Key Points
Question
Are there differences in risks and burden of second primary malignant neoplasm among patients with first potentially HPV-associated head and neck squamous cell carcinoma (HNSCC) compared with those with first non–HPV-associated HNSCC?
Findings
In this cohort analysis of 109 512 patients with HNSCC, the risk and burden of second primary malignant neoplasm was high overall; however, the risk and burden among patients with first potentially-HPV-associated HNSCC was lower than those with first non–HPV-associated HNSCC.
Meaning
Strategies that prevent or detect second primary malignant neoplasm early in patients with HNSCC are warranted.
This population-based cohort study examines risk of second primary malignant neoplasms in adult patients diagnosed with HPV-associated vs non–HPV-associated head and neck squamous cell carcinoma.
Abstract
Importance
Second primary malignant neoplasms (SPMNs) are the leading cause of death in survivors of head and neck squamous cell carcinoma (HNSCC). Recently, human papillomavirus (HPV) has emerged as a risk factor for oropharyngeal squamous cell carcinoma and has different prognosis from classic tobacco/alcohol-associated HNSCC. This suggests that there also may be different risks and burden of SPMNs among patients who’s HNSCC were from HPV or tobacco and/or alcohol.
Objective
To assess SPMN risks and burden in a large US cohort of patients with a first potentially HPV-associated HNSCC vs non–HPV-associated HNSCC.
Design, Setting, and Participants
In this population-based retrospective cohort study, 109 512 adult patients diagnosed with HNSCC between 2000 and 2014 were identified from the Surveillance, Epidemiology, and End Results registry.
Exposures
HPV-relatedness based on whether patients’ first HNSCC was potentially associated with HPV. Patients were grouped into 2 cohorts: potentially HPV-associated HNSCC, and non–HPV-associated HNSCC.
Main Outcomes and Measures
The primary outcome was incidence of SPMN (defined as the first subsequent primary cancer occurring at least 2 months after first cancer diagnosis). Excess SPMN risk was calculated using relative (standardized incidence ratios [SIRs]) and absolute (excess absolute risk [EAR] per 10 000 person-years at risk [PYR]).
Results
A total of 109 512 patients with HNSCC (mean [SD] age, 61.9 [12.1] years; 83 305 [76.1%] men) were identified. The overall SIR was 2.18 (95% CI, 2.14-2.22) corresponding to 160 excess cases per 10 000 PYR. The risk among patients with first potentially HPV-associated HNSCC (SIR, 1.98; EAR, 114 excess cases per 10 000 PYR) was lower than those with first non–HPV-associated HNSCC (SIR, 2.28; EAR, 188 excess cases per 10 000 PYR). Overall, the largest SIRs and EARs were observed for cancers of the head and neck, lung, and esophagus. However, the risks of SPMN were lower among potentially HPV-associated HNSCC patients.
Conclusions and Relevance
Patients diagnosed with HNSCC experience excess risk of SPMN, which was higher among those with non–HPV-associated HNSCC than from potentially HPV-associated HNSCC. Clinicians should implement strategies that prevent or detect SPMN early in patients with HNSCC.
Introduction
There was an estimated 63 000 new cases of head and neck cancer in the United States in 2017, accounting for about 4% of new cancers.1 Patients with head and neck squamous cell carcinoma (HNSCC) experience elevated risk of developing second primary malignant neoplasms (SPMNs).2,3,4,5,6,7 These are of increasing concern because the number of survivors of HNSCC has been growing owing to early detection and advances in cancer treatments.8 There are an estimated 430 000 HNSCC survivors in the United States, and the number is expected to continue to rise.9 The incidence of SPMN in HNSCC patients is about 3% to 7% per year, with an estimated 20-year cumulative risk of 36%.5 Approximately one-third of HNSCC deaths are attributable to SPMNs. Second primary malignant neoplasms are the cause of death in 3 times as many patients with HNSCC compared with those who die of metastatic disease.10,11,12
Tobacco and excessive alcohol use, as well as human papillomavirus (HPV) infection, are the main causal factors associated with HNSCC, HPV being mostly associated with a subset of patients with HNSCC called oropharyngeal squamous cell carcinoma (SCC).13,14,15 Although there has been a steady decrease in the incidence of HNSCC associated with tobacco and alcohol, HPV-associated oropharyngeal SCC has increased about 225% in the United States in the past 3 decades and is projected to surpass cervical cancer as the most common HPV-associated cancer by 2020.16 Human papillomavirus-associated HNSCC, especially oropharyngeal SCC, affects young males, whites, and has a good prognosis, whereas non-HPV-associated HNSCC typically affects older patients and has poor prognosis.16,17,18,19 Therefore, anatomical subsites of SPMNs following diagnosis of a first HPV-associated HNSCC may also be different from a first non–HPV-associated HNSCC.
Patients with a first HNSCC have an elevated risk of developing an SPMN of the lung, esophagus, and head and neck sites.3,5,7,20,21,22 However, studies have not examined the incidence and sites of SPMNs stratified by a first HPV-associated HNSCC compared with non–HPV-associated HNSCC. Describing subsite-specific risks of SPMNs may have implications for the pattern and intensity of screening and other preventive efforts in patients with HNSCC after diagnosis.
In this study, we quantified the risk of SPMN among patients with a first HPV-associated HNSCC vs non–HPV-associated HNSCC, and described the anatomical sites of these SPMNs.
Methods
Data Source and Population
A population-based cohort of all patients (>20 years) with a first HNSCC diagnosed between 2000 and 2014 from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) 18 database, which covers approximately 30% of the US population and includes cases diagnosed from 2000 to 2014, were used for this study.23 SEER is a publicly available, nationally representative population-based cancer database.23 It has been described as the premier cancer statistics source in the United States,24,25 and covers approximately 97% of all incident cancers in its registry areas.26 All cancers, primary and subsequent, occurring among residents of geographic registries comprising SEER are reportable, and the program has near-universal follow-up.27 Patients with HNSCC included invasive squamous cell carcinoma (International Classification of Diseases for Oncology, third edition [ICD-O-3]), histology codes (codes 8070-8076 and 8078),28 and topographic codes for head and neck subsites (oral cavity, oropharynx, larynx, and hypopharynx). Patients were excluded if the only documentation of their cancer was from a death certificate or autopsy, or if cause of death was unknown or listed as alive but had no survival time. The National Cancer Institute does not require institutional review board approval for use of this deidentified data set.
SPMN Identification
Coding of multiple primary tumors followed the common set of rules proposed by the International Agency for Research on Cancer (IARC). These rules define multiple primary tumors as 2 or more tumors arising in different sites, or at the same site when the histologic findings are different.29 An SPMN was defined as the first subsequent primary cancer occurring at least 2 months after first cancer diagnosis, similar to other studies.7,21,30 Consequently, the person-year at risk (PYR) for each individual began at 2 months of follow-up, and ended at the date of SPMN diagnosis, last known vital status, death, or the end of the study period of follow-up (December 2014); whichever came first. Extensions, recurrences, metastasis, and third and subsequent cancers were not included.
