Key Points
Question
Does the quality of total mesorectal excision plane affect clinical outcomes in patients with rectal cancer that was treated with preoperative chemoradiotherapy and adjuvant treatment?
Findings
In this secondary end point analysis of a phase 3 randomized clinical trial, the quality of the total mesorectal excision plane was an independent prognostic factor for the local recurrence in rectal cancer.
Meaning
These data add high-level clinical evidence on the importance of pathology work-up and surgical skills in rectal cancer.
Abstract
Importance
Previous retrospective studies have shown that surgical quality affects local control in rectal cancer..
Objective
In this secondary end point analysis, we evaluated the prognostic effect of the total mesorectal excision (TME) plane in the CAO/ARO/AIO-04 phase 3 randomized clinical trial.
Design, Setting, and Participants
The CAO/ARO/AIO-04 trial enrolled 1236 patients with cT3-4 and/or node-positive rectal adenocarcinoma from 88 centers in Germany between July 25, 2006, and February 26, 2010.
Interventions
Patients were randomized to receive treatment with standard fluorouracil-based preoperative chemoradiotherapy (CRT) alone (control arm) or oxaliplatin (experimental arm) followed by TME and adjuvant chemotherapy.
Main Outcomes and Measures
The TME quality (mesorectal, intramesorectal, and muscularis propria plane) was prospectively assessed in 1152 operation specimens. An assessment was performed independently by pathologists and surgeons. The results were correlated with clinicopathologic data and the clinical outcome was tested, including multivariable analysis with the Cox regression model.
Results
Of 1152 German Caucasian participants, 332 (28.8) were women and the mean age was 63 years. The plane of TME was mesorectal in 930 patients (80.7%), intramesorectal in 169 (14.7%), and muscularis propria in 53 (4.6%). In a univariable analysis, the TME plane was significantly associated with 3-year disease-free survival (mesorectal vs intramesorectal vs muscularis propria, 95% CI, 73.1-78.8 vs 61.6-76.0 vs 55.6-81.3, respectively; P = .01), cumulative incidence of local and distant recurrences (mesorectal vs intramesorectal vs muscularis propria, 95% CI, 2.0-4.5 vs 1.2-8.1 vs 2.5-20.5, respectively; P < .001; and mesorectal vs intramesorectal vs muscularis propria, 95% CI, 17.0-22.4 vs 18.3-32.0 vs 14.2-39.0, respectively; P = .03, respectively), and overall survival (mesorectal vs intramesorectal vs muscularis propria, 95% CI, 88.3-92.3 vs 79.7-91.0 vs 81.6-98.7, respectively; P = .02). In contrast to the pathologist-based evaluation, the assessment of TME plane by the operating surgeon failed to demonstrate prognostic significance for any of these clinical end points. In a multivariable analysis, the plane of surgery (mesorectal vs muscularis propria TME) constituted an independent factor for local recurrence (P = .002).
Conclusions and Relevance
This phase 3 randomized clinical trial confirms the long-term clinical effect of TME plane quality on local recurrence, as initially reported in the MRC CR07 study. The data highlight the key role of pathologists and surgeons in the multidisciplinary management of rectal cancer.
Trial Registration
ClinicalTrials.gov Identifier: NCT00349076.
This secondary analysis of a randomized clinical trial investigates the prognostic role of the total mesorectal excision plane quality in patients with rectal cancer who were treated within the CAO/ARO/AIO-04 phase 3 randomized clinical trial.
Introduction
Randomized clinical trials have shown that preoperative chemoradiotherapy (CRT) or short-course radiotherapy (RT) before surgery improve local control in locally advanced rectal cancer.1,2,3 Total mesorectal excision (TME) constitutes the current surgical treatment of choice for rectal cancer.4 The pathology studies by Quirke and colleagues5,6,7 have demonstrated variability in the completeness of resected TME specimens. Three grades of TME quality have been defined, including the mesorectal plane (good-quality, complete, and smooth), intramesorectal plane (moderate-quality, nearly complete, and moderately irregular), and muscularis propria plane (poor-quality, incomplete, and severely irregular).6 Several studies have revealed that the quality of TME greatly determines long-term local control, but most studies consisted of retrospective series, whereas, to our knowledge, phase 3 trial evidence was only provided in the CR07 study.8,9,10,11,12,13
The clinical relevance of the circumferential resection margin (CRM) after TME in rectal cancer has been described in several series.8,14,15,16,17 In a meta-analysis of more than 17 500 patients, Nagtegaal and Quirke18 showed that a tumor involvement of CRM (≤1 mm) was a predictor for both local and distant recurrence and overall survival (OS).18 The Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY) study showed that high-resolution magnetic resonance imaging (MRI) enabled accurate staging, including the detection of CRM involvement that strongly correlated with worse disease-free survival (DFS) and local control rates.19
However, most previous reports were characterized by a relatively small sample size and retrospective nature, and phase 3 trial confirmation of the long-term effect of TME quality on local control, as initially reported in the CR07 trial, is lacking. In this study, we aimed to investigate the association of TME quality with clinical and pathologic parameters and its association with the clinical outcome as part of the large CAO/ARO/AIO-04 phase 3 trial. This randomized clinical trial assessed the clinical effect following the addition of oxaliplatin to 5-fluorouracil–based preoperative CRT and adjuvant chemotherapy.20,21 The quality of TME was recorded prospectively in both arms of the trial using the system by Quirke and colleagues.5,6,7
Methods
Study Design and Participants
The CAO/ARO/AIO-04 trial was a multicenter, 2-arm randomized phase 3 study. Its design and details have been previously reported.20,21 The trial had approval by the local ethics committee of the University of Erlangen, Germany. Participants provided written informed consent. A description is shown in the eMethods in Supplement 1 and the eFigure 1 in Supplement 1 illustrates the treatment plan. The full trial protocol is provided in Supplement 2.
Pathologic Examination of Resected Specimen
A standardized pathology protocol for resected specimens was developed by the committee of reference pathologists (C.W., A.H., and P.S.).20 Preparation, processing and analysis of the resected specimens has been reported in detail before.22,23 The quality of TME plane (mesorectal, intramesorectal, and muscularis propria plane) was scored according to Quirke et al.8 Examples of TME planes are shown in eFigure 2 in Supplement 1. In addition to the pathologist-based evaluation, the quality of TME plane was also prospectively scored by the operating surgeon. A central pathology review of resected specimen was not performed. The follow-up process has been described in detail in eMethods in Supplement 1.
