and image quality index.
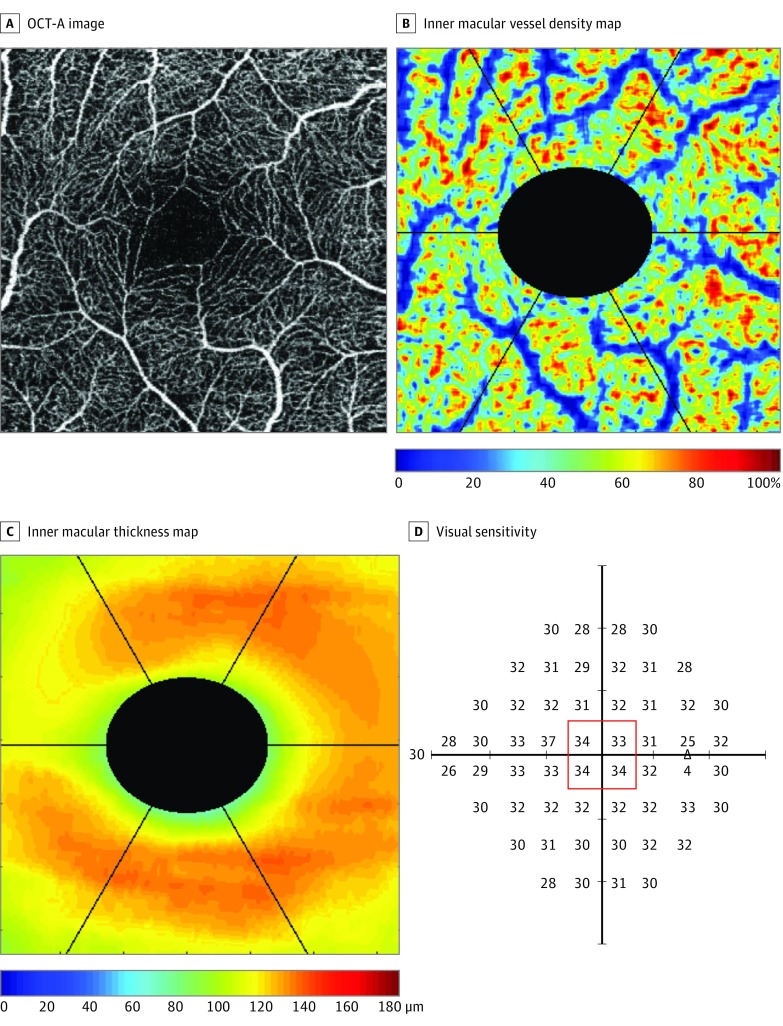
Figure 2. Example of Optical Coherence Tomography Angiography (OCT-A) and OCT Measurements in a Healthy Eye.
The OCT-A images (A), color-coded inner macular vessel density (B) and inner macular thickness (C) maps, and the corresponding visual sensitivity (outlined in red) (D) at the central 3 x 3-mm2 macular region. The triangle represents the blind spot.
Figure 3. Example of Optical Coherence Tomography Angiography (OCT-A) and OCT Measurements in an Eye With Mild Glaucoma (Visual Field Mean Deviation, −4.95 dB).
The OCT-A images (A), color-coded inner macular vessel density (B) and inner macular thickness (C) maps, and the corresponding visual sensitivity (outlined in red) (D) at the central 3 x 3-mm2 macular region. The triangle represents the blind spot.