HPV-Relatedness in Patients With HNSCC
HPV-relatedness was based on whether patients’ first HNSCC was potentially associated with HPV. Since the SEER database does not record HPV status, patients with a first potentially HPV-associated HNSCCs were identified using methodology previously described,14,15,31,32 which is based on ICD-O-3 topography: oropharynx (C100-109), tonsil (lingual and palatine; C024, and C090-099, respectively), base of tongue (C019), and Waldeyer’s ring (C142). Patients with a first non–HPV-associated HNSCC were other ICD-O-3 sites, including oral cavity (tongue, C020-023); gum, C030-039; palate, C050-059; other mouth areas, C060-069; lip, C000-009; and floor of mouth, C040-049), hypopharynx (C130-139), and larynx (C320-329).
Statistical Analyses
The standard person-year approach to describe relative and absolute excess risk of developing SPMN was used.21,33 Following this method, the number of observed SPMNs was compared with the number of expected cancers if patients with a first HNSCC had experienced the same cancer rates as the general population. The number of expected SPMNs was calculated for a noncancer patient cohort of identical age, sex, race, and time period. Expected numbers of secondary cancers was estimated by multiplying sex-, age-, race-, and calendar year-specific SEER cancer incidence rates (available at http://seer.cancer.gov) with the accumulated person-years at risk. Standardized incidence ratio (SIR), described by Schoenberg and Myers34 and adapted to cancer registry analysis by Begg,35 is a relative measure of the strength of association between 2 cancers. The SIR was defined as the ratio of observed to expected SPMNs. Confidence intervals for SIRs were calculated with Byar’s approximation to the Poisson distribution.33 The excess absolute risk (EAR)21 is an absolute measure of the burden of additional cancer occurrences in a given population. The EAR was calculated as the excess (observed to expected) number of SPMNs per 10 000 PYR.
Because the typical HNSCC survivor with a first HPV-associated oropharyngeal SCC tends to be a white male in their 20s to 50s,16,18,36,37,38 a sensitivity analysis was conducted selecting only patients that fit the age, race, and sex criteria above to examine the incidence of SPMNs. All tests were 2-tailed and α was set at .05. The SIR and EAR values were calculated in SEER Stat (version 8.3.4, Surveillance Research Program, National Cancer Institute). Other analyses were performed using SAS statistical software (version 9.4, SAS Institute).
Results
Patient Population
In all, 109 512 patients with first HNSCC were identified in the SEER registry between 2000 and 2014. Table 1 shows that mean (SD) age at first HNSCC diagnosis was 61.9 (12.1) years, and the mean (SD) time between the first HNSCC and SPMN was 3.9 (3.7) years. Median follow-up time for patients with HNSCC was 31 months, 83 305 (76.1%) were men, and 91 699 (83.7%) were white. Among 109 512 patients with first HNSCC, 13 517 (12.3%) developed SPMN (9.6% for patients diagnosed with a first potentially HPV-associated HNSCC and 14.0% for patients with a first non–HPV-associated HNSCC).
Table 1. Patient and Tumor Characteristics, Overall and by HPV Relatedness, SEER 2000 to 2014.
| Characteristic | Frequency, No. (%)a | ||
|---|---|---|---|
| Total (n = 109 512) |
First Potentially HPV-Associated HNSCC (n = 41 682) |
First Non–HPV-Associated HNSCC (n = 67 830) |
|
| HPV relatedness | |||
| First potentially HPV-associated HNSCC | 41 682 (38.1) | NA | NA |
| First non–HPV-associated HNSCC | 67 830 (61.9) | NA | NA |
| Anatomic sites | |||
| Oropharynx | 41 682 (38.1) | NA | NA |
| Oral cavity | 30 464 (27.8) | NA | NA |
| Larynx | 31 802 (29.0) | NA | NA |
| Hypopharynx | 5564 (5.08) | NA | NA |
| Second primary tumor | |||
| No | 95 995 (87.7) | 37 686 (90.4) | 58 309 (86.0) |
| Yes | 13 517 (12.3) | 3996 (9.6) | 9521 (14.0) |
| Age at index HNSCC diagnosis, y | |||
| ≤49 | 15 860 (14.5) | 7347 (17.6) | 8513 (12.6) |
| 50-59 | 33 049 (30.2) | 15 242 (36.6) | 17 807 (26.2) |
| 60-69 | 31 676 (28.9) | 11 887 (28.5) | 19 789 (29.2) |
| ≥70 | 28 927 (26.4) | 7206 (17.3) | 21 721 (32.0) |
| Sex | |||
| Female | 26 207 (23.9) | 8254 (19.8) | 17 953 (26.5) |
| Male | 83 305 (76.1) | 33 428 (80.2) | 49 877 (73.5) |
| Race | |||
| White | 91 699 (83.7) | 35 804 (85.9) | 55 895 (82.4) |
| Black | 12 158 (11.1) | 4009 (9.6) | 8149 (12.0) |
| Other | 5655 (5.2) | 1869 (4.5) | 3786 (5.6) |
| Marital status at HNSCC diagnosis | |||
| Married | 56 974 (52.0) | 22 835 (54.8) | 34 139 (50.3) |
| Divorced/separated | 15 187 (13.9) | 6083 (14.6) | 9104 (13.4) |
| Widowed | 10 272 (9.4) | 2671 (6.4) | 7601 (11.2) |
| Never married | 19 742 (18.0) | 7668 (18.4) | 12 074 (17.8) |
| Unknown | 7337 (6.7) | 2425 (5.8) | 4912 (7.2) |
| Year of diagnosis of first HNSCC | |||
| 2000 to 2004 | 34 224 (31.2) | 10 857 (26.0) | 23 367 (34.4) |
| 2005 to 2009 | 36 534 (33.4) | 14 157 (34.0) | 22 377 (33.0) |
| 2010 to 2014 | 38 754 (35.4) | 16 668 (40.0) | 22 086 (32.6) |
| Histologic grade | |||
| Well | 14 649 (13.4) | 2549 (6.1) | 12 100 (17.8) |
| Moderately | 45 674 (41.7) | 14 409 (34.6) | 31 265 (46.1) |
| Poor | 26 764 (24.4) | 15 056 (36.1) | 11 708 (17.3) |
| Undifferentiated/unknown | 22 425 (20.5) | 9668 (23.2) | 12 757 (18.8) |
| Stage | |||
| Localized | 41 826 (38.2) | 6137 (14.7) | 35 689 (52.6) |
| Regional | 46 322 (42.3) | 26 910 (64.6) | 19 412 (28.6) |
| Distant | 17 233 (15.7) | 7192 (17.2) | 10 041 (14.8) |
| Unstaged/unknown | 4131 (3.8) | 1443 (3.5) | 2688 (4.0) |
| Surgery performed | |||
| Yes | 54 981 (50.5) | 17 182 (41.4) | 37 799 (56.0) |
| No | 53 995 (49.5) | 2030 (58.6) | 29 720 (44.0) |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; NA, not applicable; SEER, Surveillance, Epidemiology, and End Results.
