This national UK transplant registry study examines the corneal transplant replacement survival rates for the 3 main indications and types of regraft surgery.
Key Points
Question
Is actuarial survival of replacement corneal transplants associated with primary corneal diagnosis and type of regraft donor (lamellar or penetrating)?
Findings
In a national transplant registry study in the United Kingdom of 9925 regrafts, the proportion of endothelial keratoplasty regrafts increased to 38.0% in the most recent year of analysis, and stratification of 5-year survival was found for successive grafts in the same eye. For first regrafts in keratoconus and pseudophakic bullous keratopathy but not Fuchs dystrophy, no differences in survival after lamellar and penetrating keratoplasty procedures were identified.
Meaning
Survival of replacement grafts had greater association with the number of prior grafts than primary corneal diagnosis or type of regraft procedure (penetrating or lamellar).
Abstract
Importance
An increasing proportion of corneal transplant procedures are undertaken for replacement of a failed previous graft. The proportion of lamellar transplant procedures has significantly increased. There are limited large-scale reports on regraft procedures that may help guide surgeons and patients in their choice of surgery.
Objective
To examine the corneal transplant replacement survival rates for the 3 main indications and types of regraft surgery.
Design, Setting, and Participants
This national transplant registry study examined surgery and follow-up data on all corneal transplants performed in the United Kingdom from April 1, 1999, through March 31, 2016.
Main Outcomes and Measures
Actuarial regraft 5-year survival rates were compared for the 3 main indications and types of graft: penetrating keratoplasty (PK) and deep anterior lamellar keratoplasty for keratoconus, PK and endothelial keratoplasty (EK) for Fuchs endothelial dystrophy (FED), and pseudophakic bullous keratopathy (PBK).
Results
A total of 9925 regrafts were analyzed during the 17-year study period. Penetrating keratoplasty represented 7261 cases (73.2%) in the cohort. Endothelial keratoplasty increased by 1361.5%, from 12 (2.6%; 95% CI, 1.3%-4.5%) of all 467 regrafts during 2005-2006 to 292 (38.0%; 95% CI, 34.6%-41.6%) of 768 during 2015-2016. The median time to first regraft for all graft types was 28 months (interquartile range, 10-64 months). When examining all graft types performed for all indications, stratification of 5-year survival was found for successive grafts, with a difference in survival of 25 270 (72.5%; 95% CI, 71.7%-73.2%) from the first graft to 4224 (53.4%; 95% CI, 51.4%-55.4%) from the second graft and 1088 (37.3%; 95% CI, 33.4%-41.3%) from the second to third graft. For first regrafts in keratoconus and PBK, survival after lamellar and PK procedures was similar. For FED, there was a higher regraft survival after PK (375 [70.8%]; 95% CI, 64.6%-76.1%) compared with EK (303 [54.7%]; 95% CI, 45.8%-62.8%) (P < .001). For FED and PBK, there was no difference in first regraft survival identified between EK followed by PK vs PK followed by PK or EK followed by EK vs PK followed by EK.
Conclusions and Relevance
In this large registry-based analysis of corneal regraft survival, regraft survival was found to vary with indication for first graft surgery and for FED with type of regraft procedure performed. For FED and PBK, the permutation of graft and subsequent first regraft procedure were not associated with any survival benefit for the first regraft. These reported outcomes may assist decision-making in management of a failed corneal transplant.
