Key Points
Question
What is the maximum tolerated dosage of tucatinib combined with ado-trastuzumab emtansine in the treatment of patients with ERBB2/HER2-positive metastatic breast cancer with and without brain metastases?
Findings
In this phase 1b study of 57 patients with metastatic or unresectable locally advanced ERBB2/HER2-positive breast cancer treated previously with trastuzumab and a taxane, the maximum tolerated dosage of tucatinib combined with ado-trastuzumab emtansine was determined to be 300 mg administered orally twice daily; the objective response rate was 48%; and median progression-free survival was 8.2 months.
Meaning
Tucatinib in combination with ado-trastuzumab emtansine had acceptable toxicity and showed preliminary antitumor activity among heavily pretreated patients with ERBB2/HER2-positive metastatic breast cancer with and without brain metastases.
Abstract
Importance
Treatment options for patients with disease progression after treatment with trastuzumab, pertuzumab, and ado-trastuzumab emtansine (T-DM1) are limited. Tucatinib is an oral, potent, human epidermal growth factor receptor 2 (HER2)-specific tyrosine kinase inhibitor (TKI) being developed as a novel treatment for ERBB2/HER2-positive breast cancer.
Objective
To determine the maximum tolerated dosage of tucatinib in combination with T-DM1 in the treatment of patients with ERBB2/HER2-positive metastatic breast cancer with and without brain metastases.
Design, Setting, and Participants
In this phase 1b open-label, multicenter, clinical trial, 57 participants enrolled between January 22, 2014, and June 22, 2015, were 18 years of age or older with ERBB2/HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane. Data were analyzed between January and March 2018.
Interventions
Tucatinib 300 mg or 350 mg administered orally twice per day for 21 days and T-DM1 3.6 mg/kg administered intravenously once every 21 days.
Main Outcomes and Measures
Safety assessments, pharmacokinetics, and response were assessed using RECIST 1.1 every 2 cycles for 6 cycles, followed by every 3 cycles.
Results
Fifty-seven T-DM1-naive patients (median [IQR] 51 [44.0-63.0] years of age) who had undergone a median of 2 earlier HER2 therapies (range, 1-3) were treated. The tucatinib maximum tolerated dosage was determined to be 300 mg administered twice per day with dose-limiting toxic reactions seen at 350 mg twice per day. Pharmacokinetic analysis showed that there was no drug-drug interaction with T-DM1. Adverse events seen among the 50 patients treated at the maximum tolerated dosage regardless of causality included nausea (36 patients; 72%), diarrhea (30 patients; 60%), fatigue (28 patients; 56%), epistaxis (22 patients; 44%), headache (22 patients; 44%), vomiting (21 patients; 42%), constipation (21 patients; 42%), and decreased appetite (20 patients; 40%); the majority of adverse events were grade 1 or 2. Tucatinib-related toxic reactions that were grade 3 and above included thrombocytopenia (7 patients; 14%) and hepatic transaminitis (6 patients; 12%).
Conclusions and Relevance
In this study, tucatinib in combination with T-DM1 appeared to have acceptable toxicity and to show preliminary antitumor activity among heavily pretreated patients with ERBB2/HER2-positive metastatic breast cancer with and without brain metastases.
Trial Registration
ClinicalTrials.gov Identifier: NCT01983501
This clinical trial investigates the maximum tolerated dosage of tucatinib combined with ado-trastuzumab emtansine in the treatment of patients with ERBB2/HER2-positive metastatic breast cancer with and without brain metastases.