P < .001 for all.
Risk of Solid Tumor SPMN
Table 2 shows that 12 618 patients developed an SPMN, compared with 6519 expected cancers if these patients had had the same risk of cancer as the general population. The SIR of developing an SPMN in all HNSCC patients was 2.07 (95% CI, 2.04-2.11) and the EAR was 164.3 cases per 10 000 PYR. The SIR of SPMN in first potentially HPV-associated HNSCC patients was 1.88 (95% CI, 1.83-1.94; corresponding to an EAR of 116.1 cases per 10 000 PYR), was lower than patients with first non–HPV-associated HNSCC (SIR, 2.16; 95% CI, 2.12-2.21; corresponding to EAR, 193.9 cases per 10 000 PYR). Patients with first non–HPV-associated HNSCC had 77 more cases per 10 000 PYR than those with first potentially HPV-associated HNSCC. The risk of SPMN was significantly increased for patients with first hypopharynx cancer, younger age, female sex, and those diagnosed initially with distant tumors.
Table 2. Risk of Solid Tumor SPMN According to the Characteristics of the First HNSCC in 12 618 Patients, SEER 2000 to 2014.
| Characteristic | Observed | Expected | PYR | SIR Rate (95% CI) | EAR per 10 000 PYR |
|---|---|---|---|---|---|
| All HNSCC patients | 12 618 | 6519.42 | 425 793.49 | 2.18 (2.14-2.22) | 160.34 |
| HPV-relatedness | |||||
| First potentially HPV-associated HNSCC | 3996 | 2120.94 | 161 538.00 | 1.88 (1.83-1.94) | 116.08 |
| First non-HPV-associated HNSCC | 9521 | 4398.48 | 264 255.48 | 2.16 (2.12-2.21) | 193.85 |
| Anatomic sites | |||||
| Oropharynx | 3996 | 2120.94 | 161 538.00 | 1.88 (1.83-1.94) | 116.08 |
| Larynx | 4635 | 2288.18 | 129 327.93 | 2.03 (1.97-2.08) | 181.46 |
| Oral cavity | 4217 | 1875.17 | 120 792.16 | 2.25 (2.18-2.32) | 193.87 |
| Hypopharynx | 669 | 235.12 | 14 135.39 | 2.85 (2.63-3.07) | 306.95 |
| Age at index HNSCC diagnosis, y | |||||
| ≤49 | 1264 | 351.12 | 81 423.47 | 3.60 (3.40-3.80) | 112.12 |
| 50-59 | 3785 | 1523.29 | 138 734.03 | 2.48 (2.41-2.57) | 163.02 |
| 60-69 | 4606 | 2349.34 | 117 776.61 | 1.96 (1.90-2.02) | 191.61 |
| ≥70 | 3862 | 2295.67 | 87 859.39 | 1.68 (1.63-1.74) | 178.28 |
| Sex | |||||
| Male | 10 403 | 5308.88 | 326 375.27 | 1.96 (1.92-2.00) | 156.08 |
| Female | 3114 | 1210.54 | 99 418.21 | 2.57 (2.48-2.66) | 191.46 |
| Race | |||||
| White | 11 510 | 5675.76 | 366 507.63 | 2.03 (1.99-2.07) | 159.18 |
| Black | 1503 | 611.10 | 37 408.02 | 2.46 (2.34-2.59) | 238.42 |
| Other | 502 | 177.75 | 18113.23 | 2.58 (2.58-3.08) | 179.01 |
| Marital status at HNSCC diagnosis | |||||
| Married | 7528 | 3919.39 | 248 968.45 | 1.92 (1.88-1.96) | 144.94 |
| Divorced/separated/widowed | 3060 | 1362.00 | 82 461.23 | 2.25 (2.17-2.33) | 205.92 |
| Never married | 2121 | 804.02 | 66 199.91 | 2.64 (2.53-2.75) | 198.94 |
| Unknown | 808 | 434.01 | 28 163.90 | 1.86 (1.74-1.99) | 132.79 |
| Follow-up | |||||
| 2 mo to <1 y | 2369 | 1141.65 | 79 218.86 | 2.08 (1.99-2.16) | 154.93 |
| 1 y to <5 y | 6788 | 3250.73 | 216 689.35 | 2.09 (2.04-2.14) | 163.24 |
| 5 y to <10 y | 3619 | 1728.80 | 106 793.78 | 2.09 (2.03-2.16) | 176.99 |
| ≥10 y | 741 | 398.24 | 23 091.49 | 1.83 (1.73-2.00) | 148.44 |
| Year of diagnosis of first HNSCC | |||||
| 2000 to 2004 | 6445 | 3216.18 | 199 344.70 | 2.00 (1.96-2.05) | 161.97 |
| 2005 to 2009 | 4962 | 2340.54 | 157 967.48 | 2.12 (2.06-2.18) | 165.95 |
| 2010 to 2014 | 2110 | 962.70 | 68 481.31 | 2.19 (2.10-2.29) | 167.53 |
| Histologic grade | |||||
| Well | 2025 | 1077.49 | 65 905.33 | 1.88 (1.80-1.96) | 143.77 |
| Moderately | 6001 | 2664.55 | 174 807.39 | 2.25 (2.20-2.31) | 190.86 |
| Poor | 2839 | 1424.75 | 99 121.66 | 1.99 (1.92-2.07) | 142.68 |
| Undifferentiated/unknown | 2652 | 1352.64 | 85 959.10 | 1.96 (1.89-2.04) | 151.16 |
| Stage | |||||
| Localized | 6613 | 3361.49 | 199 430.93 | 1.97 (1.92-2.02) | 163.04 |
| Regional | 4968 | 2301.29 | 168 134.36 | 2.16 (2.10-2.22) | 158.61 |
| Distant | 1433 | 593.83 | 41 649.55 | 2.41 (2.29-2.54) | 201.48 |
| Unstaged/unknown | 503 | 262.81 | 16 578.65 | 1.91 (1.75-2.09) | 144.88 |
| Surgery performed | |||||
| Yes | 7567 | 3690.99 | 246 741.46 | 2.05 (2.00-2.10) | 157.09 |
| No | 5819 | 2769.61 | 175 087.55 | 2.10 (2.05-2.16) | 174.16 |
| Unknown | 131 | 58.83 | 3964.48 | 2.23 (1.86-2.64) | 182.05 |
Abbreviations: EAR, excess absolute risk; HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; PYR, person-year at risk; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio; SPMN, second primary malignant neoplasm.