Introduction
During the past 2 decades, there has been international adoption of lamellar transplant procedures, in particular endothelial keratoplasty (EK), as an alternative to full-thickness penetrating keratoplasty (PK). Surgeons now have options as to which type of transplant and transplant replacement procedure to offer patients, whether PK, deep anterior lamellar keratoplasty (DALK), Descemet stripping automated endothelial keratoplasty (DSAEK), or Descemet membrane endothelial keratoplasty (DMEK). Although failed previous corneal grafts account for approximately 20% of PK procedures undertaken1 and it is well established that replacement PK survival rates decrease with successive PK regraft procedures,1,2,3,4 little information is available from large data sets on lamellar replacement transplants for common corneal disorders. One single-center study5 analyzed 113 eyes that underwent a second PK or EK after a failed PK and in which the primary corneal diagnosis was pseudophakic bullous keratopathy (PBK). Endothelial keratoplasty provided better regraft survival outcomes compared with PK regraft (86% vs 51% survival at 5 years), with a second PK being a significant risk factor for graft failure compared with DSAEK. In contrast, an additional single-center study6 of 112 eyes undergoing regraft for a failed first therapeutic PK for infectious keratitis reported that EK and PK regraft survival was not statistically different. Keane et al7 reported outcomes from 400 eyes in which a second graft (PK or EK) had been performed after a failed first PK, indicating that a second PK may offer better graft survival rates than EK in keratoconus (KC) or PBK. Kitzmann et al8 evaluated the outcomes of 17-second PKs vs 7 DSAEKs for PK grafts that failed because of corneal edema and found that there was no statistically significant difference in the graft survival rates at 3 years between the groups (58% [second PK] vs 69% [DSAEK], P = .51). However, they observed better postoperative visual acuity, a lower postoperative complication rate, and a higher graft survival rate in the DSAEK group compared with the second PK group. Mitry et al9 reported that DSAEK after a failed PK had an overall 5-year graft survival rate of approximately 50%, which was comparable to that after an additional PK, and found that a rejection episode before PK failure was a marker of subsequent DSAEK rejection and failure. Several other studies10,11,12,13,14,15,16 have also examined EK (DSAEK and DMEK) as an option for treating a failed PK.
In their meta-analysis examining EK vs second PK after failed PK in the 4 cohort studies cited above,5,6,7,8 Wang et al17 concluded that EK demonstrated a significantly lower risk of graft rejection than second PK but that there were no significant differences observed in graft survival or visual acuity. Feizi et al18 reported that the 11 PK regrafts and 1 DALK regraft in their KC patient group who had undergone initial DALK were clear at a mean (SD) of approximately 4 (2) years of follow-up from their regraft operation and concluded that there was no difference in visual outcomes between eyes with clear first grafts and regraft eyes that underwent PK or additional DALK. Additional DMEK has also been reported as a feasible regraft management option for primary DMEK failure, achieving acceptable visual outcomes.19
This study was an exploratory investigation of survival of replacement penetrating or lamellar corneal transplants for the 3 most common primary disease indications: KC, Fuchs endothelial dystrophy (FED), and PBK. Lamellar transplants have quantitatively less alloantigen than full-thickness donor corneas and would, for this reason, be expected to have higher rates of survival. Patients would also have lower risk of allorecognition and immunologic rejection. Current limitations in research are that survival of regrafts is often based on a small number of transplants. The benefit of this study is that it extracted large-scale data from the UK Transplant Registry, a national transplant database. This analysis could help inform decision making by surgeons and patients in regard to the type of regraft with the highest likelihood of survival.
Methods
Data are collected by National Health Service Blood and Transplant (NHSBT) on all corneal transplants performed in the United Kingdom from a transplant surgery record form and follow-up forms at 1, 2, and 5 years after transplant. The provision of these data is a requirement of all surgeons undertaking corneal transplantation in the United Kingdom. Data collection commenced on April 1, 1999, and this study analyzed all regrafts performed from April 1, 1999, through March 31, 2016. In cases in which there was a significant difference in regraft survival among graft types, any grafts that failed within the first 6 months were excluded and an analysis was performed again. Patients were excluded if they received a graft in the fellow eye because it is known that such grafts can have a different survival profile.20 After submission of the proposed analysis by the NHSBT, the research was approved by the Audit and Clinical Research subgroup of the Ocular Tissue Advisory Group. Patients gave general consent for use of their data for research and governance purposes.21 Data were not deidentified, but all analysis was performed by the NHSBT and data were not shared.
All statistical analyses were performed using SAS Enterprise Guide software, version 6.1 (SAS Institute Inc). Regrafts analyzed were those in the main 3 primary indications for corneal transplant and the main 3 types of graft, as appropriate: PK and DALK for KC, PK and EK for FED, and PK and EK for PBK. Kaplan-Meier estimates were used to compare differences in graft survival at 5 years between graft number and graft type for KC, FED, and PBK. Survival estimates are quoted along with 95% CIs. Differences in survival were tested using a log-rank test.
