Abstract
Importance
A headache is a symptom of a migraine, but not all patients with migraine have headaches. It is still unclear whether a migraine might increase the risk of cochlear disorders, even though a migraine does not occur concurrently with cochlear disorders.
Objective
To investigate the risk of cochlear disorders for patients with a history of migraines.
Design, Setting, and Participants
This study used claims data from the Taiwan Longitudinal Health Insurance Database 2005 to identify 1056 patients with migraines diagnosed between January 1, 1996, and December 31, 2012. A total of 4224 controls were also identified from the same database based on propensity score matching. Statistical analysis was performed from January 23, 1996, to December 28, 2012.
Main Outcomes and Measures
The incidence rate of cochlear disorders (tinnitus, sensorineural hearing impairment, and/or sudden deafness) was compared between the cohorts by use of the Kaplan-Meier method. The Cox proportional hazards regression model was also used to examine the association of cochlear disorders with migraines.
Results
Of the 1056 patients with migraines, 672 were women and 384 were men, and the mean (SD) age was 36.7 (15.3) years. Compared with the nonmigraine cohort, the crude hazard ratio for cochlear disorders in the migraine cohort was 2.83 (95% CI, 2.01-3.99), and the adjusted hazard ratio was 2.71 (95% CI, 1.86-3.93). The incidence rates of cochlear disorders were 81.4 (95% CI, 81.1-81.8) per 1 million person-years for the migraine cohort and 29.4 (95% CI, 29.2-29.7) per 1 million person-years for the nonmigraine cohort. The cumulative incidence of cochlear disorders in the migraine cohort (12.2%) was significantly higher than that in the matched nonmigraine cohort (5.5%). Subgroup analysis showed that, compared with the nonmigraine cohort, the adjusted hazard ratios in the migraine cohort were 3.30 (95% CI, 2.17-5.00) for tinnitus, 1.03 (95% CI, 0.17-6.41) for sensorineural hearing impairment, and 1.22 (95% CI, 0.53-2.83) for sudden deafness.
Conclusions and Relevance
In this population-based study, the risk of cochlear disorders, especially for tinnitus, was found to be significantly higher among patients with a history of migraines. This finding may support the presence and/or concept of “cochlear migraine.”
This cohort study used claims data from the Taiwan Longitudinal Health Insurance Database 2005 to investigate the risk of tinnitus and other cochlear disorders inr patients with a history of migraines.
Key Points
Question
Does a history of migraines increase the risk of tinnitus and other cochlear disorders?
Findings
In this cohort study of claims data among patients in Taiwan, 1056 patients with a history of migraines and 4224 controls were identified. The cumulative incidence of cochlear disorders, especially tinnitus, was found to be significantly higher among patients with history of migraines than those without a history of migraines.
Meaning
A history of migraines may increase the risk of tinnitus and other cochlear disorders.
Introduction
Migraine is not a synonym for headache. A headache can be a symptom of a migraine, but not all patients with a migraine have headaches. Active migraine symptoms are associated with abnormal sensory perception, including vision, hearing, smell, and somatosensation.1 The basilar-type migraine aura comprises diplopia, vertigo, tinnitus, bilateral visual symptoms, hyperacusis, ataxia, dysarthria, bilateral paresthesias, and decreased level of consciousness.2 Intense emotional stimuli and sleep disorders were the most common triggering factors for active migraine symptoms. Also, low socioeconomic status was associated with an increased frequency of migraines.3
Dizziness, vertigo, phonophobia, tinnitus, and hearing loss were the most commonly reported symptoms in patients with migraines in one study.4 In another study, the association between tinnitus and active migraine symptoms was stronger for students with migraines with auras than for those with migraines without auras.5 The association between active migraine symptoms and sudden deafness was also presented in one case report6 and a population-based study.7 In addition, Viirre and Baloh8 presented 13 cases of individuals with sudden deafness who met the diagnostic criteria for a migraine.
However, it is still unclear whether migraines might increase the risk of other cochlear disorders, including tinnitus and/or sensorineural hearing impairment, even though migraines do not occur concurrently with cochlear disorders. Therefore, the aim of this study was to examine the risk of cochlear disorders for patients with a history of migraines.
