Key Points
Question
Is the combination of the anti–epidermal growth factor receptor antibody panitumumab and neoadjuvant chemotherapy safe and effective in patients with primary human epidermal growth factor receptor 2 (HER2)-negative inflammatory breast cancer (IBC)?
Findings
In this single-arm, open-label trial, the combination of panitumumab and neoadjuvant chemotherapy produced pathologic complete response rates of 14% in patients with HER2-negative, hormone receptor–positive disease and 42% in patients with triple-negative IBC. Treatment-related hematological and dermatological toxic effects were substantial but transient, and there were no treatment-related deaths.
Meaning
The combination of panitumumab and neoadjuvant chemotherapy for primary HER2-negative IBC had significant efficacy, particularly in patients with triple-negative IBC.
This single-arm, open-label trial evaluates the safety and efficacy of the anti–epidermal growth factor receptor antibody panitumumab plus neoadjuvant chemotherapy (nab-paclitaxel and carboplatin followed by fluorouracil, epirubicin, and cyclophosphamide) in patients with primary HER2-negative inflammatory breast cancer.
Abstract
Importance
Combining conventional chemotherapy with targeted therapy has been proposed to improve the pathologic complete response (pCR) rate in patients with inflammatory breast cancer (IBC). Epidermal growth factor receptor (EGFR) expression is an independent predictor of low overall survival in patients with IBC.
Objective
To evaluate the safety and efficacy of the anti-EGFR antibody panitumumab plus neoadjuvant chemotherapy in patients with primary human epidermal growth factor receptor 2 (HER2)-negative IBC.
Design, Setting, and Participants
Women with primary HER2-negative IBC were enrolled from 2010 to 2015 and received panitumumab plus neoadjuvant chemotherapy. Median follow-up time was 19.3 months. Tumor tissues collected before and after the first dose of panitumumab were subjected to immunohistochemical staining and RNA sequencing analysis to identify biomarkers predictive of pCR.
Intervention
Patients received 1 dose of panitumumab (2.5 mg/kg) followed by 4 cycles of panitumumab (2.5 mg/kg), nab-paclitaxel (100 mg/m2), and carboplatin weekly and then 4 cycles of fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2) every 3 weeks.
Main Outcomes and Measures
The primary end point was pCR rate; the secondary end point was safety. The exploratory objective was to identify biomarkers predictive of pCR.
Results
Forty-seven patients were accrued; 7 were ineligible. The 40 enrolled women had a median age of 57 (range, 23-68) years; 29 (72%) were postmenopausal. Three patients did not complete therapy because of toxic effects (n = 2) or distant metastasis (n = 1). Nineteen patients had triple-negative and 21 had hormone receptor–positive IBC. The pCR and pCR rates were overall, 11 of 40 (28%; 95% CI, 15%-44%); triple-negative IBC, 8 of 19 (42%; 95% CI, 20%-66%); and hormone receptor–positive/HER2-negative IBC, 3 of 21 (14%; 95% CI, 3%-36%). During treatment with panitumumab, nab-paclitaxel, and carboplatin, 10 patients were hospitalized for treatment-related toxic effects, including 5 with neutropenia-related events. There were no treatment-related deaths. The most frequent nonhematologic adverse event was skin rash. Several potential predictors of pCR were identified, including pEGFR expression and COX-2 expression.
Conclusions and Relevance
This combination of panitumumab and chemotherapy showed the highest pCR rate ever reported in triple-negative IBC. A randomized phase 2 study is ongoing to determine the role of panitumumab in patients with triple-negative IBC and to further validate predictive biomarkers.
Trial Registration
ClinicalTrials.gov Identifier: NCT01036087
Introduction
Inflammatory breast cancer (IBC), which accounts for 3% to 5% of all breast cancers, is characterized by aggressive progression and metastasis. Pathologic complete response (pCR) to neoadjuvant chemotherapy (NAC) is associated with improved progression-free and overall survival; however, the rate of pCR to NAC is low for IBC (15.2%).1
Evidence from preclinical and clinical studies indicates that epidermal growth factor receptor (EGFR) might be a promising therapeutic target for IBC. EGFR is overexpressed in 18% of breast cancer cases and up to 50% of IBC cases.2,3,4 Preclinical studies showed that suppression of EGFR signaling in breast cancer controls tumor growth by enhancing apoptosis5,6 and suppressing the cancer stem cell population.7,8 Targeting the EGFR pathway shifted the phenotype of IBC cells from mesenchymal to epithelial and inhibited tumor growth and metastatic progression.9 Furthermore, we have shown that EGFR regulates IBC cells expressing cancer stem cell markers through COX-2 and Nodal has been identified as a potential driver of EGFR/COX-2–mediated cancer stem cell regulation in IBC cells.10 Indeed, in patients with IBC, EGFR expression is independently associated with a high rate of recurrence and shorter survival.2
To our knowledge, before this report, no study had investigated NAC including an anti-EGFR antibody for IBC. To determine the efficacy and safety of such therapy, we conducted a phase 2 trial of NAC with the anti-EGFR monoclonal antibody panitumumab, nab-paclitaxel, and carboplatin (PNC) followed by an anthracycline-containing regimen in patients with primary human epidermal growth factor receptor 2 (HER2)-negative IBC.
