This study evaluates the vestibuloocular reflex gain and change in fall risks after administration of the video head impulse test among patients with subacute or chronic dizziness.
Key Points
Questions
Is the video head impulse test associated with a reduction of fall risk?
Findings
This case series study of 38 patients with subacute or chronic dizziness found that the change in the vestibuloocular reflex gain measured by the video head impulse test was associated with a change in fall risk as evaluated by the dynamic gait index, a test of dynamic balance. Improvement of vestibuloocular reflex gain was associated with a reduction of fall risk, whereas the opposite was not true.
Meaning
The video head impulse test may be useful in the follow-up of patients for direct measurement of gaze stability and its association with fall risk and dynamic balance.
Abstract
Importance
It is important to know whether recovery of the vestibuloocular reflex (VOR) as measured by the video head impulse test (vHIT) is associated with the recovery of dynamic balance. It is also critical to know how much change in VOR gain is clinically relevant for establishing the recovery of dynamic balance.
Objectives
To investigate the association between improved VOR gain as measured by the vHIT and improved dynamic balance (reduced fall risk) as measured by the dynamic gait index (DGI) and to calculate the minimal clinically important difference of VOR gain.
Design, Setting, and Participants
This retrospective case series study was performed at a tertiary referral center at the Johns Hopkins University School of Medicine. Thirty-eight consecutive patients with subacute or chronic dizziness from January 1, 2014, through May 31, 2017, who visited the vestibular physical therapy clinic were included in the study.
Interventions
Each patient was evaluated with room light and video-infrared oculomotor examination, vHIT, and balance testing before and after vestibular physical therapy.
Main Outcomes and Measures
Gain of the lesioned VOR and score on the DGI.
Results
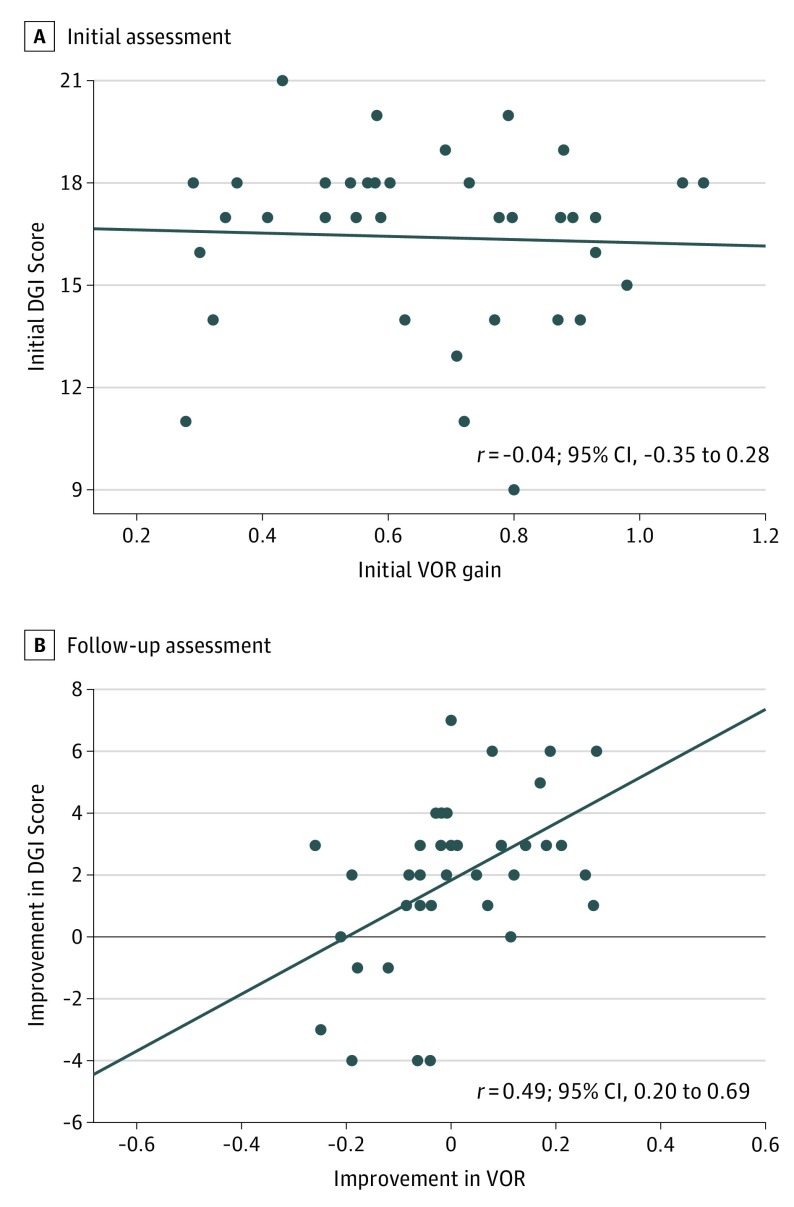
Among the 38 patients (25 women [66%]; mean [SD] age, 65 [14] years), the mean (SD) initial lesioned VOR gain was 0.66 (0.23) and initial DGI score was 16 (3). No correlation was found between initial VOR gain and initial DGI score (r = −0.04; 95% CI, −0.35 to 0.28). At follow-up, 15 patients (39%) had an improved VOR gain and 30 (79%) had an improved DGI score, which was correlated (r = 0.49; 95% CI, 0.20-0.69). In those 15 patients with improved VOR gain, 14 (93%) had improvement of DGI score. In the 23 patients without improvement of VOR gain, 16 (70%) still showed improvement in their DGI score. When using VOR gain to estimate improvement of DGI, the minimal clinically important difference of VOR gain was −0.06.
Conclusions and Relevance
The change of VOR gain in the vHIT was moderately associated with the change of DGI score. Improved VOR gain was associated with a high probability of improved dynamic balance. However, in most of the patients whose VOR gains did not improve, balance improvement occurred putatively through sensory reweighting strategies.