Results
During the 17-year study period from April 1, 1999, through March 31, 2016, a total of 37 098 primary grafts and 9925 regrafts were reported. Regrafts accounted for a mean of 584 (21.1%) of the total corneal transplants in this study period. The median time to first regraft for all graft types was 28 months (interquartile range, 10-64 months). The most common cause of failure of the first graft was endothelial decompensation (defined as endothelial failure that occurs in the absence of observed complications to which it might be secondary, eg, rejection or glaucoma) (n = 1060 [36.4%]), followed by irreversible rejection (defined as failure to restore graft transparency with a high-frequency topical corticosteroid) (n = 601 [20.6%]) and primary graft failure (defined as failure of graft function within 1 month of transplantation) (n = 438 [15.0%]).
The number of regrafts increased during the study period from 370 during 1999-2000 to more than 700 during each of the last 6 years. Penetrating keratoplasty remained the most common regraft type during the 17-year period, accounting for 7261 regrafts (73.2%). There was an increase in the number and proportion of regrafts by EK, and overall this regraft type represented 1710 regrafts (17.2%) during the 17-year period. Few EK regrafts were performed in the first few years of this study, increasing by 1361.5% from only 12 (2.6%; 95% CI, 1.3%-4.5%) of 467 regrafts during 2005-2006 to 292 (38.0%; 95% CI, 34.6%-41.6%) of 768 regrafts during 2015-2016. Few DALK procedures were performed as a regraft, with 5 in the first reported year (1999-2000) and then fluctuating between 11 and 35 per year thereafter.
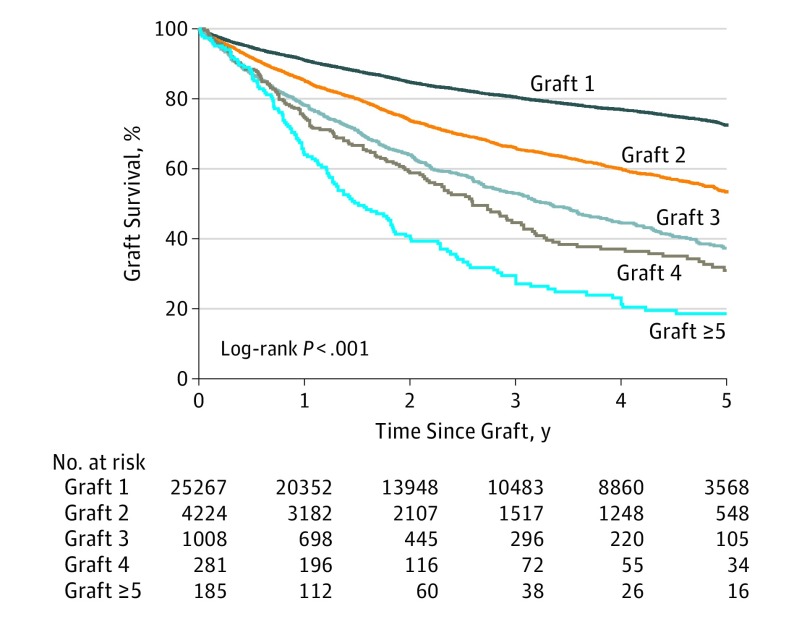
When examining all graft types performed for all indications, stratification of 5-year survival was found for successive regrafts in the same eye (Figure 1). There was a difference in survival between the first grafts of 25 270 (72.5%; 95% CI, 71.7%-73.2%) compared with 4224 (53.4%; 95% CI, 51.4%-55.4%) of the second grafts (P < .001), 1008 (37.3%; 95% CI, 33.4%-41.3%) of the second and third grafts (P < .001), 281 (30.9%; 95% CI, 24.2%-37.9%) of the fourth grafts, and 1185 (18.6%; 95% CI, 12.5%-25.6%) of the fifth or subsequent grafts (P < .001). There was no difference in survival between the third and fourth graft (Figure 1).
Figure 1. Five-Year Graft Survival According to Number of Grafts in the Same Eye, Including Penetrating and Lamellar Grafts for All Indications .
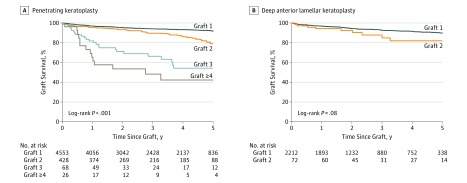
For survival after PK according to the number of previous grafts for KC, there was a difference in 5-year graft survival between first grafts of 4553 (91.9%; 95% CI, 90.9%-92.9%), second grafts of 428 (79.1%; 95% CI, 73.1%-83.9%), and second and third grafts of 68 (54.2%; 95% CI, 38.5%-67.5%) (P < .001); there was no difference in survival between the third and fourth or subsequent grafts of 26 (42.2%; 95% CI, 21.8%-61.3%) for PK grafts performed because of similar outcomes in the first year (P = .16). There was no difference in 5-year survival of second grafts (72 [82.0%]; 95% CI, 67.2%-90.6%) compared with first DALK transplants in KC (2212 [89.8%]; 95% CI, 87.9%-91.4%) (P = .08) (Figure 2).