Incidence and Location of SPMN Among First Potentially HPV-Associated HNSCC
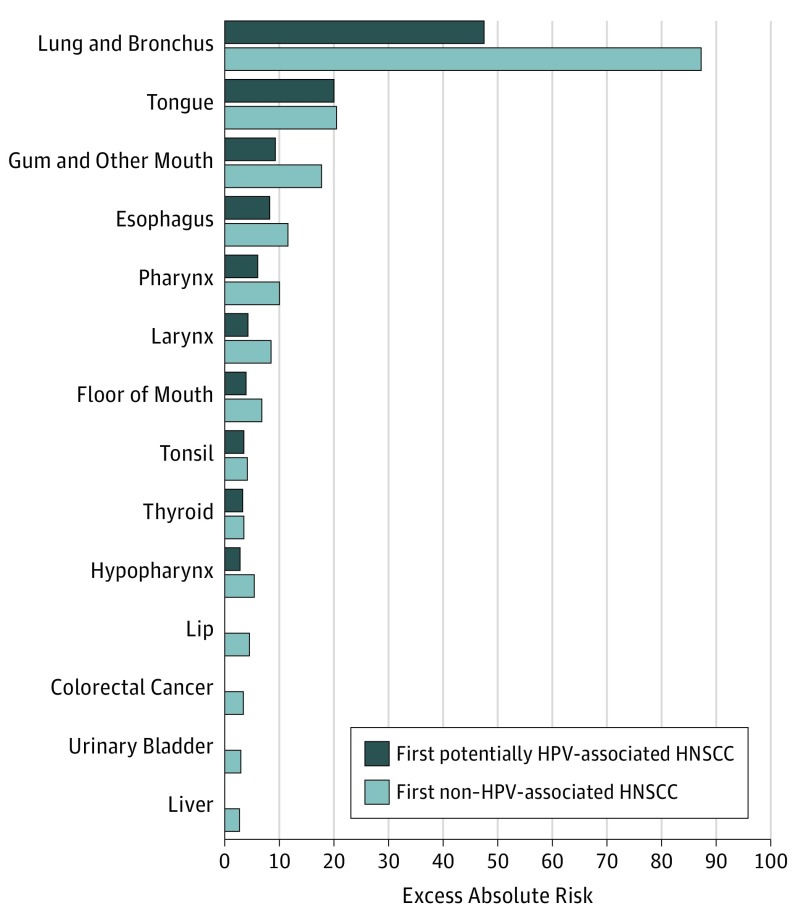
Table 3 and the Figure show that 3742 SPMNs were observed among patients diagnosed with first potentially HPV-associated HNSCC, leading to a SIR of 1.98 (95% CI, 1.91-2.04) and an EAR of 114.48 excess cases per 10 000 PYR. The highest SIR of SPMN was observed for head and neck cancers: gum and other mouth (SIR, 21.56; 95% CI, 18.32-25.21), floor of mouth (SIR, 18.29; 95% CI, 14.17-23.22), tongue (SIR, 16.18; 95% CI, 14.52-17.99), and hypopharynx (SIR, 11.16; 95% CI, 8.28-14.71). After head and neck sites, the other cancers with higher SIRs were for second primary esophageal cancer (SIR, 5.47; 95% CI, 4.66-6.38), followed by lung and bronchus cancer (SIR, 3.63; 95% CI, 3.41-3.85), and thyroid cancer (SIR, 2.95; 95% CI, 2.35-3.67). The excess burden of disease, as measured by EAR in number of excess cases per 10 000 PYR, was highest for lung and bronchus (EAR, 47.5), followed by tongue (EAR, 20.0), gum and other mouth (EAR, 9.3), and esophagus (EAR, 8.2) (Figure, A).
Table 3. Anatomic Site of SPMN According to First HNSCC by HPV-Relatedness in 12 618 Patients, SEER 2000 to 2014.
| Site of SPMNa | Observed | SIR Rate (95% CI) |
|---|---|---|
| First potentially HPV-associated HNSCC | ||
| All solid tumors | 3742 | 1.98 (1.91-2.04) |
| Gum and other mouth | 157 | 21.56 (18.32-25.21) |
| Floor of mouth | 67 | 18.29 (14.17-23.22) |
| Other oral cavity and pharynx | 28 | 17.26 (11.47-24.95) |
| Tongue | 345 | 16.18 (14.52-17.99) |
| Oropharynx | 35 | 11.99 (8.35-16.68) |
| Hypopharynx | 50 | 11.16 (8.28-14.71) |
| Pharynx | 107 | 10.85 (8.89-13.11) |
| Nasopharynx | 22 | 8.92 (5.59-13.50) |
| Esophagus | 162 | 5.47 (4.66-6.38) |
| Tonsil | 71 | 5.11 (3.99-6.44) |
| Larynx | 92 | 3.94 (3.18-4.83) |
| Lung and bronchus | 1059 | 3.63 (3.41-3.85) |
| Thyroid | 81 | 2.95 (2.35-3.67) |
| First non–HPV-associated HNSCC | ||
| All solid tumors | 8876 | 2.28 (2.23-2.32) |
| Gum and other mouth | 485 | 32.58 (29.75-35.62) |
| Floor of mouth | 187 | 29.92 (25.78-34.53) |
| Other oral cavity and pharynx | 68 | 23.66 (18.37-29.99) |
| Hypopharynx | 151 | 18.12 (15.34-21.25) |
| Tongue | 574 | 16.91 (15.55-18.35) |
| Lip | 130 | 14.83 (12.39-17.61) |
| Oropharynx | 67 | 14.1 (10.92-17.90) |
| Pharynx | 242 | 14.02 (12.31-15.90) |
| Larynx | 311 | 7.02 (6.26-7.85) |
| Tonsil | 128 | 6.87 (5.73-8.17) |
| Esophagus | 365 | 6.26 (5.63-6.93) |
| Salivary gland | 56 | 4.53 (3.42-5.88) |
| Lung and bronchus | 2969 | 4.47 (4.31-4.63) |
| Thyroid | 137 | 3.02 (2.53-3.57) |
| Liver | 150 | 1.94 (1.65-2.28) |
| Stomach | 116 | 1.46 (1.21-1.76) |
| Colorectal cancer | 402 | 1.29 (1.17-1.43) |
| Urinary bladder | 368 | 1.28 (1.15-1.42) |
Abbreviations: HNSCC, head and neck squamous cell carcinoma; HPV, human papillomavirus; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio; SPMN, second primary malignant neoplasm.
Second primary malignant neoplasms are those with at least 1 excess case per 10 000 person-year at risk.
Figure. Excess Absolute Risk of Second Primary Malignant Neoplasm, by HPV-Relatedness: SEER 2000 to 2014.
HNSCC indicates head and neck squamous cell carcinoma; HPV, human papillomavirus. Second primary malignant neoplasms are those with at least 2 excess cases per 10 000 person-year at risk. SEER indicates Surveillance, Epidemiology, and End Results.
The sensitivity analysis (eTable 1 in the Supplement) by limiting the study population to white males in their 20s to 50s showed that the same anatomical sites were at elevated risks of developing SPMN.