Introduction
Breast cancer is the most common cancer among women worldwide and is the second leading cause of cancer-related death in the United States.1,2 Approximately 20% of breast cancers overexpress human epidermal growth factor receptor 2 (HER2).3,4 Human epidermal growth factor receptor 2 (expressed by ERBB2/HER2) is a transmembrane tyrosine kinase receptor that mediates cell growth, differentiation, and survival. Cancers associated with overexpression of HER2 are more aggressive and, before the introduction of ERBB2/HER2-targeted agents, were associated with poorer overall survival compared with ERBB2/HER2 negative cancers.5
The introduction of antibody-based ERBB2/HER2-targeted therapies has led to significant improvements in outcomes in the neoadjuvant, adjuvant, and metastatic settings.6,7,8,9,10 Lapatinib, 1 of 2 currently approved tyrosine kinase inhibitors (TKIs), has shown modest benefit in the metastatic setting but is associated with epidermal growth factor receptor (EGFR) off-target toxic reactions, including rash and diarrhea.11 Similarly, neratinib, a pan-Erb TKI approved for use in the extended adjuvant setting, is also associated with EGFR-related toxic reactions.12
Treatment options for patients who experience disease progression after treatment with trastuzumab, pertuzumab, and T-DM1 are limited. Although there are no direct comparator trials, treatment guidelines currently recommend use of either lapatinib plus capecitabine, trastuzumab plus a cytotoxic chemotherapy, or lapatinib plus trastuzumab.4 Although lapatinib plus capecitabine is an approved regimen, it has not been studied in the setting of disease progression after earlier treatment with trastuzumab, pertuzumab, and T-DM1, nor has it been shown to improve overall survival.4 At this time, no single regimen is considered to be standard of care in this setting, and treatment options with better efficacy and improved tolerability for late-stage breast cancer are needed.
Tucatinib is an oral, potent, reversible HER2-specific TKI that is being developed as a novel treatment for ERBB2/HER2-positive breast cancer. Tucatinib selectively inhibits ERBB2/HER2, a feature that differentiates it from the other small-molecule ERBB2/HER2-targeted TKIs, all of which are dual inhibitors of both EGFR and ERBB2/HER2. Tucatinib is active as a single agent and in combination with either chemotherapy or trastuzumab in murine xenograft models of ERBB2/HER2-positive breast cancer, including intracranial tumor xenograft models.13
This study is an open-label multidose phase 1b trial of the combination of tucatinib and T-DM1 among T-DM1-naive patients with advanced metastatic breast cancer with or without brain metastases.
Methods
Study Design and Treatment
The primary objective of this open-label phase 1b study with dose-escalation and expansion cohorts was to determine the maximum tolerated dosage of tucatinib in combination with T-DM1. Secondary objectives were to determine the safety, tolerability, pharmacokinetics, and preliminary antitumor activity of this combination as assessed by objective response rate and progression-free survival. The institutional review boards of all participating sites approved the trial, which has been registered at ClinicalTrials.gov (NCT01983501). All patients provided written informed consent before the initiation of study-related treatment or procedures. The trial protocol is available in Supplement 1.
In the dose-escalation phase, a modified 3 + 3 dose-escalation design was used to determine the maximum tolerated dosage of tucatinib. On the basis of the results of the single-agent ARRAY-380-101 study, the starting dosage was selected as 300 mg twice daily using a tablet formulation.13 The design allowed at least 6 evaluable patients to be enrolled per tucatinib dosage level, with an expansion cohort at the maximum tolerated dosage. In addition to the maximum tolerated dosage expansion cohort, an additional brain metastases expansion cohort was enrolled that consisted of patients with either untreated or previously treated progressing brain metastases not requiring immediate central nervous system–directed therapy.
Dosage-limiting toxic reactions were defined as any adverse event during the first cycle not attributable to the patient’s disease in the dosage escalation cohort. The dosage-limiting toxic reactions included hematologic toxic reactions of grade 3 or greater, neutropenia with fever, grade 4 neutropenia for more than 7 days, grade 4 thrombocytopenia, grade 3 or greater thrombocytopenia associated with significant bleeding, and grade 4 anemia; nonhematologic toxic reactions of grade 3 or greater toxicity considered related to tucatinib or the combination; interruption of dosing for more than 2 weeks if secondary to an adverse event; a dose reduction because of a toxic reaction related to tucatinib or the combination; grade 4 hypokalemia; and grade 3 or greater elevation of hepatic transaminase levels (alanine aminotransferase [ALT] or aspartate aminotransferase [AST]) or bilirubin levels (irrespective of transaminase levels). Events not considered to be dosage-limiting toxic reactions were grade 3 fatigue (lasting 3 days or less); nausea, diarrhea, or vomiting without optimal use of antiemetics or antidiarrheals; and grade 3 rash without maximal use of corticosteroids or anti-infective agents.