Introduction
The head impulse test, first described by Halmagyi and Curthoys1 in 1988, is a critical and accessible bedside vestibular test of the high-frequency vestibuloocular reflex (VOR). In the past decade, the development of the video head impulse test (vHIT) has provided quantitative data of the VOR.2 When applied in the emergency department, the vHIT is valuable for helping differentiate stroke from vestibular neuritis.3 When applied in the neuro-otology clinic, the vHIT can accurately measure the severity of unilateral or bilateral vestibular hypofunction.2 When applied in the vestibular rehabilitation clinic, the vHIT can examine recovery of the gain of the VOR (eye velocity/head velocity) as well as adaptation of the compensatory saccade latency and frequency.4,5,6,7,8
Although VOR gain is useful to measure, its functional relevance is gaze instability, which is less common than the report of postural and gait instability in patients with vestibular hypofunction.9 Monitoring the improvement of postural balance and gait is critical and directly associated with the patients’ quality of life and fall risk. Whether recovery of the VOR gain is associated with the recovery of balance is unknown, as is how much change in VOR gain is clinically relevant for establishing recovery of balance function.
Common clinical tests for assessing postural balance in patients with vestibular hypofunction include the modified clinical tests of sensory interaction and balance,10,11 computerized dynamic posturography,12 and the dynamic gait index (DGI).13 Although the modified clinical test of sensory interaction and balance and posturography are used for evaluating static balance function, the DGI estimates fall risk on the basis of tasks related to dynamic balance. When applied in vestibular disorders, the DGI is a useful indicator of fall risk with moderate interrater reliability, incorporates head-body movements into the gait assessment (ie, yaw/pitch head rotation, whole-body rotation), and documents the functional recovery of dynamic balance.13
The purpose of this study was to investigate the association between improved VOR gain (as measured by the vHIT) and improved dynamic balance (as measured by the DGI) in patients who underwent vestibular physical therapy. Furthermore, we calculated the minimal clinically important difference (MCID) of VOR gain using the anchor-based method.14 We also report the optimal sensitivity and specificity.
Methods
We collected data using retrospective medical record review. Data were from consecutive patients with subacute or chronic dizziness from January 1, 2014, through May 31, 2017, who visited the vestibular physical therapy clinic of the tertiary referral center at Johns Hopkins University School of Medicine and were prescribed a home exercise program of vestibular physical therapy.6 This study was approved by the institutional review board of the Johns Hopkins University, which waived the need for informed consent for the medical record review.
All patients underwent a structured examination. Clinical oculomotor examination included tests of spontaneous and gaze-evoked nystagmus, cover test for skew deviation, smooth pursuit, saccade, slow head rotation, and VOR suppression test. Video infrared testing included spontaneous nystagmus, Valsalva maneuver (pinched nose and closed glottis), head shaking nystagmus test, skull-vibration nystagmus test, Dix-Hallpike test, and the supine-roll test. In addition, the vHIT, the modified clinical test of sensory interaction and balance, and DGI were performed.
Only patients assessed with the vHIT and DGI before and after vestibular physical therapy were included in this study. According to the patient histories, the clinical oculomotor and vestibular examination results, and the laboratory vestibular function test results (video-oculography, caloric test, and vestibular evoked myogenic potentials), we identified the diagnoses of all included patients and classified their functional diagnoses as (1) unilateral vestibular hypofunction (UVH), (2) bilateral vestibular hypofunction (BVH), and (3) other dizziness.
The vHIT data were collected using a commercial device that records the head and right eye velocity at a frame rate of 250 Hz (GN Otometrics). We used the term lesioned VOR gain to represent the patients’ VOR function and defined the lesioned VOR gain as such:
For UVH, the lesioned VOR gain was the ipsilesional horizontal semicircular canal or, in the case of unilateral superior canal dehiscence, the ipsilesional superior semicircular canal.
For BVH, the lesioned VOR gain was the mean VOR gain of both horizontal semicircular canals, except bilateral superior canal dehiscence in which the lesioned VOR gain was the mean VOR gain of both superior semicircular canals.
For other dizziness (eg, vestibular migraine), the lesioned VOR gain was the mean VOR gain of both horizontal canals.
For analysis of the improvement in VOR gain and DGI after vestibular physical therapy, we defined ∆VOR as the difference in lesioned VOR gain after and before vestibular physical therapy and ∆DGI as the difference in DGI score after and before vestibular physical therapy. Positive values indicated improvement, and zero or negative values indicated no improvement. Existing studies15,16 suggest that a change of at least 4 points of the DGI score is considered to be clinically significant.
For the MCID of VOR gain, we used the anchor-based method, which requires a moderate correlation (r>0.30) between the anchor and the outcome changes.17 Based on the correlation between ∆VOR and ∆DGI, we selected 2 dichotomies for the ∆DGI. The first dichotomy was to define a ∆DGI of at least 1 as balance improved and a ∆DGI of 0 or less as balance not improved; the second was to define ∆DGI of at least 4 as balance significantly improved and ∆DGI of 3 or less as balance not significantly improved. Next, we plotted the receiver operating characteristic (ROC) curve for calculating the MCID of VOR gain and its sensitivity and specificity for estimating improvement and significant improvement of balance. The MCID chosen was defined as the optimum cutoff point of the ROC curve selected using the Youden index.
Statistical Analysis
Descriptive statistics were reported for the mean (SD) of VOR gains and DGI scores and for the improving rates of VOR gain and DGI score in all the patients and the patients in each of the 3 functional diagnosis groups (ie, UVH, BVH, or other dizziness). Next, we compared the rate of change in VOR gain with the DGI score using the McNemar test (P < .05 indicated significance) and reporting the effect size with 95% CI around the effect size. Pearson correlation coefficient (with 95% CI) and linear regression were used to analyze correlations between the initial VOR gain and initial DGI score (before vestibular physical therapy) and between ∆VOR and ∆DGI. In addition, we conducted a subanalysis using Pearson correlation analyses between ∆VOR and ∆DGI for each group classified by the functional diagnosis. Finally, we used the ROC curve for measuring the anchor-based MCID of VOR gain, as well as its sensitivity and specificity. We used SPSS version 23 (SPSS Inc) for all analyses.
