Key Points
Question
Have there been meaningful improvements in survival rates among patients who were treated for metastatic uveal melanoma?
Finding
In this cohort study of patients who were treated between 1982 and 2009 and received a diagnosis of metastasis through 2011, there were similar differences in survival rates between patients who received treatment for metastasis and those who did not compared with patients who were treated for uveal melanoma between 1975 and 1987 who developed metastasis through 1988.
Meaning
Despite the development of new therapeutics, these findings suggest that significant treatment advances for metastatic uveal melanoma have not been made; further research to identify effective treatments is necessary.
Abstract
Importance
Despite high rates of local tumor control in patients who are treated for uveal melanoma, most patients will eventually die of metastasis. When metastasis develops, the liver is involved in most cases, and hepatic metastases are particularly refractory to treatment. Finding effective treatments has been challenging. A comparison of survival rates in patients who were treated for metastasis over approximately 30 years may offer insights into progress that has been made in prolonging survival.
Objective
To compare survival after treatment for metastasis in a cohort of patients who were treated for uveal melanoma at the Massachusetts Eye and Ear Infirmary (MEE) during an approximately 30-year period with an earlier analysis to determine if there was meaningful improvement in survival rates after treatment for metastasis.
Design, Setting, and Participants
This review included patients (n = 661) who received a diagnosis of metastasis from uveal melanoma who were identified from a cohort of 3063 patients treated at MEE between January 1982 and December 2009 and followed up through December 2011. They were compared with findings from a previous study of patients treated between 1975 and 1987.
Main Outcomes and Measures
Survival rates in patients who received treatment for metastasis were compared with those who did not receive treatment. The differences in survival rates were compared with an earlier analysis that was completed at MEE. A comparison of patients with hepatic metastases and extrahepatic metastases was also completed. Kaplan-Meier analysis was used to calculate survival rates and the log rank test was used to test for statistically significant differences between the groups.
Results
Of 620 patients with race information available, 615 (97.3%) were white; the mean (SD) age of patients was 59.71 (13.23) years and 307 (47.3%) were women. The median time from the initial treatment of the tumor to metastasis was 3.45 years (interquartile range [IQR], 2.0-5.57). Overall, the median survival time was poor (3.9 months [IQR, 1.6-10.1]). Patients who received treatment fared better than those who did not receive treatment (median survival after metastasis diagnosis, 6.3 months [IQR, 2.96-14.41] vs 1.7 months [IQR, 0.66-3.5]). This finding was similar to that of our earlier study in which median survival was 5.2 months and 2 months for treated and untreated patients, respectively.
Conclusions and Relevance
These findings suggest that advances in treatments that lead to clinically meaningful improvements in survival times have not been realized. Similar survival rates in patients who were treated for metastasis were observed in this recent analysis compared with our earlier study. Adjuvant therapies that are initiated at the time of melanoma diagnosis may be the most effective way to prolong survival.
This study compares survival rates in patients who were treated for uveal melanoma.
Introduction
Up to 50% of patients who receive a diagnosis of uveal melanoma will die of metastasis after treatment of the tumor.1,2,3 The prognosis is particularly poor for patients who exhibit clinical1 and/or genetic4 characteristics that increase risk. Overall, nearly 80% of patients with class 2 tumors, as determined by gene expression profiling (GEP), develop metastasis by 5 years after diagnosis.5 Melanoma-associated mortality depends on GEP class and tumor size; the probability of dying of metastatic melanoma by 5 years may be as low as 10% for patients with small tumors (largest basal diameter, <12 mm) and as high as 70% for patients with larger (≥12 mm in basal diameter) tumors.6 In 90% of cases, hepatic metastasis occurs.7,8
We previously evaluated survival after metastasis in a small group of patients with uveal melanoma who were treated with proton irradiation between 1975 and 1987.8 Patients who received treatment for metastasis had a somewhat longer survival time after metastasis diagnosis than patients who did not receive treatment. However, patient survival rates were low and the survival time after initial diagnosis of the tumor was similar in treated and untreated patients.8 To our knowledge, over the past 30 years, few data exist to indicate that changes in surveillance9 or treatments10,11 have led to improvements in patient outcomes after the onset of metastasis. Surveillance protocols have varied widely over this period, as have treatments for metastasis. Treatment depends on many factors, including the tumor burden at time of diagnosis, patient age, the health status of the patient, and the preferences of the treating physician and patient.10 In this article, we examined survival rates in a much larger cohort of patients who were treated with proton beam radiation over more than 30 years at a single institution (Massachusetts Eye and Ear Infirmary). The goal of our study was to determine empirically if there have been meaningful changes in survival time in patients who developed metastasis during this period.