Methods
Study Design and Participants
This single-arm, open-label phase 2 trial was designed to assess the efficacy and safety of PNC followed by fluorouracil, epirubicin hydrochloride, and cyclophosphamide (FEC) as NAC in patients with operable HER2-negative IBC. The study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. Written informed consent was obtained from all patients. The trial protocol is available in Supplement 1.
Eligibility criteria included age at least 18 years, histologic confirmation of breast carcinoma, clinical diagnosis of HER2-negative IBC,11 Eastern Cooperative Oncology Group performance status score of 0 or 1, and adequate hematologic, hepatic, renal, and cardiac functions. Patients with distant metastases amenable to radiotherapy and/or surgery based on consultation with our IBC multidisciplinary team were eligible. Pregnant and lactating women were excluded, as were patients with previous radiotherapy or chemotherapy, HER2-positive breast carcinoma, recurrent breast cancer, history of other malignant neoplasm, positive test for HIV infection or acute or chronic active hepatitis B or C virus infection, history of extensive interstitial lung disease, other significant medical or psychiatric condition that would make assessment of toxic effects or efficacy difficult, uncontrolled intercurrent illness, or peripheral neuropathy more than grade 2. Hormone receptor (HR) positivity was defined as positive staining in at least 10% of cells.
Procedures
The first 17 patients received 1 dose of panitumumab (2.5 mg/kg) on day 1 and, starting 1 week later, 4 cycles of PNC (panitumumab, 2.5 mg/kg; paclitaxel-protein bound [nab-paclitaxel], 5 mg/mL intravenous piggyback 100 mg/m2; and carboplatin intravenous piggyback at a dose of 2.0 area under the concentration time curve) weekly for 3 weeks. As a result of unacceptable hematological toxic effects, the PNC cycle for the remaining 23 patients was changed to 3 weeks of weekly treatment followed by 1 week off to allow bone marrow recovery. During cycle 4, patients received panitumumab weekly for 2 weeks and carboplatin and nab-paclitaxel weekly for 3 weeks. After PNC, all patients received 4 cycles of FEC consisting of fluorouracil (500 mg/m2), epirubicin hydrochloride (100 mg/m2), and cyclophosphamide (500 mg/m2) weekly for 3 weeks (eFigure 1 in Supplement 2). For correlative studies, tumor core needle biopsy was performed before and after the first dose of panitumumab (at the beginning of week 2 of PNC). Radiologists attempted to collect the pre- and postpanitumumab tumor samples from neighboring areas. Biopsy was optional but recommended to all patients, especially during the second half of study accrual. After NAC, all patients underwent modified radical mastectomy with axillary node dissection followed by irradiation of the chest wall and draining lymphatics.
Clinical breast examination and adverse events assessment were performed before each cycle of PNC and FEC.12 Toxic effects were graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 3.0. Dose modifications were made according to the organ system with the highest-grade of toxic effects; dose modifications were specified for skin- and nail-related toxic effects (eTable 1 in Supplement 2). When toxic effects occurred, treatment delay was allowed for up to 3 weeks for resolution of acute toxic effects and 4 weeks for resolution of chronic toxic effects (except alopecia, skin changes, and anemia). Dose modifications for panitumumab were as described in eFigure 2 in Supplement 2. Dose modifications for FEC were based on standard practice.
Identification of Biomarkers for pCR
To identify potential biomarkers for pCR, we examined protein and gene expression in tumor core needle biopsy samples obtained before and after the first dose of panitumumab and analyzed the relationship between (1) protein expression before and after first dose of panitumumab and pCR and (2) change in protein and gene expression and pCR.