Results
Thirty-eight patients with vHIT and DGI data before and after vestibular physical therapy (13 men [34%] and 25 women [66%]; mean [SD] age, 65 [14] years) were included in this study (Table 1). Eighteen patients were diagnosed with UVH, 11 patients were diagnosed with BVH, and 9 patients were classified as having other dizziness. The UVH group included 4 patients with vestibular neuritis, 2 with labyrinthitis (acute unilateral vestibular loss comorbid with hearing loss), 5 with acoustic neuroma (3 of which were seen postoperatively), 4 with Meniere disease (3 of which were seen after intratympanic gentamicin injection), 2 with postoperative superior canal dehiscence, and 1 with vestibular hypofunction with temporal arteritis. The BVH group included 2 patients with ototoxic effects of gentamicin, 1 with bilateral cholesteatoma requiring surgical excision, 1 with bilateral superior canal dehiscence with surgery, and 7 with idiopathic bilateral vestibulopathy. The group of patients with other dizziness included 6 with vestibular migraine based on International Classification of Vestibular Disorders criteria,18 2 with persistent postural-perceptual dizziness,19 and 1 without a clear cause.
Table 1. Demographic Data of Patients Who Underwent Vestibular Physical Therapy.
| Patient No./Sex/Age, y | Clinical Diagnosis | Functional Diagnosis | Interval, moa | Initial VOR Gain | ∆VORb | Initial DGI | ∆DGIc |
|---|---|---|---|---|---|---|---|
| 1/M/70s | Right vestibular neuritis | UVH | 2 | 0.63 | 0.17 | 14 | 5 |
| 2/F/70s | Left vestibular neuritis | UVH | 1 | 0.79 | 0.18 | 17 | 3 |
| 3/F/50s | Left SCD with canal plugging | UVH | 12 | 0.3 | −0.06 | 16 | 3 |
| 4/F/50s | Left acoustic neuroma | UVH | 9 | 0.78 | −0.06 | 17 | 1 |
| 5/M/50s | Right MD with IT gentamicin | UVH | 1 | 0.69 | 0 | 19 | 3 |
| 6/F/60s | Left labyrinthitis | UVH | 1 | 0.41 | 0.14 | 17 | 3 |
| 7/F/30s | Left acoustic neuroma with surgery | UVH | 2 | 0.55 | −0.26 | 17 | 3 |
| 8/F/80s | Right vestibular loss/ temporal arteritis | UVH | 4 | 0.5 | −0.04 | 18 | 1 |
| 9/F/60s | Right acoustic neuroma with surgery | UVH | 1 | 0.36 | 0 | 18 | 3 |
| 10/M/70s | Right MD | UVH | 4 | 0.89 | 0 | 17 | 3 |
| 11/F/50s | Left acoustic neuroma with surgery | UVH | 18 | 0.59 | 0.12 | 17 | 2 |
| 12/M/60s | Left acoustic neuroma | UVH | 2 | 0.58 | −0.02 | 18 | 4 |
| 13/F/60s | Right vestibular neuritis | UVH | 4 | 0.34 | 0.05 | 17 | 2 |
| 14/F/70s | Left MD with IT gentamicin | UVH | 2 | 0.5 | 0.01 | 17 | 3 |
| 15/M/80s | Left vestibular neuritis | UVH | 2 | 0.54 | −0.01 | 18 | 2 |
| 16/F/60s | Left SCD with canal plugging | UVH | 15 | 0.43 | −0.08 | 21 | 2 |
| 17/F/60s | Right labyrinthitis | UVH | 1 | 0.77 | 0.19 | 14 | 6 |
| 18/F/50s | Right MD with IT gentamicin | UVH | 9 | 0.73 | −0.21 | 18 | 0 |
| 19/M/80s | Bilateral vestibulopathy | BVH | 4 | 0.63 | −0.25 | 14 | −3 |
| 20/F/70s | Bilateral vestibulopathy | BVH | 23 | 0.87 | −0.12 | 14 | −1 |
| 21/M/60s | Bilateral SCD with canal plugging | BVH | 30 | 0.6 | −0.18 | 18 | −1 |
| 22/F/60s | Cholesteatoma with surgery | BVH | 28 | 0.58 | −0.04 | 20 | −4 |
| 23/M/40s | Ototoxic effects of gentamicin | BVH | 2 | 0.28 | 0.28 | 11 | 6 |
| 24/F/70s | Bilateral vestibulopathy | BVH | 1 | 0.29 | 0.27 | 18 | 1 |
| 25/F/80s | Bilateral vestibulopathy | BVH | 2 | 0.8 | 0.10 | 9 | 3 |
| 26/M/80s | Bilateral vestibulopathy | BVH | 9 | 0.57 | 0.21 | 18 | 3 |
| 27/F/80s | Bilateral vestibulopathy | BVH | 5 | 0.32 | 0.26 | 14 | 2 |
| 28/M/80s | Bilateral vestibulopathy | BVH | 12 | 0.9 | −0.02 | 14 | 3 |
| 29/F/70s | Ototoxic effects of gentamicin | BVH | 3 | 0.72 | −0.09 | 11 | 1 |
| 30/F/30s | Vestibular migraine | Other dizziness | 2 | 0.79 | 0.12 | 20 | 0 |
| 31/F/60s | Vestibular migraine | Other dizziness | 11 | 0.88 | −0.06 | 19 | 2 |
| 32/M/70s | PPPD | Other dizziness | 11 | 0.71 | 0.08 | 13 | 1 |
| 33/M/60s | Vertigo with unknown origin | Other dizziness | 2 | 0.93 | 0 | 16 | 7 |
| 34/F/80s | Vestibular migraine | Other dizziness | 9 | 1.07 | −0.19 | 18 | −4 |
| 35/F/50s | Vestibular migraine | Other dizziness | 20 | 0.88 | −0.07 | 17 | −4 |
| 36/F/50s | PPPD | Other dizziness | 23 | 0.93 | −0.02 | 17 | 4 |
| 37/F/40s | Vestibular migraine | Other dizziness | 2 | 0.98 | 0.08 | 15 | 6 |
| 38/M/70s | Vestibular migraine | Other dizziness | 1 | 1.1 | −0.19 | 18 | 2 |
Abbreviations: BVH, bilateral vestibular hypofunction; DGI, dynamic gait index; DZ, other dizziness; IT, intratympanic; MD, Meniere disease; PPPD, persistent postural-perceptual dizziness; SCD, superior canal dehiscence; UVH, unilateral vestibular hypofunction; VOR, vestibuloocular reflex.