Incidence and Location of SPMN Among First Non–HPV-Associated HNSCC
In all, 8876 SPMNs were observed among patients diagnosed with first non–HPV-associated HNSCC (Table 3), leading to an SIR of 2.28 (95% CI, 2.23-2.32) and EAR of 188.38 excess cases per 10 000 PYR. The highest SIR of SPMN was observed for second head and neck cancers: gum and other mouth (SIR, 32.58; 95% CI, 29.75-35.62), floor of mouth (SIR, 29.92; 95% CI, 25.78-34.53), hypopharynx (SIR, 18.12; 95% CI, 15.34-21.25), and tongue (SIR, 16.91; 95% CI, 15.55-18.35). After second head and neck sites, the other cancers with higher SIRs were second primary esophageal cancer (SIR, 6.26; 95% CI, 5.63-6.93), followed by lung and bronchus cancer (SIR, 4.47; 95% CI, 4.31-4.63), and thyroid cancer (SIR, 3.02; 95% CI, 2.53-3.57). The excess burden of disease, as measured by EAR in number of excess cases per 10 000 PYR, was highest for lung and bronchus (EAR, 87.2), followed by tongue (EAR, 20.4), gum and other mouth (EAR, 17.8), and finally esophagus (EAR, 11.6) (Figure, B).
Incidence and Location of SPMN by Anatomical Subsite
Table 4 shows that the SIR of a second solid tumor at any site was highest for patients with a first cancer of the hypopharynx (SIR, 3.05; 95% CI, 2.81-3.29), and lowest for a first oropharynx (SIR, 1.98; 95% CI, 1.91-2.04) SCC. Similarly, the absolute number of excess second solid cancers per 10 000 PYR was highest in patients with a first hypopharynx (EAR, 303.1), and lowest for a first oropharynx (EAR, 114.5) SCC. The sites with higher SPMN risk were similar among all sites even though the rates and excess risks varied. They were mainly second head and neck (gum and other mouth, floor of mouth, tongue, lip, pharynx, larynx, oropharynx, and hypopharynx), esophagus, lung and bronchus, thyroid, colorectal, liver, and stomach cancers.
Table 4. Anatomic Site of SPMN According to Site of First HNSCC in 12 618 Patients, SEER 2000 to 2014.
| Site of First HNSCC | Site of SPMNa | Observed | SIR Rate (95% CI) | EAR Per 10 000 PYR |
|---|---|---|---|---|
| All HNSCC | ||||
| All solid tumors | 12 618 | 2.18 (2.14-2.22) | 160.34 | |
| Lung and bronchus | 4028 | 4.21 (4.08-4.35) | 72.15 | |
| Tongue | 919 | 16.63 (15.57-17.74) | 20.29 | |
| Gum and other mouth | 642 | 28.96 (26.77-31.29) | 14.56 | |
| Esophagus | 527 | 5.99 (5.49-6.52) | 10.31 | |
| Larynx | 403 | 5.96 (5.39-6.57) | 7.88 | |
| Pharynx | 349 | 12.87 (11.55-14.29) | 7.56 | |
| Floor of mouth | 254 | 25.62 (22.57-28.97) | 5.73 | |
| Hypopharynx | 201 | 15.68 (13.59-18.01) | 4.42 | |
| Tonsil | 199 | 6.12 (5.30-7.03) | 3.91 | |
| Thyroid | 218 | 2.99 (2.61-3.42) | 3.41 | |
| Lip | 144 | 11.14 (9.39-13.11) | 3.08 | |
| Oropharynx | 102 | 13.30 (10.84-16.14) | 2.22 | |
| Other oral cavity and pharynx | 96 | 21.35 (17.29-26.07) | 2.15 | |
| Colorectal cancer | 533 | 1.20 (1.10-1.31) | 2.09 | |
| Liver | 207 | 1.68 (1.46-1.93) | 1.98 | |
| Urinary bladder | 477 | 1.16 (1.06-1.27) | 1.53 | |
| Stomach | 169 | 1.47 (1.26-1.71) | 1.28 | |
| Salivary gland | 72 | 3.99 (3.12-5.03) | 1.27 | |
| Oropharynx | ||||
| All solid tumors | 3742 | 1.98 (1.91-2.04) | 114.48 | |
| Lung and bronchus | 1059 | 3.63 (3.41-3.85) | 47.49 | |
| Tongue | 345 | 16.18 (14.52-17.99) | 20.04 | |
| Gum and other mouth | 157 | 21.56 (18.32-25.21) | 9.27 | |
| Esophagus | 162 | 5.47 (4.66-6.38) | 8.19 | |
| Pharynx | 107 | 10.85 (8.89-13.11) | 6.01 | |
| Larynx | 92 | 3.94 (3.18-4.83) | 4.25 | |
| Floor of mouth | 67 | 18.29 (14.17-23.22) | 3.92 | |
| Tonsil | 71 | 5.11 (3.99-6.44) | 3.53 | |
| Thyroid | 81 | 2.95 (2.35-3.67) | 3.32 | |
| Hypopharynx | 50 | 11.16 (8.28-14.71) | 2.82 | |
| Oropharynx | 35 | 11.99 (8.35-16.68) | 1.99 | |
| Other oral cavity and pharynx | 28 | 17.26 (11.47-24.95) | 1.63 | |
| Nasopharynx | 22 | 8.92 (5.59-13.50) | 1.21 | |
| Oral cavity | ||||
| All solid tumors | 3903 | 2.36 (2.28-2.43) | 186.07 | |
| Lung and bronchus | 895 | 3.18 (2.98-3.40) | 50.80 | |
| Gum and other mouth | 425 | 64.35 (58.37-70.77) | 34.64 | |
| Tongue | 420 | 29.85 (27.06-32.85) | 33.61 | |
| Floor of mouth | 144 | 56.28 (47.47-66.27) | 11.71 | |
| Esophagus | 146 | 6.27 (5.29-7.37) | 10.16 | |
| Lip | 113 | 29.72 (24.50-35.74) | 9.04 | |
| Pharynx | 105 | 15.62 (12.77-18.91) | 8.14 | |
| Larynx | 101 | 6.00 (4.88-7.29) | 6.97 | |
| Tonsil | 77 | 10.23 (8.08-12.79) | 5.75 | |
| Hypopharynx | 56 | 17.70 (13.37-22.99) | 4.37 | |
| Salivary gland | 44 | 8.23 (5.98-11.05) | 3.20 | |
| Thyroid | 58 | 2.67 (2.03-3.45) | 3.00 | |
| Oropharynx | 36 | 19.47 (13.63-26.95) | 2.83 | |
| Colorectal cancer | 165 | 1.20 (1.02-1.40) | 2.26 | |
| Other oral cavity and pharynx | 27 | 23.48 (15.47-34.17) | 2.14 | |
| Liver | 52 | 1.68 (1.26-2.21) | 1.75 | |
| Larynx | ||||
| All solid tumors | 4335 | 2.13 (2.07-2.20) | 177.99 | |
| Lung and bronchus | 1804 | 5.19 (4.95-5.44) | 112.62 | |
| Larynx | 184 | 7.39 (6.36-8.54) | 12.30 | |
| Esophagus | 165 | 5.19 (4.42-6.04) | 10.30 | |
| Tongue | 125 | 6.96 (5.79-8.29) | 8.28 | |
| Pharynx | 116 | 12.20 (10.08-14.63) | 8.23 | |
| Hypopharynx | 82 | 17.53 (13.94-21.76) | 5.98 | |
| Urinary system | 312 | 1.29 (1.15-1.44) | 5.44 | |
| Urinary bladder | 216 | 1.40 (1.22-1.60) | 4.81 | |
| Thyroid | 72 | 3.37 (2.64-4.24) | 3.92 | |
| Colorectal cancer | 203 | 1.29 (1.12-1.48) | 3.55 | |
| Gum and other mouth | 49 | 6.53 (4.83-8.64) | 3.21 | |
| Liver | 81 | 1.94 (1.54-2.41) | 3.04 | |
| Other oral cavity and pharynx | 37 | 23.70 (16.68-32.67) | 2.74 | |
| Tonsil | 43 | 4.30 (3.11-5.79) | 2.55 | |
| Floor of mouth | 31 | 9.31 (6.32-13.21) | 2.14 | |
| Hypopharynx | ||||
| All solid tumors | 638 | 3.04 (2.81-3.29) | 303.08 | |
| Lung and bronchus | 270 | 7.63 (6.75-8.60) | 165.99 | |
| Esophagus | 54 | 16.72 (12.56-21.82) | 35.92 | |
| Tongue | 29 | 15.16 (10.15-21.78) | 19.16 | |
| Larynx | 26 | 10.15 (6.63-14.87) | 16.58 | |
| Pharynx | 21 | 20.38 (12.61-31.15) | 14.13 | |
| Colorectal cancer | 34 | 2.12 (1.47-2.96) | 12.68 | |