Statistical Analysis
Disease-free survival was defined as the time between patient randomization and either the formation of a macroscopically visible tumor following surgery (R2 resection), locoregional recurrence after an R0/1 resection, distant metastases or progression, or death from any cause, whichever occurred first. The cumulative incidence of locoregional and distant recurrences were defined as the time between patient randomization and the occurrence of any locoregional or distal recurrence, irrespective of whether this was a first event. The time from patient randomization to death from any cause was used to calculate OS.
The correlation of TME plane with clinicopathologic factors was assessed using the χ2 test. This test was used to test the association of pathologist-based evaluation with surgeon-based evaluation of TME plane. A univariable analysis using the log-rank test was performed to investigate the prognostic value of TME, the treatment arm, pretreatment clinicopathologic factors, and postsurgical pathologic factors. The hazard ratios (HRs) and corresponding P values of the TME intergroup comparisons of intramesorectal vs mesorectal TME and muscularis propria vs mesorectal TME were calculated using a Cox regression model.
A multivariable analysis for OS, DFS, locoregional, and distant recurrence was conducted using Cox regression models. Patients with missing values in 1 or more of the variables (TME, treatment arm, local resection status, or pathologic stage) were excluded. A P value of less than .05 was considered significant. Statistical analyses were performed with SPSS, version 20 (SPSS Inc) and R, version 3.4 (R Foundation).
Results
Patient Characteristics and Association of TME With Clinicopathologic Factors
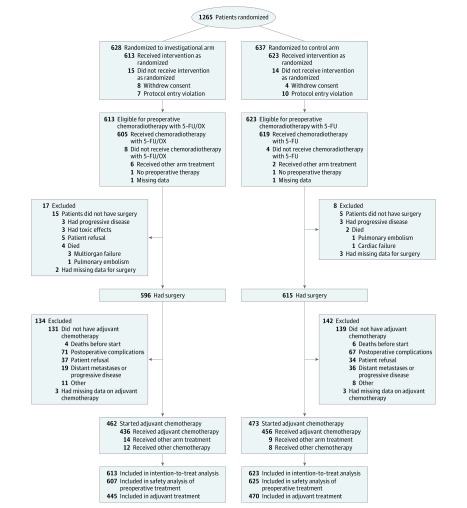
In total, 1265 patients were recruited for trial between July 25, 2006, and February 26, 2010. The Consolidated Standards of Reporting Trials diagram is shown in Figure 1. Total mesorectal excision quality as assessed by the pathologist was available for 1152 patients (eTable 1 in Supplement 1). The plane of surgery was comparable in the 2 treatment arms (mesorectal vs intramesorectal vs muscularis propria: 5-fluorouracil [FU], 80.8% vs 14.3% vs 4.9%, respectively; 5-FU + oxaliplatin, 80.7% vs 15.1% vs 4.3%, respectively; P = .82). Total mesorectal excision quality was significantly worse in patients with a more advanced cT category (mesorectal vs intramesorectal vs muscularis propria: cT2, 78.7% vs 21.3% vs 0%, respectively; cT3, 81.9% vs 14.1% vs 4.0%, respectively; cT4, 69.2% vs 16.7% vs 14.1%, respectively; missing, 40.0% vs 16.1% vs 4.8%, respectively; P < .001), lymph node involvement (mesorectal vs intramesorectal vs muscularis propria: cN0, 85.8% vs 0.4% vs 3.8%, respectively; cN1, 79.1% vs 16.1% vs 4.8%, respectively; P = .04), and tumors in the lower third of the rectum (mesorectal vs intramesorectal vs muscularis propria: distance from the anal verge of 0-5 cm, 72.6% vs 19.9% vs 7.4%, respectively; P < .001). There was no correlation between TME plane and any of the other factors (eTable 1 in Supplement 1).
Figure 1. Consolidated Standards of Reporting Trials Diagram of the CAO/ARO/AIO-04 Phase 3 Randomized Clinical Trial.
FU indicates fluorouracil; OX, oxaliplatin.
We next examined the correlation between pathologist-based evaluation and surgeon-based evaluation of TME plane quality (eTable 2 in Supplement 1). Compared with the pathologist-based mesorectal plane TME, surgeon-based evaluation showed agreement in 827 cases (86.4%). In contrast, a significant discrepancy was observed for intermesorectal plane, as only 68 surgeon-based evaluations (56.2%) of TME plane were in agreement with the pathologist assessment. For muscularis propria, agreement in TME plane quality evaluation occurred in 15 cases (75%).
Low anterior resection was associated with a significantly better plane of surgery compared with intersphincteric or abdominoperineal resection (mesorectal vs intramesorectal vs muscularis propria: anterior resection, 86.0% vs 11.3% vs 2.7%, respectively; abdominoperineal extirpation, 67.0% vs 23.0% vs 10.0%, respectively; intersphincter resection, 86.7% vs 13.3% vs 0%, respectively; other, 64.0% vs 24.0% vs 12.0%, respectively; P < .001). Similarly, the plane of surgery was significantly worse in patients with advanced ypT category (mesorectal vs intramesorectal vs muscularis propria: ypT0, 83.6 vs 12.2% vs 4.2%, respectively; ypT1/Tis, 90.5% vs 8.3% vs 1.2%, respectively; ypT3, 78.4% vs 16.1% vs 5.6%, respectively; ypT4, 50.0% vs 30.6% vs 19.4%, respectively; P < .001) and advanced ypN category (mesorectal vs intramesorectal vs muscularis propria: ypN, 82.2% vs 13.3% vs 4.5%, respectively; ypN1, 80.2% vs 16.3% vs 3.5%, respectively; ypN2, 69.8% vs 21.9% vs 8.3%, respectively; missing, 100.0% vs 0% vs 0%, respectively; P = .04) (Table 1).
