Abstract
Importance
Problems with speech in patients with facial paralysis are frequently noted by both clinicians and the patients themselves, but limited research exists describing how facial paralysis affects verbal communication.
Objective
To assess the influence of facial paralysis on communicative participation.
Design, Setting, and Participants
A nationwide online survey of 160 adults with unilateral facial paralysis was conducted from March 1 to June 1, 2017. To assess communicative participation, respondents completed the Communicative Participation Item Bank (CPIB) Short Form questionnaire and the Facial Clinimetric Evaluation (FaCE) Scale.
Main Outcomes and Measures
The CPIB Short Form and the correlation between the CPIB Short Form and FaCE Scale. In the CPIB, the level of interference in communication is rated on a 4-point Likert scale (where not at all = 3, a little = 2, quite a bit = 1, and very much = 0). Total scores for the 10 items range from 0 (worst) to 30 (best). The FaCE Scale is a 15-item instrument that produces an overall score ranging from 0 (worst) to 100 (best), with higher scores representing better function and higher quality of life.
Results
Of the 160 respondents, 145 (90.6%) were women and 15 were men (mean [SD] age, 45.1 [12.6] years). Most respondents reported having facial paralysis for more than 3 years. Causes of facial paralysis included Bell palsy (86 [53.8%]), tumor (41 [25.6%]), and other causes (33 [20.6%]), including infection, trauma, congenital defects, and surgical complications. The mean (SD) score on the CPIB Short Form was 0.16 (0.88) logits (range, –2.58 to 2.10 logits). The mean (SD) score of the FaCE Scale was 40.92 (16.05) (range, 0-83.3). Significant correlations were observed between the CPIB Short Form and overall FaCE Scale scores, as well as the Social Function, Oral Function, Facial Comfort, and Eye Comfort subdomains of the FaCE Scale, but not with the Facial Movement subdomain.
Conclusions and Relevance
Patients with facial paralysis in this study sample reported restrictions in communicative participation that were comparable with restrictions experienced by patients with other known communicative disorders, such as laryngectomy and head and neck cancer. We believe that communicative participation represents a unique domain of dysfunction and can help quantify the outcome of facial paralysis and provide an additional frame of reference when assessing treatment outcomes.
This survey study assesses the association of facial paralysis with communicative participation among US adults with unilateral facial paralysis caused by Bell palsy, tumor, and other causes.
Key Points
Question
What is the influence of facial paresis or paralysis on speech and communicative participation?
Findings
In this nationwide online survey of patients with unilateral facial paresis or paralysis, respondents reported restrictions in communicative participation that were comparable with restrictions experienced by patients with known causes of communication disorders, such as laryngectomy and head and neck cancers.
Meaning
Impaired communicative participation may represent a unique domain of dysfunction in facial paralysis; measurement of communicative participation can help quantify the influence of facial paralysis on daily living as well as provide an additional frame of reference when setting goals of care and assessing outcomes of treatment.
Introduction
Facial paralysis alters facial appearance, affects the patient’s ability to express emotions, and may cause oral incompetence, difficulty manipulating a food bolus, and problems with articulation of bilabial sounds. It has been well established that these sequelae of facial paralysis significantly affect daily living and have an adverse influence on quality of life.1,2,3,4,5,6,7
Although problems with speech in patients with facial paralysis are commonly noted by clinicians and the patients themselves, there has been limited research into how facial paralysis affects the broader concept of communication. A recent study by Movérare et al8 reported significantly decreased lip force, which impaired articulation of labial consonants and speech intelligibility (particularly with labial consonants) among patients with facial paralysis, highlighting the detrimental effects of facial paralysis on speech production. However, the ways in which problems with speech in individuals with facial paralysis influence communicative function and quality of life are still poorly understood. Although several instruments have been developed to assess facial function and quality of life in patients with facial paralysis, the most commonly used instruments in practice today assess deficits in communication only peripherally.9,10,11,12,13
In addition to difficulties in mechanical speech production due to impaired facial movement, the influence of facial paralysis on communication is far reaching and likely has many physical, psychological, and environmental factors contributing to decreased communicative function.8,14 We hypothesize that patients with facial paralysis experience a global dysfunction in communication that significantly restricts their participation in circumstances requiring verbal communication. As such, we sought to examine the influence of facial paralysis on communicative participation. Communicative participation is defined as “taking part in life situations in which knowledge, information, ideas, or feelings are exchanged.”15(p1191) Restrictions in communicative participation can affect multiple domains of life, including personal care, employment, and community engagement, and reflects a significant area of functioning and disability. An evaluation of communicative participation specifically addresses aspects of communication that are required in various life situations and reflects a person’s ability to fulfill his or her life roles.16
The purpose of this study is to investigate the influence of facial paralysis on communicative participation as measured by a validated, patient-reported outcome measure, the Communicative Participation Item Bank (CPIB) Short Form questionnaire.16 Secondarily, we sought to examine associations between communicative participation and patient characteristics. Finally, we explored associations between communicative participation and a facial paralysis–specific quality-of-life assessment instrument, the Facial Clinimetric Evaluation (FaCE) Scale.11
Methods
Participants and Data Collection
A nationwide online survey of adults (age ≥18 years) with self-reported facial paralysis was conducted from March 1 to June 1, 2017. Participants reporting bilateral facial paralysis were excluded from this study. This study was approved by the University of Southern California Institutional Review Board (HS-17-00086). The need for informed consent was waived by the University of Southern California Institutional Review Board owing to limited risk and the use of a deidentified data set.