| Hypopharynx | 13 | 26.23 (13.96-44.86) | 8.85 | |
| Liver | 17 | 3.74 (2.18-5.98) | 8.81 | |
| Floor of mouth | 12 | 33.28 (17.18-58.14) | 8.23 | |
| Gum and other mouth | 11 | 14.12 (7.04-25.26) | 7.23 | |
| Stomach | 13 | 3.01 (1.60-5.14) | 6.14 | |
| Tonsil | 8 | 7.23 (3.11-14.25) | 4.88 | |
| Kidney | 14 | 1.77 (0.97-2.97) | 4.31 | |
| Oropharynx | 6 | 21.59 (7.89-47.00) | 4.05 | |
| Thyroid | 7 | 3.05 (1.22-6.28) | 3.33 | |
| Other oral cavity and pharynx | 4 | 24.51 (6.59-62.75) | 2.71 | |
| Urinary bladder | 18 | 1.22 (0.72-1.93) | 2.32 |
Abbreviations: EAR, excess absolute risk; HNSCC, head and neck squamous cell carcinoma; PYR, person-year at risk; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio; SPMN, second primary malignant neoplasm.
Second primary malignant neoplasms are those with at least 1 excess case per 10 000 PYR.
Discussion
To our knowledge, this is the first population-based analysis of SPMN risk in the United States that examined differences in SPMN by HPV relatedness of the first HNSCC. The burden of SPMN was high in patients with HNSCC, with 160 excess second solid tumors developing per 10 000 PYR. The anatomic sites—head and neck, lung, esophagus—at elevated risk for SPMN were the same irrespective of HPV relatedness of the first HNSCC. However, the risks and excess of SPMNs were lower among patients with a first potentially HPV-associated HNSCC (114 excess per 10 000 PYR) than those with a first non–HPV-associated HNSCC (188 excess per 10 000 PYR). The lower relative risk and absolute excess among patients with a first potentially HPV-associated HNSCC could be explained by the fact that most patients with HPV-positive SCC tend to have better prognosis and are less likely to use tobacco and alcohol.16,17
Among patients with first potentially HPV-associated HNSCC, lung cancer accounted for the largest proportion of excess second cancer burden followed by tongue, gum, and esophagus, which is consistent with previous findings.3,4 There were strongly elevated risk ratios for the development of a head and neck SPMN (gum, floor of mouth, tongue), with SIR values greater than 15. Oncogenic HPV types are associated with cervical, anal, vaginal, vulvar, and penile cancers.14,39 A previous study reported an association between oropharyngeal SCC and the risk of second cancers in these sites,40 but the present study did not find significantly elevated risks in these sites, consistent with what Neumann et al found.4 These could be owing to the low prevalence of these cancers in the United States. Also, it could be that the burden of smoking is high among HNSCC patients41,42 irrespective of HPV status, which might explain why tobacco-associated cancers such as lung, rather than HPV-associated anogenital cancers, made up most of the SPMNs found in this study.
Among patients with first non–HPV-associated HNSCC, there were strongly elevated risk ratios for the development of a head and neck SPMN (gum and floor of mouth), with SIR values of 30 or higher. Second primary lung, tongue, gum, and esophagus cancers accounted for the largest proportion of excess second cancer burden; a finding consistent with previously published population-based studies including an international multicenter study from 13 population-based cancer registries.4,5,7,20 When looking at subsites separately, patients with index hypopharyngeal SCC had relative risks for SPMN that were highest of any subsite (SIR, 3.0) as well as excess risk (EAR, 303). All subsites—oral cavity, laryngeal, and hypopharyngeal SCCs—were strongly associated with SPMNs in the head and neck with SIRs being as high as 64 for gum among patients with oral cavity SCC. However, largest proportions of excess SPMNs were observed in the lung for all subsites followed by head and neck and esophagus; possibly owing to tobacco and alcohol use. Tobacco and alcohol consumption are estimated to be responsible for 72% of head and neck cancers in the general population.43 The association of the first HNSCC with SPMNs, particularly with head and neck cancers, has been described in the context of a field cancerization effect,44 in which carcinogenic effects from tobacco and alcohol on the aerodigestive tract may simultaneously act on other parts, this is believed to elevate epithelial cancer risk through the head and neck, lung, and esophagus. Smoking and drinking habits of patients before the first primary diagnosis play a role in the SPMN occurrence; it will be important to inform cancer patients who continued tobacco or tobacco-related and alcohol use habits of their increase risk of SPMNs, including lung cancer, which subsequently leads to poorer survival.
Implications
Our findings have implications for cancer survivors, clinicians, and public health practitioners. Second primary malignat neoplasms are associated with a poor prognosis, are the leading cause of long-term morbidity and mortality10,11,12 and represent a considerable obstacle to improvements in survival in patients with HNSCC.45 Better understanding of the threat of SPMN to cancer survivorship is important to influence clinical guidelines in the treatment of patients with HNSCC. The identification of anatomic sites at high risk of SPMNs may be useful for screening, prevention strategies, and counseling. Our study findings are in alignment with current National Comprehensive Cancer Network46 guidelines to screen first HNSCC patients at risk for SPMNs. In addition, our study shows that the risks of SPMNs are elevated independent of potential HPV-association of first HNSCC, hence, clinical guidelines should reflect that follow-up recommendations for SPMN surveillance should cover all first HNSCC anatomical sites, including those primarily associated with HPV. Future studies targeting the prevention of SPMNs in cancer survivors are needed as well as the development of specific risk prediction tools for SPMNs detection.