Figure 2. Five-Year Survival of Transplants for Keratoconus (KC) According to Number of Grafts in the Same Eye .
Regrafts for FED
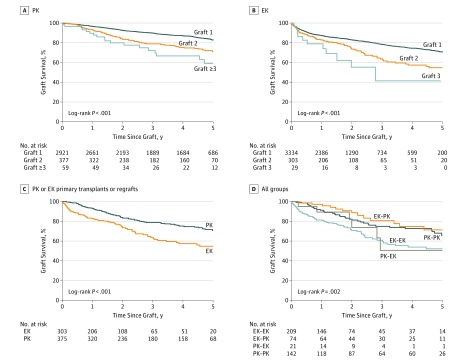
For FED, 5-year survival after PK was lower, with 2921 (82.9%; 95% CI, 81.2%-84.4%) after first grafts and 377 (71.0%; 95% CI, 64.9%-76.3%) after second grafts (P < .001), but survival after second grafts and third grafts (59 [59.1%]; 95% CI, 41.6%-72.9%) was not different (P = .12) (Figure 3A). Five-year survival after EK graft for FED also differed from 3336 (70.8%; 95% CI, 68.2%-73.3%) for first grafts to 303 (54.7%; 95% CI, 45.8%-62.8%) for second grafts (P < .001). Survival for third grafts was 29 (41.4%; 95% CI, 14.3%-67.1%) but was not different when compared with second grafts (P = .13), probably because of the small number of third grafts (11 failed grafts and 29 third grafts) (Figure 3B).
Figure 3. Five-Year Survival of First Transplants and Regrafts for Fuchs Endothelial Dystrophy (FED) According to Number of Grafts in the Same Eye.
A, Survival rates after penetrating keratoplasty (PK). B, Survival rates after first, second, and third PK. C, Survival in groups in which primary transplants and regrafts were performed by PK or endothelial keratoplasty (EK). D, Survival after first regrafts for the 4 permutations of primary and regraft procedures (eg, PK followed by PK [PK-PK]).
For first regrafts performed for FED, there was longer regraft survival among eyes that underwent PK compared with EK regrafts (Figure 3C). This analysis was performed again for first regraft PK vs first regraft EK, in which grafts that failed within the first 6 months after transplant were excluded. Thus, failures that may have been caused by surgeon inexperience were excluded. Survival in the PK group was not different compared with that in the EK group, with 5-year survival being 368 grafts (72.1%; 95% CI, 65.9%-77.4%; failed = 75) for PK and 269 grafts (61.8%; 95% CI, 51.8%-70.3%; failed = 53) for EK (P = .07).
In analysis of FED, 5-year graft survival for first regraft as analyzed by sequential keratoplasty type, for example, for EK followed by PK (EK-PK) vs PK followed by PK (PK-PK), there was no difference in survival between the 74 eyes that underwent EK-PK (71.4%; 95% CI, 54.6%-82.9%) and the 142 eyes that underwent PK-PK (65.6%; 95% CI, 54.6%-74.6%) (P = .30). This result was also the case for the 209 eyes that underwent EK-EK (52.2%; 95% CI, 41.9%-61.5%) compared with the 21 eyes that underwent PK-EK (50.6%; 95% CI, 18.6%-75.9%) (P = .80) (Figure 3D).