Table 1. Association of the Pathologist-Based Quality of Total Mesorectal Excision (TME) With Posttreatment Patient and Tumor Factors in Patients Who Underwent an Operation and Received Preoperative 5-Fluorouracil–Based Chemoradiotherapy With or Without Oxaliplatin.
| Characteristic | No. (%) | P Value | |||
|---|---|---|---|---|---|
| Total | Mesorectal | Intramesorectal | Muscularis Propria | ||
| Surgery method | |||||
| Anterior resection | 776 (67.4) | 667 (86.0) | 88 (11.3) | 21 (2.7) | <.001a |
| Abdominoperineal extirpation | 291 (25.3) | 195 (67.0) | 67 (23.0) | 29 (10.0) | |
| Intersphincter resection | 60 (5.2) | 52 (86.7) | 8 (13.3) | 0 (0.0) | |
| Other | 25 (2.2) | 16 (64.0) | 6 (24.0) | 3 (12.0) | |
| Local resection status | |||||
| R0 | 1102 (95.7) | 898 (81.5) | 158 (14.3) | 46 (4.2) | .18 |
| R1 | 21 (1.8) | 16 (76.2) | 5 (23.8) | 0 (0.0) | |
| R2 | 13 (1.1) | 9 (69.2) | 2 (15.4) | 2 (15.4) | |
| Missing | 16 (1.4) | 7 (43.8) | 4 (25.0) | 5 (31.2) | |
| CRMb | |||||
| ≤1 mm | 62 (5.4) | 39 (62.9) | 13 (21.0) | 10 (16.1) | <.001a |
| >1 mm | 803 (69.7) | 655 (81.6) | 117 (14.6) | 31 (3.9) | |
| pCR | 178 (15.5) | 148 (83.1) | 22 (12.4) | 8 (4.5) | |
| Missing | 109 (9.5) | 88 (80.7) | 17 (15.6) | 4 (3.7) | |
| Pathologic stage | |||||
| 0 | 185 (16.1) | 151 (81.6) | 26 (14.1) | 8 (4.3) | .11 |
| I | 296 (25.7) | 257 (86.8) | 30 (10.1) | 9 (3.0) | |
| II | 291 (25.3) | 224 (77.0) | 48 (16.5) | 19 (6.5) | |
| III | 311 (27.0) | 244 (78.5) | 53 (17.0) | 14 (4.5) | |
| IV | 53 (4.6) | 40 (75.5) | 10 (18.9) | 3 (5.7) | |
| Missing | 16 (1.4) | 14 (87.5) | 2 (12.5) | 0 (0.0) | |
| ypT category | |||||
| ypT0 | 189 (16.4) | 158 (83.6) | 23 (12.2) | 8 (4.2) | <.001a |
| ypT1/Tis | 84 (7.3) | 76 (90.5) | 7 (8.3) | 1 (1.2) | |
| ypT2 | 321 (27.9) | 269 (83.8) | 44 (13.7) | 8 (2.5) | |
| ypT3 | 522 (45.3) | 409 (78.4) | 84 (16.1) | 29 (5.6) | |
| ypT4 | 36 (3.1) | 18 (50.0) | 11 (30.6) | 7 (19.4) | |
| ypN category | |||||
| ypN0 | 798 (69.3) | 656 (82.2) | 106 (13.3) | 36 (4.5) | .04a |
| ypN1 | 257 (22.3) | 206 (80.2) | 42 (16.3) | 9 (3.5) | |
| ypN2 | 96 (8.3) | 67 (69.8) | 21 (21.9) | 8 (8.3) | |
| Missing | 1 (0.1) | 1 (100.0) | 0 (0.0) | 0 (0.0) | |
Abbreviations: 5-FU, 5-fluorouracil; CRM, circumferential resection margin; pCR, pathological complete response; TME, total mesorectal excision.
Statistically significant.
TME quality was unknown in 5 patients with CRM involvement.
Involvement of CRM (≤1 mm) was found in 35 patients (6.2%) who were treated in the standard arm and in 32 patients (6.1%) in the experimental arm. An involved CRM occurred in 39 of 930 patients (3.7%) after mesorectal TME surgery, in 13 of 169 (7.6%) after intramesorectal, and in 10 of 53 patients (18.9%) after muscularis propria, respectively (P < .001) (Table 2). Circumferential resection margin involvement occurred in 31 of 729 patients (4.3%) who received low anterior resection, 26 of 276 patients (9.4%) after undergoing abdominoperineal resection, 3 of 52 patients (5.8%) after undergoing intersphincteric resection, and in 7 of the 29 patients (24%) who underwent an operation conducted with another surgical method (P < .001).
Table 2. Association of Posttreatment Clinical and Pathologic Factors With 3-Year Outcomes After Preoperative 5-Fluorouracil Chemoradiotherapy With or Without Oxaliplatin and Surgery.
| Characteristic | No. | 3-Y DFS | 3-Y Cumulative Incidence of Distant Metastases | 3-Y Cumulative Incidence of Local Recurrences After Local R0/R1 Resection | 3-Y OS | ||||
|---|---|---|---|---|---|---|---|---|---|
| % (95% CI) | P Value | % (95% CI) | P Value | % (95% CI) | P Value | % (95% CI) | P Value | ||
| Pathologist-based TME | |||||||||
| Mesorectal | 930 | 75.9 (73.1-78.8) | .01a | 19.7 (17.0-22.4) | .03a | 3.3 (2.0-4.5) | <.001a | 90.3 (88.3-92.3) | .02a |
| Intramesorectal | 169 | 68.4 (61.6-76.0) | 25.5 (18.3-32.0) | 4.8 (1.2-8.1) | 85.1 (79.7-91.0) | ||||
| Muscularis propria | 53 | 67.2 (55.6-81.3) | 27.6 (14.2-39.0) | 12.0 (2.5-20.5) | 89.7 (81.6-98.7) | ||||
| Surgeon-based TME | |||||||||
| Mesorectal | 957 | 75.3 (72.6-78.2) | .18 | 19.7 (17.0-22.3) | .23 | 3.5 (2.3-4.7) | .10 | 89.2 (87.2-91.3) | .31 |
| Intramesorectal | 121 | 69.9 (62.0-78.8) | 24.0 (15.6-31.5) | 7.3 (2.3-12.1) | 89.5 (84.0-95.3) | ||||
| Muscularis propria | 20 | 68.8 (50.9-93.0) | 31.2 (7.0-49.1) | 5.3 (0.0-14.8) | 94.7 (85.2-100) | ||||
| Type of surgery | |||||||||