Patient Population
Patients 18 years of age or older with progressive ERBB2/HER2-positive metastatic breast cancer previously treated with trastuzumab and a taxane were eligible for the study. HER2 positivity was documented by fluorescent in situ hybridization amplification or by 3+ immunohistochemistry staining using local assays. Other key inclusion criteria included evaluable lesions as defined according to RECIST 1.1, Eastern Cooperative Oncology Group performance status 0 or 1, adequate organ function, and normal left ventricular ejection fraction (LVEF). Key exclusion criteria included earlier exposure to doxorubicin at a dosage of more than 360 mg/m2; earlier treatment with T-DM1, neratinib, or afatinib; and the presence of leptomeningeal metastases. Patients with treated progressive brain metastases were excluded from the dosage escalation cohort; however, patients with treated stable brain metastases who did not require corticosteroids were included. Patients with untreated brain metastases or treated and progressive brain metastases not requiring immediate central nervous system–directed therapy were included in the brain metastases expansion cohort.
Statistical Analysis
This study was designed to assess the safety, tolerability, and maximum tolerated dosage of tucatinib given in combination with T-DM1. No formal statistical comparisons between dose cohorts were performed.
Demographic characteristics, baseline characteristics, adverse events, laboratory toxicities, and dosage-limiting toxicities were summarized among patients in the safety analysis set (ie, patients who received at least 1 dose of either tucatinib or T-DM1) using descriptive statistics. Progression-free survival, defined as the time from the date of the first dose of the study treatment to the date of documented disease progression or death from any cause, was evaluated among patients in the safety analysis set with use of Kaplan-Meier methods. Duration of response, defined as the time from the first objective response to documented disease progression, was evaluated among patients in the safety analysis set who had an objective response with use of Kaplan-Meier methods. Objective response, defined as achieving a best overall response of complete or partial response, was evaluated for assessable patients in the safety analysis set (ie, patients who had at least 1 identifiable target and/or nontarget lesion at baseline and had at least 1 postbaseline disease assessment). Patients were not followed-up to determine overall survival after completion of the study treatment.
Safety Assessments
Safety was monitored throughout the study, and assessments included all adverse events, dosage-limiting toxic reactions (in cycle 1 for dose escalation cohorts only), clinical laboratory parameters, electrocardiogram findings, Eastern Cooperative Oncology Group performance status, vital signs, physical examination findings, and LVEF. Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
Efficacy Assessments
Contrast computed tomography or magnetic resonance imaging scans of all areas of known disease were obtained at baseline, every 2 treatment cycles through cycle 6, and every 3 treatment cycles thereafter until disease progression, initiation of a new therapy, or withdrawal of consent. All patients underwent screening brain magnetic resonance imaging before the first study dose. Scans were locally assessed with RECIST 1.1 to determine the objective response rate. For patients with brain metastases, exploratory assessments that considered only lesions in the brain with use of modified RECIST 1.1 criteria were performed.14
Pharmacokinetic Assessments
Plasma and serum samples were collected to assess the association between combination treatment and the pharmacokinetics of both tucatinib and T-DM1. Levels of tucatinib and its metabolite as well as of the T-DM1 metabolite, DM1, were measured.15 On binding to HER2, T-DM1 is internalized intracellularly, and DM1 is presumably cleaved from the antibody by lysosomal enzymes. The DM1 can also be released from the antibody-drug conjugate by plasma proteases. Tucatinib pharmacokinetics were assessed at the start of treatment (cycle 1, day 1) and again at the beginning of cycle 2 (cycle 2, day 1). Trough tucatinib samples were collected on the first day of cycles 3 through 6. Samples to evaluate DM1 levels were collected before and after T-DM1 administration for the first 2 cycles, starting on cycle 1, day 2. In addition, samples were collected to assess DM1 levels on a weekly basis for the first 6 weeks.
Results
Patient Characteristics
Between January 22, 2014, and July 22, 2015, 57 patients (median age, 51 years [interquartile range, 44.0-63.0 years]; all patients were female) were enrolled at 11 sites in the United States and Canada. Data cutoff for the analysis was September 30, 2017, and data analysis was performed between January and March 2018. Fifty patients were treated at a dosage of 300 mg twice daily (including 8 patients treated in the initial dosage escalation cohort of 300 mg twice daily), 23 in the maximum tolerated dosage expansion cohort, and 19 in the brain metastases expansion cohort (Figure 1). Sixty percent of the patients (30 of 50 patients) treated at the combination maximum tolerated dosage had baseline brain metastases. Seven additional patients were enrolled and treated in the 350 mg twice daily dosage escalation cohort before that dosage level was declared dosage limiting. All patients received earlier trastuzumab, 46% (23 of 50 patients) received earlier pertuzumab, and 20% (10 of 50 patients) received earlier lapatinib. Patient characteristics are summarized in Table 1. Only a single patient treated in the 300-mg tucatinib arm had metastatic disease only in the bones.