Indicates the time from the first to the last visits.
Calculated as the difference between lesioned VOR gain after and before vestibular physical therapy.
Calculated as the difference between DGI score after and before vestibular physical therapy.
For the UVH group, the mean (SD) lesioned VOR gain at the initial visit was 0.58 (0.17) (contralesional VOR gain, 0.88 [0.09]). In the BVH group, the mean (SD) lesioned VOR gain at the initial visit was 0.60 (0.22) (right VOR gain, 0.60 [0.27]; left VOR gain, 0.58 [0.23]). In the group with other dizziness, the mean (SD) lesioned VOR gain was 0.92 (0.12) (right VOR gain, 0.93 [0.16]; left VOR gain, 0.90 [0.12]). Overall, the initial mean (SD) lesioned VOR gain for all patients was 0.66 (0.23). The initial mean DGI score for all patients was 16 (3) (UVH group, 17 [2]; BVH group, 15 [4]; other dizziness group, 17 [2]). No correlation occurred between the initial VOR gain and initial DGI score (r = −0.04; 95% CI, −0.35 to 0.28) (Figure 1A).
Figure 1. Correlation Analysis Between Vestibuloocular Reflex (VOR) Gain and Dynamic Gait Index (DGI).
A, No significant correlation occurred between initial VOR gain and initial DGI score. B, During follow-up, there was a moderate correlation between the improvement of VOR gain and the improvement of DGI score, with improvement measured as the difference between measurements after and before vestibular physical therapy. Circles indicate individual data points; horizontal line, linear regression.
All 38 patients underwent home vestibular physical therapy. The mean (range) follow-up time was 215 days (14-897 days). After vestibular physical therapy, 15 patients (39%) had improvement in VOR gain (UVH group, 7 of 18 [39%]; BVH group, 5 of 11 [45%]; other dizziness group, 3 of 9 [33%]). However, approximately twice that number of patients (30 of 38 [79%]) showed improvement in their DGI score (UVH group, 17 of 18 [94%]; BVH group, 7 of 11 [64%]; other dizziness group, 6 of 9 [67%]). Seven patients (18%) reached a ∆DGI of at least 4 (UVH group, 3 of 18 [17%]; BVH group, 1 of 11 [9%]; other dizziness group, 3 of 9 [33%]). Overall, the difference in the rate of subjects who displayed an improvement in DGI score (30 of 38 [79%]) and VOR gain (15 of 38 [39%]) was 39% (95% CI, 17%-56%), a clinically meaningful difference significantly higher than the rate of improvement in VOR gain (P < .001, McNemar test).
Moderate correlation occurred between ∆DGI and ∆VOR (r = 0.49; 95% CI, 0.20-0.69) (Figure 1B). In the 15 patients with improvement in VOR gain, 14 (93% sensitivity; 95% CI, 70%-99%) also improved in DGI as well (4 of 15 [27%] with ∆DGI≥4). On the other hand, in the 23 patients without improvement in VOR, 16 (70%) still showed an improved DGI (3 of 23 [13%] with ∆DGI≥4), whereas 7 (30% specificity; 95% CI, 16%-51%) did not (Table 2).
Table 2. Number of Patients With VOR and DGI Improvement.
| ∆DGIa | ∆VOR, No. (%) of Patientsb | Total No. | |
|---|---|---|---|
| >0 | ≤0 | ||
| ≥1 | 14 (37) | 16 (42) | 30 (79) |
| ≥4 | 4 (11) | 3 (8) | 7 (18) |
| 1-3 | 10 (26) | 13 (34) | 23 (61) |
| ≤0 | 1 (3) | 7 (18) | 8 (21) |
| All | 15 (39) | 23 (61) | 38 (100) |
Abbreviations: DGI, dynamic gait index; VOR, vestibuloocular reflex.
Calculated as the difference between DGI score after and before vestibular physical therapy.
Calculated as the difference between lesioned VOR gain after and before vestibular physical therapy.