| Anterior resection | 814 | 77.4 (74.5-80.4) | <.001a | 18.0 (15.2-20.7) | .002a | 3.0 (1.8-4.3) | .01a | 90.7 (88.6-92.8) | <.001a |
| Abdominoperineal resection | 303 | 66.5 (61.2-72.2) | 28.6 (23.1-33.8) | 5.7 (2.8-8.5) | 87.0 (83.1-91.1) | ||||
| Intersphincteric | 61 | 80.8 (71.2-91.7) | 16.0 (5.8-25.1) | 5.4 (0.0-11.2) | 90.9 (83.6-98.8) | ||||
| Other | 33 | 56.5 (40.9-78.1) | 26.5 (8.5-41.0) | 12.3 (0.0-24.5) | 69.7 (53.9-90.1) | ||||
| Local resection status | |||||||||
| R0 | 1151 | 76.4 (73.9-79.0) | <.001a | 19.5 (17.1-21.9) | <.001a | 3.2 (2.1-4.3) | <.001a | 90.9 (89.1-92.6) | <.001a |
| R1 | 24 | 47.0 (30.0-73.4) | 40.1 (15.3-57.6) | 13.4 (0.0-29.8) | 54.9 (37.5-80.4) | ||||
| R2 | 15 | NA | 62.7 (25.1-81.4) | 30.4 (0.4-51.3) | 50.6 (30.1-84.8) | ||||
| CRM | |||||||||
| ≤1 mm | 67 | 41.4 (30.8-55.6) | <.001a | 45.9 (31.8-57.1) | <.001a | 22.8 (10.2-33.6) | <.001a | 68.9 (58.1-81.7) | <.001a |
| >1 mm | 834 | 72.0 (68.9-75.2) | 23.3 (20.3-26.3) | 3.2 (1.9-4.5) | 88.9 (86.7-91.2) | ||||
| pCR | 185 | 94.2 (90.7-97.8) | 2.9 (0.4-5.4) | 0.0 (0.0-0.0) | 96.5 (93.7-99.3) | ||||
| ypT category | |||||||||
| ypT0 | 197 | 92.9 (89.3-96.7) | <.001a | 3.9 (1.0-6.6) | <.001a | 0.0 (0.0-0.0) | <.001a | 96.2 (93.4-99.0) | <.001a |
| ypT1/Tis | 86 | 86.5 (79.4-94.3) | 10.1 (3.2-16.5) | 1.3 (0.0-3.8) | 95.0 (90.2-99.9) | ||||
| ypT2 | 343 | 83.1 (79.0-87.3) | 12.7 (9.0-16.4) | 1.9 (0.4-3.4) | 93.6 (91.0-96.4) | ||||
| ypT3 | 538 | 62.6 (58.5-67.0) | 32.0 (27.7-36.0) | 6.0 (3.8-8.1) | 85.1 (81.9-88.3) | ||||
| ypT4 | 43 | 42.8 (30.1-60.9) | 44.1 (26.2-57.6) | 22.8 (7.5-35.6) | 62.7 (49.3-79.7) | ||||
| ypN category | |||||||||
| ypN0 | 839 | 82.7 (80.1-85.4) | <.001a | 12.5 (10.2-14.8) | <.001a | 2.2 (1.2-3.3) | <.001a | 92.6 (90.8-94.5) | <.001a |
| ypN1 | 264 | 63.8 (57.9-70.2) | 32.1 (25.9-37.9) | 4.0 (1.4-6.5) | 88.8 (84.9-93.0) | ||||
| ypN2 | 102 | 32.8 (24.8-43.6) | 58.8 (47.6-67.7) | 20.3 (11.1-28.6) | 64.1 (55.1-74.6) | ||||
| Pathologic stage | |||||||||
| 0 | 193 | 94.4 (91.1-97.8) | <.001a | 2.8 (0.3-5.2) | <.001a | 0.0 (0.0-0.0) | <.001a | 96.6 (94.0-99.3) | <.001a |
| I | 316 | 89.0 (85.5-92.6) | 7.9 (4.7-10.9) | 0.7 (0.0-1.6) | 95.6 (93.2-98.0) | ||||
| II | 302 | 72.8 (67.7-78.2) | 20.4 (15.4-25.1) | 3.7 (1.4-6.0) | 89.5 (85.9-93.2) | ||||
| III | 323 | 63.3 (58.0-69.0) | 30.6 (25.1-35.8) | 7.8 (4.6-10.8) | 86.0 (82.1-90.1) | ||||
| IV | 54 | NA | NA | 11.0 (1.4-19.7) | 50.5 (38.1-67.0) | ||||
Abbreviations: 5-FU, 5-fluorouracil; CRM, circumferential resection margin; DFS, disease-free survival; NA, not applicable; OS, overall survival; pCR, pathological complete response; TME, total mesorectal excision.
Statistically significant.
Prognostic Value of TME for Clinical Outcomes
The median follow-up was 50 (interquartile range, 38-61) months. Treatment in the experimental arm was a predictor for a superior 3-year DFS (5-FU CRT vs 5-FU/oxaliplatin CRT, 71.2% vs 75.9%, respectively; P = .03), the cumulative incidence of distant metastases (23.1% vs 19.2%; P = .04), and the cumulative incidence of local recurrences after R0/R1 resection (4.9% vs 3.2%; P = .01). A significantly better 3-year DFS and cumulative incidence of distant metastases correlated significantly with an Eastern Cooperative Oncology Group (ECOG) performance status of 0 (ECOG 0 vs ECOG 1 +2, 76.8% vs 63.4%, respectively, and 19.0% vs 29.15, respectively; P < .001 for both), early cT category (cT2 vs cT3 vs cT4, 77.7% vs 74.8% vs 56.7%, respectively, and 12.9% vs 20.4% vs 34.85, respectively; P < .001 for both) and low tumor grading (G1 vs G2 vs G3, 68.2% vs 75.7% vs 56.8%, respectively, and 21.8% vs 19.4% vs 35.9%, respectively; P < .001 and P = .001, respectively), whereas tumors located 0 to 5 cm from the anus were associated with a higher metastatic propensity (0-5 cm vs 5-10 cm vs >10 cm, 25.3% vs 19.2% vs 18.8%, respectively; P = .04). Age that was younger or equal to the median (age <63.6 years vs >63.6 years, 86.1% vs 90.5%, respectively; P = .01), an ECOG performance status of 0 (ECOG 0 vs ECOG 1 + 2, 90.8% vs 79.3%, respectively; P < .001), early cT category (cT2 vs cT3 vs CT4, 87.7% vs 89.1% vs 80.3%, respectively; P = .01), and low tumor grading (G1 vs G2 vs G3, 93.1% vs 89.9% vs 71.7%, respectively; P < .001) were associated with significantly better 3-year OS. A higher incidence of local recurrence was observed in patients with worse ECOG status (ECOG 0 vs ECOG 1+ 2, 3.4% vs 7.2%, respectively; P = .01) (eTable 3 in Supplement 1).