All participants were recruited through the websites and social media outlets for the Acoustic Neuroma Association and the Facial Paralysis & Bell’s Palsy Foundation, necessarily restricting the conditions examined to patient self-report of these diagnoses. An open access link that directed participants to an online REDCap (Research Electronic Data Capture) survey portal was posted to the respective webpages. All responses were recorded through an encrypted REDCap online database.
CPIB Short Form
To assess how the participants functioned in various communication settings, all participants completed the CPIB Short Form.16 The CPIB Short Form is a 10-item instrument derived from a longer, 46-item question bank and is intended for use in a clinical setting. The authors of the CPIB reported a strong correlation (r = 0.971) between the full-length CPIB and the CPIB Short Form and suggested that scores from the short form are nearly identical to the scores on the full-length item set.16
The CPIB Short Form was designed to measure the amount of interference that community-dwelling adults with various speech-related disorders experience while conversing or engaging in discussions with various audiences.16 The CPIB was selected to assess everyday communication to provide a broad overview of function, encompassing many factors, such as situational context and speaking partners.15 The CPIB is not specific for assessment of any one communication disorder; it is applicable across a broad range of communication disorders, allowing the ability to compare restrictions in communicative participation among different pathologic conditions.
In the CPIB, the level of interference in communication is rated on a 4-point Likert scale (where not at all = 3, a little = 2, quite a bit = 1, and very much = 0). Total scores for the 10 items range from 0 (worst) to 30 (best). Higher scores are more favorable, as they reflect higher levels of communicative participation and, conversely, lower levels of interference with communication. The authors used Item Response Theory to create this shortened 10-item scale with a single dimension. Conversion of the numerical score into logits is recommended, as is customary in Item Response Theory applications. However, to calculate correlations between the CPIB and FaCE instrument, total CPIB scores (nonlogit) were used.
FaCE Scale
Participants were also asked to complete the FaCE Scale, which is a disease-specific, patient-reported outcome measure that assesses self-perception of overall functioning and quality of life in patients with facial paralysis.11 The FaCE Scale is a 15-item instrument that produces an overall score ranging from 0 (worst) to 100 (best), with higher scores representing better function and higher quality of life. In addition to the overall score, the FaCE Scale produces scores in 6 subdomains: Facial Movement, Facial Comfort, Oral Function, Eye Comfort, Lacrimal Control, and Social Function.
Demographic Data
Self-reported participant demographics (current age, sex, cause of facial paralysis, duration of facial paralysis, and prior treatment for facial paralysis) were also surveyed. Respondents indicated the duration of facial paralysis in the following 4 categories: less than 1 year, 1 to 2 years, 2 to 3 years, or more than 3 years.