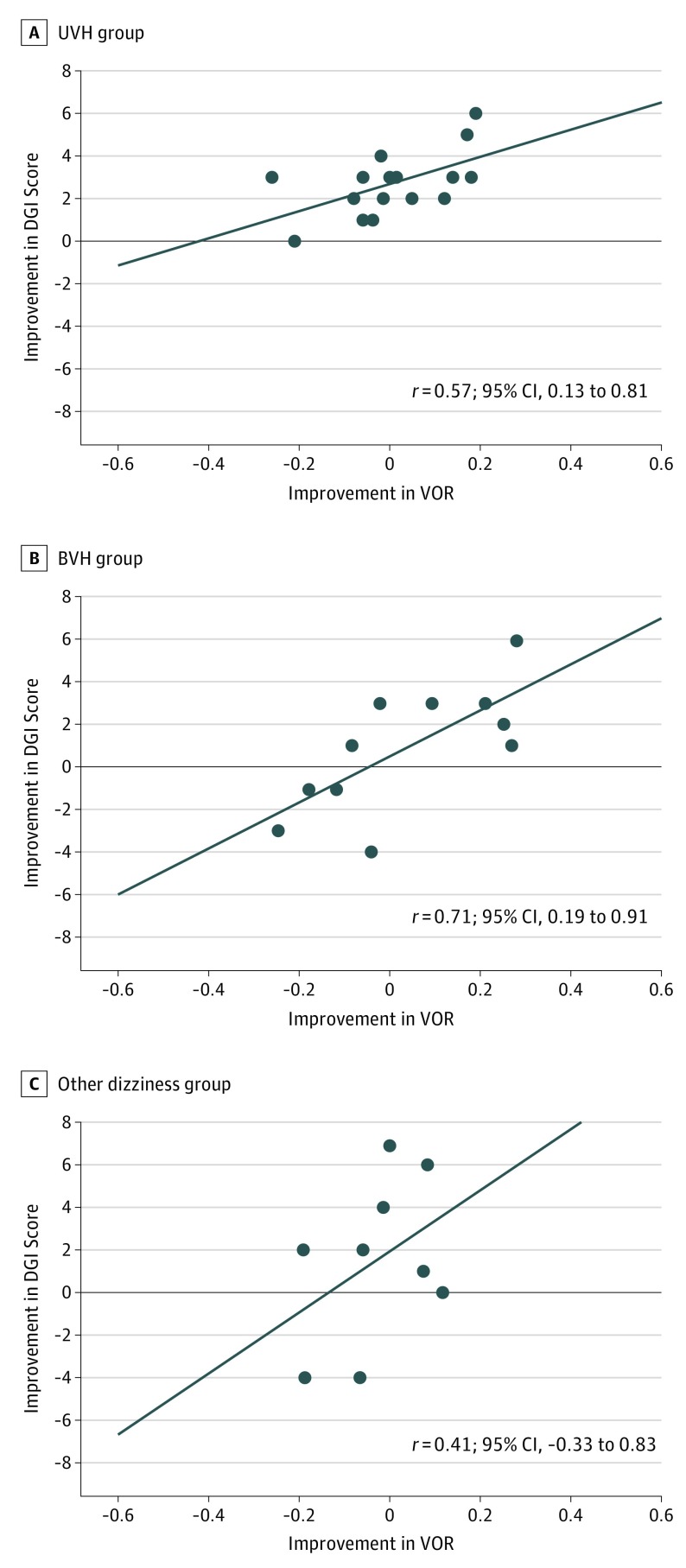
Intragroup subanalysis to examine the correlation across the functional diagnoses revealed the ∆VOR and ∆DGI were moderately correlated in the UVH group (r = 0.57; 95% CI, 0.13 to 0.81), highly correlated in the BVH group (r = 0.71; 95% CI, 0.19 to 0.91), and not correlated in the other dizziness group (r = 0.41; 95% CI, −0.33 to 0.83) (Figure 2). When the 3 patients with superior canal dehiscence surgery were excluded, change of VOR gain was still moderately correlated with change of DGI (r = 0.48; 95% CI, 0.17 to 0.70).
Figure 2. Intragroup Correlation Analysis .
Data are presented as the correlation between the improvement in lesioned vestibuloocular reflex (VOR) gain and the improvement in the dynamic gait index (DGI) score, with improvement measured as the difference between measurements after and before vestibular physical therapy (ΔVOR and ΔDGI). Patients are stratified by diagnosis of unilateral vestibular hypofunction (UVH) (n = 18), bilateral vestibular hypofunction (BVH) (n = 11), and other dizziness (n = 9). A, ∆VOR was moderately correlated with ∆DGI in the UVH group. B, ∆VOR was highly correlated with ∆DGI in the BVH group. C, ∆VOR was not significantly correlated with ∆DGI in the other dizziness group. Circles indicate individual data points; horizontal line, linear regression.
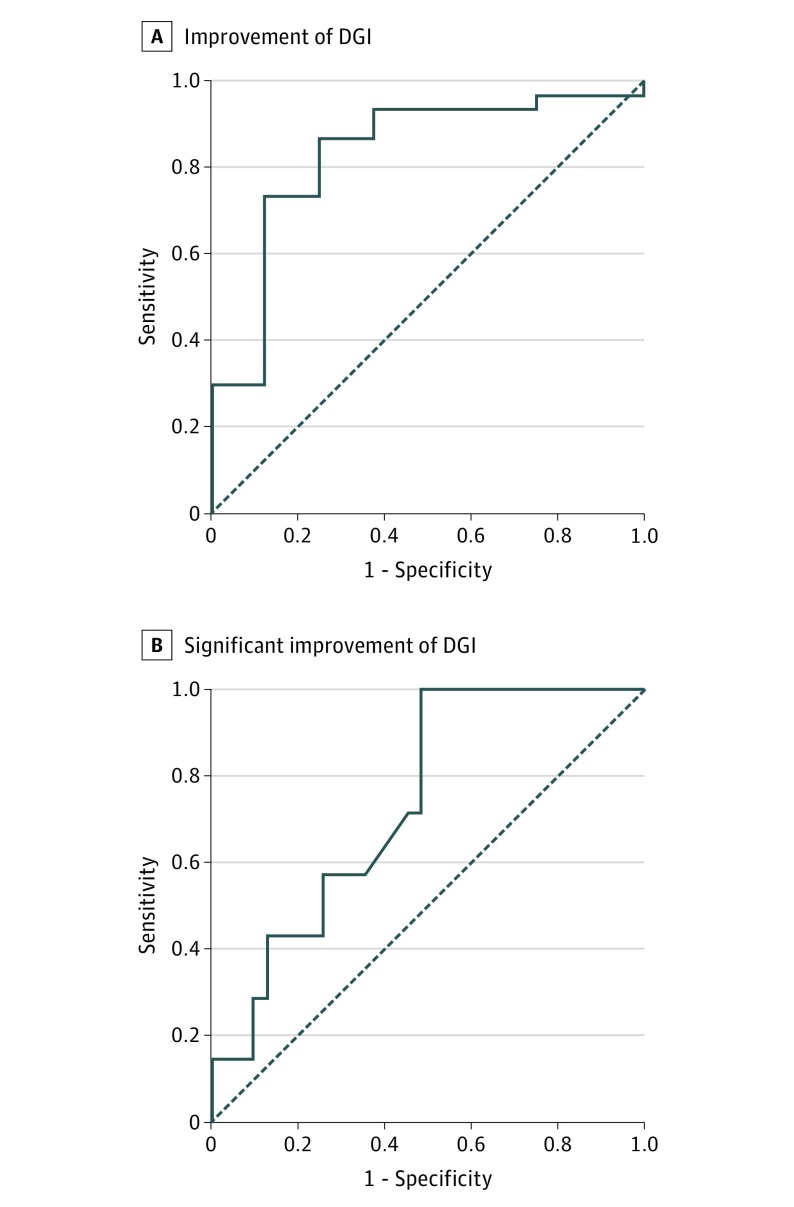
Because the correlation between ∆DGI and ∆VOR exceeded the threshold of r = 0.30, the anchored DGI was eligible to estimate an MCID of VOR gain. Figure 3A shows the ROC curve for detecting improvement of DGI (∆DGI≥1). The area under the curve was 0.83. The MCID of VOR gain (ie, the optimum cutoff point of the ROC curve) was −0.06. This negative MCID value means that after completion of vestibular physical therapy, a decrement of VOR gain by less than 0.06 is associated with an improvement of DGI score. Under this optimum cutoff point, the sensitivity for detecting DGI improvement was 86.7% (95% CI, 70.3%-94.7%), and the specificity was 75.0% (95% CI, 40.9%-92.9%). In addition, Figure 3B illustrates the ROC curve for detecting significant improvement of DGI (∆DGI, ≥4). The area under the curve was 0.74, MCID was −0.02, and sensitivity and specificity were 100% (95% CI, 64.6%-100%) and 51.6% (95% CI, 34.8%-68.0%), respectively.
Figure 3. Receiver Operating Characteristic (ROC) Analysis for Detecting Improvement and Significant Improvement of Dynamic Gait Index (DGI) by Vestibuloocular Reflex (VOR) Gain.
Improvement of DGI indicates a difference (ΔDGI) of at least 1; significant improvement, a ΔDGI of at least 4. Solid line indicates ROC curve; broken line, reference line. A, When improvement of DGI indicates a significant difference (ΔDGI) of at least 1, the area under ROC curve (AUC) is 0.83. The optimum cutoff point is −0.06 with sensitivity of 86.7% (95% CI, 70.3%-94.7%) and specificity of 75% (95% CI, 40.9%-92.9%). B, When the ΔDGI indicates a significant difference of at least 4, the AUC is 0.74 and the optimum cutoff point is −0.02 with sensitivity of 100% (95% CI, 64.6%-100%) and specificity of 51.6% (95% CI, 34.8%-68.0%). Solid line indicates ROC curve; broken line denotes reference line.
Discussion