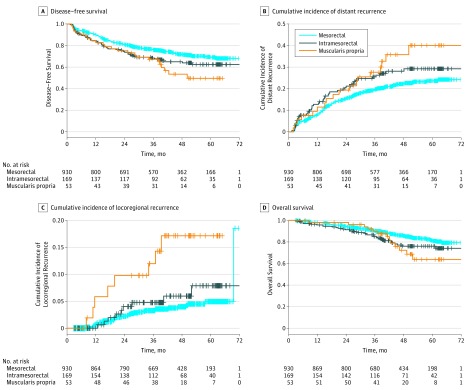
We next examined the prognostic role of post-CRT patient and tumor factors for all 4 clinical end points in a univariable analysis (Table 3). The quality of TME plane was significantly associated with 3-year DFS (mesorectal vs intramesorectal vs muscularis propria, 75.9% vs 68.4% vs 67.2%, respectively; P = .01, Figure 2A) the 3-year cumulative incidence of distant metastases (mesorectal vs intramesorectal vs muscularis propria, 19.7% vs 25.5% vs 27.6%, respectively; P = .03; Figure 2B), local recurrence after undergoing an R1/R0 resection (mesorectal vs intramesorectal vs muscularis propria, 3.2% vs 4.8% vs 12.0%, respectively; P < .001, Figure 2C) and OS (mesorectal vs intramesorectal vs muscularis propria, 90.3% vs 85.1% vs 89.7%, respectively; P = .02, Figure 2D). Notably, mesorectal vs muscularis propria TME was associated with significantly lower local recurrence rates (HR = 4.19; P < .001), whereas local recurrence after mesorectal TME was not significantly different compared with intramesorectal quality (HR = 1.47; P = .30). In total, 185 patients (16.0%) presented with pathological complete response but the univariate analysis failed to demonstrate a significant association of TME quality with either DFS (mesorectal vs intramesorectal vs muscularis propria TME, 93.2 % vs 86.4% vs 87.5%, respectively; P = .75), the cumulative incidence of distant metastases (mesorectal vs intramesorectal vs muscularis propria TME, 3.4% vs 9.1% vs 12.5%, respectively; P = .41), local recurrence (mesorectal vs intramesorectal vs muscularis propria TME, 4.1% vs 4.5% vs 12.5%, respectively; P = .12) or OS (mesorectal vs intramesorectal vs muscularis propria TME, 95.9% vs 100% vs 87.5%, respectively; P = .28) in this subgroup.
Table 3. Multivariate Analysis of Different Covariables on 3-Year Outcomes After Preoperative 5-Fluorouracil Chemoradiotherapy With or Without Oxaliplatin and Surgerya.
| Characteristic | DFS | Cumulative Incidence of Distant Metastases | Cumulative Incidence of Local Recurrences After Local R0/R1 Resection | OS | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Pathologist-based TME | ||||||||
| Mesorectal vs Intramesorectal | 1.15 (0.86-1.56) | .35 | 1.18 (0.84-1.65) | .34 | 1.06 (0.48- 2.32) | .89 | 1.39 (0.94-2.06) | .10 |
| Mesorectal vs Muscularis propria | 1.55 (0.98-2.48) | .06 | 1.41 (0.83-2.41) | .20 | 3.72 (1.59- 8.71) | .002 | 1.37 (0.72-2.58) | .34 |
| ypN category | ||||||||
| ypN+ vs ypN0 | 2.28 (1.81-2.88) | <.001 | 2.87 (2.20-3.74) | <.001 | 2.54 (1.40- 4.63) | .002 | 1.99 (1.45-2.75) | <.001 |
| ypT category | ||||||||
| ypT3 vs ypT0-2 | 2.07 (1.61-2.66) | <.001 | 2.34 (1.74-3.15) | <.001 | 1.89 (0.95- 3.75) | .07 | 2.14 (1.50-3.05) | <.001 |
| ypT4 vs ypT0-2 | 3.75 (2.19-6.42) | <.001 | 3.69 (1.95-6.98) | <.001 | 8.14 (2.99-22.14) | <.001 | 4.23 (2.11-8.50) | <.001 |
| Treatment arm | ||||||||
| 5-FU/Ox-CRT vs 5-FU CRT | 0.76 (0.61-0.95) | .016 | 0.80 (0.62-1.03) | .08 | 0.43 (0.23- 0.80) | .01 | 0.93 (0.68-1.26) | .64 |
| Resection status | ||||||||
| R1-2 vs R0 | 4.21 (2.73-6.51) | <.001 | 2.13 (1.23-3.69) | .01 | 4.75 (1.90-11.89) | <.001 | 3.26 (1.85-5.75) | <.001 |
Abbreviations: CRT, chemoradiotherapy; DFS, disease-free survival; HR, hazard ratio; OS, overall survival; Ox, oxaliplatin; TME, total mesorectal excision; 5-FU, 5-fluorouracil.
Patients with R2-resection were also included in the multivariate analysis for cumulative incidence of distant metastases and overall survival.
Figure 2. Prognostic Significance of the Quality of Total Mesorectal Excision (TME) Plane in Rectal Cancer.
A, Disease-free survival (intramesorectal vs mesorectal TME: hazard ratio [HR], 1.35; 95% CI, 1.01-1.80; P = .04; muscularis propria vs mesorectal TME: HR, 1.73; 95% CI, 1.13-2.66; P = .01; global P = .01). B, Cumulative incidence of distant metastases (intramesorectal vs mesorectal TME: HR, 1.34; 95% CI, 0.97-1.86; P = .08; muscularis propria vs mesorectal TME: HR, 1.69; 95% CI, 1.04-2.75; P = .03; global P = .03). C, Cumulative incidence of local recurrence (intramesorectal vs mesorectal TME: HR, 1.47; 95% CI, 0.71-3.06; P = .3; muscularis propria vs mesorectal TME: HR, 4.19; 95% CI, 1.94-9.05; P = .0003; global P < .001). D, Overall survival according to the TME plane (intramesorectal vs mesorectal TME: HR, 1.52; 95% CI, 1.04-2.21; P = .03; muscularis propria vs mesorectal TME: HR, 1.77; 95% CI, 1.00-3.14; P = .05; global P = .02). A univariable analysis was performed using the log-rank test. The HRs and corresponding P values of the TME intergroup comparisons (intramesorectal vs mesorectal TME, indicated by the blue lines; muscularis propria vs mesorectal TME, indicated by the red lines) were calculated using a Cox regression model.