Figure 1. Maximum Tolerated Dosage of Tucatinib Combined With Ado-Trastuzumab Emtansine (T-DM1) Trial Profile.
A flow diagram showing the progress of patients throughout the trial.
Table 1. Patient Characteristics.
| Characteristic | Patients, No. (%) | |
|---|---|---|
| 300 mg Twice Daily (n = 50) | 350 mg Twice Daily (n = 7) | |
| Age, median (range), y | 51 (30-72) | 51 (31-66) |
| Sex | ||
| Female | 50 (100) | 7 (100) |
| Race/ethnicity | ||
| White | 37 (74) | 6 (86) |
| Black/African American | 6 (12) | 0 |
| Asian | 6 (12) | 1 (14) |
| Unknown | 1 (2) | 0 |
| ECOG performance status | ||
| 0 | 20 (40) | 2 (29) |
| 1 | 30 (60) | 5 (71) |
| No. of previous HER2 agents, median (range) | 2 (1-3) | 1 (1-2) |
| Brain metastases | ||
| Yes | 30 (60) | 2 (29) |
| No | 20 (40) | 5 (71) |
| Most recent previous radiotherapy for brain metastases | ||
| Whole brain | 10 (20) | 2 (29) |
| Stereotactic radiosurgery | 9 (18) | 0 |
| None | 31 (62) | 5 (71) |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Dosage Escalation, Dosage-Limiting Toxicities, and Maximum Tolerated Dosage
The initial dosage level for tucatinib was 300 mg administered orally twice daily (tablet formulation), which is 50% of the maximum tolerated dosage for the tucatinib powder in capsule formulation.13 Additional dosage escalation cohorts were originally planned with 450 and 600 mg administered orally twice daily. However, pharmacokinetic data from the 300-mg cohort demonstrated that the maximum concentration (Cmax) and area under the curve (AUC) of the tablet formulation of tucatinib were indistinguishable from those of the powder in capsule formulation at the maximum tolerated dosage of 600 mg twice daily.13 Consequently, the dosage escalation schema was amended to include the dosages of 300, 350, and 400 mg twice daily of tucatinib.
A total of 4 patients experienced dosage-limiting toxic reactions (all grade 3) in the dose-finding phase (1 of 8 patients at the 300-mg dose level; 3 of 7 patients at the 350-mg dose level) (Table 2). Because of 3 dosage-limiting toxic reactions (drug hypersensitivity, fatigue, and vomiting) observed in 7 patients at the 350-mg dose level, the 300 mg administered orally twice daily dosage level was determined to be the maximum tolerated dosage.
Table 2. Dosage-Limiting Toxic Reactions Among the Dosage-Finding Cohorts.
| Event | Patients, No. (%) | |
|---|---|---|
| 300 mg Twice Daily (n = 8) | 350 mg Twice Daily (n = 7) | |
| Any dosage-limiting toxic reaction | 1 (13) | 3 (43) |
| Abnormal liver function test result | 1 (13) | 0 |
| Drug hypersensitivity | 0 | 1 (14) |
| Fatigue | 0 | 1 (14) |
| Vomiting | 0 | 1 (14) |
Safety and Tolerability
Treatment-emergent adverse events and tucatinib-related adverse events are summarized in eTable 1 in Supplement 2. The most commonly reported treatment-emergent adverse events (all grades) at the maximum tolerated dosage were nausea (36 of 50 patients [72%]), diarrhea (30 patients [60%]), fatigue (28 patients [56%]), epistaxis (22 patients [44%]), headache (22 patients [44%]), constipation (21 patients [42%]), thrombocytopenia (7 patients [14%]), vomiting (21 patients [42%]), and decreased appetite (20 patients [40%]). Adverse events assessed by the investigator to be related to tucatinib and reported for 20% or more patients receiving the maximum tolerated dosage were (in decreasing frequency) nausea, diarrhea, fatigue, increased AST levels, vomiting, decreased appetite, increased ALT levels, and hypokalemia.