Methods
Data Source
This retrospective cohort study used claims data from the Longitudinal Health Insurance Database 2005, a subset of the National Health Insurance Research Database of Taiwan, to identify patients with migraines diagnosed between January 1, 1996, and December 31, 2012. The study was approved by the institutional review board of the Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan (No. B10202022). Since the Longitudinal Health Insurance Database 2005 files contain only deidentified secondary data, the review board of Dalin Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taiwan, waived the requirement for obtaining informed consent from the patients.
Study Population
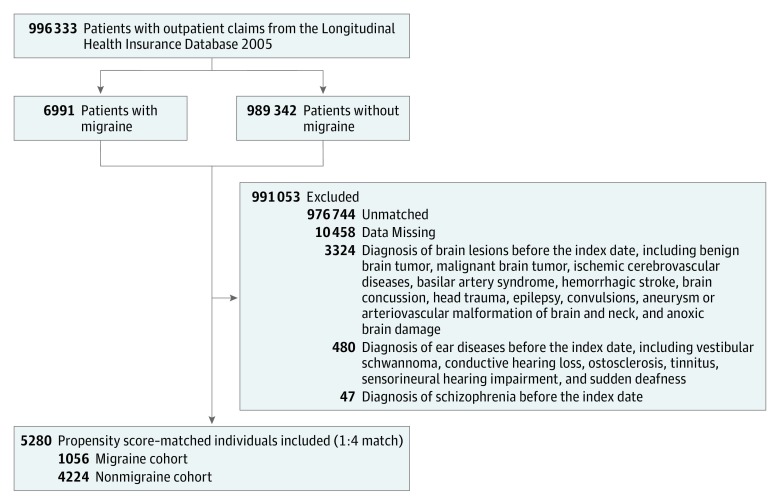
We first identified 996 333 patients who received a diagnosis of migraine based on outpatient claims (Figure 1). During this period, we identified 6991 patients with a history of migraines who received a diagnosis twice within 3 months. We excluded patients who had some preexisting diseases before the index date (eTable 1 in the Supplement), as well as patients with a history of migraines who could not be matched with controls. Finally, a total of 1056 patients with a newly diagnosed migraine were identified as the migraine cohort.
Figure 1. Flow Diagram of the Enrollment Process.
For each patient in the migraine cohort, 4 individuals were selected from the patients without a history of migraines according to propensity score matching using baseline variables, including age, sex, comorbidities, and year of index date of cases. Finally, the matched nonmigraine cohort included 4224 patients, and the index date was the selected date.
Main Outcome Measurement
Both cohorts were followed from the index date to the first diagnosis of a cochlear disorder (tinnitus, sensorineural hearing impairment, or sudden deafness), death, or the end of 2012, whichever came first. Death was defined as a withdrawal of a patient from the National Health Insurance program.
Potential Confounders
We defined the presence of comorbidities according to the presence of International Classification of Diseases, Ninth Revision (ICD-9) codes in the database before or on the index date, including sleep disorders (ICD-9 codes 780.50, 780.51, 780.52, 780.53, 780.57, and 307.40), heart diseases (ICD-9 codes 413.9, 414, 410-429, and 402), hypertension (ICD-9 codes 401-405), diabetes (ICD-9 code 250), hyperlipidemia (ICD-9 code 272), chronic kidney disease (ICD-9 codes 585 and 586), chronic hepatitis (ICD-9 codes 070, 571.4, 571, 571.2, 571.5, and 571.6), anxiety (ICD-9 codes 300.0, 300.00, 300.02, 300.09, 309.21, and 293.84), depression (ICD-9 codes 296.30, 296.20, 311, and 300.4), pregnancy (ICD-9 code 633), and menopause (ICD-9 codes 627.4, 627.8, and 627.9), as well as chronic obstructive pulmonary disease (ICD-9 codes 490-496) as a proxy for cigarette smoking.9 Use of oral contraceptives was also considered a possible confounder. Geographic region of residence and urbanization level were included to minimize potential confounding due to differences in urban vs rural location in accessibility to medical care in Taiwan.10 Also, enrollee category (EC), an indicator for socioeconomic status, was included and was classified as the following 4 subgroups: EC1 (highest socioeconomic status [eg, civil servants or full-time or regularly paid personnel in governmental agencies and public schools]), EC2 (employees of privately owned enterprises or institutions), EC3 (self-employed, other employees or paid personnel, and members of the farmers or fishers associations), and EC4 (lowest socioeconomic status [eg, substitute service draftees, members of low-income families, and veterans]).10
Statistical Analysis
Statistical analysis was performed from January 23, 1996, to December 28, 2012. We calculated the incidence rate of cochlear disorders between the cohorts by using the Kaplan-Meier method and the log-rank test. After ensuring the assumptions of proportional hazards, we used the Cox proportional hazards regression model to examine the association of combined and individual cochlear disorders with migraines, with adjustment for all covariates. We analyzed all data with SAS, version 9.4 (SAS Institute Inc), and SPSS, version 20.0 (IBM Corp), and considered a 2-sided P < .05 as statistically significant.