Statistical Analysis
All data analysis was completed using SPSS, version 24.0 (IBM Corp). Descriptive analyses were completed for demographic data, including means, ranges, and SDs for continuous variables and frequencies for categorical variables. Differences on the FaCE Scale and subdomains by demographic variables (sex, cause of facial paralysis, and prior treatment) were assessed using multivariate analysis of variance, with age as a covariate. If significant, a one-way analysis of variance was performed to identify the main effect for each domain. Differences on the CPIB Short Form by demographic variables were assessed using analysis of variance, with age as a covariate. Finally, the associations between FaCE subscales and CPIB were evaluated using Pearson correlations. Partial η2, describing the proportion of variance attributable to the factor, is reported by main effect, and 95% CIs are reported for mean differences.17 To interpret the partial η2 effect sizes, a value of 0.0099 is considered a small effect size, 0.0588 a medium effect size, and 0.1379 a large effect size.18
Results
Sample Characteristics
During the 3-month study period, 257 responses were recorded. Responses with incomplete data (n = 86) and those reporting bilateral facial paralysis (n = 11) were excluded. A total of 160 participants were included in the study. Most respondents were female (145 [90.6%]) (Table 1). There were too few men in the sample to analyze for differences by sex on either instrument. The mean (SD) age of study participants was 45.1 (12.6) years (range, 18-78 years). Most participants indicated that their facial paralysis had lasted for more than 3 years. The most common self-reported cause of facial paralysis was Bell palsy (86 [53.8%]), followed by tumor (41 [25.6%]) and other causes (33 [20.6%]), which included infection, trauma, congenital defects, and surgical complications. Twenty-five participants reported prior treatment for facial paralysis; treatments included nerve transfers, muscle transfers, eyelid weights, hyaluronic acid fillers, and facial implants.
Table 1. Study Demographics.
| Characteristic | No. (%) |
|---|---|
| Sex | |
| Male | 15 (9.4) |
| Female | 145 (90.6) |
| Age, mean (SD), y | 45.1 (12.6) |
| Self-reported facial palsy duration, y | |
| <1 | 19 (11.9) |
| 1-2 | 14 (8.8) |
| 2-3 | 27 (16.9) |
| >3 | 100 (62.5) |
| Self-reported cause of facial paralysis | |
| Bell palsy | 86 (53.8) |
| Tumor | 41 (25.6) |
| Other | 33 (20.6) |
| Self-reported prior treatments | |
| Yes | 25 (15.6) |
| No | 135 (84.4) |
FaCE Results
The overall mean (SD) score of the FaCE Scale was 40.92 (16.05), with scores ranging between 0 and 83.3 (Table 2). The overall multivariate analysis of variance of the FaCE subdomains by prior treatment (yes or no), duration of condition, possible cause, and current age as a covariate showed that age was not a significant covariate (partial η2 = 0.051). Overall, prior treatments (partial η2 = 0.122) and possible cause (partial η2 = 0.125) accounted for variation on FaCE subscale mean scores (eTable 1 in the Supplement). Simple effects analysis showed that having received prior treatment was associated with lower scores in Oral Function, Eye Comfort, and Social Function subdomains (eTable 2 in the Supplement). Simple effects analysis showed that possible cause was significantly associated with the mean scores for the Facial Movement, Facial Comfort, Oral Function, and Eye Comfort subscales (eTable 3 in the Supplement).
Table 2. Summary of Results of the FaCE Scale and CPIB Short Form.
| Characteristic | No. | Score, Mean (SD) | |
|---|---|---|---|
| FaCE | CPIB, Logits | ||
| Sex | |||
| Male | 15 | 44.44 (11.96) | 0.23 (0.84) |
| Female | 145 | 40.55 (16.41) | 0.16 (0.88) |
| Duration of facial paralysis, y | |||
| <1 | 19 | 40.26 (21.83) | 0.05 (1.01) |
| 1-2 | 14 | 41.19 (14.00) | 0.13 (0.93) |
| 2-3 | 27 | 40.68 (15.93) | −0.02 (0.79) |
| >3 | 100 | 41.07 (15.32) | 0.24 (0.87) |
| Possible cause | |||
| Bell palsy | 86 | 40.04 (15.96) | 0.14 (0.88) |
| Tumor | 41 | 39.43 (15.39) | 0.03 (0.88) |
| Other causes | 33 | 45.05 (16.90) | 0.39 (0.83) |
| Prior surgical treatment | |||
| Yes | 25 | 35.33 (16.78) | 0.004 (1.01) |
| No | 135 | 41.95 (15.76) | 0.19 (0.85) |
| Age range, y | |||
| <20 | 1 | 23.33 | −1.10 |
| 20-29 | 20 | 46.58 (16.73) | 0.22 (0.58) |
| 30-39 | 30 | 44.00 (15.10) | 0.12 (0.99) |
| 40-49 | 49 | 38.50 (16.65) | 0.30 (0.84) |
| 50-59 | 36 | 40.69 (16.64) | 0.32 (0.92) |
| 60-69 | 19 | 37.46 (13.50) | −0.37 (0.89) |
| 70-80 | 5 | 41.67 (16.03) | −0.07 (0.31) |
Abbreviations: CPIB, Communicative Participation Item Bank; FaCE, Facial Clinimetric Evaluation Scale.