eTable 1 in Supplement 2 shows the incidence of grade 3 or higher treatment-emergent adverse events and tucatinib-related adverse events. At the maximum tolerated dosage, the grade 3 or higher treatment-emergent adverse events reported for 10% or more of the patients (in decreasing frequency) were thrombocytopenia, hypokalemia, increased ALT levels, increased AST levels, fatigue, and hypophosphataemia. The tucatinib-related adverse events of grade 3 or higher that were reported for 10% or more of the patients were thrombocytopenia, increased ALT levels, and increased AST levels.
One patient died during the study owing to drowning, and 2 additional patients (4%) died owing to disease progression within 30 days after their last dose of tucatinib. Overall, 21 of 57 patients (37%) developed serious adverse events; in 6 patients, these adverse events were considered to be related to receipt of tucatinib. These adverse events were cardiac failure in 2 patients (both grade 1; asymptomatic decrease in LVEF) and fatigue, pyrexia and drug hypersensitivity, vomiting and hypokalemia, and pneumonia in 1 patient each.
Overall, 32 of 57 patients (56%) required interruption of tucatinib treatment. Tucatinib therapy was successfully reinitiated for 22 of 32 patients (69%), 18 of whom underwent a dosage reduction. Five patients discontinued tucatinib therapy because of an adverse event.
Elevation of hepatic transaminase levels (mostly grade 1) occurred in more than 70% of patients who were treated at the maximum tolerated dosage. In instances of grade 3 or 4 elevation of AST levels (7 of 57 patients [12%]) and/or ALT levels (8 of 57 patients [14%]), all elevated levels were reversible to baseline grade after administration of study drugs was interrupted, except in 2 cases where patients were documented to have progression of hepatic metastases. Two patients discontinued tucatinib treatment because of elevated transaminase levels: 1 patient with grade 4 elevation of AST levels discontinued the study as mandated by the protocol, and 1 patient had tucatinib administration interrupted because of grade 3 ALT level elevation and was subsequently found to have disease progression in the liver before tucatinib therapy was restarted.
Two patients experienced grade 1 cardiac failure. In 1 patient, the decrease in LVEF did not resolve on repeated testing, and the patient discontinued the study. In the second patient, the LVEF decrease resolved, and the patient restarted therapy with tucatinib but not with T-DM1 and did not have a recurrence of the event while participating in the study.
Pharmacokinetics
The mean steady state Cmax, median time to maximum concentration (Tmax), and median AUC over the dosing interval (AUCtau) for the 300-mg twice daily dosage were 790 ng/mL (SD, 329 ng/mL), 2 hours (range, 0-6 hours), and 4540 hours × ng/mL (range, 2600-7270 hours × ng/mL), respectively (eTable 2 in Supplement 2). At 350 mg twice daily, the mean Cmax, median Tmax, and median AUCtau were 1120 ng/mL (SD, 525 ng/mL), 1 hour (range, 1-3 hours), and 7120 hours × ng/mL (range, not calculated), respectively (eTable 2 in Supplement 2). The last time point for pharmacokinetic sampling was 6 hours, such that the AUCtau value was extrapolated. Pharmacokinetic data demonstrated that the mean plasma tucatinib concentrations and AUC were higher at the 350-mg twice daily dosage but with a similar Tmax, which suggests a slower elimination profile for the drug at a higher dosage. The exposure to tucatinib increased after repeated dosing, with an accumulation factor of approximately 1.5. The intersubject variability in pharmacokinetic parameters ranged between 29% and 47%. The mean plasma concentration of DM1, the metabolite of T-DM1, was low and in the nanograms per milliliter range, which is consistent with findings reported in the literature and indicates no association between tucatinib and DM1 drug levels.