Methods
We evaluated 3063 patients with uveal melanoma who were treated between January 1982 and December 2009 and followed up through December 31, 2011. Institutional review board approval was obtained from the Massachusetts Eye and Ear Infirmary, which included a waiver of consent for this study. Many of the patients were also participants in a uveal melanoma repository, for which they provided written consent. The median follow-up was 8.9 years (interquartile range [IQR], 1.3 months-29.6 years). A metastatic workup was completed at the time of diagnosis of the primary tumor, which included laboratory tests (alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubin, lactate dehydrogenase, gamma-glutamyl transferase [which was discontinued in 2003], and 5’- nucleotidase [which was discontinued in 2003]), and chest radiography. If abnormal results were found, further evaluation by computed tomography, magnetic resonance imaging, ultrasonography, and, as necessary, a liver biopsy was completed. The recommended surveillance protocol included an annual hepatic panel and other liver function tests. Depending on the primary care physician or oncologist providing the patient’s care, liver imaging was also performed. In patients for whom liver imaging was not routinely performed and there were elevated liver enzymes, further testing with ultrasonography, magnetic resonance imaging, or a computed tomography scan was recommended to rule out metastasis.
Data regarding metastasis were collected through active patient follow-up or medical records obtained from patients’ referring physicians. The following data were ascertained regarding metastasis: symptoms before diagnosis, the time of diagnosis, the site of metastasis, and the treatments received. For patients lost to follow-up, the cause of death was determined through the National Death Index, a federal repository that includes data from state cancer registries and is available to qualified researchers.
The time from metastasis diagnosis to death and survival rates after a metastasis diagnosis were calculated using the Kaplan-Meier method and compared between treated and untreated patients over the entire period and within each of 3 decades (1982-1991, 1992-2001, and 2002-2009). This sensitivity analysis was completed to determine how robust our overall results were and to test for possible treatment associations in subgroups during more well-defined treatment periods. P values were not adjusted for multiple analyses. Additionally, survival rates were compared between patients with hepatic (liver-only and liver + other sites) vs extrahepatic metastasis. A multivariable Cox regression analysis was completed to determine if there was an independent association of treatment with survival rates. Statistical significance was set at P = .05.
Results
Metastasis was diagnosed in 661 patients (21.6%) in the cohort. Of these, 12 patients (1.8%) developed metastasis within 6 months of diagnosis of the primary tumor and were excluded from analysis. This was done because it was likely that the metastasis was present at the time of diagnosis in these cases, and the decision to treat the patient for metastasis may have been influenced by this. The mean (SD) age of patients was 59.71 (13.23) years, and 307 (47.3%) were women. Of 620 patients with race information available, 615 (97.3%) were white.
The date of metastasis was unavailable for 87 patients (13.2%). Of the remaining 562 patients, the median time to metastasis after proton irradiation was 3.45 years (IQR, 2-5.56). The median survival time after metastasis diagnosis was 3.9 months (IQR, 1.64-10.07). Overall, the 1-year survival rate was 21.2% and the 3-year survival rate was 4.3%, and only 62 patients (11.1%) survived 1.5 years or more. The difference in 1-year survival rates between patients who received treatment and patients who were untreated was 24.6% (95% CI, 22.46-26.61; P < .001) (Table 1).