Bonferroni-corrected simple effects of possible cause on each of the FaCE subscales showed a complex pattern of results. For the Facial Movement subscale, patients with tumors were more impaired relative to those with Bell palsy but were no different from patients with other causes of facial paralysis. Conversely, on the Facial Comfort subscale, patients with tumors had less impairment relative to those with Bell palsy but were no different from patients with other causes of facial paralysis. On the Oral Function subscale, patients with other causes of facial paralysis had significantly better scores than patients with Bell palsy and those with tumors. Finally, on the Eye Comfort subscale, patients with tumors were more impaired relative to those with Bell palsy and other causes of facial paralysis (eTable 3 in the Supplement).
CPIB Results
The overall mean (SD) score on the CPIB Short Form for the study sample was 0.16 (0.88) logits (range, –2.58 to 2.10 logits) (Table 2). Univariate analysis of variance by prior treatment (yes or no), duration of condition, possible cause, and current age showed no significant effects, indicating that the mean CPIB Short Form scores did not differ by disease factor or age (corrected R2 = 0.012).
Association Between the CPIB and the FaCE Scale
A significant Pearson correlation was observed between total CPIB score and total FaCE scores (r = 0.47) (Table 3), which represents approximately 22% variance shared between the 2 instruments. Higher scores are indicative of higher function for both the CPIB and FaCE Scale, indicating that the better a respondent rated his or her facial function, the better communication participation he or she perceived.
Table 3. Pearson r Correlation Among CPIB Short Form and FaCE Total and Subdomains With 95% CIs for 160 Respondents.
| FaCE Scale | 1. CPIB | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 2. Overall FaCE Scale | 0.47 (0.37 to 0.57)a | 1 [Reference] | NA | NA | NA | NA | NA |
| 3. Facial Movement | 0.06 (−0.11 to −0.20) | 0.42 (0.28 to 0.55)a | 1 [Reference] | NA | NA | NA | NA |
| 4. Facial Comfort | 0.29 (0.15 to −0.41)a | 0.65 (0.56 to 0.73)a | −0.03 (−0.18 to 0.15) | 1 [Reference] | NA | NA | NA |
| 5. Oral Functiona | 0.47 (0.35 to 0.59) | 0.74 (0.67 to 0.80) | 0.24 (0.08 to 0.39) | 0.42 (0.28 to 0.55) | 1 [Reference] | NA | NA |
| 6. Eye Comfort | 0.19 (0.04 to 0.36)a | 0.54 (0.41 to 0.64)a | 0.24 (0.09 to 0.38)a | 0.13 (−0.02 to 0.27) | 0.40 (0.26 to 0.52)a | 1 [Reference] | NA |
| 7. Lacrimal Control | 0.11 (−0.05 to 0.27) | −0.33 (0.18 to 0.47)a | −0.06 (−0.20 to 0.09) | 0.19 (0.03 to 0.34)a | 0.14 (−0.03 to 0.29) | −0.04 (−0.20 to 0.13) | 1 [Reference] |
| 8. Social Functiona | 0.49 (0.39 to 0.60) | 0.86 (0.81 to 0.89) | 0.19 (0.02 to 0.35) | 0.46 (0.33 to 0.57) | 0.67 (0.58 to 0.74) | 0.32 (0.16 to 0.46) | 0.26 (0.10 to 0.41) |
Abbreviations: CPIB, Communicative Participation Item Bank; FaCE, Facial Clinimetric Evaluation Scale; NA, not applicable.
Indicates significant correlation.
When examining the association between total CPIB score and the FaCE subdomains, statistically significant correlations were observed for the Facial Comfort, Oral Function, Eye Comfort, and Social Function subdomains. No associations were found between the total CPIB score and the Facial Movement or Lacrimal Control subdomains (Table 3). Also reported in Table 3 are the intercorrelations among the FaCE subscales, indicating good coherence among the subscales, with several exceptions: the Lacrimal Control subscale seems to provide different information than the other subscales and the Facial Movement subscale is not related to the Facial Comfort subscale.