Efficacy
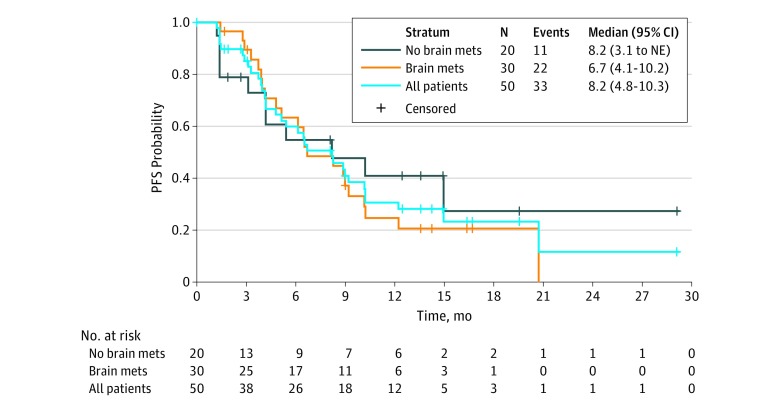
Fifty patients treated with tucatinib plus T-DM1 at the maximum tolerated dosage had a median progression-free survival of 8.2 months (95% CI, 4.8-10.3 months) (Figure 2). Among patients previously treated with both trastuzumab and pertuzumab (n = 20), the median progression-free survival was 6.5 months (95% CI, 4.1-9.2 months). Thirty-four of 50 patients (68%) treated with the maximum tolerated dosage had measurable disease and were evaluable for response with an objective response rate of 47% (1 patient with complete response, 15 patients with partial response, 14 patients with stable disease, and 4 patients with disease progression). The clinical benefit rate (patients with complete response, partial response, and stable response for >6 months) among 48 evaluable patients with postbaseline cancer scans was 58% (28 patients). Among patients whose disease responded to treatment, the median duration of response was 6.9 months (95% CI, 2.8-19.8 months).
Figure 2. Kaplan-Meier Plot of Progression-Free Survival (PFS) Among Patients Treated With the Maximum Tolerated Dosage of Tucatinib Combined With Ado-Trastuzumab Emtansine.
Abbreviations: BM, brain metastases; Mets, metastases; NBM, no brain metastases; NE, nonevaluable.
Thirty of 50 patients (60%) treated with the maximum tolerated dosage had brain metastases at study entry. Of these, 21 of 30 patients (70%) had either untreated or previously treated and progressive brain metastases. Median progression-free survival among patients with brain metastases was 6.7 months (95% CI, 4.1-10.2 months) with a median duration of overall response according to RECIST 1.1 of 6.9 months (95% CI, 1.45-19.48 months).
Activity in brain metastases was assessed using modified RECIST criteria (brain-specific criteria) considering only lesions in the brain. Brain metastases were measurable in 14 of 30 patients (47%). Among the 14 patients with measurable brain metastases, the brain-specific objective response rate was 36% (2 patients had complete response, 3 had partial response, 7 had stable disease, and 2 were nonevaluable).
Discussion
Although the use of ERBB2/HER2-targeted therapy has improved the outlook for patients with ERBB2/HER2-positive breast cancer, almost all patients with metastatic breast cancer ultimately experience disease progression. There is evidence that dual targeting of ERBB2/HER2, either through the combination of 2 different ERBB2/HER2-targeted antibodies or through an antibody-based therapy and a TKI, can lead to further improvements in efficacy.8 This study, combining T-DM1 and tucatinib, demonstrated the feasibility of the combination, which provided dual inhibition of ERBB2/HER2, potentially improving on the efficacy of single-agent therapy with T-DM1 through use of an alternative mechanism of receptor inhibition. The greater selectivity of tucatinib compared with other ERBB2/HER2-targeted TKIs offers the potential to provide dual HER2 blockade with potentially fewer toxic reactions than are associated with agents that also inhibit EGFR. A ERBB2/HER2-targeted TKI like tucatinib with brain metastases activity may also improve the treatment of brain metastases compared with antibody-based therapy.
The study established that the tucatinib tablet formulation maximum tolerated dosage was 300 mg administered orally twice daily, and this is the recommended dosage of tucatinib to be used in subsequent and ongoing clinical trials. Furthermore, pharmacokinetic analyses indicated no drug-drug interaction between tucatinib and T-DM1.