Immunohistochemistry
Immunohistochemical staining of EGFR, pEGFR, E-cadherin, vimentin, COX-2, and Nodal in tumor biopsy samples was performed at the MD Anderson Research Histopathology Facility. The staining was interpreted and recorded by S.K. Protein expression levels were scored by multiplying the staining intensity score (1, weak; 2, moderate; or 3, strong) by the percentage of epithelial tumor cells with positive staining. Individuals performing immunostaining and scoring were blinded to clinical outcome. Matched pairs of tumor biopsy samples obtained before and after first panitumumab dose were identified. The change in protein expression for each pair was calculated as postpanitumumab level of expression minus prepanitumumab level of expression.
RNA Sequencing
RNA was extracted from matched pairs of samples and sequenced on an Illumina HiSeq2000 at the MD Anderson Sequencing and Microarray Facility. Sequencing reads of samples that passed FastQC checks were aligned with tophat2 to the UCSC hg19 reference genome (Illumina iGenomes repository).13 The concordant pair alignment rate of all samples was greater than 80%. Gene-level read counts were summarized using htseq-count.
Statistical Analysis
Pathologic complete response was defined as absence of invasive carcinoma in the breast, axillary lymph nodes, and skin and absence of tumor emboli within the surgical field.14 Patient characteristics examined for possible association with pCR included race, age, menopausal status, clinical N category, TNM stage at presentation, nuclear grade, and primary tumor subtype. Continuous variables were summarized using mean (standard deviation) and median (range). Categorical variables were summarized in frequency tables. χ2 test or Fisher exact test was used to compare categorical variables between patients with and without pCR. Wilcoxon rank sum test was used to compare continuous variables between patients with and without pCR. Disease-free survival was defined as the time from surgery to the first disease recurrence or death. Overall survival was defined as the time from date of surgery to death or last follow-up. The Kaplan-Meier method was used to estimate the distributions of disease-free and overall survival. The log-rank test was used to compare survival distributions between patients with and without pCR. The intent-to-treat population included all patients who received at least 1 dose of PNC. The evaluable population included all patients who underwent NAC plus surgery, postoperative radiotherapy, and, for HR-positive patients, adjuvant hormonal therapy.
Associations between prepanitumumab protein expression and pCR status, protein expression at week 2 and pCR status, and change in protein expression and pCR status were assessed using Wilcoxon rank sum test. Statistical significance was defined as 2-sided P < .05. SAS, version 9.4 (SAS Institute, Inc), was used for data analysis.
For RNA sequencing analysis, to compare expression across samples, the log-transformed gene-level read counts of each sample were first normalized to total library size and then fitted with a generalized linear model with an identity using the R package edgeR.15 A random effect for each participant was used to accommodate dependency between samples from the same participant. Groups were compared using likelihood ratio test adjusted by the Benjamini-Hochberg procedure.
Results
Patient Characteristics
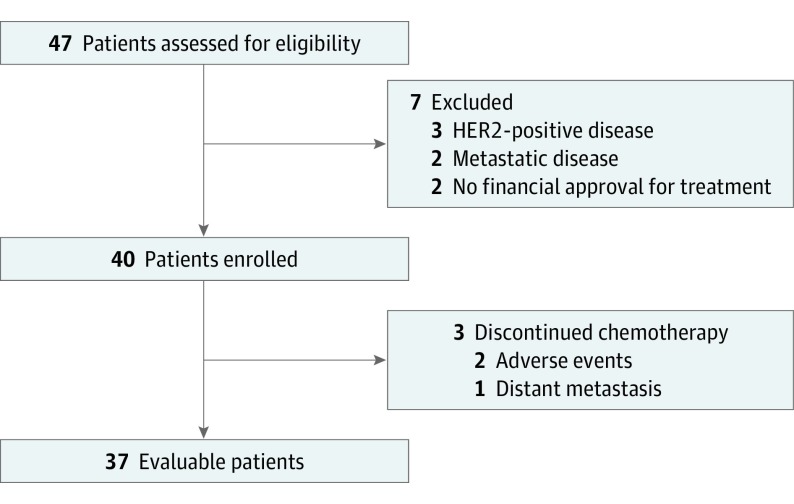
From November 2010 through July 2015, 47 patients were accrued and assessed for eligibility. Seven patients were ineligible and excluded (3 had HER2-positive disease, 2 had metastatic disease, and 2 did not receive financial approval for treatment). Of the 40 patients who started PNC, 3 discontinued treatment early because of adverse events (n = 2) or distant metastasis (n = 1) (Figure). Baseline characteristics of the 40 enrolled patients are presented in Table 1. The median age was 57 years (range, 23-68 years). Twenty-one patients (53%) had HR-positive/HER2-negative IBC, and 19 (47%) had triple-negative (TN)-IBC.