Our study revealed that the recovery of the high-frequency VOR in the vHIT was moderately associated with the recovery of dynamic balance and reduction of fall risk. The study further demonstrated 2 important phenomena. First, with time and after participating in vestibular physical therapy, 15 of 38 patients (39%) showed an improved high-frequency VOR gain, but 30 of 38 (79%) showed improved dynamic balance. Second, an improved VOR gain as determined by vHIT was associated with an improved DGI, but the opposite was not true (ie, a lack of improved vHIT does not mean that the DGI will not improve). These results imply that for some patients, recovery of vestibular function concurrently leads to recovery of high-frequency VOR and balance function, either through restoration of peripheral vestibular function or through vestibular adaptation. However, regardless of whether the high-frequency VOR recovered, dynamic balance recovered, which may reflect the contribution of other compensatory pathways, such as the substitution by somatosensory and visual systems.20,21
To our knowledge, this study is the first to investigate the association between vHIT and dynamic balance. Prior studies have correlated vHIT-determined VOR gain with the search-coil head impulse test, the caloric test, the dizziness handicap inventory, and the vestibular evoked myogenic potential.2,22,23,24,25 These studies revealed high correlations between vHIT and the search-coil measured head impulse test2 but poor correlation with the caloric test or the dizziness handicap inventory.22,23,24 In addition, vHIT was significantly correlated with ocular vestibular evoked myogenic potential in vestibular neuritis but not with vertigo of central origin or unspecified dizziness.25 In our study, the initial VOR gain of vHIT was poorly correlated with the initial DGI score. This result is not surprising, given that multiple sensory systems are involved in maintaining dynamic balance and that peripheral vestibular function is only one of the contributing afferent inputs. Thus, the DGI does not isolate the relative peripheral vestibular contribution. Instead, it represents a more gross function of balance and fall risk after vestibular loss, which is closely linked to quality of life. However, the change of VOR gain in vHIT (as captured at the follow-up visit) was associated with the change of DGI. These data suggest that the recovery of vestibular function is associated with improvement of dynamic balance and the degree of improvement can be quantified with DGI.
In patients with surgical vestibular deafferentation, VOR gain and asymmetry during lower-velocity rotary chair testing usually improve over time.26,27 Whether the high-frequency VOR can be similarly improved is a matter for debate.21,22 However, more recent studies using a specific VOR adaptation protocol (incremental velocity error signal)28 have shown that the high-frequency VOR gain (measured using head impulses) can improve after training.8 Furthermore, a study in monkeys with unilateral labyrinthectomy showed an increase in the high-frequency VOR gain and reduced gain asymmetry after a training paradigm that involved ipsilesional-only rotation.29 Together, these data in humans8,28 and monkeys29 reveal that the high-frequency VOR gain after vestibular loss is modifiable and dependent on the unique training methods.