The combination of tucatinib and T-DM1 was well tolerated at the study-derived maximum tolerated dosage and schedule. Most toxic reactions encountered were attributable to T-DM1 and were consistent with those observed in earlier studies that used T-DM1 as monotherapy.9,16,17,18 Most adverse events were manageable with supportive therapy, interruption of therapy, or dosage reduction. Of note, no significant EGFR-related adverse events were observed, which is consistent with the selectivity for HER2 of tucatinib. Despite no preventative antidiarrheal regimen being used in this study, only 2 patients experienced grade 3 tucatinib-related diarrhea. Similarly, no cases of tucatinib-related rash were observed. The infrequency of EGFR-related toxic reactions seen with tucatinib and T-DM1 therapy makes this combination a potentially attractive treatment option.
Hepatotoxicity is observed with single-agent T-DM1 therapy as well as with tucatinib therapy. In this trial, hepatotoxicity was infrequent and reversible, which is consistent with reported single-agent studies of either agent. Cardiotoxicity, a known on-target adverse event associated with ERBB2/HER2-targeted agents, was uncommon in this study, and cardiotoxicity rates in this study were consistent with historical rates of cardiotoxicity associated with these agents.
The combination of tucatinib and T-DM1 demonstrated preliminary activity in pretreated patients with ERBB2/HER2-positive metastatic breast cancer as reflected by a median progression-free survival of 8.2 months (95% CI, 4.8-10.3 months) among patients treated with the maximum tolerated dosage. These results compare favorably with those of retrospective trials assessing response to T-DM1 therapy after multiple lines of ERBB2/HER2-based therapy, among which the median progression-free survival is approximately 6 months.18,19,20,21 The median progression-free survival among patients with brain metastases was 6.7 months (95% CI, 4.1-10.2 months), which is encouraging compared with other systemic therapies used to treat a similar patient population.22
Limitations
Nonetheless, data regarding activity among patients with brain metastases are based on a relatively small sample size. Moreover, most patients with brain metastases in the trial had either untreated brain metastases or progressive brain metastases, and such patients are typically excluded from clinical trials of systemic therapy in metastatic breast cancer. Up to 50% of patients with ERBB2/HER2-positive metastatic breast cancer will develop brain metastases during the course of their disease.23,24,25,26 The current standard therapy for brain metastases mostly uses central nervous system–directed therapy with surgery and/or radiation, both of which have the potential for treatment-related central nervous system injury that may impact cognition or elementary neurologic function, such as ambulation. There is no approved systemic regimen with significant activity in ERBB2/HER2-positive brain metastases. Several current ERBB2/HER2-targeted regimens have purported activity in brain metastases, including single-agent T-DM1; however, there are no randomized data to identify the most active systemic therapy among this patient population.27,28,29,30 A randomized trial of tucatinib in combination with T-DM1 is necessary to confirm the preliminary efficacy of this regimen among patients with brain metastases and to determine the added benefit of tucatinib in combination with T-DM1 among patients overall.
Conclusions
Tucatinib represents a promising new therapy with highly selective ERBB2/HER2 targeting that offers a favorable adverse event profile and demonstrates preliminary systemic and brain metastases activity when used in combination with standard-dosage T-DM1 for the treatment of ERBB2/HER2-positive metastatic breast cancer. Additional studies with tucatinib combinations are warranted.
Trial protocol.
eTable 1. Incidence of treatment-emergent and tucatinib-related adverse events.
eTable 2. Steady-state pharmacokinetic parameters of tucatinib.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. , eds. Surveillance, Epidemiology, and End Results (SEER) cancer statistics review, 1975-2012. Bethesda, MD: National Cancer Institute; 2015. https://seer.cancer.gov/csr/1975_2012/. Accessed July 9, 2017.
- 2.US Cancer Statistics Working Group United States Cancer Statistics: 1999–2009 Incidence and Mortality Web-based Report Atlanta, GA: Centers for Disease Control and Prevention and National Cancer Institute, US Dept of Health and Human Services; 2013. https://www.cdc.gov/cancer/npcr/pdf/uscs_factsheet.pdf. Accessed July 9, 2017.