Discussion
Facial paralysis is well known to cause a variety of functional, aesthetic, and psychological consequences. Several studies have demonstrated that facial paralysis significantly affects quality of life.1,2,3,5,6,7 However, there is only limited research that examines the influence of facial paralysis on speech and functional behaviors, such as communication.8 We report a large internet survey of persons with self-reported facial paralysis and their responses to a validated instrument, the CPIB Short Form, assessing the interference of facial paralysis with communication.
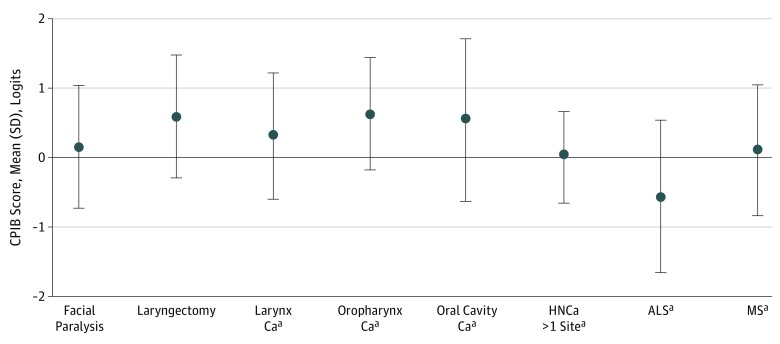
The results of this study suggest that at least some patients with facial paralysis experience significant restriction in communicative participation as reflected by their CPIB Short Form scores. To provide a context for these findings, we compared our results with prior research examining communicative participation in patient samples with known communication disorders. A study by Eadie et al19 that examined communicative participation in patients who had undergone a total laryngectomy reported levels of communicative participation (CPIB Short Form mean [SD] score, 0.60 [0.88] logits) that were comparable with the facial paralysis sample in this study. Similarly, other studies examining communicative participation in other patients (including those with head and neck cancer, multiple sclerosis, and amyotrophic lateral sclerosis) using a longer, 46-item version of the CPIB reported levels of communicative participation that were also comparable with the facial paralysis sample represented in this study.20,21,22 A comparison of communicative participation between these samples is shown in the Figure.19,20,21,22 Although we are cautious in making direct comparisons between these different patient groups (as they may have differing perspectives and expectations regarding their communicative function), it is surprising to see that the sample in this study subjectively reported levels of communicative participation that are comparable with those in patients with known, severe communicative disorders, such as those who have undergone laryngectomy. These findings are consistent with prior qualitative research that reported that patients with various disorders that affect verbal communication (spasmodic dysphonia, multiple sclerosis, Parkinson disease, and amyotrophic lateral sclerosis) experienced similar types of restrictions in communicative participation despite different mechanisms of speech impairment.14
Figure. Comparison of Communicative Participation Between Our Sample of Self-reported Facial Paralysis and Other Samples From the Literature.
Samples from the literature included laryngectomy,19 head and neck cancer (HNCa),20 amyotrophic lateral sclerosis (ALS),21 and multiple sclerosis (MS).22 Error bars indicate SD.
aPatients evaluated with the full-length, 46-item version of the Communicative Participation Item Bank (CPIB) as opposed to the Short Form. Higher logit scores indicate better communicative function. Ca indicates cancer.
In this study sample, those who reported Bell palsy as the cause of their facial paralysis scored better on the Facial Movement subscale but lower on the Oral Function, Facial Comfort, and Eye Comfort subscales when compared with those with facial palsy as a result of a tumor. This finding is comparable with that from a study by Saito and Cheung23 that similarly reported that patients with idiopathic facial paralysis reported lower levels of social functioning despite better facial movement when compared with patients with facial paralysis as a result of vestibular schwannoma. In addition, a history of prior treatment and cause of facial paralysis had a significant effect on FaCE Scale scores. Specifically, having previously received treatment was associated with lower scores in the Oral Function, Eye Comfort, and Social Function subdomains. This finding is consistent with a previous report that patients with facial paralysis who received prior treatment reported lower scores in both the overall FaCE Scale and its subdomains.1 In contrast, the CPIB Short Form scores did not differ by previous treatments or cause of paralysis. Although the CPIB Short Form score and FaCE Scale scores were significantly correlated, certain factors associated with lower FaCE Scale scores did not apply to the results of the CPIB Short Form. We therefore believe that the 2 instruments measure different aspects of the constellation of symptoms, behaviors, and social context of facial paralysis today.