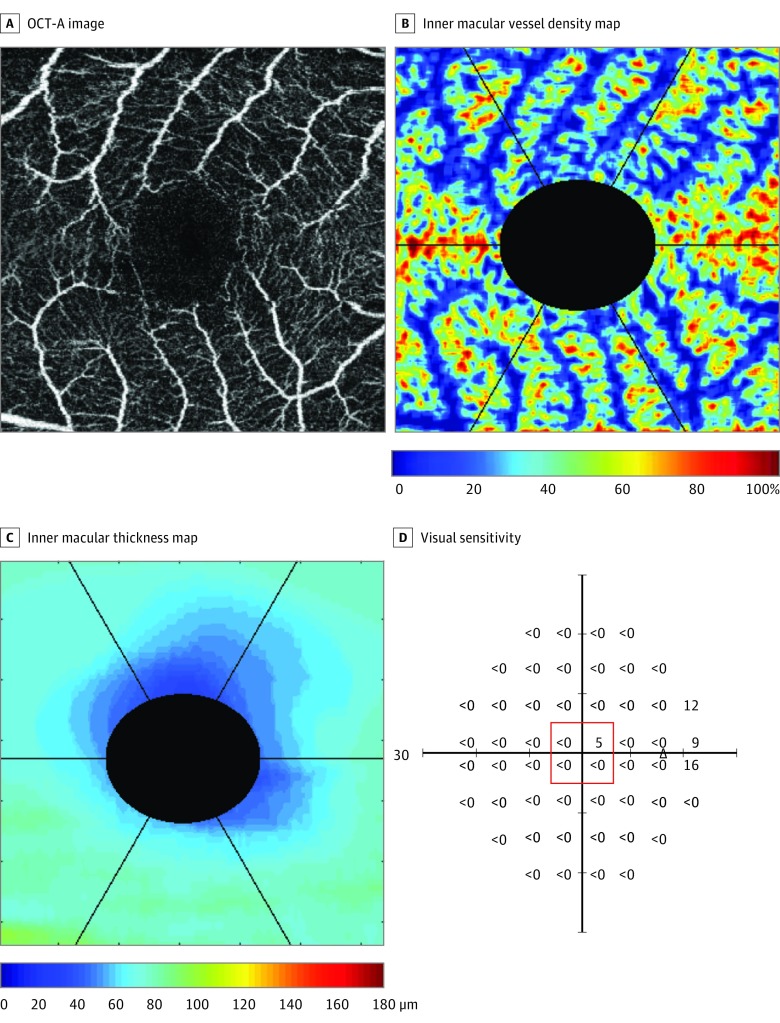
Figure 4. Example of Optical Coherence Tomography Angiography (OCT-A) and OCT Measurements in an Eye With Advanced Glaucoma (Visual Field Mean Deviation, −32.96 dB).
The OCT-A images (A), color-coded inner macular vessel density (B) and inner macular thickness (C) maps, and the corresponding visual sensitivity (outlined in red) (D), at the central 3 x 3-mm2 macular region. The triangle represents the blind spot.
Structure-Function Association of OCT-A and OCT Measurements
The mean inner macular vessel density and the mean inner macular thickness were both associated with the mean visual sensitivity over the 3 × 3-mm2 macular area in the linear and nonlinear (second-order polynomial) regression analyses (n = 150) (P < .001) (eTable 5 in the Supplement). The strength of structure-function association of the mean inner macular thickness (R2 = 0.59 for both linear and nonlinear regression analyses) was stronger compared with the mean inner macular vessel density (R2 = 0.21 for linear and R2 = 0.23 for nonlinear regression analysis) (difference, 0.38; 95% CI, 0.22-0.54; P < .001 and 0.36; 95% CI, 0.21-0.51; P < .001 for linear and nonlinear regression, respectively) (eFigure 3 in the Supplement).
Discussion
Standardizing the region of interest and the retinal layers for measurements of inner macular thickness and inner macular vessel density, we show that the former attains a higher diagnostic performance for detection of glaucoma and a stronger structure-function association than the latter measurement. Together with a shorter scan duration and a lower incidence of poor-quality images, OCT measurement of inner macular thickness is preferable to OCT-A measurement of inner macular density in the diagnostic evaluation of glaucoma.
We selected the macula for comparisons of diagnostic performance between OCT-A and OCT measurements because the macula is largely devoid of the influence of parapapillary atrophy (PPA), and the inner macular thickness has been shown to have a high discriminating power to differentiate glaucomatous from healthy eyes.18,19,20,21 The relatively low sensitivity of inner macular vessel density measurements contrasts with some studies reporting a high diagnostic performance of the macular and parapapillary vessel density for glaucoma detection.6,7,22,23,24,25,26,27 One factor accounting for the discrepancy is the application of customized software in our study to standardize the macular area and the macular layers with exclusion of the fovea to compare the diagnostic performance between inner macular vessel density and inner macular thickness. Parapapillary vessel density has been measured over a 3 × 3-mm2, 4.5 × 4.5-mm2, or 6.0 × 6.0-mm2 parapapillary region or using an elliptical annulus extended from the optic disc margin, all of which would contain beta-zone PPA (if present), which may increase with the severity of glaucoma.28,29,30 It is plausible that PPA would confound the measurement of parapapillary vessel density because segmentation of the retinal layers is often problematic over the region of PPA. For this reason, circumpapillary RNFL thickness is conventionally measured with a 3.46-mm-diameter scan circle beyond the PPA. Excluding the area of PPA may be necessary in future studies comparing the performance of OCT-A and OCT measurements at the parapapillary region for detection of glaucoma.