- 3.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissues. Clin Breast Cancer. 2004;5(1):1214-1220. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Temin S, Kirshner JJ, et al. ; American Society of Clinical Oncology . Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(19):2078-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177-182. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. . Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792. [DOI] [PubMed] [Google Scholar]
- 7.Geyer CE, Forster J, Lindquist D, et al. . Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355(26):2733-2743. [DOI] [PubMed] [Google Scholar]
- 8.Baselga J, Cortés J, Kim SB, et al. ; CLEOPATRA Study Group . Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma S, Miles D, Gianni L, et al. ; EMILIA Study Group . Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Minckwitz G, Procter M, de Azambuja E, et al. ; APHINITY Steering Committee and Investigators . Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377(2):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Capri G, Chang J, Chen SC, et al. . An open-label expanded access study of lapatinib and capecitabine in patients with HER2-overexpressing locally advanced or metastatic breast cancer. Ann Oncol. 2010;21(3):474-480. [DOI] [PubMed] [Google Scholar]
- 12.Martin M, Holmes FA, Ejlertsen B, et al. ; ExteNET Study Group . Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700. [DOI] [PubMed] [Google Scholar]
- 13.Moulder SL, Borges VF, Baetz T, et al. . Phase I study of ONT-380, a HER2 inhibitor, in patients with HER2+-advanced solid tumors, with an expansion cohort in HER2+ metastatic breast cancer (MBC). Clin Cancer Res. 2017;23(14):3529-3536. [DOI] [PubMed] [Google Scholar]
- 14.Lin NU, Lee EQ, Aoyama H, et al. ; Response Assessment in Neuro-Oncology (RANO) group . Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16(6):e270-e278. [DOI] [PubMed] [Google Scholar]
- 15.Girish S, Gupta M, Wang B, et al. . Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody-drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69(5):1229-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diéras V, Miles D, Verma S, et al. . Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(6):732-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krop IE, Kim SB, González-Martín A, et al. ; TH3RESA study collaborators . Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(7):689-699. [DOI] [PubMed] [Google Scholar]
- 18.Krop IE, Kim SB, Martin AG, et al. . Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18(6):743-754. [DOI] [PubMed] [Google Scholar]
- 19.Fabi A, Giannarelli D, Moscetti L, et al. . Ado-trastuzumab emtansine (T-DM1) in HER2+ advanced breast cancer patients: does pretreatment with pertuzumab matter? Future Oncol. 2017;13(30):2791-2797. [DOI] [PubMed] [Google Scholar]
- 20.Vici P, Pizzuti L, Michelotti A, et al. . A retrospective multicentric observational study of trastuzumab emtansine in HER2 positive metastatic breast cancer: a real-world experience. Oncotarget. 2017;8(34):56921-56931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dzimitrowicz H, Berger M, Vargo C, et al. . T-DM1 activity in metastatic human epidermal growth factor receptor 2-positive breast cancers that received prior therapy with trastuzumab and pertuzumab. J Clin Oncol. 2016;34(29):3511-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laakmann E, Müller V, Schmidt M, Witzel I. Systemic treatment options for HER2-positive breast cancer patients with brain metastases beyond trastuzumab: a literature review. Breast Care (Basel). 2017;12(3):168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clayton AJ, Danson S, Jolly S, et al. . Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91(4):639-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldhirsch A, Gelber RD, Piccart-Gebhart MJ, et al. ; Herceptin Adjuvant (HERA) Trial Study Team . 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): an open-label, randomised controlled trial. Lancet. 2013;382(9897):1021-1028. [DOI] [PubMed] [Google Scholar]
- 25.Lin NU. Breast cancer brain metastases: new directions in systemic therapy. Ecancermedicalscience. 2013;7:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pestalozzi BC, Holmes E, de Azambuja E, et al. . CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013;14(3):244-248. [DOI] [PubMed] [Google Scholar]
- 27.Jacot W, Pons E, Frenel JS, et al. . Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res Treat. 2016;157(2):307-318. [DOI] [PubMed] [Google Scholar]
- 28.Bachelot T, Romieu G, Campone M, et al. . Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64-71. [DOI] [PubMed] [Google Scholar]
- 29.Lin NU, Diéras V, Paul D, et al. . Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452-1459. [DOI] [PubMed] [Google Scholar]
- 30.Freedman RA, Gelman RS, Wefel JS, et al. . Translational Breast Cancer Research Consortium (TBCRC) 022: a phase II trial of neratinib for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2016;34(9):945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol.
eTable 1. Incidence of treatment-emergent and tucatinib-related adverse events.
eTable 2. Steady-state pharmacokinetic parameters of tucatinib.