Our study demonstrated that 14 of 15 patients with vHIT recovery (93%) had improved DGI scores. The vHIT thus appears to be associated with dynamic balance as measured by the DGI. This finding may be a direct function of the DGI, including items that involve vestibular stimuli of higher-frequency content, such as horizontal and vertical head turns and whole-body rotation. Of interest, among the patients without vHIT recovery, most (16 of 23 [70%]) still showed an improved DGI score. This improvement implies that other processes contribute to the compensation, although the high-frequency VOR does not recover. For example, after a unilateral vestibular assault, the asymmetry between resting firing rates of the second-order vestibular neurons continues to improve via commissural inhibitory pathways and the cerebellar flocculus.30,31 In addition, sensory substitution (visual and/or somatosensory systems) are known to aid in the recovery of postural balance.20,21 Our intragroup analysis appears to confirm these substitutional strategies, given that 10 of 11 patients in the UVH group with no VOR gain improvement (91%) still had an improved DGI score, whereas only 2 of 6 patients in the BVH group with no VOR gain improvement (33%) had DGI improvement. Although most of the patients in the UVH group (94%) had improved DGI at follow-up visits, their degrees of DGI improvement were positively associated with the changes of VOR gain. This association implies that although other compensatory pathways are assisting the improved balance, the high-frequency VOR still plays an important role in maintaining balance.
The MCID is the smallest change of interest that represents a clinically relevant difference.32 It is useful for clinicians to interpret the clinical meaning of changes for individual patients. Herein, we calculated 2 MCID values of VOR gain by using ROC analysis based on 2 definitions of DGI improvement (∆DGI≥1 and ∆DGI≥4). Our data suggest that a VOR gain change of −0.06 is considered to be clinically significant. Our MCIDs of VOR gain were slightly negative, owing to our finding that 70% of patients did not have recovery of their VOR gain although they had recovery of their balance. This finding is interpreted as meaning that a decrement of VOR gain by less than 0.06 is associated with a clinical improvement in dynamic balance (as assessed by the DGI). In addition, the area under the ROC curve was greater when we used ∆DGI of at least 1 compared with using ∆DGI of at least 4, implying that use of ∆DGI of at least 1 is a better anchor for estimating the change of VOR gain. Although studies suggest that a change of at least 4 points in the DGI score is clinically significant,15,16 two-thirds of our patient population had initial DGI scores greater than 17 and therefore would reach normal DGI scores with less than 4 points of change. Our data suggest that the milder improvement of DGI (∆DGI of 1-3) is still relevant when associated with improvement of VOR gain. Moreover, when we used DGI change to estimate the patients’ subjective improvement of dizziness (eTable 1 in the Supplement), ∆DGI of at least 1 was a better cutoff point than ∆DGI of at least 4 based on an improved summary statistic (Youden index is found in eTable 2 and eFigure in the Supplement).
Limitations
Our study has a few limitations. First, we performed a retrospective study with a limited sample size. The smaller sample size results in relative imprecision of estimates owing to wider 95% CIs. Second, the examiner was not blinded to the diagnoses and the previous test results. An unblinded rater may score the DGI lower before therapy and higher after therapy. Third, the time for patients to return for follow-up was long, in part owing to the study population being from a single clinic. Finally, we used a broad diagnosis of patients for determining the MCID. Although our clinical population is an accurate representation of patients seen in tertiary medical centers, a study of MCID in a single patient population may reveal different values. For example, if MCID were examined in vestibular neuritis, a negative value would be unlikely.
Conclusions
In our study, the change of VOR gain in vHIT was moderately associated with change of DGI score. Because the DGI is a reliable measure for assessing balance and fall risk, this result suggests that vHIT may be worth using in follow-up of patients, not only for measurement of gaze stability but also for its association with dynamic balance and fall risk. As a practical matter, if a patient presents with improved VOR gain at a follow-up visit, this patient has a high probability of also having improved balance and reduced fall risk (93% in our study). However, the same rule cannot be applied to the patients with unchanged VOR gains, because some of them may still have balance improvement through other compensatory pathways. Finally, using the anchor-based method with the DGI, we establish that the MCID of VOR gain is −0.06.
eTable 1. Subjective Improvement of Dizziness of the Study Patients
eTable 2. Youden Index for Different Cutoff Points of ∆VOR and ∆DGI To Predict Subjective Improvement of Dizziness
eFigure. ROC Analysis for Using ∆VOR and ∆DGI to Predict Subjective Improvement of Dizziness
References
- 1.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45(7):737-739. doi: 10.1001/archneur.1988.00520310043015 [DOI] [PubMed] [Google Scholar]
- 2.MacDougall HG, Weber KP, McGarvie LA, Halmagyi GM, Curthoys IS. The video head impulse test: diagnostic accuracy in peripheral vestibulopathy. Neurology. 2009;73(14):1134-1141. doi: 10.1212/WNL.0b013e3181bacf85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantokoudis G, Tehrani ASS, Wozniak A, et al. VOR gain by head impulse video-oculography differentiates acute vestibular neuritis from stroke. Otol Neurotol. 2015;36(3):457-465. doi: 10.1097/MAO.0000000000000638 [DOI] [PubMed] [Google Scholar]
- 4.Macdougall HG, Curthoys IS. Plasticity during vestibular compensation: the role of saccades. Front Neurol. 2012;3:21. doi: 10.3389/fneur.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantokoudis G, Schubert MC, Tehrani ASS, Wong AL, Agrawal Y. Early adaptation and compensation of clinical vestibular responses after unilateral vestibular deafferentation surgery. Otol Neurotol. 2014;35(1):148-154. [DOI] [PubMed] [Google Scholar]