Results
Table 1 shows the basic characteristics between the migraine and nonmigraine cohorts. The mean (SD) age of the migraine cohort was 36.7 (15.3) years, and 384 of the 1056 patients (36.4%) were men. The mean (SD) age (36.7 [15.3] years in the migraine group vs 35.0 [13.7] years in the nonmigraine group; P = .001), prevalence of hypertension (68 [6.4%] in the migraine group vs 179 [4.2%] in the nonmigraine group; P = .002), use of oral contraceptives (25 [2.4%] in the migraine group vs 29 [0.7%] in the nonmigraine group; P < .001), geographic region (northern, 532 [50.4%] in the migraine group vs 2240 [53.0] in the nonmigraine group; central, 211 [20.0%] in the migraine group vs 896 [21.2%] in the nonmigraine group; eastern, 16 [1.5%] in the migraine group vs 111 [2.6%] in the nonmigraine group; and southern, 297 [28.1%] in the migraine group vs 977 [23.1%] in the nonmigraine group; P = .002), urbanization level (urban, 349 [33.1%] in the migraine group vs 1233 [29.2%] in the nonmigraine group; suburban, 488 [46.2%] in the migraine group vs 2025 [47.9%] in the nonmigraine group; and rural, 219 [20.7%] in the migraine group vs 966 [22.9%] in the nonmigraine group; P = .04), and EC (EC1, 611 [57.9%] in the migraine group vs 1582 [37.5%] in the nonmigraine group; EC2, 28 [2.7%] in the migraine group vs 882 [20.9%] in the nonmigraine group; EC3, 275 [26.0%] in the migraine group vs 489 [11.6%] in the nonmigraine group; and EC4, 142 [13.4%] in the migraine group vs 1271 [30.1%] in the nonmigraine group; P < .001) were significantly different between the cohorts.
Table 1. Baseline Characteristics of Study Cohort, 1996-2012.
| Variable | Patients, No. (%) | |
|---|---|---|
| Migraine Cohort (n = 1056) | Matched Nonmigraine Cohort (n = 4224) | |
| Sex | ||
| Men | 384 (36.4) | 1665 (39.4) |
| Women | 672 (63.6) | 2559 (60.6) |
| Age, mean (SD), y | 36.7 (15.3) | 35.0 (13.7) |
| Comorbidity | ||
| Sleep disorders | 31 (2.9) | 146 (3.5) |
| Heart diseases | 44 (4.2) | 147 (3.5) |
| Hypertension | 68 (6.4) | 179 (4.2) |
| Diabetes | 18 (1.7) | 68 (1.6) |
| Hyperlipidemia | 2 (0.2) | 10 (0.2) |
| Chronic kidney disease | 4 (0.4) | 7 (0.2) |
| Chronic hepatitis | 10 (0.9) | 47 (1.1) |
| Anxiety | 17 (1.6) | 66 (1.6) |
| Depression | 10 (0.9) | 25 (0.6) |
| COPD | 47 (4.5) | 193 (4.6) |
| Pregnancy | 2 (0.2) | 11 (0.3) |
| Menopause | 2 (0.2) | 3 (0.1) |
| Oral contraceptives | 25 (2.4) | 29 (0.7) |
| Geographic region | ||
| Northern | 532 (50.4) | 2240 (53.0) |
| Central | 211 (20.0) | 896 (21.2) |
| Eastern | 16 (1.5) | 111 (2.6) |
| Southern | 297 (28.1) | 977 (23.1) |
| Urbanization level | ||
| Urban | 349 (33.1) | 1233 (29.2) |
| Suburban | 488 (46.2) | 2025 (47.9) |
| Rural | 219 (20.7) | 966 (22.9) |
| Enrollee category | ||
| 1 (Highest) | 611 (57.9) | 1582 (37.5) |
| 2 | 28 (2.7) | 882 (20.9) |
| 3 | 275 (26.0) | 489 (11.6) |
| 4 (Lowest) | 142 (13.4) | 1271 (30.1) |
Abbreviation: COPD, chronic obstructive pulmonary disease.