The construct of communicative participation addresses the extent to which a patient partakes in commonly encountered situations requiring speech.14 It specifically addresses the communication that is required to successfully engage in common life situations and reflects a person’s ability to fulfill his or her life roles. Thus, the results of this study suggest that some individuals with facial paralysis may experience significant limitations to their daily living secondary to their restricted communicative participation. We suspect that the influence of facial paralysis on certain patients’ ability to communicate may be a key driver of the decreased quality of life in those with this disease.
Communicative participation as reflected by scores on the CPIB Short Form showed a significant correlation with the overall scores on the FaCE Scale, although this correlation was limited to certain subdomains. Moderate correlations were observed between communicative participation and the Oral Function and Social Function subdomains. Significant but weak correlations were seen between the CPIB Short Form and the Facial Comfort and Eye Comfort subdomains of the FaCE Scale.
No significant correlation was observed between communicative participation and the Facial Movement subdomain of the FaCE Scale. This finding may suggest that restrictions in communicative participation experienced by patients with facial paralysis are not attributable solely to motor function of facial muscles involved in speech production. Rather, as communicative participation encompasses multiple factors relating to communicative success, including situational contexts and communication partners, it represents a more global assessment of communicative function beyond pure motor speech deficits. The observed restriction in communicative participation in patients with facial paralysis is likely multidimensional, influenced by a combination of physical, social, and environmental factors. Prior studies examining communicative participation in individuals with other types of communication disorders have similarly suggested that restrictions in communicative participation are secondary to a constellation of physical, social, and environmental factors.14,22,24,25 Although the CPIB Short Form provides broad assessment of restrictions in communicative participation, it does not necessarily identify the specific factors that contribute to lower levels of communicative participation. Further research is needed to identify specific factors associated with poor communicative participation in patients with facial paralysis.
Furthermore, there is only a moderate correlation between communicative participation and the FaCE Scale, suggesting that communicative participation represents a unique construct that is not adequately captured by existing facial paralysis–specific instruments. As currently used, facial paralysis–specific, patient-reported outcome measures provide a broad assessment of function and quality of life, but they do not directly assess function relating to speech or communication. This shortcoming can be particularly problematic in cases in which deficits in verbal communication are particularly distressing to the patient relative to other symptoms of facial paralysis. An evaluation of communicative participation provides the ability to contextualize the effect of facial paralysis on communication and daily living. In addition, the brevity of the CPIB Short Form allows it to easily be incorporated into a clinical visit. We suggest that an assessment of communicative participation can provide greater insight into the patient’s level of function and perception of his or her disease, as well as provide an additional frame of reference when evaluating treatment outcomes.
Although it is unclear whether communicative participation in patients with facial paralysis correlates with more objective, physician-graded assessments of facial function, prior research has suggested that degree of impairment is not associated with self-perceived communicative function. A previous study by Movérare et al8 reported that the degree of facial paralysis (assessed by the Sunnybrook Facial Grading System) did not demonstrate significant correlation with either articulation or the patient’s perceived communicative abilities. This finding is consistent with prior literature suggesting that patient-reported assessments of the severity of facial paralysis may show only limited correlation to physician-graded instruments, such as the House-Brackmann method or Sunnybrook Facial Grading.26,27,28 Such results indicate that patient-reported assessments can provide new insights into the influence of facial paralysis on daily living and highlight the importance of incorporating patient-reported outcome measures in the management of facial paralysis. Correlation between physician-graded instruments, such as the Sunnybrook Facial Grading system or Electronic Facial Paralysis Assessment grading system, and communicative participation in this subset of patients is a topic for future research.