Although we similarly investigated SPMN in the HNSCC population, our study is different from Morris et al3 in important ways. First, we used the latest SEER data (up to 2014), thereby ensuring that the latest information on HPV-associated HNSCC was captured. This is very important given the changing landscape in the etiology of HNSCC in the United States. There also has been a gradual shift from tobacco to HPV as the leading cause of HNSCC with HPV-associated HNSCC being the leading HPV-associated cancer in the United States.47 Our study describes how SPMN has developed in this population since 2006, the last year of data used by Morris and colleagues.3 Second, Morris et al followed patients from 1975 to 2006. However, HPV’s role in HNSCC was relatively unknown until the mid-80s when it became known that HPV may be associated with SCC.48 A recent study showed that the steep increase in the incidence of HPV-associated HNSCC may have happened in the late 90s or early 2000s.49 It is important to note that our cohort spanned from 2000 to 2014. Therefore, if HPV relatedness is associated with the development of SPMNs, our study period was designed to accurately capture this association. In contrast, the late 70s or early 80s featured in Morris et al’s3 study could not have captured this noteworthy association of HPV relatedness.
Limitations and Strengths
A limitation of this study is the lack of information about HPV status in SEER. We therefore used anatomical sites that are well established for HPV-associated oropharyngeal SCC.14,15,31,32 Because only 70% to 72% of cancers of these sites are HPV positive,16,47 this could have resulted in overestimation of SPMN risks among patients with first potentially HPV-associated HNSCC. However, our sensitivity analysis provided similar results to the main analysis; even though not every patient in the sensitivity analysis would be HPV positive. In addition, our findings are similar to those of 2 single-institution studies that used actual HPV status in patients with oropharyngeal SCC.17,50 Likewise, SEER registry data do not record risk factors such as tobacco and/or alcohol use, and we were therefore unable to incorporate these factors into a wider analysis of risk factors for SPMN. Another limitation is the 2-month delay used to define SPMN. We performed sensitivity analyses also using the 6-month definition which provided very similar results to our main results. Finally, we were limited to clinical criteria for SPMN and therefore there could be misclassifications. Lung metastases may be misclassified as SPMNs or vice versa leading to overestimation of SPMN risks. Despite these limitations, strengths of our study included a large sample size, near complete follow-up, and the high quality control of the SEER program.21,27 Risks were calculated using a large SEER reference cohort, thus maximizing both internal validity and generalizability of results.
Conclusions
Patients with first potentially HPV-associated HNSCC and also those with non–HPV-associated HNSCC are at increased risks of developing SPMN in the head and neck region as well as lung and esophagus even though the risk and burden were lower among those with potentially HPV-associated HNSCC. These findings could be useful for clinicians in the diagnosis and prevention of subsequent cancers in these patients. Efforts made by public health policy makers and oncology care professionals should be sustained to develop effective smoking cessation interventions. Future studies should evaluate if there are differences in risks of SPMN among patients with HNSCC in earlier years vs later years owing to shifting risk profiles among patients with HNSCC.
eTable. Anatomic site of SPM among white males aged 20-54 with oropharynx squamous cell carcinoma, SEER 2000-2014 (N=900)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30. doi: 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Jain KS, Sikora AG, Baxi SS, Morris LG. Synchronous cancers in patients with head and neck cancer: risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer. 2013;119(10):1832-1837. doi: 10.1002/cncr.27988 [DOI] [PubMed] [Google Scholar]
- 3.Morris LG, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29(6):739-746. doi: 10.1200/JCO.2010.31.8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann F, Jégu J, Mougin C, et al. Risk of second primary cancer after a first potentially-human papillomavirus-related cancer: A population-based study. Prev Med. 2016;90:52-58. doi: 10.1016/j.ypmed.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 5.Chuang SC, Scelo G, Tonita JM, et al. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer. 2008;123(10):2390-2396. doi: 10.1002/ijc.23798 [DOI] [PubMed] [Google Scholar]
- 6.Kwon M, Roh JL, Song J, et al. Noncancer health events as a leading cause of competing mortality in advanced head and neck cancer. Ann Oncol. 2014;25(6):1208-1214. doi: 10.1093/annonc/mdu128 [DOI] [PubMed] [Google Scholar]
- 7.Jégu J, Binder-Foucard F, Borel C, Velten M. Trends over three decades of the risk of second primary cancer among patients with head and neck cancer. Oral Oncol. 2013;49(1):9-14. doi: 10.1016/j.oraloncology.2012.06.018 [DOI] [PubMed] [Google Scholar]
- 8.Lin SS, Massa ST, Varvares MA. Improved overall survival and mortality in head and neck cancer with adjuvant concurrent chemoradiotherapy in national databases. Head Neck. 2016;38(2):208-215. doi: 10.1002/hed.23869 [DOI] [PubMed] [Google Scholar]
- 9.Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society Head and Neck Cancer Survivorship Care Guideline. CA Cancer J Clin. 2016;66(3):203-239. doi: 10.3322/caac.21343 [DOI] [PubMed] [Google Scholar]
- 10.Garavello W, Ciardo A, Spreafico R, Gaini RM. Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2006;132(7):762-766. doi: 10.1001/archotol.132.7.762 [DOI] [PubMed] [Google Scholar]
- 11.Fuller CD, Wang SJ, Thomas CR Jr, Hoffman HT, Weber RS, Rosenthal DI. Conditional survival in head and neck squamous cell carcinoma: results from the SEER dataset 1973-1998. Cancer. 2007;109(7):1331-1343. doi: 10.1002/cncr.22563 [DOI] [PubMed] [Google Scholar]
- 12.van der Schroeff MP, van de Schans SA, Piccirillo JF, Langeveld TP, Baatenburg de Jong RJ, Janssen-Heijnen ML. Conditional relative survival in head and neck squamous cell carcinoma: Permanent excess mortality risk for long-term survivors. Head Neck. 2010;32(12):1613-1618. doi: 10.1002/hed.21369 [DOI] [PubMed] [Google Scholar]
- 13.American Cancer Society Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed August 25, 2016.