Table 2 shows the crude hazard ratios (HRs) and adjusted HRs (aHRs) for combined cochlear disorders. Compared with the nonmigraine cohort, the migraine cohort had a crude HR for cochlear disorders of 2.83 (95% CI, 2.01-3.99) and an aHR was for cochlear disorders of 2.71 (95% CI, 1.86-3.93). Age and diabetes were also significantly associated with increased aHRs for cochlear disorders (1.02 [95% CI, 1.01-1.04] for age; and 3.70 [95% CI, 1.93-7.06] for diabetes).
Table 2. Crude and Adjusted HRs for Combined Cochlear Disorders.
| Variable | Crude HR (95% CI) | Adjusted HR (95% CI)a |
|---|---|---|
| Migraine (yes or no) | 2.83 (2.01-3.99) | 2.71 (1.86-3.93) |
| Sex (men or women) | 1.18 (0.83-1.67) | 0.93 (0.64-1.34) |
| Age (per year) | 1.03 (1.02-1.04) | 1.02 (1.01-1.04) |
| Comorbidity (yes or no) | ||
| Sleep disorders | 1.01 (0.44-2.29) | 1.20 (0.52-2.75) |
| Heart diseases | 1.42 (0.72-2.80) | 1.12 (0.53-2.37) |
| Hypertension | 1.45 (0.78-2.70) | 0.71 (0.35-1.45) |
| Diabetes | 4.74 (2.61-8.59) | 3.70 (1.93-7.06) |
| Hyperlipidemiab | NA | NA |
| Chronic kidney disease | 2.59 (0.36-18.55) | 1.56 (0.21-11.88) |
| Chronic hepatitis | 2.01 (0.81-4.98) | 1.21 (0.46-3.20) |
| Anxiety | 1.91 (0.84-4.37) | 1.86 (0.81-4.30) |
| Depression | 0.81 (0.11-5.80) | 0.55 (0.08-3.98) |
| COPD | 1.69 (0.97-2.96) | 1.78 (1.01-3.15) |
| Pregnancyb | NA | NA |
| Menopause | 6.58 (0.92-47.11) | 4.62 (0.63-33.90) |
| Oral contraceptives | 1.95 (0.62-6.12) | 1.86 (0.58-6.02) |
| Geographic region | ||
| Northern | 1 [Reference] | 1 [Reference] |
| Central | 1.11 (0.71-1.73) | 1.06 (0.64-1.75) |
| Eastern | 0.69 (0.17-2.82) | 0.59 (0.14-2.50) |
| Southern | 1.34 (0.91-2.00) | 1.20 (0.78-1.85) |
| Urbanization level | ||
| Urban | 1 [Reference] | 1 [Reference] |
| Suburban | 0.76 (0.51-1.13) | 0.78 (0.51-1.17) |
| Rural | 1.04 (0.67-1.62) | 1.04 (0.61-1.77) |
| Enrollee category | ||
| 1 | 1 [Reference] | 1 [Reference] |
| 2 | 0.28 (0.12-0.66) | 0.40 (0.17-0.95) |
| 3 | 1.87 (1.22-2.87) | 1.29 (0.81-2.05) |
| 4 | 1.30 (0.86-1.95) | 1.29 (0.83-2.00) |
Abbreviations: COPD, chronic obstructive pulmonary disease; HR, hazard ratio; NA, not applicable.