Limitations
There are several limitations of this study. Most participants in this study were recruited through online support groups, and this sample may not reflect the entire spectrum of patients with facial paralysis as a whole. Patients actively engaging in online support groups may represent patients who feel their impairment is more severe than those who do not attend support groups. Conversely, those in support groups may feel better about their condition relative to the facial paralysis community owing to the social support received from the group. Furthermore, the vast majority of respondents were women (145 [90.6%]). Prior studies have observed that females with facial paralysis tend to report lower levels of function and quality of life than do males.1,2 As such, the sample presented in this study may represent a group that is more biased toward negative perceptions of their communicative abilities. In addition, most patients in the study reported Bell palsy as the cause of their facial paralysis. It is important to consider the cause of disease, as the context in which facial paralysis occurs (ie, idiopathic vs tumor resection) may result in different objective levels of facial function and have different influences on a patient’s ability to cope with his or her dysfunction.23 This study did not explore the correlation between objective severity of facial paralysis and communicative participation. Finally, duration of disease was collected as a categorical variable, with a duration of more than 3 years as the maximum possible selection. Thus, this study was unable to examine the differences in communicative participation for individuals with facial palsy for more than 3 years or make inferences regarding changes in communicative function over time. These potential biases should be considered when interpreting the results of this study.
Despite these biases, we believe this study presents an important first look into the influence of facial paralysis on communication. We believe the results of this study suggest that restrictions in communicative participation represents a significant area of dysfunction in facial paralysis in a subset of patients that is not adequately explored by current standards of evaluation. Communication is a key aspect of a person’s ability to function in various life settings. As such, we believe the results of this study warrant further investigation into communicative participation in all patients with facial paralysis to assess the broader influence of facial nerve dysfunction in daily life.
Conclusions
The patients with facial paralysis in this study reported restrictions in communicative participation that were comparable with those in patients with other known communicative disorders (including laryngectomy, head and neck cancers, multiple sclerosis, and amyotrophic lateral sclerosis). Although there was a statistically significant correlation between communicative participation and facial paralysis–specific quality of life measures, this correlation was limited to certain subdomains and did not correlate with patients’ subjective rating of facial movement. Although we are unable to draw broad conclusions regarding communicative dysfunction in patients facial paralysis as a whole, we believe the construct of communicative participation may represent a unique domain of dysfunction in patients with facial paralysis that is often overlooked by commonly used facial paralysis assessments, warranting further investigations into the effects of facial paralysis on communication. We believe that assessments of communicative participation can help quantify the severity of facial paralysis as well as provide an additional frame of reference when setting goals of care and assessing outcomes of treatment.
eTable 1. Effect Size for Each Factor Entered in the FaCE Subscale MANOVA
eTable 2. Confidence Intervals (95%) for the FaCE by Subscale for Prior Treatment Effect
eTable 3. Effect Sizes and 95% CIs for the FaCE by Subscale for Effect of Etiology (Bell’s Palsy, Tumor, Other)
References
- 1.Leong SC, Lesser TH. A national survey of facial paralysis on the quality of life of patients with acoustic neuroma. Otol Neurotol. 2015;36(3):503-509. [DOI] [PubMed] [Google Scholar]
- 2.Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clin Otolaryngol. 2015;40(6):651-656. [DOI] [PubMed] [Google Scholar]
- 3.Neely JG, Neufeld PS. Defining functional limitation, disability, and societal limitations in patients with facial paresis: initial pilot questionnaire. Am J Otol. 1996;17(2):340-342. [PubMed] [Google Scholar]
- 4.Ho AL, Scott AM, Klassen AF, Cano SJ, Pusic AL, Van Laeken N. Measuring quality of life and patient satisfaction in facial paralysis patients: a systematic review of patient-reported outcome measures. Plast Reconstr Surg. 2012;130(1):91-99. [DOI] [PubMed] [Google Scholar]
- 5.Lindsay RW, Bhama P, Hadlock TA. Quality-of-life improvement after free gracilis muscle transfer for smile restoration in patients with facial paralysis. JAMA Facial Plast Surg. 2014;16(6):419-424. [DOI] [PubMed] [Google Scholar]