Table 1. Survival Rates After Diagnosis of Metastasis by Metastasis Treatment Status.
| Period | Post–Metastasis Diagnosis, mo | Participants Alive, % (95% CI) | Difference in Survival Rates, % (95% CI) | P Value | |
|---|---|---|---|---|---|
| No Metastasis Treatment | Treatment for Metastasis | ||||
| Overall Survival Rates (Initial Diagnosis and Treatment) | |||||
| 1982-2009 | 12 | 5.6 (2.89-9.62) | 30.2 (25.35-35.23) | 24.6 (22.46-25.61) | <.001 |
| 24 | 1.1 (0.22-2.86) | 12.2 (8.91-16.02) | 11.1 (8.69-13.16) | ||
| Survival Rates by Decade of Initial Diagnosis and Treatment | |||||
| 1982-1991 | 12 | 6.25 (2.76-11.76) | 29.9 (23.61-36.42) | 23.65 (20.85-24.66) | <.001 |
| 24 | 0.89 (0.22-2.86) | 11.34 (7.37-16.25) | 10.45 (7.15-13.39) | ||
| 1992-2001 | 12 | 3.67 (0.68-11.18) | 31.73 (23.05-40.74) | 28.06 (22.37-29.56) | <.001 |
| 24 | a | 14.42 (8.49-21.86) | 14.42 (8.49-21.86) | ||
| 2002-2009 | 12 | 6.52 (0.68-11.18) | 27.08 (13.07-43.24) | 20.56 (12.39-32.06) | .004 |
| 24 | 6.52 | 9.03 (1.69-24.23) | 2.51 (1.21-0.53) | ||
| Survival Rates by Decade After Metastasis Diagnosis | |||||
| 1982-1991 | 12 | 3.08 (0.58-9.51) | 22.8 (15.98-30.44) | 19.72 (15.4-20.93) | <.001 |
| 24 | 1.54 (0.13-7.28) | 6.3 (2.95-11.43) | 4.76 (2.82-4.15) | ||
| 1992-2001 | 12 | 7.35 (3.00-14.31) | 37.5 (29.41-45.56) | 30.15 (26.41-31.25) | <.001 |
| 24 | a | 17.65 (11.78-24.49) | 17.65 (11.78-24.49) | ||
| 2002-2011 | 12 | 5.68 (1.06-16.42) | 29.45 (19.03-40.62) | 23.77 (17.97-24.2) | <.001 |
| 24 | 2.84 (0.22-12.51) | 12.21 (5.49-21.79) | 9.37 (5.27-9.28) | ||
Zero participants were alive.
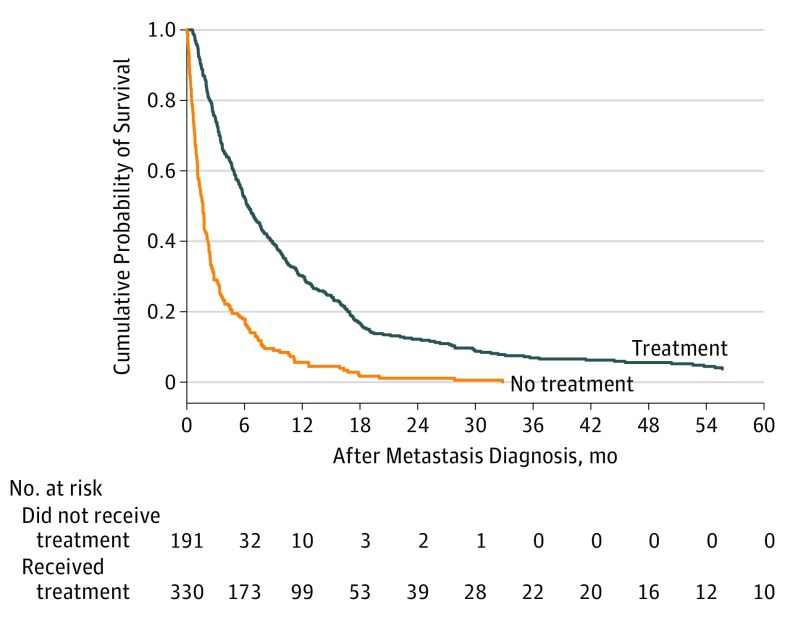
Of the 649 patients with metastasis, 344 (53.0%) received treatment. Of 521 evaluable patients, at 1 year after metastasis diagnosis, 30.2% (95% CI, 25.35-35.23) were alive compared with 5.6% (95% CI, 2.89-9.62) of untreated patients (P < .001) (Figure 1; Table 1). The median survival was 6.3 months (IQR, 2.96-14.41) for patients who received treatment compared with 1.7 months (IQR, 0.66-3.5) for patients who did not receive treatment (P < .001).
Figure 1. Cumulative Probability of Survival After Metastasis Diagnosis Stratified by Treatment Status.
Treatment status is defined as receiving any treatment or not receiving any treatment.