Regrafts for PBK
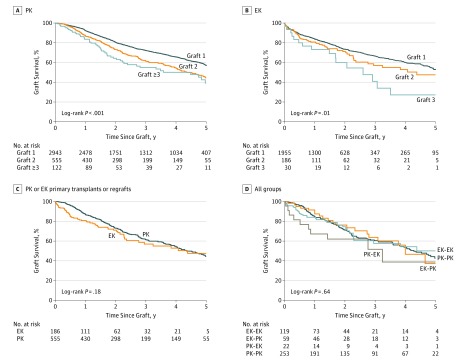
For PBK, there was a difference in 5-year survival after PK between 2943 (57.1%; 95% CI, 54.7%-59.4%) for first PK grafts and 555 (44.5%; 95% CI, 38.7%-50.0%) for second PK grafts (P < .001). However, there was no difference in survival between the second PK grafts and third PK grafts (122 [39.2%] 95% CI, 26.6%-51.6%) (P = .17) (Figure 4A). There was no also no difference in 5-year survival after EK grafts for PBK between first EK grafts (1956 [53.0%]; 95% CI, 48.8%-57.0%) and second EK grafts (186 [47.5%]; 95% CI, 35.0%-59.0%) or between second grafts and third EK grafts (30 [27.2%]; 95% CI, 9.3%-49.1%) (P = .09); however, similar to the case with FED, there were only a small number of third EK procedures (16 failed grafts, 30 EK grafts) (Figure 4B).
Figure 4. Survival of First Transplants and Regrafts for Pseudophakic Bullous Keratopathy (PBK) According to Number of Grafts in the Same Eye.
A, Five-year survival of the first, second, and third grafts after penetrating keratopathy (PK). B, Survival of the first, second, and third grafts after endothelial keratoplasty (EK). C, Survival of groups in which primary and regrafts were performed by PK or EK. D, Survival of first regrafts for the 4 permutations of primary and regraft procedures (eg, PK followed by PK [PK-PK]).
In eyes that underwent transplantation for PBK and in which the primary and regraft were the same type, there was no difference in 5-year survival of the first regraft between PK and EK (Figure 4C). There was no difference in survival after first regraft in eyes that underwent EK-PK (59 [37.2%]; 95% CI, 16.7%-58.0%) and eyes that underwent PK-PK (252 [42.6%]; 95% CI, 34.1%-50.9%) (P = .85). This finding was also true for eyes that underwent EK-EK (119 [50.1%]; 95% CI, 34.4%-63.9%) and those that underwent PK-EK (22 [38.8%]; 95% CI, 12.4%-65.0%) (P = .30) (Figure 4D).
Discussion
This registry-based analysis of corneal regraft survival by indication and procedure type included more than 700 regrafts performed per annum in each of the last 6 years of the data collection period and a total of 9925 regrafts. This study provides new large-scale registry data on regraft survival that can help guide surgeons and patients in their choice of surgery in conjunction with clinical studies examining regraft survival. In this study, we examined graft survival as the outcome measure. Although a surviving and functioning graft is a prerequisite for good visual potential, in consideration of prior grafts with endothelial failure, the speedier visual rehabilitation and other benefits of EK surgery are important advantages for patients in whom EK grafts survive. In the United Kingdom, as elsewhere, the proportion of EK procedures has steadily increased, from 2.6% during 2005-2006 to 38.0% during 2015-2016 (representing an increase of 1361.5%). Penetrating keratoplasty remains the most common corneal regraft procedure performed in the United Kingdom, accounting for 57% of corneal regrafts during 2015-2016. Although surgeon decision making in the context of a failed graft in an eye with good visual potential is made on a case-by-case basis and informed by the surgeon’s own experience, large database studies with long-term procedure outcomes will have increasing value as the number of regrafts increases over time.
It may seem intuitive that accelerated rejection would occur in successive allografts, and we found that, if not primarily attributable to rejection in all cases, at least survival was shortened with successive allografts in the overall regraft population (Figure 1). However, after subsets categorized by primary corneal diagnosis or type of transplant were examined, the association of graft number with survival of successive grafts was less clear when considering third or subsequent grafts. Although 5-year survival after PK for KC decreased from first to second graft and from second to third graft, there was no reduction in survival after DALK for KC from first to second graft, possibly because of the small number of DALK regrafts.
Five-year survival after PK and EK differed from first to second graft for FED, but there was no difference in survival from second to third graft after PK for KC. For FED, unlike PBK, there was a benefit in regraft survival after PK compared with EK. Because EK techniques were not widely adopted by UK corneal surgeons until the later years of the analysis period, one possible explanation for this finding may be that complications associated with surgeon inexperience in EK as a graft replacement procedure have a greater influence on survival than the reduced alloantigen load of a DSAEK or DMEK graft.22 However, as acknowledged above, functioning EK grafts or EK regrafts are generally associated with faster visual rehabilitation compared with PK, and surgeons should consider this factor and others in conjunction with likely graft survival in decision making on surgical management of a failed first graft for FED.