- 6.Henstrom DK, Lindsay RW, Cheney ML, Hadlock TA. Surgical treatment of the periocular complex and improvement of quality of life in patients with facial paralysis. Arch Facial Plast Surg. 2011;13(2):125-128. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay RW, Bhama P, Hohman M, Hadlock TA. Prospective evaluation of quality-of-life improvement after correction of the alar base in the flaccidly paralyzed face. JAMA Facial Plast Surg. 2015;17(2):108-112. [DOI] [PubMed] [Google Scholar]
- 8.Movérare T, Lohmander A, Hultcrantz M, Sjögreen L. Peripheral facial palsy: Speech, communication and oral motor function. Eur Ann Otorhinolaryngol Head Neck Dis. 2017;134(1):27-31. [DOI] [PubMed] [Google Scholar]
- 9.VanSwearingen JM, Brach JS. The Facial Disability Index: reliability and validity of a disability assessment instrument for disorders of the facial neuromuscular system. Phys Ther. 1996;76(12):1288-1298. [DOI] [PubMed] [Google Scholar]
- 10.Neely JG, Cherian NG, Dickerson CB, Nedzelski JM. Sunnybrook facial grading system: reliability and criteria for grading. Laryngoscope. 2010;120(5):1038-1045. [DOI] [PubMed] [Google Scholar]
- 11.Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001;111(3):387-398. [DOI] [PubMed] [Google Scholar]
- 12.Banks CA, Bhama PK, Park J, Hadlock CR, Hadlock TA. Clinician-graded electronic facial paralysis assessment: the eFACE. Plast Reconstr Surg. 2015;136(2):223e-230e. [DOI] [PubMed] [Google Scholar]
- 13.Fattah AY, Gurusinghe ADR, Gavilan J, et al. ; Sir Charles Bell Society . Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plast Reconstr Surg. 2015;135(2):569-579. [DOI] [PubMed] [Google Scholar]
- 14.Baylor C, Burns M, Eadie T, Britton D, Yorkston K. A qualitative study of interference with communicative participation across communication disorders in adults. Am J Speech Lang Pathol. 2011;20(4):269-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eadie TL, Yorkston KM, Klasner ER, et al. Measuring communicative participation: a review of self-report instruments in speech-language pathology. Am J Speech Lang Pathol. 2006;15(4):307-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baylor C, Yorkston K, Eadie T, Kim J, Chung H, Amtmann D. The Communicative Participation Item Bank (CPIB): item bank calibration and development of a disorder-generic short form. J Speech Lang Hear Res. 2013;56(4):1190-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson JTE. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6(2):135-147. [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New York, NY: Academic Press; 1969. [Google Scholar]
- 19.Eadie TL, Otero D, Cox S, et al. The relationship between communicative participation and postlaryngectomy speech outcomes. Head Neck. 2016;38(suppl 1):E1955-E1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eadie TL, Lamvik K, Baylor CR, Yorkston KM, Kim J, Amtmann D. Communicative participation and quality of life in head and neck cancer. Ann Otol Rhinol Laryngol. 2014;123(4):257-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yorkston K, Baylor C, Mach H. Factors associated with communicative participation in amyotrophic lateral sclerosis. J Speech Lang Hear Res. 2017;60(6S):1791-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yorkston KM, Baylor C, Amtmann D. Communicative participation restrictions in multiple sclerosis: associated variables and correlation with social functioning. J Commun Disord. 2014;52:196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saito DM, Cheung SW. A comparison of facial nerve disability between patients with Bell’s palsy and vestibular schwannoma. J Clin Neurosci. 2010;17(9):1122-1125. [DOI] [PubMed] [Google Scholar]
- 24.Eadie TL, Otero DS, Bolt S, Kapsner-Smith M, Sullivan JR. The effect of noise on relationships between speech intelligibility and self-reported communication measures in tracheoesophageal speakers. Am J Speech Lang Pathol. 2016;25(3):393-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McAuliffe MJ, Baylor CR, Yorkston KM. Variables associated with communicative participation in Parkinson’s disease and its relationship to measures of health-related quality-of-life. Int J Speech Lang Pathol. 2017;19(4):407-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ng JH, Ngo RY. The use of the facial clinimetric evaluation scale as a patient-based grading system in Bell’s palsy. Laryngoscope. 2013;123(5):1256-1260. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda M, Nakazato H, Hiroshige K, Abiko Y, Sugiura M. To what extent do evaluations of facial paralysis by physicians coincide with self-evaluations by patients: comparison of the Yanagihara method, the House-Brackmann method, and self-evaluation by patients. Otol Neurotol. 2003;24(2):334-338. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2007;133(1):56-60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Effect Size for Each Factor Entered in the FaCE Subscale MANOVA
eTable 2. Confidence Intervals (95%) for the FaCE by Subscale for Prior Treatment Effect
eTable 3. Effect Sizes and 95% CIs for the FaCE by Subscale for Effect of Etiology (Bell’s Palsy, Tumor, Other)