- 14.Zandberg DP, Bhargava R, Badin S, Cullen KJ. The role of human papillomavirus in nongenital cancers. CA Cancer J Clin. 2013;63(1):57-81. doi: 10.3322/caac.21167 [DOI] [PubMed] [Google Scholar]
- 15.Combes JD, Franceschi S. Role of human papillomavirus in non-oropharyngeal head and neck cancers. Oral Oncol. 2014;50(5):370-379. doi: 10.1016/j.oraloncology.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 16.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. doi: 10.1200/JCO.2011.36.4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24-35. doi: 10.1056/NEJMoa0912217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deschler DG, Richmon JD, Khariwala SS, Ferris RL, Wang MB. The “new” head and neck cancer patient-young, nonsmoker, nondrinker, and HPV positive: evaluation. Otolaryngol Head Neck Surg. 2014;151(3):375-380. doi: 10.1177/0194599814538605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758-6762. doi: 10.1158/1078-0432.CCR-09-0784 [DOI] [PubMed] [Google Scholar]
- 20.Morris LG, Sikora AG, Hayes RB, Patel SG, Ganly I. Anatomic sites at elevated risk of second primary cancer after an index head and neck cancer. Cancer Causes Control. 2011;22(5):671-679. doi: 10.1007/s10552-011-9739-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis RE, Freedman DM, Ron E, Ries LAG, Hacker DG, Edwards BK, Tucker MA, Fraumeni JF Jr. (eds). New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. National Cancer Institute. NIH Publ. No. 05-5302. Bethesda, MD, 2006. [Google Scholar]
- 22.Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States). Cancer Causes Control. 2003;14(2):131-138. doi: 10.1023/A:1023060315781 [DOI] [PubMed] [Google Scholar]
- 23.National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Overview of the SEER program. 2016. http://seer.cancer.gov/about/overview.html. Accessed April 1, 2018.
- 24.Boehmer U, Ozonoff A, Miao X. An ecological approach to examine lung cancer disparities due to sexual orientation. Public Health. 2012;126(7):605-612. doi: 10.1016/j.puhe.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Overview of the Surveillance, Epidemiology, and End Results database: evolution, data variables, and quality assurance. Curr Probl Cancer. 2012;36(4):183-190. doi: 10.1016/j.currproblcancer.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 26.Zippin C, Lum D, Hankey BF. Completeness of hospital cancer case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76(11):2343-2350. doi: [DOI] [PubMed] [Google Scholar]
- 27.Harlan LC, Hankey BF. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21(12):2232-2233. doi: 10.1200/JCO.2003.94.023 [DOI] [PubMed] [Google Scholar]
- 28.Fritz A, Percy C, Jack A, et al. International classification of diseases for oncology (ICD-O-3). 3rd ed Geneva: World Health Organization; 2000. [Google Scholar]
- 29.Muir CS, Percy C. Cancer registration: principles and methods. Classification and coding of neoplasms. IARC Sci Publ. 1991;(95):64-81. [PubMed] [Google Scholar]
- 30.Zhu G, Chen Y, Zhu Z, et al. Risk of second primary cancer after treatment for esophageal cancer: a pooled analysis of nine cancer registries. Dis Esophagus. 2012;25(6):505-511. doi: 10.1111/j.1442-2050.2011.01273.x [DOI] [PubMed] [Google Scholar]
- 31.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612-619. doi: 10.1200/JCO.2007.14.1713 [DOI] [PubMed] [Google Scholar]
- 32.Hwang TZ, Hsiao JR, Tsai CR, Chang JS. Incidence trends of human papillomavirus-related head and neck cancer in Taiwan, 1995-2009. Int J Cancer. 2015;137(2):395-408. doi: 10.1002/ijc.29330 [DOI] [PubMed] [Google Scholar]
- 33.Breslow NE, Day NE. Statistical methods in cancer research. Volume II—The design and analysis of cohort studies. IARC Sci Publ. 1987;(82):1-406. [PubMed] [Google Scholar]
- 34.Schoenberg BS, Myers MH. Statistical methods for studying multiple primary malignant neoplasms. Cancer. 1977;40(4)(suppl):1892-1898. doi: [DOI] [PubMed] [Google Scholar]
- 35.Begg CB, Zhang ZF, Sun M, Herr HW, Schantz SP. Methodology for evaluating the incidence of second primary cancers with application to smoking-related cancers from the Surveillance, Epidemiology, and End Results (SEER) program. Am J Epidemiol. 1995;142(6):653-665. doi: 10.1093/oxfordjournals.aje.a117689 [DOI] [PubMed] [Google Scholar]
- 36.Silverman S., Jr Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001;132(suppl):7S-11S. doi: 10.14219/jada.archive.2001.0382 [DOI] [PubMed] [Google Scholar]
- 37.Schantz SP, Yu GP. Head and neck cancer incidence trends in young Americans, 1973-1997, with a special analysis for tongue cancer. Arch Otolaryngol Head Neck Surg. 2002;128(3):268-274. doi: 10.1001/archotol.128.3.268 [DOI] [PubMed] [Google Scholar]
- 38.Patel SC, Carpenter WR, Tyree S, et al. Increasing incidence of oral tongue squamous cell carcinoma in young white women, age 18 to 44 years. J Clin Oncol. 2011;29(11):1488-1494. doi: 10.1200/JCO.2010.31.7883 [DOI] [PubMed] [Google Scholar]
- 39.Jemal A, Simard EP, Dorell C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105(3):175-201. doi: 10.1093/jnci/djs491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sikora AG, Morris LG, Sturgis EM. Bidirectional association of anogenital and oral cavity/pharyngeal carcinomas in men. Arch Otolaryngol Head Neck Surg. 2009;135(4):402-405. doi: 10.1001/archoto.2009.19 [DOI] [PubMed] [Google Scholar]
- 41.Osazuwa-Peters N, Adjei Boakye E, Chen BY, Tobo BB, Varvares MA. Association between head and neck squamous cell carcinoma survival, smoking at diagnosis, and marital status. JAMA Otolaryngol Head Neck Surg. 2017;(Nov):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karam-Hage M, Cinciripini PM, Gritz ER. Tobacco use and cessation for cancer survivors: an overview for clinicians. CA Cancer J Clin. 2014;64(4):272-290. doi: 10.3322/caac.21231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541-550. doi: 10.1158/1055-9965.EPI-08-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963-968. doi: [DOI] [PubMed] [Google Scholar]
- 45.Lin K, Patel SG, Chu PY, et al. Second primary malignancy of the aerodigestive tract in patients treated for cancer of the oral cavity and larynx. Head Neck. 2005;27(12):1042-1048. doi: 10.1002/hed.20272 [DOI] [PubMed] [Google Scholar]
- 46.National Comprehensive Cancer Network (NCCN) NCCN Guidelines for Detection, Prevention, & Risk Reduction. https://www.nccn.org/professionals/physician_gls/default.aspx#detection. Accessed December 3, 2017.
- 47.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus–associated cancers—United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016;65(26):661-666. doi: 10.15585/mmwr.mm6526a1 [DOI] [PubMed] [Google Scholar]
- 48.zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384(2):260-265. doi: 10.1016/j.virol.2008.11.046 [DOI] [PubMed] [Google Scholar]
- 49.Osazuwa-Peters N, Simpson MC, Massa ST, Adjei Boakye E, Antisdel JL, Varvares MA. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017;74:90-97. doi: 10.1016/j.oraloncology.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 50.Peck BW, Dahlstrom KR, Gan SJ, et al. Low risk of second primary malignancies among never smokers with human papillomavirus-associated index oropharyngeal cancers. Head Neck. 2013;35(6):794-799. doi: 10.1002/hed.23033 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Anatomic site of SPM among white males aged 20-54 with oropharynx squamous cell carcinoma, SEER 2000-2014 (N=900)