Substantially greater differences in survival rates between treated and untreated patients were not observed in patients who received a diagnosis and were treated more recently compared with those who were treated decades ago. The differences in 1-year survival rates between treated and untreated patients varied from 23.7% for patients who were treated between 1982 and 1991, to 28% for patients who were treated between 1992 and 2001 and 20.6% for patients who were treated between 2002 and 2009 (Table 1).
However, when we compared survival rates between patients who received treatment and those who did not during the same periods as described previously but classified by date of metastasis diagnosis, we found that the difference in survival rates between treated and untreated patients was somewhat higher (30.15%; 95% CI, 26.41-31.25) in the patients who developed metastasis between 1992 and 2001 than the earlier and later periods (19.72%; 95% CI, 15.4-20.93 for patients who received a diagnosis of metastasis between 1982-1991; 23.77%; 95% CI, 17.97-24.2 for those who received a diagnosis of metastasis between 2002-2011) (Table 1). A Cox regression analysis that was adjusted for the site of the metastasis revealed a similar reduction in risk that was associated with treatment for each period evaluated, with hazard ratios (95% CI) of 0.36 (0.26-0.49), 0.33 (0.24-0.44), and 0.41 (0.27-0.62) for 1982 to 1991, 1992 to 2001, and 2002 to 2011, respectively (Table 2).
Table 2. Multivariable Cox Regression Analysis of Risk Factors Associated With Mortality After Metastasis Diagnosis.
| Risk Factor | Referent | 1982-1991 | 1992-2001 | 2002-2011 | |||
|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | ||
| Treatment | No metastasis treatment | 0.36 (0.26-0.49) | <.001 | 0.33 (0.24-0.44) | <.001 | 0.41 (0.27-0.62) | <.001 |
| Hepatic metastasis | Extrahepatic metastasis | 2.13 (1.17-3.89) | .01 | 2.18 (1.31-3.61) | .003 | 2.27 (0.98-5.27) | .06 |
Chemotherapy was the most common treatment that was administered to 127 patients with metastasis, accounting for half (50%) of all interventions. One-hundred eighteen patients (34%) received more than 1 type of therapy. Surgery was performed for 22 patients (6%) and radiation for 18 patients (5%) (Table 3). All other interventions were administered to less than 5% of patients.
Table 3. Treatments Receiveda.
| Treatment Type | No. (%) |
|---|---|
| Chemotherapy/immunotherapy | 172 (50.0) |
| Surgery | 22 (6.4) |
| Radiation | 18 (5.2) |
| >1 Therapya | 118 (34.3) |
| Other | 9 (2.6) |
Also includes combination therapy.
Information regarding specific treatments was available for 103 (30%) of the 344 patients who were treated. The most frequently administered first-line therapy was single-agent chemotherapy (eg, alkylating agents, microtubule inhibitors, and anthracyclines), which was used in 53 patients (51.4%). Combination chemotherapy regimens, which were less common than single-agent regimens, were administered in 5 patients (4.85%). Patients underwent liver-directed therapies (eg, intrahepatic arterial infusion, percutaneous hepatic perfusion, hepatic artery perfusion, or hyperthermic isolated liver perfusion) or chemoembolization infrequently (10 [9.7%] and 8 [7.8%], respectively).