Adjusted for all covariates (age per year, sex, comorbidity, use of oral contraceptives, geographic region, urbanization level, and enrollee category).
Correlated with another independent factor during regression analysis.
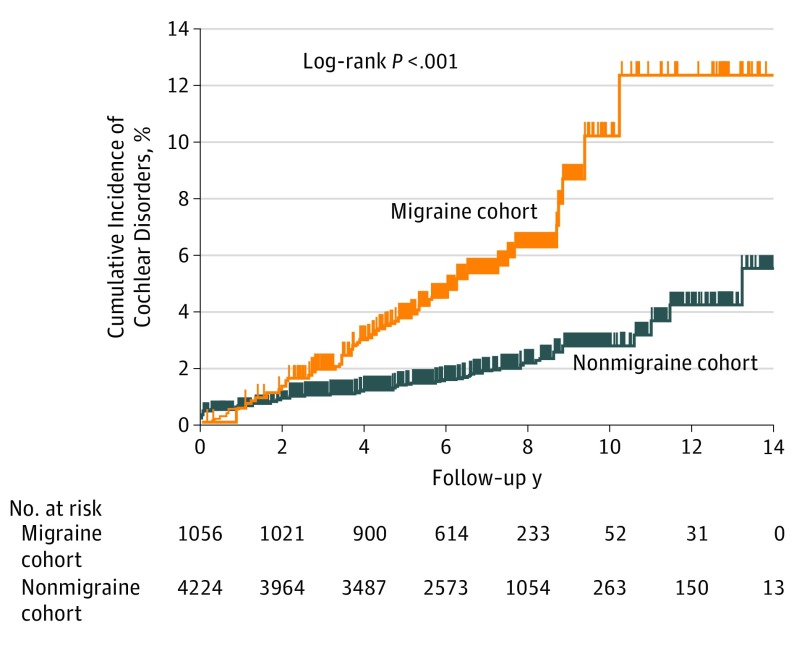
Table 3 shows the incidence rate of combined cochlear disorders by migraine status. The mean (SD) duration of follow-up was 6.39 (2.34) years for the migraine cohort and 6.52 (2.92) years for the matched nonmigraine cohort. By the end of follow-up, the incidence rate of cochlear disorders was 81.4 (95% CI, 81.1-81.8) per 1 million person-years of follow-up in the migraine cohort and 29.4 (95% CI, 29.2-29.7) per 1 million person-years of follow-up in the nonmigraine matched cohort. Kaplan-Meier estimates showed that the cumulative incidence in the migraine cohort (12.2%) was higher than that in the matched nonmigraine cohort (5.5%) (Figure 2).
Table 3. Incidence Rate of Cochlear Disorders by Migraine Status.
| Cohort | Mean Follow-up, ya | Total Follow-up, Person-yearsa | Cochlear Disorders, No. (%)b | Incidence Rateb,c |
|---|---|---|---|---|
| Migraine (n = 1056) | 6.39 | 6750 | 55 (5.2) | 81.4 |
| Nonmigraine (n = 4224) | 6.52 | 27 520 | 81 (1.9) | 29.4 |
P = .15.
P < .001.
Per 1 million person-years.
Figure 2. Cumulative Incidence of Cochlear Disorders.
The cumulative incidence of cochlear disorders in the migraine cohort (12.2%) was significantly higher than that in the matched nonmigraine cohort (5.5%).
Subgroup analysis showed that, compared with the nonmigraine cohort, the aHRs in the migraine cohort were 3.30 (95% CI, 2.17-5.00) for tinnitus, 1.03 (95% CI, 0.17-6.41) for sensorineural hearing impairment, and 1.22 (95% CI, 0.53-2.83) for sudden deafness (eTable 2 in the Supplement).