Furthermore, abdominoperineal resection was associated with significantly worse 3-year DFS (anterior resection vs abdominoperineal resection vs intersphincteric vs other, 77.4% vs 66.5% vs 80.8% vs 56.5%, respectively; P < .001), cumulative incidence of distant (presenter resection vs abdominoperineal resection vs intersphincteric vs other, 18.0% vs 28.6% vs 16.0% vs 26.5%, respectively; P = .002) and local recurrence (anterior resection vs abdominoperineal resection vs intersphincteric vs other, 3.0% vs 5.7% vs 5.4% vs 12.3%, respectively; P = .01), and OS (anterior resection vs abdominoperineal resection vs intersphincteric vs other, 90.7% vs 87.0% vs 90.9% vs 69.7%, respectively; P < .001) compared with low anterior resection or intersphincteric resection. Local resection status (R0 vs R1 vs R2: DFS, 76.4% vs 47.0%; distant metastases, 19.5% vs 40.1% vs 62.7%; local recurrences, 3.2% vs 13.4% vs 30.4%; overall survival, 90.9% vs 54.9% vs 50.6%), CRM (<1 mm vs >1 mm vs pCR: DFS, 41.4% vs 72.0% vs 94.2%; distant metastases, 45.9% vs 23.3% vs 2.9%; local recurrences, 22.8% vs 3.2% vs 0.0%; overall survival, 68.9% vs 88.9% vs 96.5%), ypT (ypT0 vs ypT1/ypT2 vs ypT3 vs ypT4: DFS, 92.9% vs 86.5% vs 62.6% vs 42.8%; distant metastases, 3.9% vs 10.1% vs 12.7% vs 32.0% vs 44.1%; local recurrences, 0% vs 1.3% vs 1.9% vs 6.0% vs 22.8%; overall survival, 96.2% vs 95.0% vs 93.6% vs 85.1% vs 62.7%), ypN (ypN0 vs ypN1 vs ypN2: DFS, 82.7% vs 63.8% vs 32.8%; distant metastases, 12.5% vs 32.1% vs 58.8%; local recurrences, 2.2% vs 4.0% vs 20.3%; overall survival, 92.6% vs 88.8% vs 64.1%), and pathologic stage 0 vs 1 vs 2 vs 3 vs 4: DFS, 94.4% vs 89.0% vs 72.8% vs 63.3%; distant metastases, 2.8% vs 7.9% vs 20.4% vs 30.6%; local recurrences, 0% vs 0.7% vs 3.7% vs 7.8% vs 11.0%; overall survival, 96.6% vs 95.6% vs 89.5% vs 86.0% vs 50.5%) comprised the prognostic factors for all 4 clinical end points (P < .001 in each case) (Table 2).
In the multivariable analysis (Table 3), the plane of surgery (mesorectal vs muscularis propria TME) was an independent factor for the cumulative incidence of local recurrence (mesorectal vs muscularis propria: HR, 3.72; 95% CI, 1.59-8.71; P = .002). Notably, we excluded CRM, as several cases were either missing or unknown. Advanced ypT category (ypT3 vs ypT0-2 and ypT4 vs ypT0-2) was an adverse prognostic factor for 3-year DFS (HR, 2.07; 95% CI, 1.61-2.66 and HR, 3.75; 95% CI, 2.19-6.42, respectively; P < .001 for both), the cumulative incidence of distant metastases (HR, 2.34; 95% CI, 1.74-3.15 and HR, 3.69; 95% CI, 1.95-6.98, respectively; P < .001 for both), the cumulative incidence of local recurrence (HR, 1.89; 95% CI, 0.95-3.75 and HR, 8.14; 95% CI, 2.99-22.14, respectively; P = .07 and P < .001, respectively), and OS (HR, 2.14; 95% CI, 1.50-3.05 and HR, 4.23; 95% CI, 2.11-8.50, respectively; P < .001, respectively). Advanced ypN category (ypN+ vs ypN0) was associated with a less favorable outcome for all 4 clinical end points (HR, 2.28; 95% CI, 1.81-2.88; HR, 2.87; 95% CI, 2.20-3.74; HR, 1.99; 95% CI, 1.45-2.75; and HR, 2.54; 95% CI, 1.40-4.63, respectively; P < .001 in all except cumulative incidence of local recurrence, which was P = .002). Adding oxaliplatin to 5-FU–based treatment correlated with better DFS (HR, 0.76; 95% CI, 0.61-0.95; P = .02) and a lower incidence of local recurrences after R0/R1 resection (HR, 0.43; 95% CI, 0.23-0.80; P = .01). Complete resection status was associated with significantly superior outcomes for all 4 clinical end points. We failed to detect any further significance in multivariable analyses (Table 3).
As CRM has been reported to majorly affect local control,18 we performed an additional multivariable analysis that also included CRM on exclusion of all missing CRM cases to avoid statistical bias (eTable 4 in Supplement 1). Importantly, TME plane quality remained an independent prognostic factor for local recurrence (mesorectal vs muscularis propria: HR, 2.62; 95% CI, 1.11-6.18; P = .03), whereas CRM involvement (>1 mm vs ≤1 mm) was a predictor for worse DFS (HR, 1.58; 95% CI, 1.08-2.31; P = .02) and local recurrence (HR, 3.60; 95% CI, 1.66-7.79;P = .001).
Discussion
Total mesorectal excision represents the surgical technique of choice for treating rectal cancer.4,24 Here, we investigated the prognostic role of the TME plane quality in patients with rectal cancer who were treated within the CAO/ARO/AIO-04 phase 3 randomized clinical trial. Of the 1152 patients with known quality of TME plane, 930 (80.7%) had pathologist-confirmed mesorectal (good) quality of surgery. This aligns with data from modern clinical trials comparing open vs laparoscopic surgery that showed mesorectal TME in approximately 80% to 90% of patients25,26 and exceeds the 52% and 56% reported in the MRC CR078 and the Dutch Colorectal Cancer Group9 phase 3 studies. Leonard et al10 reported a mesorectal TME plane in 875 of 1382 resections (63%) based on the Belgian PROCARE database. Data similar to the PROCARE analysis were also reported by Leite et al13 and Maslekar et al.12 The difference could in part be attributed to the different chronological periods of study conductance, as surgical training has evolved significantly during the last decade.