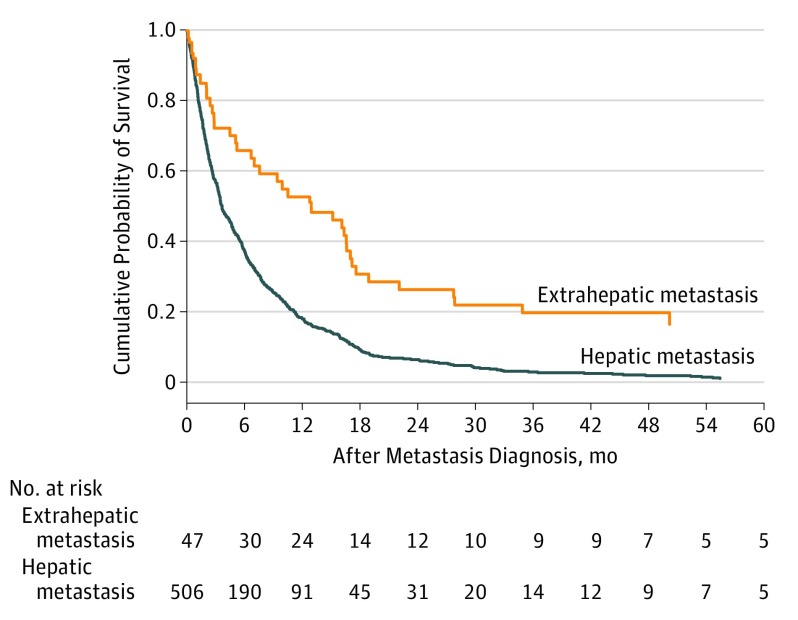
Hepatic metastases (alone or with other sites of involvement) were diagnosed in 557 patients (85.8%), whereas only extrahepatic metastases were diagnosed in 52 patients (8%). Patients with extrahepatic metastases had significantly better survival rates than those with hepatic metastases: 52.8% and 19.8% compared with 18.4% and 2.9% at 1 year and 3 years after the onset of metastatic disease (P < .001) (Figure 2). Patients with extrahepatic metastasis were more likely to receive treatment (35 [81.4%]) than those with hepatic metastasis alone (160 [53.3%]) or with other sites of involvement (147 [71.4%]).
Figure 2. Cumulative Probability of Survival After Metastasis Diagnosis Stratified by Metastasis Site.
Metastasis site is defined as metastasis with hepatic involvement or extrahepatic metastasis.
Discussion
Our earlier study8 included 145 patients who developed metastasis by December 1988, 100 (69%) of whom received treatment. The median survival was 5.2 months for patients who received treatment and 2 months for those who did not (P < .001). The 1-year survival rate was 13% overall, and less than 20% of patients who were symptomatic at the time of metastasis diagnosis and received treatment survived that long. No untreated symptomatic patients survived past 1 year. In total, only 17 patients (11.7%) survived longer than 1 year.
For patients who received treatment for metastasis, the median survival in the cohort that was analyzed in this study is not better than that of the earlier cohort (6.3 months and 5.2 months, respectively). Further, although statistically significant differences in median survival time between treated and untreated patients were found, they were similar to the differences that were found in our earlier study.
Our sensitivity analysis confirmed the overall findings of the study. Most results that were obtained in the 3 treatment period subgroups were similar, with an overlap in the 95% confidence intervals. However, a larger difference in survival rates was found in patients who received a diagnosis of metastasis between 1992 and 2001 compared with other periods. Although this is suggestive of an improvement in survival due to treatment, a closer look at the patients in this subgroup revealed that all but 1 patient who survived more than 5 years after metastasis diagnosis and more than 50% of the patients with extrahepatic metastasis were in this subgroup. These factors, and the results of the Cox regression, which controlled for the associations of the site of metastasis, make it more likely that this finding is due to chance.
It also appears that there is no survival advantage that is associated with a specific therapy, although this could not be tested because of the variety of treatments that were used and thus small numbers of patients in each group. Patients were treated with antineoplastic agents during the 1970s and 1980s; the use of cytotoxic agents, including alkylating agents (eg, carmustine, dacarbazine and temozolomide), vinca alkaloids (eg, taxol and vinblastine), and biochemotherapy (eg, interferon and interleukin) alone or in combination was common during this period.12,13,14 Theoretical advances in treatment included local administration (eg, hepatic artery perfusion and chemoembolization) of some of these same agents15,16 and treatment with newer monoclonal antibodies.17 The use of newer classes of drugs, such as methyl ethyl ketone (MEK) inhibitors,18,19 programmed cell death protein–1 inhibitors,20,21 and cytotoxic T-lymphocyte–associated antigen 4 inhibitors,22 has been tested over the past decade or more. None of these treatments has improved progression-free survival rates or overall survival in a meaningful way.
Limitations
One of the limitations of this study is the variability in the selection of patients for treatment. It can be based on several factors, many of which are subjectively measured, including the oncologist’s recommendation, patient choice, the health status of the patient, metastatic burden, and patient age. It is difficult to determine the effect of such selection bias but it is reasonable to assume that these factors have not changed during any of the periods that were compared. Although our study was completed retrospectively, most patients included in the study were followed up prospectively as part of a uveal melanoma registry, reducing the potential for ascertainment bias and allowing for a thorough characterization of patient and tumor characteristics in both treated and untreated patients.