Discussion
In this large-scale cohort study, we found increased risk of cochlear disorders, especially for tinnitus, among patients with a history of migraines. It could be suggested that the findings do not reflect the cochlea at all and may reflect a central process causing tinnitus. This possibility would need to be investigated further in studies using audiometry; however, it clearly outlines a link between migraine and tinnitus that will be influential.
Vestibular migraine (VM) has been described with recurrent episodic vertigo and migraine-related symptoms.11 Headache represented the first symptom of VM and was discovered several years before vertigo.12 Migraine might also be linked to peripheral and central auditory dysfunctions.13,14 Subjective hearing loss, aural pressure, and tinnitus were reported in 38% of patients during episodes of VM.15 Morganti et al12 also reported that 61.5% of patients with VM had auditory symptoms, with tinnitus the most common. Recently, Lai and Liu16 proposed a new concept of cochlear migraine (CM) for patients who did not meet the strict criteria for VM. Our study demonstrates that a history of migraines might increase the risk of cochlear disorders. Thus, if CM was really present, we might define migraine-related sequelae in the vestibular system as VM, migraine-related sequelae in the auditory system as CM, and migraine-related sequelae in both the vestibular and auditory systems as cochleovestibular migraine.
Some patients with CM may experience a transformation of their condition to Ménière disease once severe vertigo develops; however, for some patients, their CM and vertigo episodes mimicked Ménière disease, but they had a normal summating potential to action potential ratio on an extratympanic electrocochleogram.
According to Hwang et al,17 about 21.3% of patients with Ménière disease had a normal summating potential to action potential ratio in the ear with disease. However, Liu et al18 found bilateral endolymphatic hydrops in a patient with migraine variant without vertigo. Therefore, the association between migraine-related cochleovestibular disorders and Ménière disease may be complex and overlapping.
Migraine and cochlear disorders might share common pathophysiologic characteristics. Sleep disorders, trigeminovascular theory, neuroinflammation, and/or cortical hypersensitivity have been associated with migraine.2,19 Meanwhile, patients with tinnitus have vagal withdrawal and/or sympathetic overactivity.20 Sleep disorders, neural inflammation, and/or damage from oxidative stress could increase the risk of tinnitus and age-related hearing impairment.21,22,23,24,25 That is, these cochleovestibular disorders might result from sleep disorders, migraine-associated vasospasm, cortical hyperexcitability, neural inflammation and/or damage from oxidative stress, or enhanced activation of the sympathetic nervous system in the limbic and autonomous brain regions.
Limitations
There were some limitations to this study. All diagnoses were based on ICD-9-CM codes in the database. The definition of a migraine used in this study (the presence of 2 ICD-9-CM diagnosis codes of migraine within 3 months) might contribute to bias. We had considered alternatives; however, we believed that including patients with a single diagnosis of a migraine might increase the rate of false-positive diagnoses in the migraine group, and 3 or more diagnoses might increase the rate of false-negative diagnoses in the nonmigraine group. We had explored alternative definitions of the migraine cohort and found similar results if we included patients with 2 or more diagnoses of a migraine within the entire study period. Risk of sudden deafness was increased, in addition to tinnitus (eTables 3 and 4 in the Supplement). Nevertheless, it is possible that the outcomes of this study apply to patients with more severe migraines and may not represent the general population of patients with migraines in Taiwan. Furthermore, there may be unmeasured variables, such as use of medical services, noise exposure, or medication use, that could confound the results of this study.
Conclusions
In this large-scale cohort study, we found that patients with a history of migraine had a tendency to develop cochlear disorders, especially tinnitus. The results of this study supported the new concept and/or presence of CM.