The strong structure-function association between inner macular thickness and visual sensitivity is not unexpected, as loss in retinal ganglion cells and their axons, signified by thinning of the inner macula, translates to loss in visual sensitivity at the corresponding region. The weaker structure-function association between inner macular vessel density and visual sensitivity suggests impairment in visual sensitivity in glaucoma to be better explained by reduction in inner macular thickness than inner macular vessel density. A long-term study with standardization of the region of interest and retinal layers for segmentation is required to ascertain the temporal sequence of change in inner macular vessel density vs inner macular thickness during the development and progression of glaucoma.
Limitations
There are limitations to this study. Projection artifacts of retinal arterioles and retinal venules always obscure the visualization and measurement of the underlying capillaries. While a number of studies have advocated the removal of large retinal vessels from vessel density calculation,11,26,27,31,32 it remains unclear whether such exclusion would affect the discriminating ability of vessel density measurement. We therefore also repeated the analysis without the removal of large retinal vessels and the conclusions of the study remain unchanged (eTables 6 and 7 in the Supplement). Our finding may not be generalizable to other populations because a considerable proportion of participants (102 of 274 [37.2%]) were excluded because of poor-quality OCT-A images. The exclusion of a higher proportion of patients from the glaucoma group (93 of 228 [40.8%]) than the healthy group (9 of 46 [19.6%]) is likely attributed to the fact that patients with glaucoma are less able to maintain fixation.33 Whereas the diagnostic performance for detection of glaucoma would probably improve had a larger region of interest been selected for analysis (eg, 6 × 6 mm2),7 the concomitant reduction in scan resolution (320 × 320 pixels over 6 × 6-mm2 vs 320 × 320 pixels over 3 × 3-mm2 pixels) would decrease the signal-to-noise ratio of the OCT-A images and underestimate vessel density measurement (eFigure 4 in the Supplement). Measurement of inner macular vessel density over the 3 × 3-mm2 area was found to have a low test-retest variability, and the adoption of the same 3 × 3-mm2 macular region has facilitated a fair comparison of diagnostic performance and structure-function association between OCT-A and OCT measurements in our study. Lastly, although patients with glaucoma were younger and more myopic than the healthy participants, age and refractive errors have been demonstrated not to affect macular vessel density measurements.34 Covariate-adjusted analyses were performed to account for the demographic differences between the healthy group and the glaucoma group.
Conclusions
With a higher diagnostic sensitivity, a stronger structure-function association, and a shorter scan duration, OCT measurement of inner macular thickness outperforms OCT-A measurement of inner macular vessel density for diagnostic evaluation of glaucoma. However, our finding does not imply that studying vessel density changes is not important in glaucoma. Optimization of the instrumentation and scan protocol to decrease motion artifacts and improve the signal-to-noise ratio of OCT-A images in longitudinal studies would be relevant to investigate whether vascular changes detected by OCT-A would be a cause or a consequence of retinal ganglion cell degeneration in glaucoma.
eFigure 1. Examples of Poor Quality Optical Coherence Tomography Angiography (OCT-A) Images
eFigure 2. Analysis of Optical Coherence Tomography Angiography (OCT-A) and OCT Images for Measurements of Inner Macular Vessel Density and Inner Macular Thickness
eFigure 3. Scatterplots Between Visual Sensitivity and Inner Macular Vessel Density/Inner Macular Thickness Measurements
eFigure 4. A Comparison Between the 6x6mm2 and 3x3mm2 Optical Coherence Tomography Angiography (OCT-A) Scan Protocols
eTable 1. Types of Poor Quality Optical Coherence Tomography Angiography Images
eTable 2. Comparisons of Demographics and Visual Field Measurements Between the Included and Excluded Subjects in the Normal Group
eTable 3. Comparisons of Demographics and Visual Field Measurements Between the Included and Excluded Subjects in the Glaucoma Group
eTable 4. Demographics, Visual Field, Optical Coherence Tomography and Optical Coherence Tomography Angiography Measurements