Figure. CONSORT Flow Diagram.
Table 1. Patient Characteristics Overall and by Pathologic Complete Response (pCR).
| Characteristic | No. (%) | No. | P Value | |
|---|---|---|---|---|
| All Patients (N = 40) | Patients With pCR (n = 11) | Patients Without pCR (n = 29) | ||
| Age, median (range), y | 57 (23-68) | 57 (29-62) | 57 (23-68) | .42 |
| Race/ethnicity | ||||
| White | 35 (88) | 11 | 24 | .30 |
| Hispanic | 2 (5) | 1 | 1 | |
| African American | 2 (5) | 0 | 2 | |
| Other | 1 (2) | 1 | 0 | |
| Age, y | ||||
| <50 | 10 (25) | 4 | 6 | .42 |
| ≥50 | 30 (75) | 7 | 23 | |
| Menopausal status | ||||
| Premenopausal | 11 (28) | 3 | 8 | >.99 |
| Postmenopausal | 29 (72) | 8 | 21 | |
| Clinical N category | ||||
| N1 | 20 (50) | 5 | 15 | .88 |
| N2 | 4 (10) | 1 | 3 | |
| N3 | 16 (40) | 5 | 11 | |
| TNM stage at presentation | ||||
| III | 36 (90) | 10 | 26 | >.99 |
| IV | 4 (10) | 1 | 3 | |
| Nuclear grade | ||||
| 2 | 13 (32) | 1 | 12 | .07 |
| 3 | 27 (68) | 10 | 17 | |
| Primary tumor subtype | ||||
| HR positive HER2 negative | 21 (52) | 3 | 18 | .05 |
| HR negative HER2 negative | 19 (48) | 8 | 11 | |
Abbreviations: HER2, human epidermal growth factor receptor 2; HR, hormone receptor (estrogen receptor and/or progesterone receptor).
Efficacy
A pCR was achieved by 11 of 40 enrolled patients (28%; 95% CI, 15%-44%), 8 of 19 with TN-IBC (42%; 95% CI, 20%-66%), and 3 of 21 with HR-positive/HER2-negative IBC (14%; 95% CI, 3%-36%). The association between pCR and subtype was significant (χ2 P = .05) (Table 1). We observed no significant associations between pCR and age, menopausal status, clinical N category, or nuclear grade (Table 1). A pCR was achieved by 11 of 37 evaluable patients (30%; 95% CI, 16%-47%), 8 of 17 with TN-IBC (47%; 95% CI, 23%-72%), and 3 of 20 with HR-positive/HER2-negative IBC (15%; 95% CI, 3%-38%).
Safety
Treatment-related toxic effects are summarized in Table 2. Among the first 17 patients treated with PNC weekly regimen, 9 developed grade 3 or higher neutropenia, of whom 4 received granulocyte–colony-stimulating factor, and 4 developed grade 3 or higher thrombocytopenia, of whom 3 required platelet transfusion. Therefore, the remaining 23 patients were treated with 3 weeks of PNC followed by 1 week off, which had less intense hematological toxic effects compared with the PNC weekly regimen.
Table 2. Hematological and Nonhematological Toxic Effects Occurring in at Least 10% of Patients by Panitumumab, Nab-paclitaxel, and Carboplatin Regimen and Gradea.