eTable 1. The Inclusion and Exclusion Diseases and Their ICD-9 Codes in this Study
eTable 2. Adjusted Hazard Ratios (aHRs) for Individual Cochlear Disorders (Tinnitus, Sensorineural Hearing Impairment, and Sudden Deafness) Associated With Migraine
eTable 3. Adjusted Hazard Ratios (aHRs) for Combined Cochlear Disorders Associated With Migraine if Migraine Cases Were Defined as the Presence of ≥2 ICD-9 codes 346.01, 346.10, 346.90 During the Period 1996-2012
eTable 4. Adjusted Hazard Ratios (aHRs) for Individual Cochlear Disorders Associated With Migraine if Migraine Cases Were Defined as the Presence of ≥2 ICD-9 codes 346.01, 346.10, 346.90 During the Period 1996-2012
References
- 1.Harriott AM, Schwedt TJ. Migraine is associated with altered processing of sensory stimuli. Curr Pain Headache Rep. 2014;18(11):458. doi: 10.1007/s11916-014-0458-8 [DOI] [PubMed] [Google Scholar]
- 2.Ying G, Fan W, Li N, et al. . Clinical characteristics of basilar-type migraine in the neurological clinic of a university hospital. Pain Med. 2014;15(7):1230-1235. doi: 10.1111/pme.12402 [DOI] [PubMed] [Google Scholar]
- 3.Montagni I, Guichard E, Carpenet C, Tzourio C, Kurth T. Screen time exposure and reporting of headaches in young adults: a cross-sectional study. Cephalalgia. 2016;36(11):1020-1027. doi: 10.1177/0333102415620286 [DOI] [PubMed] [Google Scholar]
- 4.Dash AK, Panda N, Khandelwal G, Lal V, Mann SS. Migraine and audiovestibular dysfunction: is there a correlation? Am J Otolaryngol. 2008;29(5):295-299. doi: 10.1016/j.amjoto.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 5.Guichard E, Montagni I, Tzourio C, Kurth T. Association between headaches and tinnitus in young adults: cross-sectional study. Headache. 2016;56(6):987-994. doi: 10.1111/head.12845 [DOI] [PubMed] [Google Scholar]
- 6.Piovesan EJ, Kowacs PA, Werneck LC, Siow C. Oscillucusis and sudden deafness in a migraine patient. Arq Neuropsiquiatr. 2003;61(3B):848-850. doi: 10.1590/S0004-282X2003000500026 [DOI] [PubMed] [Google Scholar]
- 7.Chu CH, Liu CJ, Fuh JL, Shiao AS, Chen TJ, Wang SJ. Migraine is a risk factor for sudden sensorineural hearing loss: a nationwide population-based study. Cephalalgia. 2013;33(2):80-86. doi: 10.1177/0333102412468671 [DOI] [PubMed] [Google Scholar]
- 8.Viirre ES, Baloh RW. Migraine as a cause of sudden hearing loss. Headache. 1996;36(1):24-28. doi: 10.1046/j.1526-4610.1996.3601024.x [DOI] [PubMed] [Google Scholar]
- 9.Stolt P, Bengtsson C, Nordmark B, et al. ; EIRA study group . Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case-control study, using incident cases. Ann Rheum Dis. 2003;62(9):835-841. doi: 10.1136/ard.62.9.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen YC, Lin HY, Li CY, Lee MS, Su YC. A nationwide cohort study suggests that hepatitis C virus infection is associated with increased risk of chronic kidney disease. Kidney Int. 2014;85(5):1200-1207. doi: 10.1038/ki.2013.455 [DOI] [PubMed] [Google Scholar]
- 11.Dieterich M, Obermann M, Celebisoy N. Vestibular migraine: the most frequent entity of episodic vertigo. J Neurol. 2016;263(suppl 1):S82-S89. doi: 10.1007/s00415-015-7905-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morganti LO, Salmito MC, Duarte JA, Bezerra KC, Simões JC, Ganança FF. Vestibular migraine: clinical and epidemiological aspects. Braz J Otorhinolaryngol. 2016;82(4):397-402. doi: 10.1016/j.bjorl.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joffily L, de Melo Tavares de Lima MA, Vincent MB, Frota SM. Assessment of otoacoustic emission suppression in women with migraine and phonophobia. Neurol Sci. 2016;37(5):703-709. doi: 10.1007/s10072-016-2565-2 [DOI] [PubMed] [Google Scholar]