eTable 5. Structure Function Associations Between Mean Inner Macular Vessel Density/Mean Inner Macular Thickness and Mean Visual Sensitivity at the Central 3x3 mm2 Macular Region
eTable 6. Diagnostic Performance of Inner Macular Vessel Density (Without Removal of Retinal Arterioles and Venules) and Inner Macular Thickness for Discrimination Between Eyes With Glaucoma and Normal Healthy Eyes
eTable 7. Structure Function Association Between Mean Inner Macular Vessel Density (Without Removal of Retinal Arterioles and Venules)/Inner Macular Thickness and Mean Visual Sensitivity at the Central 3x3 mm2 Macular Region
References
- 1.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300(1):5-25. [DOI] [PubMed] [Google Scholar]
- 2.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and single-hemifield visual field defect. Ophthalmology. 2017;124(5):709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoji T, Zangwill LM, Akagi T, et al. Progressive macula vessel density loss in primary open-angle glaucoma: a longitudinal study. Am J Ophthalmol. 2017;182:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao HL, Pradhan ZS, Weinreb RN, et al. A comparison of the diagnostic ability of vessel density and structural measurements of optical coherence tomography in primary open angle glaucoma. PLoS One. 2017;12(3):e0173930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao HL, Pradhan ZS, Weinreb RN, et al. Optical coherence tomography angiography vessel density measurements in eyes with primary open-angle glaucoma and disc hemorrhage. J Glaucoma. 2017;26(10):888-895. [DOI] [PubMed] [Google Scholar]
- 6.Chen HS, Liu CH, Wu WC, Tseng HJ, Lee YS. Optical coherence tomography angiography of the superficial microvasculature in the macular and peripapillary areas in glaucomatous and healthy eyes. Invest Ophthalmol Vis Sci. 2017;58(9):3637-3645. [DOI] [PubMed] [Google Scholar]
- 7.Takusagawa HL, Liu L, Ma KN, et al. Projection-resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology. 2017;124(11):1589-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 9.Stanga PE, Tsamis E, Papayannis A, Stringa F, Cole T, Jalil A. Swept-source optical coherence tomography Angio (Topcon Corp, Japan): technology review. Dev Ophthalmol. 2016;56:13-17. [DOI] [PubMed] [Google Scholar]
- 10.Kim AY, Chu Z, Shahidzadeh A, Wang RK, Puliafito CA, Kashani AH. Quantifying microvascular density and morphology in diabetic retinopathy using spectral-domain optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT362-OCT370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geyman LS, Garg RA, Suwan Y, et al. Peripapillary perfused capillary density in primary open-angle glaucoma across disease stage: an optical coherence tomography angiography study. Br J Ophthalmol. 2017;101(9):1261-1268. [DOI] [PubMed] [Google Scholar]
- 12.Corvi F, Pellegrini M, Erba S, Cozzi M, Staurenghi G, Giani A. Reproducibility of vessel density, fractal dimension, and foveal avascular zone using 7 different optical coherence tomography angiography devices. Am J Ophthalmol. 2018;186:25-31. [DOI] [PubMed] [Google Scholar]
- 13.Buades A, Coll B, Morel JM. Image denoising methods. a new nonlocal principle. SIAM Rev. 2010;52:113-147. [Google Scholar]
- 14.Phansalkar N, More S, Sabale A, Joshi M Adaptive local thresholding for detection of nuclei in diversity stained cytology images. Paper presented at: 2011 International Conference on Communications and Signal Processing; February 10-12, 2011. [Google Scholar]
- 15.Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009;9(1):1-1. [PMC free article] [PubMed] [Google Scholar]
- 16.Janes H, Longton G, Pepe M. Accommodating covariates in ROC analysis. Stata J. 2009;9(1):17-39. [PMC free article] [PubMed] [Google Scholar]
- 17.Alonzo TA, Pepe MS. Distribution-free ROC analysis using binary regression techniques. Biostatistics. 2002;3(3):421-432. [DOI] [PubMed] [Google Scholar]
- 18.Jeoung JW, Choi YJ, Park KH, Kim DM. Macular ganglion cell imaging study: glaucoma diagnostic accuracy of spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(7):4422-4429. [DOI] [PubMed] [Google Scholar]
- 19.Mwanza JC, Budenz DL, Godfrey DG, et al. Diagnostic performance of optical coherence tomography ganglion cell–inner plexiform layer thickness measurements in early glaucoma. Ophthalmology. 2014;121(4):849-854. [DOI] [PubMed] [Google Scholar]
- 20.Kim HJ, Lee SY, Park KH, Kim DM, Jeoung JW. Glaucoma diagnostic ability of layer-by-layer segmented ganglion cell complex by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57(11):4799-4805. [DOI] [PubMed] [Google Scholar]