| Toxic Effects | No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Weekly (n = 17) |
3 Weeks On, 1 Week Off (n = 23) |
|||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Hematological | ||||||||
| Neutropenia | 0 | 3 (18) | 4 (24) | 5 (29) | 0 | 3 (13) | 10 (43) | 7 (30) |
| Leucopenia | 0 | 9 (53) | 2 (12) | 0 | 5 (22) | 11 (48) | 7 (30) | 0 |
| Anemia | 0 | 4 (24) | 3 (18) | 0 | 0 | 9 (39) | 0 | 0 |
| Thrombocytopenia | 0 | 0 | 4 (24) | 0 | 0 | 4 (17) | 1 (4) | 1 (4) |
| Hypomagnesemia | 0 | 0 | 1 (6) | 0 | 0 | 3 (13) | 1 (4) | 1 (4) |
| Nonhematological | ||||||||
| Skin rash | 6 (35) | 4 (24) | 2 (12) | 0 | 5 (22) | 10 (43) | 4 (17) | 0 |
| Skin peeling | 3 (18) | 2 (12) | 0 | 0 | 5 (22) | 0 | 0 | 0 |
| Hand-foot reaction | 4 (24) | 2 (12) | 0 | 0 | 1 (4) | 2 (9) | 0 | 0 |
| Stomatitis | 5 (29) | 3 (18) | 1 (6) | 0 | 6 (26) | 3 (13) | 0 | 0 |
| Alopecia | 2 (12) | 10 (59) | 0 | 0 | 0 | 23 (100) | 0 | 0 |
| Nausea | 4 (24) | 7 (41) | 0 | 0 | 8 (35) | 6 (26) | 1 (4) | 0 |
| Vomiting | 4 (24) | 0 | 0 | 0 | 7 (30) | 3 (13) | 1 (4) | 0 |
| Constipation | 7 (41) | 1 (6) | 0 | 0 | 6 (26) | 5 (22) | 0 | 0 |
| Fatigue | 1 (6) | 9 (53) | 2 (12) | 0 | 6 (26) | 12 (52) | 0 | 0 |
| Diarrhea | 8 (47) | 3 (18) | 0 | 0 | 4 (17) | 2 (9) | 2 (9) | 0 |
| Myalgia | 5 (29) | 3 (18) | 0 | 0 | 8 (35) | 3 (13) | 0 | 0 |
| Mucositis | 2 (12) | 0 | 0 | 0 | 5 (22) | 3 (13) | 0 | 0 |
| Infection | 0 | 1 (6) | 1 (6) | 0 | 0 | 7 (30) | 0 | 0 |
| Paresthesia | 3 (18) | 5 (29) | 0 | 0 | 6 (26) | 2 (9) | 0 | 0 |
Toxic effects possibly, probably, or definitely related to treatment with panitumumab, nab-paclitaxel, and carboplatin regimen. Grade per Common Terminology Criteria for Adverse Events, version 3.0.
Among all 40 patients treated with PNC, 12 (30%) had grade 4 neutropenia, 6 (15%) had grade 3 or 4 thrombocytopenia, and 3 (8%) had grade 3 or 4 hypomagnesemia. The major nonhematological toxic effect was skin toxic effects; 6 patients (15%) had grade 3 skin rash. All patients’ symptoms were reversible with oral and topical antibiotics.
Two patients were unable to complete PNC therapy because of toxic effects. One required hospitalization for grade 3 neutropenia and severe mucositis; the other left the study during cycle 3 of PNC because of neutropenia and thrombocytopenia that delayed reinitiation of treatment. During PNC therapy, 10 patients were hospitalized for treatment-related toxic effects: 3 for neutropenia, 2 for febrile neutropenia, and 1 each for diarrhea, pulmonary embolism, bleeding from the rectum (ischemic colitis), fever without neutropenia, and confusion of unknown origin. One patient developed possible therapy-related myelodysplastic syndrome. There were no treatment-related deaths.
Recurrence and Survival
The median follow-up time for enrolled patients was 19.3 months (95% CI, 13.8-29.5 months). Fourteen patients experienced recurrence (distant recurrence in 13; recurrence in the contralateral breast in 1), and 9 died. Of the 9 patients who died, 1 had initial stage IV disease, and 7 had a high burden of disease at diagnosis and quickly developed distant metastasis. The lung was the most common site of recurrence (n = 4), followed by bone (n = 3) and brain (n = 3). The median follow-up time for the 31 survivors was 19.2 months (range, 0.3-52.2 months). Among the 37 evaluable patients, there was no significant difference in disease-free (hazard ratio, 0.66; 95% CI, 0.18-2.40; P = .53) and overall survival (hazard ratio, 0.69; 95% CI, 0.14-3.32; P = .64) between patients with and without pCR (eFigure 3 in Supplement 2).
Biomarkers for pCR
Protein Expression
Comparison of the protein expression of EGFR, pEGFR, E-cadherin, vimentin, COX-2, and Nodal in prepanitumumab tissues between patients with and without a pCR showed that expression of pEGFR (P = .05) and COX-2 (P = .05), but not other proteins, was correlated with pCR outcome (eTable 2 in Supplement 2). Comparison of changes in protein expression between prepanitumumab and postpanitumumab samples in patients with and without a pCR showed no significant correlations with pCR outcome.