- 6.Schubert MC, Migliaccio AA, Clendaniel RA, Allak A, Carey JP. Mechanism of dynamic visual acuity recovery with vestibular rehabilitation. Arch Phys Med Rehabil. 2008;89(3):500-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert MC, Migliaccio AA, Della Santina CC. Modification of compensatory saccades after aVOR gain recovery. J Vestib Res. 2006;16(6):285-291. [PMC free article] [PubMed] [Google Scholar]
- 8.Binetti AC, Varela AX, Lucarelli DL, Verdecchia DH. Unilateral head impulses training in uncompensated vestibular hypofunction. Case Rep Otolaryngol. 2017;2017:2145173. doi: 10.1155/2017/2145173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitney SL, Marchetti GF, Pritcher M, Furman JM. Gaze stabilization and gait performance in vestibular dysfunction. Gait Posture. 2009;29(2):194-198. doi: 10.1016/j.gaitpost.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitney SL, Wrisley DM. The influence of footwear on timed balance scores of the modified clinical test of sensory interaction and balance. Arch Phys Med Rehabil. 2004;85(3):439-443. [DOI] [PubMed] [Google Scholar]
- 11.Wrisley DM, Whitney SL. The effect of foot position on the modified clinical test of sensory interaction and balance. Arch Phys Med Rehabil. 2004;85(2):335-338. [DOI] [PubMed] [Google Scholar]
- 12.Allum JH, Shepard NT. An overview of the clinical use of dynamic posturography in the differential diagnosis of balance disorders. J Vestib Res. 1999;9(4):223-252. [PubMed] [Google Scholar]
- 13.Wrisley DM, Walker ML, Echternach JL, Strasnick B. Reliability of the dynamic gait index in people with vestibular disorders. Arch Phys Med Rehabil. 2003;84(10):1528-1533. doi: 10.1016/S0003-9993(03)00274-0 [DOI] [PubMed] [Google Scholar]
- 14.de Vet HCW, Ostelo RWJG, Terwee CB, et al. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16(1):131-142. doi: 10.1007/s11136-006-9109-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall CD, Herdman SJ. Reliability of clinical measures used to assess patients with peripheral vestibular disorders. J Neurol Phys Ther. 2006;30(2):74-81. [DOI] [PubMed] [Google Scholar]
- 16.Brown KE, Whitney SL, Wrisley DM, Furman JM. Physical therapy outcomes for persons with bilateral vestibular loss. Laryngoscope. 2001;111(10):1812-1817. doi: 10.1097/00005537-200110000-00027 [DOI] [PubMed] [Google Scholar]
- 17.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102-109. doi: 10.1016/j.jclinepi.2007.03.012 [DOI] [PubMed] [Google Scholar]
- 18.Lempert T, Olesen J, Furman J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012;22(4):167-172. [DOI] [PubMed] [Google Scholar]
- 19.Thompson KJ, Goetting JC, Staab JP, Shepard NT. Retrospective review and telephone follow-up to evaluate a physical therapy protocol for treating persistent postural-perceptual dizziness: a pilot study. J Vestib Res. 2015;25(2):97-103. doi: 10.3233/VES-150551 [DOI] [PubMed] [Google Scholar]
- 20.Zennou-Azogui Y, Xerri C, Harlay F. Visual sensory substitution in vestibular compensation: neuronal substrates in the alert cat. Exp Brain Res. 1994;98(3):457-473. doi: 10.1007/BF00233983 [DOI] [PubMed] [Google Scholar]
- 21.Horak FB. Postural compensation for vestibular loss. Ann N Y Acad Sci. 2009;1164(1):76-81. doi: 10.1111/j.1749-6632.2008.03708.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCaslin DL, Jacobson GP, Bennett ML, Gruenwald JM, Green AP. Predictive properties of the video head impulse test: measures of caloric symmetry and self-report dizziness handicap. Ear Hear. 2014;35(5):e185-e191. doi: 10.1097/AUD.0000000000000047 [DOI] [PubMed] [Google Scholar]
- 23.Zellhuber S, Mahringer A, Rambold HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorhinolaryngol. 2014;271(9):2375-2383. doi: 10.1007/s00405-013-2723-6 [DOI] [PubMed] [Google Scholar]
- 24.Redondo-Martínez J, Bécares-Martínez C, Orts-Alborch M, García-Callejo FJ, Pérez-Carbonell T, Marco-Algarra J. Relationship between video head impulse test (vHIT) and caloric test in patients with vestibular neuritis. Acta Otorrinolaringol Esp. 2016;67(3):156-161. doi: 10.1016/j.otorri.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 25.Skorić MK, Adamec I, Pavičić T, et al. Vestibular evoked myogenic potentials and video head impulse test in patients with vertigo, dizziness and imbalance. J Clin Neurosci. 2017;39:216-220. doi: 10.1016/j.jocn.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 26.Li CW, Cousins V, Hooper R. Vestibulo-ocular compensation following unilateral vestibular deafferentation. Ann Otol Rhinol Laryngol. 1992;101(6):525-529. doi: 10.1177/000348949210100614 [DOI] [PubMed] [Google Scholar]
- 27.Enticott JC, O’leary SJ, Briggs RJ. Effects of vestibulo-ocular reflex exercises on vestibular compensation after vestibular schwannoma surgery. Otol Neurotol. 2005;26(2):265-269. [DOI] [PubMed] [Google Scholar]
- 28.Schubert MC, Della Santina CC, Shelhamer M. Incremental angular vestibulo-ocular reflex adaptation to active head rotation. Exp Brain Res. 2008;191(4):435-446. doi: 10.1007/s00221-008-1537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ushio M, Minor LB, Della Santina CC, Lasker DM. Unidirectional rotations produce asymmetric changes in horizontal VOR gain before and after unilateral labyrinthectomy in macaques. Exp Brain Res. 2011;210(3-4):651-660. doi: 10.1007/s00221-011-2622-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergquist F, Ludwig M, Dutia MB. Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol. 2008;586(18):4441-4452. doi: 10.1113/jphysiol.2008.155291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kitahara T, Takeda N, Saika T, Kubo T, Kiyama H. Role of the flocculus in the development of vestibular compensation: immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience. 1997;76(2):571-580. [DOI] [PubMed] [Google Scholar]
- 32.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407-415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Subjective Improvement of Dizziness of the Study Patients
eTable 2. Youden Index for Different Cutoff Points of ∆VOR and ∆DGI To Predict Subjective Improvement of Dizziness
eFigure. ROC Analysis for Using ∆VOR and ∆DGI to Predict Subjective Improvement of Dizziness