- 21.Pazos M, Dyrda AA, Biarnés M, et al. Diagnostic accuracy of Spectralis SD OCT automated macular layers segmentation to discriminate normal from early glaucomatous eyes. Ophthalmology. 2017;124(8):1218-1228. [DOI] [PubMed] [Google Scholar]
- 22.Jia Y, Wei E, Wang X, et al. Optical coherence tomography angiography of optic disc perfusion in glaucoma. Ophthalmology. 2014;121(7):1322-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Jia Y, Takusagawa HL, et al. Optical coherence tomography angiography of the peripapillary retina in glaucoma. JAMA Ophthalmol. 2015;133(9):1045-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes. Invest Ophthalmol Vis Sci. 2016;57(9):OCT451-OCT459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao HL, Kadambi SV, Weinreb RN, et al. Diagnostic ability of peripapillary vessel density measurements of optical coherence tomography angiography in primary open-angle and angle-closure glaucoma. Br J Ophthalmol. 2017;101(8):1066-1070. [DOI] [PubMed] [Google Scholar]
- 26.Scripsema NK, Garcia PM, Bavier RD, et al. Optical coherence tomography angiography analysis of perfused peripapillary capillaries in primary open-angle glaucoma and normal-tension glaucoma. Invest Ophthalmol Vis Sci. 2016;57(9):OCT611-OCT620. [DOI] [PubMed] [Google Scholar]
- 27.Chen CL, Zhang A, Bojikian KD, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography-based microangiography. Invest Ophthalmol Vis Sci. 2016;57(9):OCT475-OCT485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonas JB, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes; II: correlations. Invest Ophthalmol Vis Sci. 1989;30(5):919-926. [PubMed] [Google Scholar]
- 29.Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105(8):1541-1545. [DOI] [PubMed] [Google Scholar]
- 30.Yamada H, Akagi T, Nakanishi H, et al. Microstructure of peripapillary atrophy and subsequent visual field progression in treated primary open-angle glaucoma. Ophthalmology. 2016;123(3):542-551. [DOI] [PubMed] [Google Scholar]
- 31.Chen CL, Bojikian KD, Wen JC, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and single-hemifield visual field loss. JAMA Ophthalmol. 2017;135(5):461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee EJ, Kim S, Hwang S, Han JC, Kee C. Microvascular compromise develops following nerve fiber layer damage in normal-tension glaucoma without choroidal vasculature involvement. J Glaucoma. 2017;26(3):216-222. [DOI] [PubMed] [Google Scholar]
- 33.Birt CM, Shin DH, Samudrala V, Hughes BA, Kim C, Lee D. Analysis of reliability indices from Humphrey visual field tests in an urban glaucoma population. Ophthalmology. 1997;104(7):1126-1130. [DOI] [PubMed] [Google Scholar]
- 34.Rao HL, Pradhan ZS, Weinreb RN, et al. Determinants of peripapillary and macular vessel densities measured by optical coherence tomography angiography in normal eyes. J Glaucoma. 2017;26(5):491-497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Examples of Poor Quality Optical Coherence Tomography Angiography (OCT-A) Images
eFigure 2. Analysis of Optical Coherence Tomography Angiography (OCT-A) and OCT Images for Measurements of Inner Macular Vessel Density and Inner Macular Thickness
eFigure 3. Scatterplots Between Visual Sensitivity and Inner Macular Vessel Density/Inner Macular Thickness Measurements
eFigure 4. A Comparison Between the 6x6mm2 and 3x3mm2 Optical Coherence Tomography Angiography (OCT-A) Scan Protocols
eTable 1. Types of Poor Quality Optical Coherence Tomography Angiography Images
eTable 2. Comparisons of Demographics and Visual Field Measurements Between the Included and Excluded Subjects in the Normal Group
eTable 3. Comparisons of Demographics and Visual Field Measurements Between the Included and Excluded Subjects in the Glaucoma Group
eTable 4. Demographics, Visual Field, Optical Coherence Tomography and Optical Coherence Tomography Angiography Measurements
eTable 5. Structure Function Associations Between Mean Inner Macular Vessel Density/Mean Inner Macular Thickness and Mean Visual Sensitivity at the Central 3x3 mm2 Macular Region
eTable 6. Diagnostic Performance of Inner Macular Vessel Density (Without Removal of Retinal Arterioles and Venules) and Inner Macular Thickness for Discrimination Between Eyes With Glaucoma and Normal Healthy Eyes
eTable 7. Structure Function Association Between Mean Inner Macular Vessel Density (Without Removal of Retinal Arterioles and Venules)/Inner Macular Thickness and Mean Visual Sensitivity at the Central 3x3 mm2 Macular Region