For PBK, survival outcomes were broadly similar to those for FED. Although there was stratification of graft survival according to the number of grafts, as for regrafts for FED, 5-year survival after PK decreased from the first to second graft but not from the second to third graft. As for FED, in eyes that underwent surgery for PBK, there was no difference in first regraft survival between eyes that underwent EK-PK vs PK-PK or EK-EK vs PK-EK. This finding suggests that for FED and PBK, the permutation of graft and subsequent first regraft surgical procedure is not associated with survival benefit in the first regraft. Keane et al7 found that graft survival was reduced in the PK-EK group compared with the PK-PK group in eyes in which the original indication was KC or PBK but not FED, whereas Ang et al5 found that for PBK, PK-PK was a significant risk factor for graft failure compared with PK-EK. In contrast, our study found that for PBK and FED, there was no difference in graft survival between the PK-EK and PK-PK groups.
Limitations
This analysis gives an overview from a large data set and differs in part from published single-center reports. However, despite the large sample size in this report, some groups and surgery permutations were small (eg, 21 for PK-EK for FED and 22 for PK-EK for PBK). Furthermore, registry studies of this type are limited in general by censoring because of within-study losses to follow-up and terminal censoring. Graft failure events were also not exactly located in serial or secular time because of uneven graft follow-up intervals, and there were differences between patient groups and in receiving data from surviving vs failed grafts.7 In this study, we used statistical methods, such as the Kaplan-Meier survival log-rank analysis, to help reduce these limitations in interpreting the available data.
There are also limitations to extrapolating this study to populations outside the United Kingdom. International differences in the proportions of different ethnic groups compared with the United Kingdom may be one example of a factor that could influence graft and regraft outcomes.23 One further shortcoming of registry studies of this type is that collection of detailed and reliable information on factors such as topical corticosteroid treatment is not feasible. For this reason, an analysis of the influence of topical corticosteroid treatment on corneal regraft survival is beyond the scope of this report. A multivariate analysis of risk factors known to affect graft survival (eg, glaucoma, corticosteroid use, and surgeon grade) is beyond the scope of this descriptive article. Such individual patient factors must also be taken into account by surgeons in their decision making in the context of a failed graft in an eye with good visual potential.
Conclusions
This registry-based analysis indicates stratification of survival according to number of prior transplants in that eye. Regraft survival varied with indication for first graft surgery and in FED with type of regraft procedure performed. In both FED and PBK, the permutation of graft and subsequent first regraft surgical procedure did not confer any survival benefit in the first regraft.
References
- 1.Williams KA, Keane MC, Galettis RA, Jones VJ, Mills RAD, Coster DJ. The Australian Corneal Graft Registry 2015 Report. Adelaide, Australia: Flinders University; 2015. [Google Scholar]
- 2.Vail A, Gore SM, Bradley BA, Easty DL, Rogers CA; Corneal Transplant Follow-up Study Collaborators . Corneal graft survival and visual outcome: a multicenter study. Ophthalmology. 1994;101(1):120-127. [DOI] [PubMed] [Google Scholar]
- 3.Tan DT, Janardhanan P, Zhou H, et al. Penetrating keratoplasty in Asian eyes: the Singapore corneal transplant study. Ophthalmology. 2008;115(6):975-982, e971. [DOI] [PubMed] [Google Scholar]
- 4.Claesson M, Armitage WJ. Clinical outcome of repeat penetrating keratoplasty. Cornea. 2013;32(7):1026-1030. [DOI] [PubMed] [Google Scholar]
- 5.Ang M, Ho H, Wong C, Htoon HM, Mehta JS, Tan D. Endothelial keratoplasty after failed penetrating keratoplasty: an alternative to repeat penetrating keratoplasty. Am J Ophthalmol. 2014;158(6):1221-1227.e1. [DOI] [PubMed] [Google Scholar]