The roles of surgery and pathologists are key, as they both influence treatment decisions and clinical outcomes in rectal cancer.27 In accordance with previous studies, TME was an independent prognostic factor for local recurrence. Although several reports have previously demonstrated the importance of an optimal TME plane for clinical outcomes, to our knowledge, only the CR07 trial by Quirke and colleagues8 has reported the prognostic effect of TME plane within a phase 3 study. Short-course radiotherapy resulted in a reduced local recurrence rate by more than 50% for all 3 TME planes, whereas the reduction in local recurrence rate postradiotherapy was most pronounced in the mesorectal TME plane group (HR = 4.5), indicating that patients benefit mostly when the best surgical procedure is combined with preoperative radiotherapy.8
In the comparison of pathologist-based vs surgeon-based evaluation of TME quality, we found a relatively high agreement (86.4%) in the mesorectal TME group but a significant discrepancy for intermesorectal plane (56.2%), emphasizing the importance of pathologist training for accurate prognostication of surgical outcomes.27,28,29 To our knowledge, such a direct prospective comparison of pathologist-based vs surgeon-based evaluation of TME quality has not been reported before.
An advanced pathologic stage and abdominoperineal resection correlated with a worse quality of surgery in this trial. Some studies, including the MRC trial, have reported a lack of correlation between the pathologic stage and/or surgical technique with the plane of surgery, indicating that surgical skills greatly determine the pathologic outcome,8 whereas other series have found strong association.30 The variability observed could be attributed to the different cohorts and the training of individual surgeons.4,31,32 Also, there is an ongoing discussion as to whether more extensive surgical procedures, such as posterior pelvic exenterations, should be considered for bulky tumors of the lower third of rectum.11,16,30
We failed to observe a significant association between TME plane quality and OS in a multivariable analysis. Nagtegaal et al9 have revealed OS rates of 86% and 76% for mesorectal and muscularis propria plane, respectively (P < .05) in univariable analysis, whereas this association was not assessed in the multivariable analysis. A study by Maslekar et al12 did not find a significant association between surgical quality and OS.
Circumferential resection margin involvement strongly correlated with worse clinical outcomes in our cohort, in line with the meta-analysis data found for more than 17 000 patients by Quirke and Nagtegall.18 In that report, involved CRM was found to be more common in patients with advanced stage, ulcerative growth patterns, poor differentiation, vascular invasion, poor TME quality, abdominoperineal resection for tumors of lower third of rectum, and female sex.18 It has been hypothesized that CRM positivity possibly reflects more aggressive tumors that fail to respond to preoperative CRT. In the MERCURY study, Taylor et al19 used MRI for precise disease staging and demonstrated an adverse clinical association with positive CRM. Notably, in our series, the quality of TME plane remained an independent prognostic factor for local recurrence in the separate multivariable analysis that also included CRM.
Limitations
We acknowledge the limitations of our study. First, there was no central pathology review. The PROCARE study compared the quality of TME specimens as assessed by a central review panel and local pathologists for more than 250 patients with rectal cancer33 and found no major discrepancies, whereas the prediction of clinical outcomes was comparable. Hence, under the prerequisite of adequate pathologist training, a central review may not have led to a significant correction of individual pathology reports.28,33 Second, baseline MRI results were not compulsory in this trial and the relevance of MRI-defined staging in the context of TME quality could not be explored. Third, the trial was conducted during a period when open surgery was still standard in Germany, whereas laparoscopic or robotic surgery were only scarcely performed; hence, comparison of the outcome after open vs laparoscopic (or robotic) surgery cannot be conducted in our series.
Conclusions
Good-quality TME was associated with significantly better local control independently of other clinicopathologic factors, including CRM. This phase 3 randomized clinical trial confirms the long-term clinical association of TME plane quality with local recurrence as initially reported in the MRC CR07 study.8 Our secondary end point analysis adds high-level clinical evidence and highlights the roles of surgeons and pathologists in managing rectal cancer. Continuous training should be encouraged to further enhance surgical skills.
eMethods.
eFigure 1. Treatment schedule of the CAO/ARO/AIO-04 randomized phase 3 trial.
eFigure 2. Total mesorectal excision (TME) quality in surgical specimens. Examples of (a) mesorectal plane (TME quality: “good”) with complete and smooth surface and without coning; (b) intramesorectal plane (TME quality “moderate”) with moderately irregular surface; (c) muscularis propria plane (TME quality “poor”) with severely irregular surface.