- 14.Agessi LM, Villa TR, Carvalho DS, Pereira LD. Auditory processing in children with migraine: a controlled study. Neuropediatrics. 2017;48(2):123-126. doi: 10.1055/s-0037-1598046 [DOI] [PubMed] [Google Scholar]
- 15.Neff BA, Staab JP, Eggers SD, et al. . Auditory and vestibular symptoms and chronic subjective dizziness in patients with Ménière’s disease, vestibular migraine, and Ménière’s disease with concomitant vestibular migraine. Otol Neurotol. 2012;33(7):1235-1244. doi: 10.1097/MAO.0b013e31825d644a [DOI] [PubMed] [Google Scholar]
- 16.Lai JT, Liu TC. Proposal for a new diagnosis for cochlear migraine. JAMA Otolaryngol Head Neck Surg. 2018;144(3):185-186. doi: 10.1001/jamaoto.2017.2427 [DOI] [PubMed] [Google Scholar]
- 17.Hwang JH, Ho HC, Hsu CJ, Yang WS, Liu TC. Diagnostic value of combining bilateral electrocochleography results for unilateral Ménière’s disease. Audiol Neurootol. 2008;13(6):365-369. doi: 10.1159/000136155 [DOI] [PubMed] [Google Scholar]
- 18.Liu IY, Ishiyama A, Sepahdari AR, Johnson K, Ishiyama G. Bilateral endolymphatic hydrops in a patient with migraine variant without vertigo: a case report. Headache. 2017;57(3):455-459. doi: 10.1111/head.12976 [DOI] [PubMed] [Google Scholar]
- 19.Shimizu T. Headache in consideration of gender [in Japanese]. Nihon Rinsho. 2015;73(4):656-660. [PubMed] [Google Scholar]
- 20.Choi EJ, Yun Y, Yoo S, Kim KS, Park JS, Choi I. Autonomic conditions in tinnitus and implications for Korean medicine. Evid Based Complement Alternat Med. 2013;2013:402585. doi: 10.1155/2013/402585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koo M, Hwang JH. Risk of tinnitus in patients with sleep apnea: a nationwide, population-based, case-control study. Laryngoscope. 2017;127(9):2171-2175. doi: 10.1002/lary.26323 [DOI] [PubMed] [Google Scholar]
- 22.Hwang JH, Chen JC, Hsu CJ, Liu TC. Association of obstructive sleep apnea and auditory dysfunctions in older subjects. Otolaryngol Head Neck Surg. 2011;144(1):114-119. doi: 10.1177/0194599810390859 [DOI] [PubMed] [Google Scholar]
- 23.Hwang JH, Chen JC, Chan YC. Effects of C-phycocyanin and spirulina on salicylate-induced tinnitus, expression of NMDA receptor and inflammatory genes. PLoS One. 2013;8(3):e58215. doi: 10.1371/journal.pone.0058215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hwang JH, Chang NC, Chen JC, Chan YC. Expression of antioxidant genes in the mouse cochlea and brain in salicylate-induced tinnitus and effect of treatment with spirulina platensis water extract. Audiol Neurootol. 2015;20(5):322-329. doi: 10.1159/000381934 [DOI] [PubMed] [Google Scholar]
- 25.Chan YC, Hwang JH. Effects of spirulina on the functions and redox status of auditory system in senescence-accelerated prone-8 mice. PLoS One. 2017;12(6):e0178916. doi: 10.1371/journal.pone.0178916 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. The Inclusion and Exclusion Diseases and Their ICD-9 Codes in this Study
eTable 2. Adjusted Hazard Ratios (aHRs) for Individual Cochlear Disorders (Tinnitus, Sensorineural Hearing Impairment, and Sudden Deafness) Associated With Migraine
eTable 3. Adjusted Hazard Ratios (aHRs) for Combined Cochlear Disorders Associated With Migraine if Migraine Cases Were Defined as the Presence of ≥2 ICD-9 codes 346.01, 346.10, 346.90 During the Period 1996-2012
eTable 4. Adjusted Hazard Ratios (aHRs) for Individual Cochlear Disorders Associated With Migraine if Migraine Cases Were Defined as the Presence of ≥2 ICD-9 codes 346.01, 346.10, 346.90 During the Period 1996-2012