Gene Expression
RNA sequencing analysis of 13 pairs of matched tumor samples collected before and after first dose of panitumumab identified no genes with significant expression changes after panitumumab treatment. We divided these samples into TN-IBC (8 pairs) and HR-positive/HER2-negative IBC (5 pairs) and analyzed differential gene expression.
In the TN-IBC samples, 2 genes (POU3F3 and EGR1) were significantly downregulated and 4 genes (BBOX1, GLYATL2, MUCL1, and LCN2) were significantly upregulated after panitumumab treatment (eFigure 4A and eTable 3 in Supplement 2). We identified no gene whose change in expression after panitumumab treatment predicted pCR status of patients with TN-IBC.
In the HR-positive/HER2-negative samples, 19 genes were significantly downregulated and 10 genes were significantly upregulated after panitumumab treatment (eTable 3 in Supplement 2). Canonical pathways analysis showed that these genes contribute to metastasis, cellular immune response, inflammation, and retinoid signaling–regulated biological function (eFigure 4B in Supplement 2).
Discussion
In this single-arm phase 2 study, we evaluated the efficacy and safety of PNC followed by FEC for patients with primary HER2-negative IBC. The intent-to-treat pCR rate was 28% in all evaluable patients, 42% in patients with TN-IBC, and 14% in patients with HR-positive/HER2-negative IBC. These pCR rates compare favorably with rates previously reported for patients with IBC. Costa et al16 reported that the pCR rate in 93 patients with IBC treated with docetaxel (75 mg/m2), doxorubicin (50 mg/m2), and cyclophosphamide (500 mg/m2) was only 8.6%. Our group retrospectively reviewed the outcomes of 527 patients with primary stage III IBC treated with NAC and found that the pCR rate was 15.2% overall and 12.4% in the 139 patients with TN-IBC.1 Most of the patients in this retrospective study received an anthracycline-containing regimen. To our knowledge, the overall pCR rate in the present study, 42%, is the highest reported to date for patients with TN-IBC.
The present study is the first to indicate that an anti-EGFR antibody, panitumumab, may enhance sensitivity to NAC, as indicated by a significantly better pCR rate than the pCR rate in historical series, especially in TN-IBC. In previous reports of EGFR-targeted therapy in other types of breast cancer, the efficacy was moderate. Our findings agree with those of Nabholtz et al,17 who reported that panitumumab plus FEC followed by docetaxel resulted in a pCR rate of 46.8% (95% CI, 32.5%-61.1%) in operable TNBC (n = 47). We acknowledge that the use of nab-paclitaxel in our study may add a layer of complexity to the interpretation of our findings. The GeparSepto (German Breast Group 69) trial showed that compared with traditional paclitaxel, nab-paclitaxel significantly increased the rate of pCR following anthracycline-based chemotherapy: the rate was 38.4% (233 of 606; 95% CI, 34.6%-42.3%) in the nab-paclitaxel group vs 29.0% (174 of 600; 95% CI, 25.4%-32.6%) in the conventional paclitaxel group.18,19 However, whereas our study was limited to patients with IBC, the GeparSepto trial included few patients with IBC. We recognize that the nonrandomized nature of our study limits assessment of the direct contribution of panitumumab to pCR, especially because panitumumab was given in combination with carboplatin and nab-paclitaxel.20,21 However, our study suggests a positive impact of EGFR-targeted therapy for TN-IBC patients, and we are conducting a randomized phase 2 study (NCT02876107) to definitively determine the role of panitumumab.
The initial panitumumab-containing regimen used in our study had significant hematological toxic effects. After the schedule was modified to days 1, 8, and 15 every 28 days, toxic effects was substantially reduced. The most frequent nonhematological toxic effect was rash, which is expected with panitumumab therapy. Although 15% of patients had grade 3 skin toxic effects, symptoms were manageable. We speculate that the grade 4 hematologic and grade 3 nonhematologic adverse events, such as neuropathy, may have been caused by carboplatin. In the GeparSixto trial,20 which assessed an anthracycline-taxane-bevacizumab combination with or without carboplatin, hematological toxic effects, including grade 3 or 4 neutropenia, were significantly more common with carboplatin (65% vs 27%), and the carboplatin dose was decreased.20 Similar rates of hematologic toxic effects, particularly neutropenia, were observed in phase 2 studies of panitumumab in combination with either gemcitabine and carboplatin or paclitaxel and carboplatin in metastatic triple-negative breast cancer.22,23 The fact that 1 patient in our study developed possible therapy-related myelodysplastic syndrome underscores the need for future validation of the hematological toxic effects and optimization of the dose and schedule of chemotherapy in combination with anti-EGFR drugs.