These issues of bias were addressed in a literature review that evaluated published studies of treatments for metastatic uveal melanoma.10 Among the reported concerns was the possibility of selection bias, with the inclusion of patients more likely to respond to treatment enrolled in studies. Other obstacles included comparisons of results in treated patients with inappropriate historical controls (ie, untreated patients who were not comparable with treated patients with regard to patient or tumor characteristics) and publication bias, with poor outcomes less likely to be reported than good outcomes. However, the authors concluded that there was no compelling scientific evidence of any substantial survival benefit of any method of treatment. Notably, this review identified 24 publications with median survival times after treatment that varied from 5.2 months to 29.4 months, but, to our knowledge, there were no randomized phase 3 clinical trials available for inclusion.
Since this literature review was published, a multicenter randomized phase 2 clinical trial with a relatively large number of patients with metastatic uveal melanoma was completed to determine the efficacy of selumetinib, an MEK1 and MEK2 inhibitor.18 Patients were randomized (1:1) to receive selumetinib or chemotherapy (temozolomide or dacarbazine determined by the investigator). No improvement in overall survival rates was observed in patients who were treated with selumetinib compared with chemotherapy; the median survival after developing metastasis was 12 months for selumetinib-treated patients compared with 9 months for chemotherapy-treated patients. Additionally, there was a high rate (97%) of adverse events that often required a dose reduction. Subsequently, a phase 3 study of selumetinib + dacarbazine vs placebo + dacarbazine was completed, but the efficacy of selumetinib was not demonstrated; the median progression-free survival was 2.8 months in the selumetinib arm vs 1.8 months in the dacarbazine-alone arm.19
Similar survival rates in patients who received treatment for metastasis were observed in this recent analysis compared with our earlier study.8 These comparable findings provide further evidence to suggest that advances in treatments that lead to clinically meaningful improvements in survival times have not been realized. The rates in this study were derived from a larger cohort than the earlier study and did not consider surveillance methods or specific treatments. Our surveillance protocol has changed somewhat in the years between the earlier and current studies, with more patients in the later period undergoing imaging studies. Data regarding specific types of treatment were not always available, but most of the patients were referred to specialized cancer centers and treated with the newest therapeutic agents, which were often offered in a clinical trial setting. Any specific treatments that were offered to the later patient cohort that may have had a beneficial effect would likely have been apparent without a treatment-specific analysis.
Conclusions
Adjuvant therapies, including targeted therapies, that are initiated before overt metastases arise may be the most effective way to prolong survival, particularly for patients with high-risk characteristics (eg, GEP class 2, monosomy 3, and large tumors). Randomized clinical trials to test such therapies should be designed to identify and select those patients who are most likely to respond to treatment. Continued research to understand the determinants of the high-risk phenotype is essential for developing targeted therapies and identifying modifiable risk factors.
References
- 1.Gragoudas E, Li W, Goitein M, Lane AM, Munzenrider JE, Egan KM. Evidence-based estimates of outcome in patients irradiated for intraocular melanoma. Arch Ophthalmol. 2002;120(12):1665-1671. doi: 10.1001/archopht.120.12.1665 [DOI] [PubMed] [Google Scholar]
- 2.Kujala E, Mäkitie T, Kivelä T. Very long-term prognosis of patients with malignant uveal melanoma. Invest Ophthalmol Vis Sci. 2003;44(11):4651-4659. doi: 10.1167/iovs.03-0538 [DOI] [PubMed] [Google Scholar]
- 3.Bergman L, Seregard S, Nilsson B, Lundell G, Ringborg U, Ragnarsson-Olding B. Uveal melanoma survival in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci. 2003;44(8):3282-3287. doi: 10.1167/iovs.03-0081 [DOI] [PubMed] [Google Scholar]