eTable 1. Association of the pathologist-based quality of TME with pretreatment patient and tumor factors in operated patients receiving preoperative 5-FU based chemoradiotherapy with or without oxaliplatin
eTable 2. Correlation of pathologist-based with surgeon-based TME quality
eTable 3. Impact of pre-treatment clinical and pathologic factors on 3-year outcomes after preoperative 5-FU chemoradiotherapy +/- Oxaliplatin and surgery
eTable 4. Multivariate analysis of different covariables on 3-year outcomes after preoperative 5-FU chemoradiotherapy +/- oxaliplatin and surgery
Trial Protocol
References
- 1.Sauer R, Becker H, Hohenberger W, et al. ; German Rectal Cancer Study Group . Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):-. [DOI] [PubMed] [Google Scholar]
- 2.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. ; Dutch Colorectal Cancer Group . Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638-646. [DOI] [PubMed] [Google Scholar]
- 3.Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23(24):5644-5650. [DOI] [PubMed] [Google Scholar]
- 4.Orsini RG, Wiggers T, DeRuiter MC, et al. The modern anatomical surgical approach to localised rectal cancer. EJC Suppl. 2013;11(2):60-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quirke P. Training and quality assurance for rectal cancer: 20 years of data is enough. Lancet Oncol. 2003;4(11):695-702. [DOI] [PubMed] [Google Scholar]
- 6.Quirke P, Palmer T, Hutchins GG, West NP. Histopathological work-up of resection specimens, local excisions and biopsies in colorectal cancer. Dig Dis. 2012;30(suppl 2):2-8. [DOI] [PubMed] [Google Scholar]
- 7.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2(8514):996-999. [DOI] [PubMed] [Google Scholar]
- 8.Quirke P, Steele R, Monson J, et al. ; MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group . Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373(9666):821-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH; Cooperative Clinical Investigators of the Dutch Colorectal Cancer Group . Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20(7):1729-1734. [DOI] [PubMed] [Google Scholar]
- 10.Leonard D, Penninckx F, Laenen A, Kartheuser A; PROCARE . Scoring the quality of total mesorectal excision for the prediction of cancer-specific outcome. Colorectal Dis. 2015;17(5):O115-O122. [DOI] [PubMed] [Google Scholar]
- 11.How P, Shihab O, Tekkis P, et al. A systematic review of cancer related patient outcomes after anterior resection and abdominoperineal excision for rectal cancer in the total mesorectal excision era. Surg Oncol. 2011;20(4):e149-e155. [DOI] [PubMed] [Google Scholar]
- 12.Maslekar S, Sharma A, Macdonald A, Gunn J, Monson JR, Hartley JE. Mesorectal grades predict recurrences after curative resection for rectal cancer. Dis Colon Rectum. 2007;50(2):168-175. [DOI] [PubMed] [Google Scholar]
- 13.Leite JS, Martins SC, Oliveira J, Cunha MF, Castro-Sousa F. Clinical significance of macroscopic completeness of mesorectal resection in rectal cancer. Colorectal Dis. 2011;13(4):381-386. [DOI] [PubMed] [Google Scholar]
- 14.Birbeck KF, Macklin CP, Tiffin NJ, et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann Surg. 2002;235(4):449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adam IJ, Mohamdee MO, Martin IG, et al. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344(8924):707-711. [DOI] [PubMed] [Google Scholar]
- 16.Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P; Dutch Colorectal Cancer Group; Pathology Review Committee . Low rectal cancer: a call for a change of approach in abdominoperineal resection. J Clin Oncol. 2005;23(36):9257-9264. [DOI] [PubMed] [Google Scholar]
- 17.Wittekind C, Compton C, Quirke P, et al. A uniform residual tumor (R) classification: integration of the R classification and the circumferential margin status. Cancer. 2009;115(15):3483-3488. [DOI] [PubMed] [Google Scholar]
- 18.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol. 2008;26(2):303-312. [DOI] [PubMed] [Google Scholar]
- 19.Taylor FG, Quirke P, Heald RJ, et al. ; Magnetic Resonance Imaging in Rectal Cancer European Equivalence Study Group . Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol. 2014;32(1):34-43. [DOI] [PubMed] [Google Scholar]
- 20.Rödel C, Liersch T, Becker H, et al. ; German Rectal Cancer Study Group . Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679-687. [DOI] [PubMed] [Google Scholar]
- 21.Rödel C, Graeven U, Fietkau R, et al. ; German Rectal Cancer Study Group . Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979-989. [DOI] [PubMed] [Google Scholar]
- 22.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23(34):8688-8696. [DOI] [PubMed] [Google Scholar]
- 23.Fokas E, Liersch T, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy for locally advanced rectal carcinoma revisited: updated results of the CAO/ARO/AIO-94 trial. J Clin Oncol. 2014;32(15):1554-1562. [DOI] [PubMed] [Google Scholar]
- 24.Smith JJ, Garcia-Aguilar J. Advances and challenges in treatment of locally advanced rectal cancer. J Clin Oncol. 2015;33(16):1797-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez-Pérez A, Carra MC, Brunetti F, de’Angelis N. Pathologic outcomes of laparoscopic vs open mesorectal excision for rectal cancer: a systematic review and meta-analysis. JAMA Surg. 2017;152(4):e165665. [DOI] [PubMed] [Google Scholar]
- 26.Sandhu L, Chang GJ. Which surgical approach is best for management of rectal cancer?: does the end point tell how it ends? JAMA Surg. 2017;152(4):e165659. [DOI] [PubMed] [Google Scholar]
- 27.Bosch SL, Nagtegaal ID. The importance of the pathologist’s role in assessment of the quality of the mesorectum. Curr Colorectal Cancer Rep. 2012;8(2):90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campa-Thompson M, Weir R, Calcetera N, Quirke P, Carmack S. Pathologic processing of the total mesorectal excision. Clin Colon Rectal Surg. 2015;28(1):43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagtegaal ID, West NP, van Krieken JH, Quirke P. Pathology is a necessary and informative tool in oncology clinical trials. J Pathol. 2014;232(2):185-189. [DOI] [PubMed] [Google Scholar]
- 30.Simillis C, Baird DL, Kontovounisios C, et al. A systematic review to assess resection margin status after abdominoperineal excision and pelvic exenteration for rectal cancer. Ann Surg. 2017;265(2):291-299. [DOI] [PubMed] [Google Scholar]
- 31.Martling A, Holm T, Rutqvist LE, et al. Impact of a surgical training programme on rectal cancer outcomes in Stockholm. Br J Surg. 2005;92(2):225-229. [DOI] [PubMed] [Google Scholar]
- 32.Foster JD, Gash KJ, Carter FJ, et al. Development and evaluation of a cadaveric training curriculum for low rectal cancer surgery in the English LOREC National Development Programme. Colorectal Dis. 2014;16(9):O308-O319. [DOI] [PubMed] [Google Scholar]
- 33.Demetter P, Jouret-Mourin A, Silversmit G, et al. ; PROCARE . Review of the quality of total mesorectal excision does not improve the prediction of outcome. Colorectal Dis. 2016;18(9):883-888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eFigure 1. Treatment schedule of the CAO/ARO/AIO-04 randomized phase 3 trial.
eFigure 2. Total mesorectal excision (TME) quality in surgical specimens. Examples of (a) mesorectal plane (TME quality: “good”) with complete and smooth surface and without coning; (b) intramesorectal plane (TME quality “moderate”) with moderately irregular surface; (c) muscularis propria plane (TME quality “poor”) with severely irregular surface.
eTable 1. Association of the pathologist-based quality of TME with pretreatment patient and tumor factors in operated patients receiving preoperative 5-FU based chemoradiotherapy with or without oxaliplatin
eTable 2. Correlation of pathologist-based with surgeon-based TME quality
eTable 3. Impact of pre-treatment clinical and pathologic factors on 3-year outcomes after preoperative 5-FU chemoradiotherapy +/- Oxaliplatin and surgery
eTable 4. Multivariate analysis of different covariables on 3-year outcomes after preoperative 5-FU chemoradiotherapy +/- oxaliplatin and surgery
Trial Protocol