In our comprehensive correlative analysis to determine predictive biomarkers for pCR, neither EGFR expression before panitumumab treatment nor its change after the first dose of panitumumab was correlated with pCR status, in line with previous reports that EGFR expression does not predict responsiveness to cetuximab in colorectal cancer or gefitinib in non–small cell lung cancer.20,24,25 Our findings suggest that expression of pEGFR, an indicator of activated EGFR pathway, and COX-2, a critical molecule in inflammatory response and EGFR-regulated cancer stemlike cell population,10 are potential predictors of response to panitumumab. Our RNA sequencing analysis identified other candidate predictors of pCR to panitumumab-containing NAC in TN-IBC. These candidates have been shown to contribute to the regulation of EGFR tyrosine kinase activity,26 promotion of epithelial-mesenchymal transition, tumor growth, and metastasis,27,28,29 and regulation of resistance to EGFR-targeted therapy in breast cancer and other cancers.30,31 However, given the small number of patients in our study, molecular predictors of response could not be robustly assessed. Thus, changes in expression of these candidate genes need to be validated in IBC cells, xenografts, and more patient tumor samples collected in our ongoing randomized phase 2 trial (NCT02876107).
Limitations
Because this was a single-arm phase 2 study, the contribution of panitumumab to pCR could not be definitely determined. Because of the small number of patients, it was difficult to determine the molecular predictors of response via comparison between the pCR and non-pCR groups.
Conclusions
Treatment with PNC followed by FEC as NAC for primary HER2-negative IBC causes substantial but acceptable hematological and dermatological toxic effects and has significant efficacy, particularly in patients with TN-IBC. We are conducting a randomized phase 2 trial of the same carboplatin-containing chemotherapy with and without panitumumab in patients with TN-IBC to determine the efficacy of panitumumab (NCT02876107). Our findings warrant further validation of predictive biomarkers for EGFR-targeted therapy.
Trial Protocol
eTable 1. Dermatology/Skin/Nail Assessment (from NCI Common Toxicity Criteria for Adverse Events, version 3.0, with modifications)
eTable 2. Expression of Candidate Proteins at Baseline and Week 2 and Change in Expression of Candidate Proteins between Baseline and Week 2 by Patient pCR Status
eTable 3. Genes Significantly Changed in Patient Samples between Before and After Panitumumab Treatment
eFigure 1. Trial Design
eFigure 2. Panitumumab Dose Modification Algorithm for Toxicity
eFigure 3. Disease-free Survival (A) and Overall Survival (B) Estimates for 37 Evaluable Patients
eFigure 4. Differentially Expressed Genes Regulated by Panitumumab
References
- 1.Masuda H, Brewer TM, Liu DD, et al. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor- and HER2-defined subtypes. Ann Oncol. 2014;25(2):384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabioglu N, Gong Y, Islam R, et al. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18(6):1021-1029. [DOI] [PubMed] [Google Scholar]
- 3.Corkery B, Crown J, Clynes M, O’Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009;20(5):862-867. [DOI] [PubMed] [Google Scholar]
- 4.Matsuda N, Lim B, Wang X, Ueno NT. Early clinical development of epidermal growth factor receptor targeted therapy in breast cancer. Expert Opin Investig Drugs. 2017;26(4):463-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masuda H, Zhang D, Bartholomeusz C, Doihara H, Hortobagyi GN, Ueno NT. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res Treat. 2012;136(2):331-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa DB, Halmos B, Kumar A, et al. BIM mediates EGFR tyrosine kinase inhibitor-induced apoptosis in lung cancers with oncogenic EGFR mutations. PLoS Med. 2007;4(10):1669-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Wu YT, Hsieh HC, Yu Y, Yu AL, Chang WW. Epidermal growth factor/heat shock protein 27 pathway regulates vasculogenic mimicry activity of breast cancer stem/progenitor cells. Biochimie. 2014;104:117-126. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z, Shao N, Weng H, et al. Correlation between epidermal growth factor receptor and tumor stem cell markers CD44/CD24 and their relationship with prognosis in breast invasive ductal carcinoma. Med Oncol. 2015;32(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang D, LaFortune TA, Krishnamurthy S, et al. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res. 2009;15(21):6639-6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Reyes ME, Zhang D, et al. EGFR signaling promotes inflammation and cancer stem-like activity in inflammatory breast cancer. Oncotarget. 2017;8(40):67904-67917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouad TM, Barrera AMG, Reuben JM, et al. Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol. 2017;18(4):e228-e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 13.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796-1804. [DOI] [PubMed] [Google Scholar]