- 4.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596-1603. doi: 10.1016/j.ophtha.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbour JW. A prognostic test to predict the risk of metastasis in uveal melanoma based on a 15-gene expression profile. Methods Mol Biol. 2014;1102:427-440. doi: 10.1007/978-1-62703-727-3_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol. 2016;134(7):734-740. doi: 10.1001/jamaophthalmol.2016.0913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diener-West M, Reynolds SM, Agugliaro DJ, et al. ; Collaborative Ocular Melanoma Study Group Report 23 . Screening for metastasis from choroidal melanoma: the Collaborative Ocular Melanoma Study Group Report 23. J Clin Oncol. 2004;22(12):2438-2444. doi: 10.1200/JCO.2004.08.194 [DOI] [PubMed] [Google Scholar]
- 8.Gragoudas ES, Egan KM, Seddon JM, et al. Survival of patients with metastases from uveal melanoma. Ophthalmology. 1991;98(3):383-389. doi: 10.1016/S0161-6420(91)32285-1 [DOI] [PubMed] [Google Scholar]
- 9.Augsburger JJ, Corrêa ZM, Trichopoulos N. Surveillance testing for metastasis from primary uveal melanoma and effect on patient survival. Am J Ophthalmol. 2011;152(1):5-9.e1. doi: 10.1016/j.ajo.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 10.Augsburger JJ, Corrêa ZM, Shaikh AH. Effectiveness of treatments for metastatic uveal melanoma. Am J Ophthalmol. 2009;148(1):119-127. doi: 10.1016/j.ajo.2009.01.023 [DOI] [PubMed] [Google Scholar]
- 11.Carvajal RD, Schwartz GK, Tezel T, Marr B, Francis JH, Nathan PD. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol. 2017;101(1):38-44. doi: 10.1136/bjophthalmol-2016-309034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaherty LE, Unger JM, Liu PY, Mertens WC, Sondak VK. Metastatic melanoma from intraocular primary tumors: the Southwest Oncology Group experience in phase II advanced melanoma clinical trials. Am J Clin Oncol. 1998;21(6):568-572. doi: 10.1097/00000421-199812000-00008 [DOI] [PubMed] [Google Scholar]
- 13.Nathan FE, Berd D, Sato T, et al. BOLD+interferon in the treatment of metastatic uveal melanoma: first report of active systemic therapy. J Exp Clin Cancer Res. 1997;16(2):201-208. [PubMed] [Google Scholar]
- 14.Kivelä T, Suciu S, Hansson J, et al. Bleomycin, vincristine, lomustine and dacarbazine (BOLD) in combination with recombinant interferon alpha-2b for metastatic uveal melanoma. Eur J Cancer. 2003;39(8):1115-1120. doi: 10.1016/S0959-8049(03)00132-1 [DOI] [PubMed] [Google Scholar]
- 15.Agarwala SS, Panikkar R, Kirkwood JM. Phase I/II randomized trial of intrahepatic arterial infusion chemotherapy with cisplatin and chemoembolization with cisplatin and polyvinyl sponge in patients with ocular melanoma metastatic to the liver. Melanoma Res. 2004;14(3):217-222. doi: 10.1097/01.cmr.0000129377.22141.ea [DOI] [PubMed] [Google Scholar]
- 16.Leyvraz S, Piperno-Neumann S, Suciu S, et al. Hepatic intra-arterial versus intravenous fotemustine in patients with liver metastases from uveal melanoma (EORTC 18021): a multicentric randomized trial. Ann Oncol. 2014;25(3):742-746. doi: 10.1093/annonc/mdt585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guenterberg KD, Grignol VP, Relekar KV, et al. A pilot study of bevacizumab and interferon-α2b in ocular melanoma. Am J Clin Oncol. 2011;34(1):87-91. doi: 10.1097/COC.0b013e3181d2ed67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carvajal RD, Sosman JA, Quevedo JF, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014;311(23):2397-2405. doi: 10.1001/jama.2014.6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komatsubara KM, Manson DK, Carvajal RD. Selumetinib for the treatment of metastatic uveal melanoma: past and future perspectives. Future Oncol. 2016;12(11):1331-1344. doi: 10.2217/fon-2015-0075 [DOI] [PubMed] [Google Scholar]
- 20.Kottschade LA, McWilliams RR, Markovic SN, et al. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016;26(3):300-303. doi: 10.1097/CMR.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 21.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344-3353. doi: 10.1002/cncr.30258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luke JJ, Callahan MK, Postow MA, et al. Clinical activity of ipilimumab for metastatic uveal melanoma: a retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer. 2013;119(20):3687-3695. doi: 10.1002/cncr.28282 [DOI] [PMC free article] [PubMed] [Google Scholar]