- 6.Ramamurthy S, Reddy JC, Vaddavalli PK, Ali MH, Garg P. Outcomes of repeat keratoplasty for failed therapeutic keratoplasty. Am J Ophthalmol. 2016;162:83-88.e2. [DOI] [PubMed] [Google Scholar]
- 7.Keane MC, Galettis RA, Mills RA, Coster DJ, Williams KA; for Contributors to the Australian Corneal Graft Registry . A comparison of endothelial and penetrating keratoplasty outcomes following failed penetrating keratoplasty: a registry study. Br J Ophthalmol. 2016;100(11):1569-1575. [DOI] [PubMed] [Google Scholar]
- 8.Kitzmann AS, Wandling GR, Sutphin JE, Goins KM, Wagoner MD. Comparison of outcomes of penetrating keratoplasty versus Descemet’s stripping automated endothelial keratoplasty for penetrating keratoplasty graft failure due to corneal edema. Int Ophthalmol. 2012;32(1):15-23. [DOI] [PubMed] [Google Scholar]
- 9.Mitry D, Bhogal M, Patel AK, et al. Descemet stripping automated endothelial keratoplasty after failed penetrating keratoplasty: survival, rejection risk, and visual outcome. JAMA Ophthalmol. 2014;132(6):742-749. [DOI] [PubMed] [Google Scholar]
- 10.Price FW, Price MO, Arundhati A. Descemet stripping automated endothelial keratoplasty under failed penetrating keratoplasty: how to avoid complications. Am J Ophthalmol. 2011;151(2):187-188, e182. [DOI] [PubMed] [Google Scholar]
- 11.Anshu A, Price MO, Price FW Jr. Descemet’s stripping endothelial keratoplasty under failed penetrating keratoplasty: visual rehabilitation and graft survival rate. Ophthalmology. 2011;118(11):2155-2160. [DOI] [PubMed] [Google Scholar]
- 12.Heitor de Paula F, Kamyar R, Shtein RM, Sugar A, Mian SI. Endothelial keratoplasty without Descemet stripping after failed penetrating keratoplasty. Cornea. 2012;31(6):645-648. [DOI] [PubMed] [Google Scholar]
- 13.Nottage JM, Nirankari VS. Endothelial keratoplasty without Descemet’s stripping in eyes with previous penetrating corneal transplants. Br J Ophthalmol. 2012;96(1):24-27. [DOI] [PubMed] [Google Scholar]
- 14.Chaurasia S, Murthy S, Ramappa M, Mohamed A, Garg P. Outcomes of Descemet’s stripping endothelial keratoplasty in eyes with failed therapeutic penetrating keratoplasty. Acta Ophthalmol. 2014;92(2):167-170. [DOI] [PubMed] [Google Scholar]
- 15.Heinzelmann S, Böhringer D, Eberwein P, Lapp T, Reinhard T, Maier P. Descemet membrane endothelial keratoplasty for graft failure following penetrating keratoplasty. Graefes Arch Clin Exp Ophthalmol. 2017;255(5):979-985. [DOI] [PubMed] [Google Scholar]
- 16.Lavy I, Liarakos VS, Verdijk RM, et al. Outcome and histopathology of secondary penetrating keratoplasty graft failure managed by Descemet membrane endothelial keratoplasty. Cornea. 2017;36(7):777-784. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Zhang T, Kang YW, He JL, Li S-M, Li S-W. Endothelial keratoplasty versus repeat penetrating keratoplasty after failed penetrating keratoplasty: a systematic review and meta-analysis. PLoS One. 2017;12(7):e0180468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feizi S, Javadi MA, Khajuee-Kermani P, Jafari R. Repeat keratoplasty for failed deep anterior lamellar keratoplasty in keratoconus: incidence, indications, and outcomes. Cornea. 2017;36(5):535-540. [DOI] [PubMed] [Google Scholar]
- 19.Baydoun L, van Dijk K, Dapena I, et al. Repeat Descemet membrane endothelial keratoplasty after complicated primary Descemet membrane endothelial keratoplasty. Ophthalmology. 2015;122(1):8-16. [DOI] [PubMed] [Google Scholar]
- 20.Steger B, Curnow E, Cheeseman R, et al. ; National Health Service Blood and Transplant Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Audit Study 21) . Sequential bilateral corneal transplantation and graft survival. Am J Ophthalmol. 2016;170:50-57. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.Greenrod EB, Jones MN, Kaye S, Larkin DF. Center and surgeon effect on outcomes of endothelial keratoplasty versus penetrating keratoplasty in the United Kingdom. Am J Ophthalmol. 2014;158(5):957-966, e951. [DOI] [PubMed] [Google Scholar]
- 23.Ling JD, Mehta V, Fathy C, et al. Racial disparities in corneal transplantation rates, complications, and outcomes. Semin Ophthalmol. 2016;31(4):337-344. [DOI] [PubMed] [Google Scholar]