- 15.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa SD, Loibl S, Kaufmann M, et al. Neoadjuvant chemotherapy shows similar response in patients with inflammatory or locally advanced breast cancer when compared with operable breast cancer: a secondary analysis of the GeparTrio trial data. J Clin Oncol. 2010;28(1):83-91. [DOI] [PubMed] [Google Scholar]
- 17.Nabholtz JM, Abrial C, Mouret-Reynier MA, et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane-based chemotherapy in operable triple-negative breast cancer: identification of biologically defined signatures predicting treatment impact. Ann Oncol. 2014;25(8):1570-1577. [DOI] [PubMed] [Google Scholar]
- 18.Schneeweiss A, Jackisch C, Schmatloch S, et al. Survival analysis of the prospectively randomized phase III GeparSepto trial comparing neoadjuvant chemotherapy with weekly nab-paclitaxel with solvent-based paclitaxel followed by anthracycline/cyclophosphamide for patients with early breast cancer – GBG69. Cancer Res. 2017;78(4):abstr GS3-05. [Google Scholar]
- 19.Untch M, Jackisch C, Schneeweiss A, et al. ; German Breast Group (GBG); Arbeitsgemeinschaft Gynäkologische Onkologie—Breast (AGO-B) Investigators . Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345-356. [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol. 2014;15(7):747-756. [DOI] [PubMed] [Google Scholar]
- 21.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowherd S, Miller LD, Melin SA, et al. A phase II clinical trial of weekly paclitaxel and carboplatin in combination with panitumumab in metastatic triple negative breast cancer. Cancer Biol Ther. 2015;16(5):678-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yardley DA, Ward PJ, Daniel BR, et al. Panitumumab, gemcitabine, and carboplatin as treatment for women with metastatic triple-negative breast cancer: a Sarah Cannon Research Institute phase II trial. Clin Breast Cancer. 2016;16(5):349-355. [DOI] [PubMed] [Google Scholar]
- 24.Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (the IDEAL 1 Trial) [published correction appears in J Clin Oncol. 2004;22(23):4863]. J Clin Oncol. 2003;21(12):2237-2246. [DOI] [PubMed] [Google Scholar]
- 25.Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149-2158. [DOI] [PubMed] [Google Scholar]
- 26.Agelaki S, Spiliotaki M, Markomanolaki H, et al. Caveolin-1 regulates EGFR signaling in MCF-7 breast cancer cells and enhances gefitinib-induced tumor cell inhibition. Cancer Biol Ther. 2009;8(15):1470-1477. [DOI] [PubMed] [Google Scholar]
- 27.Chung IH, Wu TI, Liao CJ, et al. Overexpression of lipocalin 2 in human cervical cancer enhances tumor invasion. Oncotarget. 2016;7(10):11113-11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding G, Fang J, Tong S, et al. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate. 2015;75(9):957-968. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Bielenberg DR, Rodig SJ, et al. Lipocalin 2 promotes breast cancer progression. Proc Natl Acad Sci U S A. 2009;106(10):3913-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irwin ME, Johnson BP, Manshouri R, Amin HM, Chandra J. A NOX2/Egr-1/Fyn pathway delineates new targets for TKI-resistant malignancies. Oncotarget. 2015;6(27):23631-23646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y, Luo Y, Zheng Y, et al. Exploring the mechanism of non-small-cell lung cancer cell lines resistant to epidermal growth factor receptor tyrosine kinase inhibitor. J Cancer Res Ther. 2016;12(1):121-125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Dermatology/Skin/Nail Assessment (from NCI Common Toxicity Criteria for Adverse Events, version 3.0, with modifications)
eTable 2. Expression of Candidate Proteins at Baseline and Week 2 and Change in Expression of Candidate Proteins between Baseline and Week 2 by Patient pCR Status
eTable 3. Genes Significantly Changed in Patient Samples between Before and After Panitumumab Treatment
eFigure 1. Trial Design
eFigure 2. Panitumumab Dose Modification Algorithm for Toxicity
eFigure 3. Disease-free Survival (A) and Overall Survival (B) Estimates for 37 Evaluable Patients
eFigure 4. Differentially Expressed Genes Regulated by Panitumumab